Quantitative and qualitative features of carotid and coronary atherosclerotic plaque among men and women
- 1Department of Radiology, Azienda Ospedaliero-Universitaria (A.O.U.), di Cagliari—Polo di Monserrato, Cagliari, Italy
- 2Department of Medical Sciences and Public Health, Università Degli Studi di Cagliari, Cagliari, Italy
- 3Department of Radiology, Fondazion Monasterio/CNR, Pisa, Italy
- 4Department of Radiology, San Luca Hospital, Istituto Auxologico Italiano IRCCS, University of Milano-Bicocca, Milan, Italy
- 5Stroke Diagnostic and Monitoring Division, AtheroPoint™, United States and Advanced Knowledge Engineering Centre, Global Biomedical Technologies Inc. (GBTI), Roseville, CA, United States
- 6Department of Radiology, Antwerp University Hospital, Antwerp, Belgium
- 7Experimental Imaging Center, Istituto di Ricovero e Cure a Carattere Scientifico (IRCCS) Ospedale San Raffaele, Milan, Italy
- 8School of Medicine, Vita-Salute San Raffaele University, Milan, Italy
Cardiovascular diseases (CVDs), particularly ischemic heart disease (IHD) and stroke, present epidemiologically in a different way among sexes. The reasons of these sex-based differences should be delved into sex-specific cardiovascular (CV) risk factors and different mechanisms of atherosclerotic progression. Imaging techniques of both carotid and coronary atherosclerotic plaques represent a tool to demonstrate sex-related features which might be used to further and better assess CV risk of male and female population. The aim of this review is to evaluate current knowledge on sex-specific qualitative and quantitative plaque features of coronary and carotid atherosclerosis. We also discuss the clinical implication of a sex-based plaque phenotype, evaluated with non-invasive imaging techniques, such as CT-angiography and MRI-angiography, to stratify CV risk.
Introduction: Patho-physiology of sex-related differences in atherosclerosis
Atherosclerosis is a progressive disease that affects arteries, characterized by the accumulation of lipids, macrophages, fibrous elements and smooth muscle cells within the vessel, forming the atherosclerotic plaque. Atherosclerosis is a chronic inflammatory condition that can lead to acute clinical events by plaque rupture and/or thrombosis, thus causing major events such as acute myocardial infarction (AMI), heart failure (HF), within the galaxy of ischemic heart disease (IHD), and stroke (1). These two cardiovascular diseases (CVDs) together represent the most common cause of death globally, with IHD and stroke responsible for 16 and 11% of the world’s total deaths, respectively, and that is why atherosclerosis is a condition of global interest (2, 3).
In the past decades, several studies have proven that atherosclerosis is strongly associated with several risk factors, such as hypertension, smoking, dyslipidemia, diabetes, obesity, age and family history, leading to the concept of vulnerable patient, at higher risk of developing acute CV events in the near future (4–6). Nonetheless, it is well known that CVDs presents differently among men and women: IHD in women develops after 7–10 years compared to men, in fact men are three times more likely to develop acute coronary syndromes (ACS) under the age of 60, while this tendency decreases with age and the likelihood among sexes equalizes over 75 years. With regards to cerebrovascular events, women over the age of 85 have a higher risk of developing stroke than men, leading to greater disability, mortality and case fatality in this group (7). Additionally, stroke is more likely to be the first manifestation of CVD in women, while IHD tends to be the first in men.
This knowledge has progressively led to the establishment of sex-specific CV risk assessment (8, 9), with sex referring to the biological characteristics of individuals as opposed to gender which indicates a broader concept rooted into society. Even though gender can influence health by reflecting the economic resources and healthcare access of the population, and despite the impossibility to define sex and gender in a binary way, the literature offers very few examples of non-binary trials or gender-related studies (10, 11). Thus, in this review we will use the term sex, but we emphasize its limitations.
Regarding sex-specific CV risk-factors, attention should be paid also to the modern role of women in the Western society: nowadays working patterns and activities are similar among sexes, but with women often having family responsibilities on top of time- and energy-consuming working roles. This joint social burden led to increased psychosocial stressors which further increase CV risk (12). Thus, modern female lifestyle and under-recognized risk factors, such as anxiety, depression, physical and psychological abuse, socioeconomic status and health literacy should be taken into consideration when adopting CV risk prevention strategies, especially during and after Sars-CoV-2 pandemic which exacerbated these risk factors (13).
The mechanisms underlying sex as a variable in atherosclerosis are constantly under-study, but the main finding is that until menopause women are protected by estrogens which seem to have an athero-protective role. In particular, estrogens have a pluripotent effect on the cardiovascular system, affecting the endothelium, coagulation, inflammation and adhesion (14). 17beta-estradiol (E2) is the most common form of circulating estrogen and by binding with estrogen receptors (ERs) it triggers a signaling cascade that alter gene expression affecting atherogenesis: For example, in hepatocytes the ER signaling activation is crucial to reverse cholesterol transport and protects against lipid accumulation in women. Furthermore, ER inhibits the proliferation of vascular smooth muscle cells in case of high level of glucose and, considering that those cells are a source of reactive oxygen species (ROS), which advances atherosclerosis, estrogen-ER complex performs its protective role. Finally, ER decreases differentiation of vascular muscle cells in osteoblastic-like cells, thus reducing occurrence of calcification within atherosclerotic lesions (15).
The important role of sex hormones also drove attention to sex hormone-binding globulin (SHBG), a protein that binds to and regulates available testosterone and estradiol. SHBG seems to be a potential risk stratification tool for predicting CV risk. In particular, there is an inverse association between serum SHBG levels and vascular risk factors (insulin resistance, diabetes, metabolic syndrome for example) and outcomes (IHD and stroke), which might be linked to either high free testosterone with consequent downstream pro-androgenic effects, or activation of inflammatory pathways (16, 17).
Moreover, proteomic studies have shown that female and male endothelial cell secretome responds differently to cellular stress induced by the same injury. Endothelial cells seem to adopt different strategies: in male more commonly apoptosis, while in female cells autophagy. Among the proteins secreted during apoptosis, PTX3 was found to have a crucial role in male-specific endothelial response to stressors. These results suggest a novel mechanism for sex-specific pathophysiological responses and identify PTX3 as a possible pharmacological target, considering its role as regulator of pro- and anti-inflammatory signals at the vascular bed, which should be further studied (18).
