Protective Potential of Maresins in Cardiovascular Diseases
- Advanced Institute for Medical Sciences, Dalian Medical University, Dalian, China
Cardiovascular diseases are the leading causes of global mortality. Growing evidence suggests that unresolved inflammation contributes to the chronicity, progression and morbidity of many cardiovascular diseases, thus emphasizing the urgent need to illuminate the mechanisms controlling inflammation and its resolution, for the sake of new effective therapeutic options. Macrophage mediators in resolving inflammation (Maresins) are a family of specialized pro-resolving lipid mediators (SPMs) derived from the ω-3 fatty acid docosahexaenoic acid (DHA). Studies have indicated that Maresins play critical role in initiating the pro-resolving functions of phagocytes, decreasing the magnitude of the overall inflammatory response, and thereby protecting against inflammation-related disorders. In this review, we summarize the detailed actions and the therapeutic potential of Maresins, with a particular emphasis on Maresin-1 (MaR1), in cardiovascular diseases. We hope this review will lead to new avenues to Maresins-based therapies for inflammation-associated cardiovascular diseases.
Introduction
Cardiovascular diseases (including atherosclerosis, aortic aneurysm, myocardial infarction, and so on) are the leading causes of death and disability in the world. It is known that inflammation is an important feature of many cardiovascular disorders, whereas acute inflammation upon tissue injury would activate adaptive immunity, repair damaged tissue, and eventually restore homeostasis. However, while acute and self-limited inflammatory reaction is protective, the persistent and uncontrolled inflammation amplifies tissue damage and drives the development of various undesirable results. Hence, understanding the mechanisms that control the resolution of inflammation may provide insight into preventing and treating inflammation-associated cardiovascular diseases (1, 2).
In recent years, researchers have demonstrated that the resolution of inflammation is not a passive return to homeostasis, but rather an active process governed by endogenous chemical mediators namely specialized pro-resolving mediators (SPMs) (3). SPMs are a group of fatty acids, including ω-6 arachidonic acid (AA) derived lipoxins, and ω-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) derived Resolvins, Protectins and Maresins, as well as n-3 docosapentaenoic acid (DPA) derived SPMs (4–7). In the midst of known SPMs, Maresins were firstly discovered by Serhan et al. in the year 2009 and had displayed widely protective potential in inflammation resolving, wound healing, tissue regeneration and organ protection (8–10). Here, we review the biosynthesis, receptors, and potential protective effects and mechanisms of Maresins in cardiovascular diseases, which may open a new window for seeking optimized anti-inflammatory therapeutic options and improving cardiovascular disease management strategies.
Biosynthesis and Receptors of Maresins
Maresins are a series of polyhydroxy and polyunsaturated conjugated double bond molecules synthesized from DHA through a series of enzymatic conversion of ω-3 essential fatty acids. At present, the Maresins family mainly includes Maresin-1 (MaR1), Maresin-2 (MaR2), Maresin conjugate in tissue regeneration (MCTRs), and Maresin-like lipid mediators (MaR-Ls). They are named differently according to the position and conformation of hydroxyl and double bond. As reflected in their nomenclature “Macrophage mediators in resolving inflammation,” macrophages are the source of Maresins in their original identification, while neutrophils, platelets and other cell types have afterwards been reported to be able to produce Maresins as well.
Briefly, after a series of complex desaturation and elongation reactions, ω-3 fatty acids (e.g., a-linolenic acid) is transformed to their higher unsaturated derivatives, EPA and DHA (11). DHA is converted by 12-lipoxygenase (12-LOX) to 14-hydroperoxy-docosahexaenoic acid (14S-HpDHA), and then to the 13,14-epoxide intermediate by the same enzyme denoted 13S,14S-epoxy-Maresin, which is further enzymatically transformed to the bioactive MaRs and MCTRs (12) (Figure 1A). MaR1 is the first member of Maresins family which is produced by enzymatic hydrolysis of 13S,14S-epoxy-Maresin. Whereas, when the epoxide intermediate is followed by conversion via soluble epoxide hydrolase (sEH), it is instantly converted to MaR2 (13). In the biosynthesis of MCTRs, the epoxide intermediate is converted to MCTR1 by leukotriene C4 synthase (LTC4S) or glutathione S-transferase mu 4 (GSTM4) and the conversion of MCTR1 to MCTR2 is catalyzed by γ-glutamyl transferase (GGT). The biosynthesis of MCTR3 is mediated by dipeptidases (DPEP) which cleaved the cysteinyl-glycinyl bond of MCTR2 (14). Different from MaRs and MCTRs, 12-LOX-initiated 14S-hydroxylation or cytochrome P450 (CYP450) catalyzed 14R-hydroxylation and CYP450-initiated ω (22)-hydroxylation are required for MaR-Ls biosynthesis. Leukocytes and platelets convert DHA to 14S-hydroperoxy-DHA (14S-HDHA) via 15-LOX or 12-LOX. 14S-HDHA is further transformed to 14, 22-dihydroxydocosahexaenoic acids (14S, 22-diHDHA) named MaR-L1 through CYP450-catalyzed ω (22)-oxidation. Alternatively, when DHA is transformed to 14R-HDHA by CYP450, 14R-HDHA will be converted to 14R, 22-diHDHA (MaR-L2) (15).