Female-specific risk factors, both modifiable and not, should also be taken into consideration. Adverse pregnancy outcomes (i.e., gestational hypertension, preterm delivery, preeclampsia/eclampsia) are associated with increased long-term CV risk of the mother and the mechanism seems to be an altered inflammatory state which affects maternal vasculature (19, 20). Lifetime estrogen exposure, early menarche and short reproductive life span in particular, represent another unmodifiable risk factor, as shown in a recent meta-analysis that highlights how a reproductive life span < 33 years is associated with higher rate of CVD events in midlife (21). Among the modifiable female-specific risk factors use of combined oral contraceptive and menopausal hormone therapy play an important role that is still under study (22). Even though systemic autoimmune disease is not a sex-specific risk factor, it should be taken into consideration that females are disproportionally affected, accounting for the 78% of patients (23). On one hand, chronic inflammation caused by autoimmune disease is associated with endothelial dysfunction and atherosclerosis progression, on the other hand steroid used to treat these conditions worsen hyperglycemia and dyslipidemia (24).
Clinical and pathological evaluation has revealed that males develop atherosclerotic plaque earlier, with the atherogenesis starting at a younger age in men than women; intima-media thickness (IMT) is usually greater in men until the age of 75, when we assist to the late catch-up phenomenon in women. Plaque inflammatory state seems to be greater in males than females and more vulnerable/unstable features are seen in non-invasive imaging of the male population. Moreover, plaque burden is greater in males while individual stenosis is greater in females, but plaque burden, associated with vulnerability, better predicts adverse ischemic events (25).
In this setting, non-invasive imaging techniques such as ultrasound, computed tomography angiography (CTA) and magnetic resonance angiography (MRA) can provide a useful tool to analyze plaque features among men and women. Thus, the aim of this review is to evaluate the current knowledge on sex-related differences of atherosclerosis in order to better stratify CV risk and target a preventive and effective therapy to the sub-population.
State of the art: Quantitative and qualitative aspects of carotid and coronary atherosclerotic plaque among men and women
Sex-related features in coronary atherosclerosis
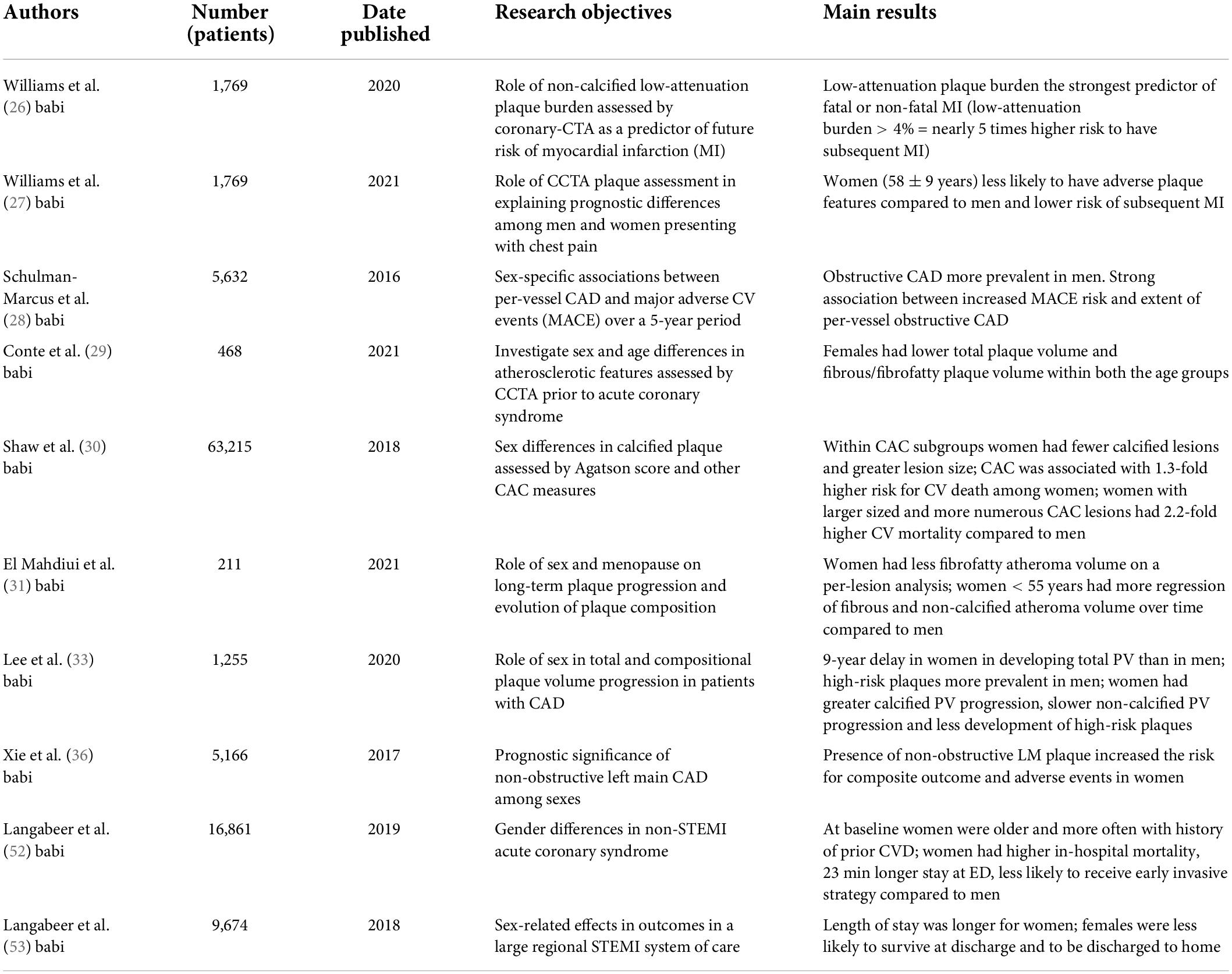
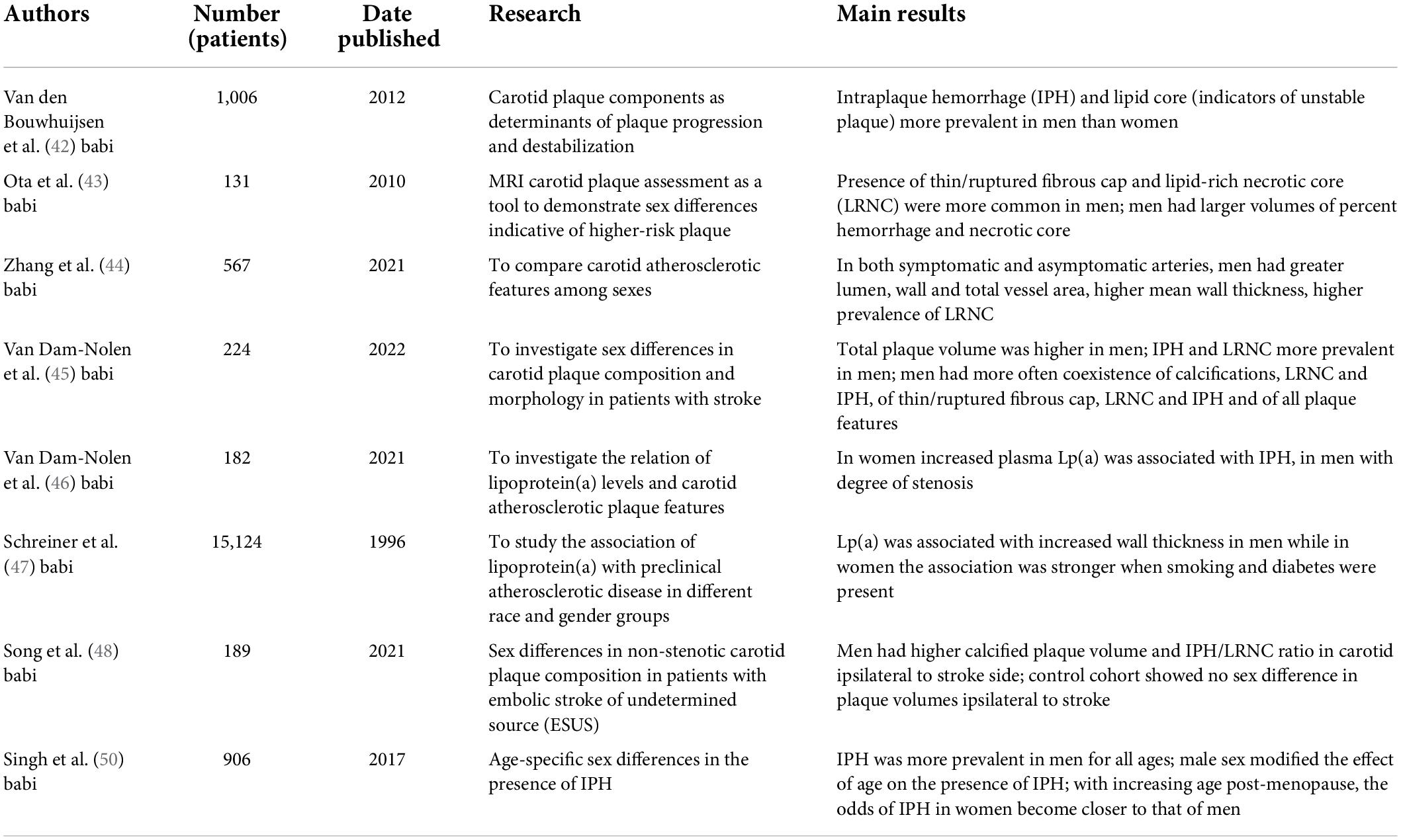
CTA has proven to be a valid technology that can easily identify patients at risk of subsequent CV events, and it can guide treatment management, improving outcome (Table 1). Recent studies have shown that a quantitative analysis of atherosclerotic plaque burden on cardiac-CTA (CCTA) can stratify the risk of future events better than traditional CV risk factors, coronary artery calcification (CAC) and coronary artery stenosis severity (26). In particular, low-attenuation plaque burden proved to be the strongest predictor of fatal or non-fatal myocardial infarction and with a low-attenuation plaque burden greater than 4% the risk of having a myocardial infarction is 5 times higher. Thus, CCTA adds important prognostic information but whether this assessment has an equal prognostic value in men and women is still under study. In fact, as Williams et al.(27) hypothesized based on the SCOT-HEART multicenter randomized controlled trial, sex differences in CAD may be explained by CT plaque assessment, which included evaluation of adverse plaque characteristics: positive remodeling, low-attenuation plaque, spotty calcification and the “napkin ring” sign (Figure 1). They discovered that women had lower CAC score, less frequent adverse plaque features, less obstructive CAD and overall lower quantitatively assessed plaque burden, leading to fewer subsequent MI compared to men. These findings, in agreement with the CONFIRM registry (28) and the ICONIC (29) study results, suggest that, while women presenting with stable chest pain have a specific, sex-based, plaque phenotype, symptomatic patients presenting with ACS have no sex difference in quantitative plaque features in culprit lesions. Indeed, there was no difference in necrotic core volume despite women having lower fibrous/fibrofatty plaque volume compared to men. Thus, even though women with stable angina presents with a different set of plaque features, when it comes to culprit lesions causing MI, both sexes seem to have similar plaque characteristics. Recent studies also showed that, within a given CAC score group, women tend to have smaller number, but larger size and density of calcified plaques compared to men, reflecting a more advanced atherosclerotic state/higher levels of inflammation, in line with less frequent obstructive disease but higher CVD mortality among women (mean age: 56.2 years) compared with men (30) (Figure 2).
Figure 1. Thirty-six years old male showing fibrofatty plaque with positive remodeling on mid-lef anterior descending artery (arrow, A). Plaque analysis confirmed the fibrofatty composition of coronary plaque demonstrating the positive remodeling (arrowhead, B,C).
Figure 2. Eighty-five years old male showing mixed coronary plaque on proximal left anterior descending artery (arrow, A). Composition of plaque was composed by calcium (arrow, B,C) and fibrofatty portion (arrowhead B,C).
Additionally, El Mahdiui et al. (31) in their study evaluated plaque composition by serial CTA taking into consideration the influence of menopause. What they demonstrated is that men had more fibrofatty percentage atheroma volume (PAV), but pre-menopausal women, younger than 55 years, had greater regression of fibrous and non-calcified PAV over time compared to men of the same age, and given the positive association between non-calcified plaques with ischemia and ACS, these findings are in agreement with the lower risk of symptomatic CAD in young women (32). On the contrary, the lack of this regression in post-menopausal women suggests a progressive match to the non-calcified PAV of men with consequent increased risk of CAD in accordance with epidemiological data.
The PARADIGM study (33), a dynamic multinational observational registry of patients who underwent clinically indicated serial CCTA, also showed that total and compositional plaque volume (PV) progression rate differed among sexes: women had lower overall atherosclerotic burden in all age groups, reduced development of high-risk plaques and a PV progression mainly driven by calcified PV (faster calcified and slower non-calcified progression) while men had more non-calcified PV progression. The study demonstrated a 9-year delay in women for developing the total coronary atherosclerotic burden of men. The protective role of estrogen, until menopause, on development and progression have been suggested, especially taken into consideration the inhibitory effect of estrogen on vascular calcification (34). However, estrogen influence seems to be over-taken by the presence of risk factors resulting in worse outcome and prognosis for young women presenting with acute coronary syndrome compared to young men (35).
Finally, Xie et al. (36) found that non-obstructive left-main plaque was associated with 50% higher risk for adverse events among women, independently of CAD burden in other vessels, whereas this association was not significantly present among men; similarly, they found that women had a risk for future events 1.8-fold higher than men. Finding these sex-specific differences in prognosis provides an important risk and prognostic marker that should be considered during risk stratification of women. Thus, when evaluating prognosis, location of non-obstructive plaque should be considered.