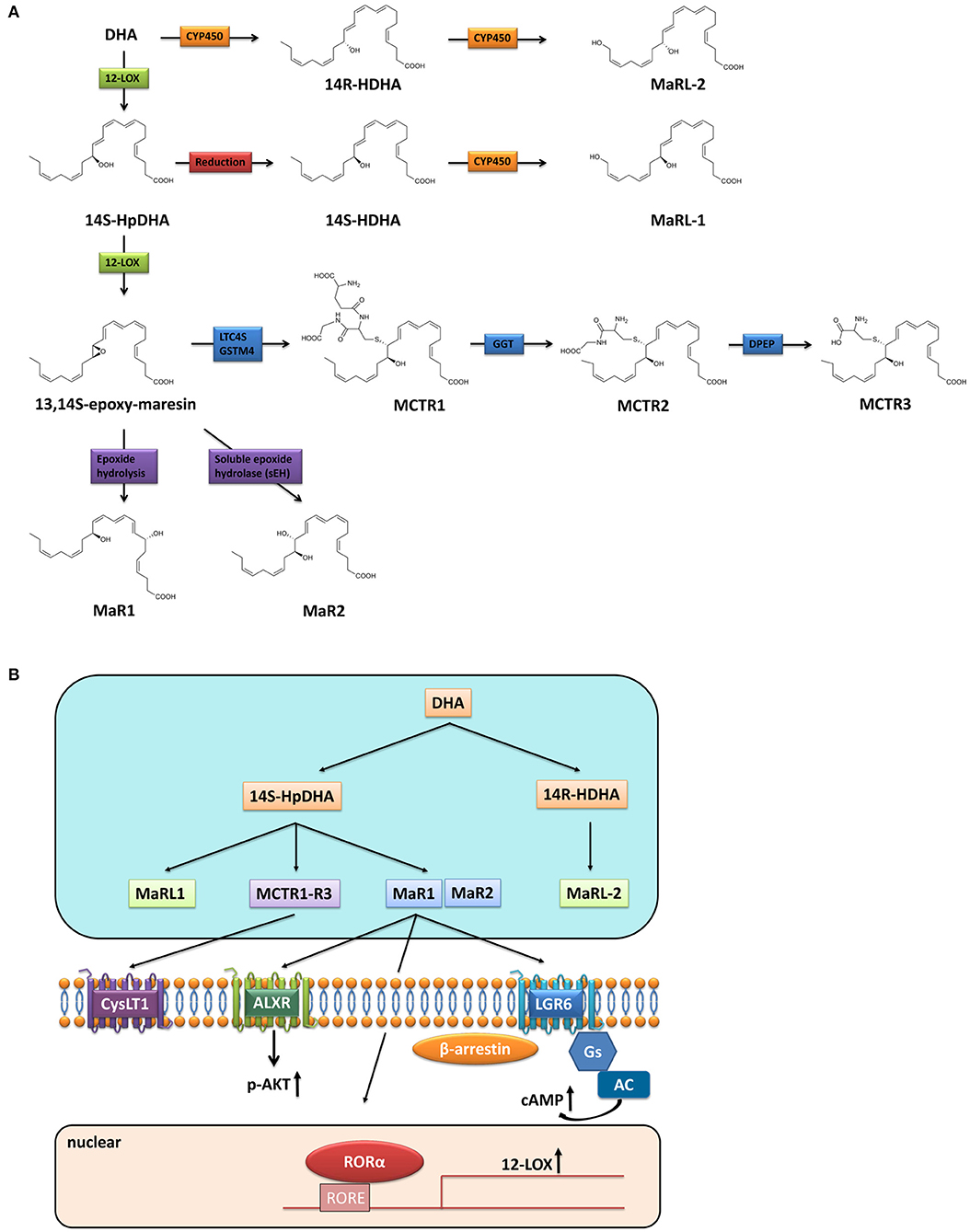
Figure 1. Biosynthesis and receptors of Maresins. (A) Biosynthesis of Maresins; (B) Receptors of Maresins.
Extensive studies have illustrated that pro-resolving activities of SPMs occur through activation of one or more G protein-coupled receptors (GPCRs) (16, 17). Similarly, after screening hundreds of GPCRs, Serhan et al. found that MaR1 is a ligand for leucine-rich repeat containing G protein-coupled receptor 6 (LGR6), whereas MaR1 stimulates efferocytosis, enhances phagocytosis, and reduces chemotaxis of polymorphonuclear leukocytes and monocytes/macrophages in LGR6-dependent manners via coupling a Gαs protein to stimulate cAMP (4). Besides, studies have shown that MaR1 exerts its function via interacting with lipoxin A4 receptor (ALXR) in several inflammatory disease model, and the salutary effect of MaR1 could be blocked by ALXR antagonist (18, 19), thus ALXR might be another GPCR for MaR1, although the direct binding of MaR1 to ALXR has not been explored. Furthermore, MaR1 is also considered as an endogenous ligand for nuclear receptor retinoic acid-related orphan receptor α (RORα), thus MaR1 enhances the expression and transcriptional activity of RORα and thereby increases the M2 polarity of liver macrophages (20). As a consequence, MaR1 presents cell-type and receptor-dependent actions (Figure 1B). While MCTRs do not directly activate LGR6 (4), they functionally interacts with human cysteinyl-LT receptor (CysLT1) to reduce leukotriene D4 (LTD4) signals (21). Unlike MaR1, the receptor of MaR2 has not been identified yet. There is also no information on the receptors of MaR-Ls and this warrants further investigation.