New insights have been given by arterial and plaque characterization through intravascular ultrasound (IVUS), particularly concerning endothelial shear stress (ESS) which, with lower values, induces endothelial cell dysfunction and plaque progression. As Wentzel et al. (37) suggested in their study, which analyzed data from the PREDICTION study, coronary arteries and plaques were significantly smaller in females compared to males but ESS and ESS-related plaque progression were similar in both sexes. Only after stratifying for age they found that ESS-related plaque growth was more marked in women < 55 years and that female population showed a continuous reduction in magnitude of ESS-dependent plaque progression until 75 years of age, partially explaining the “catch up phenomenon” in female atherosclerosis.
Sex-related features in carotid atherosclerosis
Epidemiology tells us that men have higher lifetime risk of stroke than women, but if women suffer from a stroke it is usually more severe, leading to greater disability (38). These epidemiological dissimilarities may have root in a sex-based pattern of carotid atherosclerosis, which is constantly under study especially through plaque imaging, including ultrasound and CTA, but mainly MRI (Table 2). It is well known that imaging biomarkers of vulnerability exist and that they predict stroke risk: intraplaque hemorrhage (IPH), best seen with MRI (39) but recently studied with CT as well (40); lipid-rich necrotic core (LRNC) either on CT and MRI; thin-rupture fibrous cap (TRFC), with contrast-enhanced MRI; carotid plaque thickness and surface morphology with all three imaging modalities; plaque volume with CT because of its high spatial resolution (41). These imaging features of vulnerability present differently among men and women. Indeed, men tend to have vulnerable plaques more frequently than women, as seen in the Rotterdam study (42) where they demonstrated that in a population with carotid wall thickening, IPH and LRNC, indicating plaque vulnerability, are highly frequent and more prevalent in men (Figure 3).
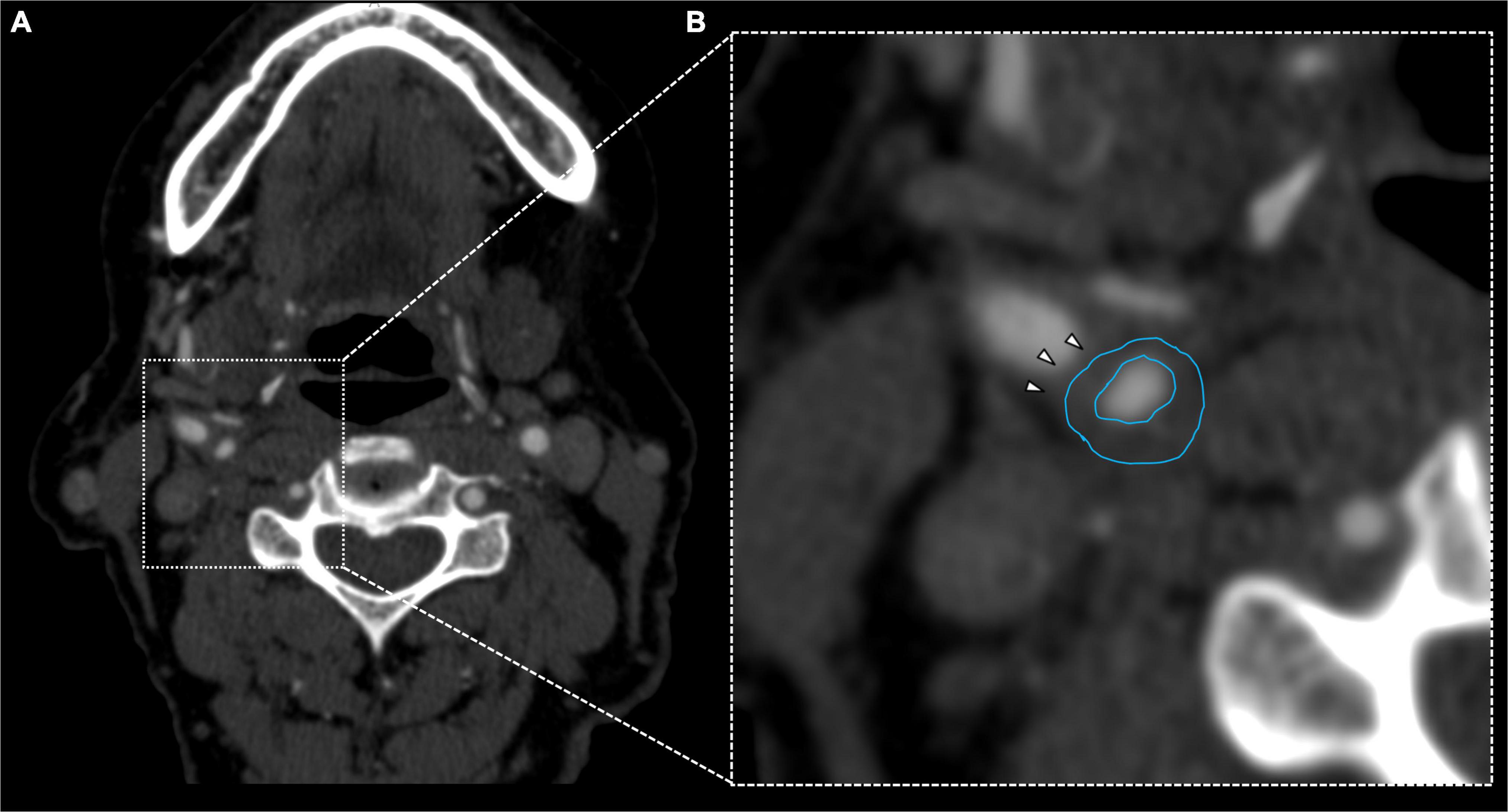
Figure 3. Fifty-six years old female showing fibrofatty plaque with concentric remodeling on right ICA (A). Plaque analysis confirmed the fatty composition of the plaque (B, white arrowheads).
Similarly, Ota et al. (43) assessed plaque characteristics in asymptomatic patients using 3-T MRI and they discovered that men present more frequently with LRNC, TRFC, larger percent volume of lipid core and IPH compared to women. With this features, atherosclerotic plaque is more prone to rupture, leading to subsequent ischemic events: hence, in their study Ota et al. suggested that 3-T carotid plaque MRI can identify high-risk phenotype and patients, and that their findings are consistent with epidemiological data, explaining why men age < 75 years have higher incidence of stroke than women.
Recently, in a study by Zhang et al. (44), both asymptomatic and symptomatic patients underwent 3-T carotid MRI. While in symptomatic carotid arteries, men showed similar plaque burden, despite larger vessel size, higher prevalence of LRNC and IPH compared to women, in asymptomatic carotid arteries men had more vulnerable features, such as higher percent LRNC and TRFC. Their findings are in agreement with the previous studies, indicating that asymptomatic male population tend to have vulnerable plaque features more frequently than women while plaque burden is similar in both sexes.
New insights have been given by the Plaque At Risk (PARISK) study (45), a prospective multicenter cohort study of patients with a high recurrent stroke risk with < 70% carotid stenosis. In particular, van Dam-Nolen et al. (46) examined patients with recent ischemic cerebrovascular symptoms and ipsilateral carotid stenosis < 70%, who underwent carotid MRI and CTA. They confirmed that men tend to have larger plaque volume, more vulnerable plaque composition (IPH, LRNC, TRFC) and more frequent coexistence of vulnerable features. They also suggested that sex-based plaque differences may not be related just to larger plaque burden in men: in fact, after adjusting the findings for total plaque volume, the prevalence of IPH and LRNC remained higher in men than in women, while the difference in IPH and LRNC volume disappeared, indicating that plaque burden does not fully explain the sex differences in carotid atherosclerosis and that sex plays an important role in the development of a vulnerable plaque rather than in the size of its components. This knowledge may lead to a sex-specific management of stroke and transient ischemic attack, with men benefitting from a carotid endarterectomy more than women (45).
Moreover, another work of van Dam-Nolen et al. (46) on the PARISK study, further evaluated the known importance of lipoprotein(a) as an independent risk factor for CVD and recurrent stroke (47). Not only they confirmed the positive association between Lp(a) levels and atherosclerosis, but also they suggested that Lp(a) distribution, as well as carotid plaque composition, differ between men and women and that Lp(a) levels peak during late perimenopause/postmenopause. Notably, they identified new associations between Lp(a) concentrations and plaque features: in male population elevated Lp(a) levels were associated with higher degree of stenosis, whilst in female population high Lp(a) levels tend to be associated with higher prevalence of IPH. This difference suggests that Lp(a) levels in women might be a stronger risk indicator for developing severe carotid atherosclerosis.
Additionally, Song et al. (48) examined sex differences in non-stenotic carotid plaque composition in patients with embolic stroke of undetermined source (ESUS) who underwent CTA. What they discovered is that in the atrial fibrillation cohort, used as control population, there was no significant sex difference in plaque volume and features, while among the ESUS cohort men had significantly higher IPH volume and IPH/LRNC ratio ipsilateral to stroke side compared to women, suggesting a differential contribution of atheroembolism from carotid plaque among men and women presenting with ESUS. Furthermore, they suggested IPH/LRNC ratio as a possible predictor of plaque rupture, stronger than LRNC alone, which may not always progress to rupture. In fact, IPH and increased neovascularity within a lipid-rich necrotic core can facilitate inflammation and core expansion, leading to greater rupture risk. Similarly, Saba et al. (49) observed that the ratio between IPH, indicated by Hounsfield units < 25 on CTA, and lipid volume is significantly associated with cerebrovascular events, hence representing a strong parameter for future events. Another study, by Singh et al. (50), also revealed that in patients with low-grade carotid stenosis the presence of IPH occurs more frequently with age, affecting less than 1% of patients before the age of 55 until a maximum of 12% by the age of 75 years, and with sex, affecting more men than women. An interesting result of this study was also the absence of IPH in females before the age of 65 years, which was progressively replaced with age, indicating that sex modifies the effect of age on carotid IPH. Hence, the infrequency of IPH in women before the age of 75 might explain the lower incidence of stroke among women of this age group when compared to men.
Discussion
Atherosclerosis differs among sexes, both from a physio-pathological point of view and clinically as well. Epidemiology shows that women presents with IHD after an average of 7–10 years compared to men; men under the age of 60 are three times more likely to present with ACS, ST-segment elevation myocardial infarction (STEMI) and NSTEMI (51); while this tendency decreases over 75 years, we see an increase of morbidity and mortality rate in women, with 7.4% in-hospital mortality for STEMI in women vs. 4.6% in men and 4.8 vs. 3.9%, respectively, for NSTEMI (52, 53). Moreover, women over the age of 85 have a higher risk of developing stroke than men, with 55,000 more females having a stroke than males every year, indicating that women might be treated less aggressively in primary and secondary prevention for this disease (7). Other data from the Heart Disease and Stroke Statistics—2022 Update (54) revealed that stroke is more prevalent in men until the age of 80 years, but over 80 this tendency inverts, and that more females than men die of stroke each year, indicating that the cause might be this higher prevalence in elderly women.
What the literature reveals is that these epidemiological disparities have a physio-pathological counterpart which can be studied histologically and with invasive methods, but also with non-invasive techniques. In this context, radiology with CTA, MRA and ultrasound provides useful tools that may be used not only for academic purposes but also for clinical ones, giving information regarding plaque characteristics that can be linked to outcome and prognosis (Table 3). Indeed, CTA provides information regarding plaque features of vulnerability (positive remodeling, low-attenuation plaque, spotty calcification, “napkin ring” sign) and thus prognosis. In fact, low-attenuation plaque volume (26) and IPH/LRNC ratio (49) appeared to be two strong prognostic markers that can predict fatal and non-fatal future MI the former, and cerebrovascular events the latter. In addition, through CCTA it is possible to understand the female-specific coronary plaque phenotype, which partially explains the epidemiological data mentioned above. Women before menopause tend to have lower CAC score, less high-risk plaque features, less plaque volume, less obstructive CAD (27) and greater fibrous and non-calcified PAV regression (31). After menopause, the literature seems to identify a different female-specific coronary plaque phenotype, which tends to be more similar to that one of men: Less non-calcified PAV regression (31) and smaller number but larger size and higher density of calcified plaque (30), progressively matching, with a 9-year delay, the total coronary atherosclerotic burden of men (33). Additionally, taken into consideration that non-calcified PV seems to be influenced mainly by environmental factors, while CAC and calcified PV are linked to genetics (55), CCTA might be a useful tool to early detect non-calcified plaque in order to start an early lifestyle intervention, thus preventing coronary plaque formation.
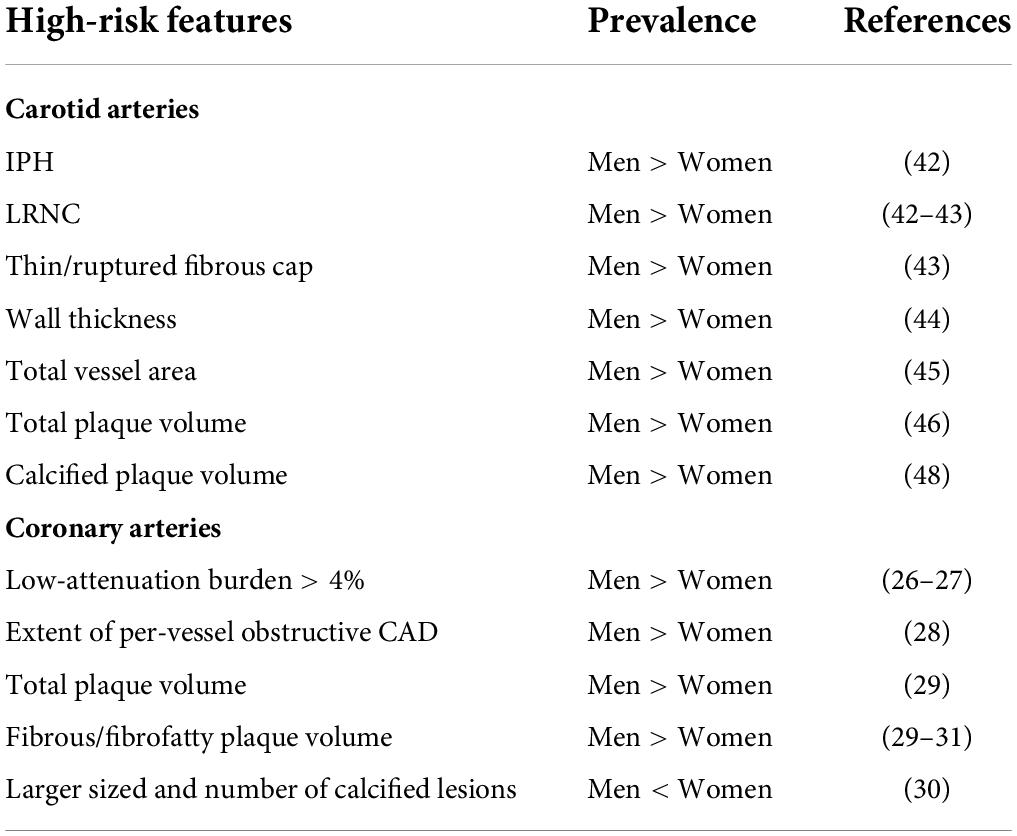
Table 3. Difference between men and women of high-risk plaque features in carotid and coronary arteries.
Notably, the literature (27) shows that women presents with this specific, sex-based plaque phenotype more evidently when they are asymptomatic or in case of stable angina. Indeed, the above-mentioned sex-based features tend to flatten when ACS occurs, and no significant difference among men and women can be found in culprit lesions causing MI. The necrotic core volume is in fact similar among sexes. This finding might suggest the role of CCTA for risk stratification, before ACS occur, and the importance of primary prevention in order to treat aggressively those women presenting with high-risk features or markers of worse prognosis. With this regard, an interesting insight has been given by Xie et al. (36) who suggested that location of CAD should be assessed with CCTA in order to stratify the risk of future events in women: particularly, they found that non-obstructive left main plaque has a great prognostic implication, with a 50% higher risk for females of having adverse events, independently from CAD burden in other vessels.
Finally, a female-specific plaque phenotype can be seen in CT/MRI evaluation of carotid atherosclerosis as well. Women tend to have less features of vulnerability compared to men: less IPH, LRNC and calcified PV in particular, in accordance with a less frequent tendency to rupture compared to male carotid plaques (42, 43). Thus, CT and MRI represent valid techniques that can detect high-risk features, in order to discriminate which group of patients might benefit from an aggressive primary prevention therapy. Moreover, the ratio of IPH/LRNC seems to be a predictive marker of future plaque rupture and consequent cerebrovascular events, and various studies showed that the ratio is usually higher in the male population (48, 49). Ultrasound is worth mentioning because of its availability and cost feasibility; in addition, it offers accurate and reproducible imaging evaluation of lumen and vessel wall, particularly IMT. However, it has a low sensitivity and specificity for detection of other plaque features, such as fibrous cap, ulceration, plaque inflammation, IPH and LRNC (56). Ultrasound does not distinguish IPH and LRNC because they both appear hypoechogenic, making it difficult to evaluate the ratio, and it is affected by calcified components that create acoustic shadow. For these reasons, ultrasound is not useful for the assessment of most quantitative measurements of carotid plaque and it is not considered the imaging modality of choice (41).
As suggested by Nasir et al. (57) the question is how and if this information will modify medical management, especially if the assessment of plaque phenotype will lead to consideration of a lipid-lowering therapy of various intensities and/or whether the presence of high-risk plaque characteristics will influence the decision-making process of starting antiplatelet treatment.
Further studies should be carried forward in order to fully comprehend how these insights can impact the clinical management of atherosclerosis in men and women, but what we know so far from the literature is that there are undeniable differences among sexes in atherosclerotic presentation, that these phenotypes can be successfully evaluated throughout non-invasive imaging techniques and that this type of plaque assessment should be strengthened and enhanced because of its prognostic implication.
Author contributions
LS, CCD, and AE contributed to the conception and design of the study. CO and FCa wrote the first draft of the manuscript. GM, SA, JS, FC, SS, and RS wrote sections of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
Conflict of interest
JS was employed by company Global Medical Technologies Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
4. Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation. (2003) 108:1664–72. doi: 10.1161/01.CIR.0000087480.94275.97
5. Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part II. Circulation. (2003) 108:1772–8. doi: 10.1161/01.CIR.0000087481.55887.C9
6. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. (2004) 364:937–52. doi: 10.1016/S0140-6736(04)17018-9
7. Persky RW, Turtzo LC, McCullough LD. Stroke in women: Disparities and outcomes. Curr Cardiol Rep. (2010) 12:6–13. doi: 10.1007/s11886-009-0080-2
8. SCORE2 Working Group and ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. (2021) 42:2439–54. doi: 10.1093/eurheartj/ehab309
9. Maffei S, Guiducci L, Cugusi L, Cadeddu C, Deidda M, Gallina S, et al. Women-specific predictors of cardiovascular disease risk – new paradigms. Int J Cardiol. (2019) 286:190–7. doi: 10.1016/j.ijcard.2019.02.005
10. Pelletier R, Khan NA, Cox J, Daskalopoulou SS, Eisenberg MJ, Bacon SL, et al. Sex versus gender-related characteristics: Which predicts outcome after acute coronary syndrome in the young? J Am Coll Cardiol. (2016) 67:127–35. doi: 10.1016/j.jacc.2015.10.067
11. Norris CM, Johnson NL, Hardwicke-Brown E, McEwan M, Pelletier R, Pilote L. The contribution of gender to apparent sex differences in health status among patients with coronary artery disease. J Womens Health (Larchmt). (2017) 26:50–7. doi: 10.1089/jwh.2016.5744
12. Sciomer S, Moscucci F, Maffei S, Gallina S, Mattioli AV. Prevention of cardiovascular risk factors in women: The lifestyle paradox and stereotypes we need to defeat. Eur J Prev Cardiol. (2019) 26:609–10. doi: 10.1177/2047487318810560
13. Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, et al. The lancet women and cardiovascular disease commission: Reducing the global burden by 2030. Lancet. (2021) 397:2385–438. doi: 10.1016/S0140-6736(21)00684-X
14. Baker L, Meldrum KK, Wang M, Sankula R, Vanam R, Raiesdana A, et al. The role of estrogen in cardiovascular disease. J Surg Res. (2003) 115:325–44. doi: 10.1016/s0022-4804(03)00215-4
15. Aryan L, Younessi D, Zargari M, Banerjee S, Agopian J, Rahman S, et al. The role of estrogen receptors in cardiovascular disease. Int J Mol Sci. (2020) 21:4314. doi: 10.3390/ijms21124314
16. Madsen TE, Luo X, Huang M, Park KE, Stefanick ML, Manson JE, et al. Circulating SHBG (sex hormone-binding globulin) and risk of ischemic stroke: Findings from the WHI. Stroke. (2020) 51:1257–64. doi: 10.1161/STROKEAHA.120.028905
17. Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, Cook NR, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. (2003) 108:1688–93. doi: 10.1161/01.CIR.0000091114.36254.F3
18. Cattaneo MG, Banfi C, Brioschi M, Lattuada D, Vicentini LM. Sex-dependent differences in the secretome of human endothelial cells. Biol Sex Differ. (2021) 12:7. doi: 10.1186/s13293-020-00350-3
19. Søndergaard MM, Hlatky MA, Stefanick ML, Vittinghoff E, Nah G, Allison M, et al. Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women. JAMA Cardiol. (2020) 5:1390–8. doi: 10.1001/jamacardio.2020.4097
20. O’Kelly AC, Michos ED, Shufelt CL, Vermunt JV, Minissian MB, Quesada O, et al. Pregnancy and reproductive risk factors for cardiovascular disease in women. Circ Res. (2022) 130:652–72. doi: 10.1161/CIRCRESAHA.121.319895
21. Rexrode KM, Madsen TE, Yu AYX, Carcel C, Lichtman JH, Miller EC. The impact of sex and gender on stroke. Circ Res. (2022) 130:512–28. doi: 10.1161/CIRCRESAHA.121.319915
22. Mishra SR, Chung HF, Waller M, Dobson AJ, Greenwood DC, Cade JE, et al. Association between reproductive life span and incident nonfatal cardiovascular disease: A pooled analysis of individual patient data from 12 studies. JAMA Cardiol. (2020) 5:1410–8. doi: 10.1001/jamacardio.2020.4105
23. Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol. (2008) 173:600–9. doi: 10.2353/ajpath.2008.071008
24. Young L, Cho L. Unique cardiovascular risk factors in women. Heart. (2019) 105:1656–60. doi: 10.1136/heartjnl-2018-314268
25. Man JJ, Beckman JA, Jaffe IZ. Sex as a biological variable in atherosclerosis. Circ Res. (2020) 126:1297–319. doi: 10.1161/CIRCRESAHA.120.315930
26. Williams MC, Kwiecinski J, Doris M, McElhinney P, D’Souza MS, Cadet S, et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: Results from the multicenter SCOT-HEART trial (Scottish computed tomography of the heart). Circulation. (2020) 141:1452–62. doi: 10.1161/CIRCULATIONAHA.119.044720
27. Williams MC, Kwiecinski J, Doris M, McElhinney P, D’Souza MS, Cadet S, et al. Sex-Specific computed tomography coronary plaque characterization and risk of myocardial infarction. JACC Cardiovasc Imaging. (2021) 14:1804–14. doi: 10.1016/j.jcmg.2021.03.004
28. Schulman-Marcus J, Hartaigh BÓ, Gransar H, Lin F, Valenti V, Cho I, et al. Sex-specific associations between coronary artery plaque extent and risk of major adverse cardiovascular events: The CONFIRM long-term registry. JACC Cardiovasc Imaging. (2016) 9:364–72. doi: 10.1016/j.jcmg.2016.02.010
29. Conte E, Dwivedi A, Mushtaq S, Pontone G, Lin FY, Hollenberg EJ, et al. Age- and sex-related features of atherosclerosis from coronary computed tomography angiography in patients prior to acute coronary syndrome: Results from the ICONIC study. Eur Heart J Cardiovasc Imaging. (2021) 22:24–33. doi: 10.1093/ehjci/jeaa210
30. Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: Observations from the CAC Consortium. Eur Heart J. (2018) 39:3727–35. doi: 10.1093/eurheartj/ehy534
31. El Mahdiui M, Smit JM, van Rosendael AR, Neglia D, Knuuti J, Saraste A, et al. Sex differences in coronary plaque changes assessed by serial computed tomography angiography. Int J Cardiovasc Imaging. (2021) 37:2311–21. doi: 10.1007/s10554-021-02204-4
32. Chang HJ, Lin FY, Lee SE, Andreini D, Bax J, Cademartiri F, et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol. (2018) 71:2511–22. doi: 10.1016/j.jacc.2018.02.079
33. Lee SE, Sung JM, Andreini D, Al-Mallah MH, Budoff MJ, Cademartiri F, et al. Sex differences in compositional plaque volume progression in patients with coronary artery disease. JACC Cardiovasc Imaging. (2020) 13:2386–96. doi: 10.1016/j.jcmg.2020.06.034
34. Osako MK, Nakagami H, Koibuchi N, Shimizu H, Nakagami F, Koriyama H, et al. Estrogen inhibits vascular calcification via vascular RANKL system: Common mechanism of osteoporosis and vascular calcification. Circ Res. (2010) 107:466–75. doi: 10.1161/CIRCRESAHA.110.216846
35. Chandrasekhar J, Mehran R. Sex-based differences in acute coronary syndromes: Insights from invasive and noninvasive coronary technologies. JACC Cardiovasc Imaging. (2016) 9:451–64. doi: 10.1016/j.jcmg.2016.02.004
36. Xie JX, Eshtehardi P, Varghese T, Goyal A, Mehta PK, Kang W, et al. Prognostic significance of nonobstructive left main coronary artery disease in women versus men: Long-term outcomes from the CONFIRM (coronary CT angiography evaluation for clinical outcomes: An international multicenter) registry. Circ Cardiovasc Imaging. (2017) 10:e006246. doi: 10.1161/CIRCIMAGING.117.006246
37. Wentzel JJ, Papafaklis MI, Antoniadis AP, Takahashi S, Cefalo NV, Cormier M, et al. Sex-related differences in plaque characteristics and endothelial shear stress related plaque-progression in human coronary arteries. Atherosclerosis. (2022) 342:9–18. doi: 10.1016/j.atherosclerosis.2021.12.013
38. Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: A systematic review. Stroke. (2009) 40:1082–90. doi: 10.1161/STROKEAHA.108.540781
39. Schindler A, Schinner R, Altaf N, Hosseini AA, Simpson RJ, Esposito-Bauer L, et al. Prediction of stroke risk by detection of hemorrhage in carotid plaques: Meta-analysis of individual patient data. JACC Cardiovasc Imaging. (2020) 13:395–406. doi: 10.1016/j.jcmg.2019.03.028
40. Saba L, Francone M, Bassareo PP, Lai L, Sanfilippo R, Montisci R, et al. CT attenuation analysis of carotid intraplaque hemorrhage. AJNR Am J Neuroradiol. (2018) 39:131–7. doi: 10.3174/ajnr.A5461
41. Saba L, Saam T, Jäger HR, Yuan C, Hatsukami TS, Saloner D, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. (2019) 18:559–72. doi: 10.1016/S1474-4422(19)30035-3
42. van den Bouwhuijsen QJ, Vernooij MW, Hofman A, Krestin GP, van der Lugt A, Witteman JC. Determinants of magnetic resonance imaging detected carotid plaque components: The Rotterdam Study. Eur Heart J. (2012) 33:221–9. doi: 10.1093/eurheartj/ehr227
43. Ota H, Reeves MJ, Zhu DC, Majid A, Collar A, Yuan C, et al. Sex differences in patients with asymptomatic carotid atherosclerotic plaque: In vivo 3.0-T magnetic resonance study. Stroke. (2010) 41:1630–5. doi: 10.1161/STROKEAHA.110.581306
44. Zhang L, Zhu L, Lu M, Zhao X, Li F, Cai J, et al. Comparison of carotid plaque characteristics between men and women using magnetic resonance vessel wall imaging: A Chinese atherosclerosis risk evaluation study. J Magn Reson Imaging. (2021) 54:646–54. doi: 10.1002/jmri.27576
45. van Dam-Nolen DHK, van Egmond NCM, Dilba K, Nies K, van der Kolk AG, Liem MI, et al. Sex differences in plaque composition and morphology among symptomatic patients with mild-to-moderate carotid artery stenosis. Stroke. (2022) 53:370–8. doi: 10.1161/STROKEAHA.121.036564
46. van Dam-Nolen DHK, van Dijk AC, Crombag GAJC, Lucci C, Kooi ME, Hendrikse J, et al. Lipoprotein(a) levels and atherosclerotic plaque characteristics in the carotid artery: The plaque at RISK (PARISK) study. Atherosclerosis. (2021) 329:22–9. doi: 10.1016/j.atherosclerosis.2021.06.004
47. Schreiner PJ, Heiss G, Tyroler HA, Morrisett JD, Davis CE, Smith R. Race and gender differences in the association of Lp(a) with carotid artery wall thickness. The atherosclerosis risk in communities (ARIC) study. Arterioscler Thromb Vasc Biol. (1996) 16:471–8. doi: 10.1161/01.atv.16.3.471
48. Song JW, Cao Q, Siegler JE, Thon JM, Woo JH, Cucchiara BL. Sex differences in carotid plaque composition in patients with embolic stroke of undetermined source. J Am Heart Assoc. (2021) 10:e020143. doi: 10.1161/JAHA.120.020143
49. Saba L, Micheletti G, Brinjikji W, Garofalo P, Montisci R, Balestrieri A, et al. Carotid intraplaque-hemorrhage volume and its association with cerebrovascular events. AJNR Am J Neuroradiol. (2019) 40:1731–7. doi: 10.3174/ajnr.A6189
50. Singh N, Moody AR, Zhang B, Kaminski I, Kapur K, Chiu S, et al. Age-specific sex differences in magnetic resonance imaging-depicted carotid intraplaque hemorrhage. Stroke. (2017) 48:2129–35. doi: 10.1161/STROKEAHA.117.017877
51. Vakhtangadze T, Singh Tak R, Singh U, Baig MS, Bezsonov E. Gender differences in atherosclerotic vascular disease: From lipids to clinical outcomes. Front Cardiovasc Med. (2021) 8:707889. doi: 10.3389/fcvm.2021.707889
52. Langabeer JR II, Champagne-Langabeer T, Fowler R, Henry T. Gender-based outcome differences for emergency department presentation of non-STEMI acute coronary syndrome. Am J Emerg Med. (2019) 37:179–82. doi: 10.1016/j.ajem.2018.05.005
53. Langabeer JR II, Henry TD, Fowler R, Champagne-Langabeer T, Kim J, Jacobs AK. Sex-based differences in discharge disposition and outcomes for ST-segment elevation myocardial infarction patients within a regional network. J Womens Health (Larchmt). (2018) 27:1001–6. doi: 10.1089/jwh.2017.6553
54. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: A report from the American heart association. Circulation. (2022) 145:e153–639. doi: 10.1161/CIR.0000000000001052
55. Drobni ZD, Kolossvary M, Karady J, Jermendy AL, Tarnoki AD, Tarnoki DL, et al. Heritability of coronary artery disease: Insights from a classical twin study. Circ Cardiovasc Imaging. (2022) 15:e013348. doi: 10.1161/CIRCIMAGING.121.013348
56. Spanos K, Tzorbatzoglou I, Lazari P, Maras D, Giannoukas AD. Carotid artery plaque echomorphology and its association with histopathologic characteristics. J Vasc Surg. (2018) 68:1772–80. doi: 10.1016/j.jvs.2018.01.068
Keywords: atherosclerosis, sex-based differences, coronary imaging, carotid imaging, MRA (magnetic resonance angiography), CTA (computed tomographic angiography), gender
Citation: Onnis C, Cadeddu Dessalvi C, Cademartiri F, Muscogiuri G, Angius S, Contini F, Suri JS, Sironi S, Salgado R, Esposito A and Saba L (2022) Quantitative and qualitative features of carotid and coronary atherosclerotic plaque among men and women. Front. Cardiovasc. Med. 9:970438. doi: 10.3389/fcvm.2022.970438
Received: 15 June 2022; Accepted: 25 July 2022;
Published: 13 September 2022.
Edited by:
Sabina Gallina, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Saverio Muscoli, Policlinico Tor Vergata, ItalyFederica Moscucci, Sapienza University of Rome, Italy
Copyright © 2022 Onnis, Cadeddu Dessalvi, Cademartiri, Muscogiuri, Angius, Contini, Suri, Sironi, Salgado, Esposito and Saba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Cadeddu Dessalvi, cadedduc@unica.it
 Carlotta Onnis
Carlotta Onnis Christian Cadeddu Dessalvi
Christian Cadeddu Dessalvi Filippo Cademartiri
Filippo Cademartiri Giuseppe Muscogiuri
Giuseppe Muscogiuri Simone Angius2
Simone Angius2  Rodrigo Salgado
Rodrigo Salgado