MaR1 and Atherosclerosis
Atherosclerosis is a multifocal chronic inflammatory disease of medium sized and large arteries which is the primary cause of fatal cardiovascular diseases (22). Analysis of atherosclerotic lesions in patients with acute myocardial infarction demonstrated that inflammation is the crucial factor leading to plaque rupture and surface plaque erosion (23). A critical aspect is the failure of inflammation resolution, which contains the inhibition of inflammatory cell influx, promotion of inflammatory cell egress, and clearance of apoptotic cells (24). A clear understanding of the process of inflammation and resolution is important to comprehend the complex process of atherosclerosis progression.
Consistent to the concept that an imbalance between specialized pro-resolving and pro-inflammatory lipid mediators promotes instability of atherosclerotic plaques, Viola et al. and Fredma et al. recently reported that there is a significant decrease of pro-resolving MaR1 and/or Resolvin D1/2 (RvD1/2) and increase of the pro-inflammatory leukotriene B4 (LTB4) through the development of early and advanced stages of atherosclerosis in mice (25, 26). By measuring the lesion and necrotic core sizes, macrophage and smooth muscle cell contents, collagen and fibrous cap thickness, Viola et al. demonstrated a significant positive correlation for LTB4 and a negative correlation for MaR1 and RvD2 with vulnerability plaque index. Moreover, therapeutic delivery of RvD1 or a combination of MaR1 and RvD2 both markedly halted atheroprogression and reestablished the balance of SPM to pro-inflammatory leukotrienes. It is well-known that alternatively activated macrophages (M2 macrophages) play critical role in suppressing inflammation and endocytosing cellular debris (24). Dalli et al. have showed that MaR1 has the function of affecting the phenotype of macrophages, tending to polarized toward M2 type, increasing efferocytosis, phagocytic ability, as well as increasing tissue regeneration (6, 27), implying that MaR1 may retard atherogenesis by promoting the inflammation/resolution balance shifted toward resolution. Indeed, by staining M1 and M2 type macrophage specific markers in aortic root sections, an increasing M2 macrophage fraction was shown upon MaR1/RvD2 treatment. Moreover, in vitro experiments showed that MaR1 and RvD2 didn't influence the expression of collagen production as well as inflammation genes in smooth muscle cells, while when smooth muscle cells were co-cultured of TNF-activated macrophages, MaR1 and RvD2 increased the mRNA levels of collagen production related genes. These data are consistent with the concept that MaR1 and RvD2 generally act on macrophages, modulating macrophage polarization toward a pro-resolving phenotype, thus suppressing local inflammatory phenotype. This in turn acts on the adjacent smooth muscle cells and stimulates collagen production and stabilizes atherosclerotic plaque (25). Noteworthy, although administration of resolving mediators MaR1 and RvD2 in combination displays promising effects on atheroprogression, given that the possible distinct roles for these two lipid mediators in addition to leukocyte-related functions (28), clarifying the specific role of MaR1 in atherosclerosis is necessarily required.
In addition to the mechanisms mentioned above with restoration of pro-resolving macrophages, in vitro experiments demonstrated that MaR1 can also ameliorate LPS-induced pro-inflammatory response, endoplasmic reticulum stress, and cell apoptosis in cultured human umbilical vein endothelial cells (29). Moreover, via up-regulation of cAMP and down-regulation of NF-κB activation, MaR1 significant reduced TNF-α induced monocyte adhesion, reactive oxygen species generation, and pro-inflammatory mediators release in cultured human vascular endothelial and vascular smooth muscle cells (30). These data suggest that MaR1 may also protect against atherosclerotic reactions in vascular endothelial and/or smooth muscle cell-dependent manner. However, the exact role of MaR1 in vascular function during atherogenesis is still not clear.
Increasing evidence has also shown that lymphocytes, particularly T cells, are critical players for the pathogenesis of atherosclerosis. MaR1 indeed has been recognized as an important modulator on adaptive immunity, whereas it suppressed the induction of effector T cells and promoted the induction Treg, thus decreased the production of pro-inflammatory cytokines and increased the production anti-inflammatory cytokines (31). The correlation between MaR1 and Treg/Th17 cell balance has also been investigated in rheumatoid arthritis and other inflammatory diseases (32, 33). However, the direct link between the action of MaR1 on lymphocytes and atherogenesis and other cardiovascular diseases is so far unknown and requires future interpretation. Nevertheless, MaR1 is most likely to be beneficial to atherosclerosis and represents a new window for therapeutic intervention for atherosclerotic diseases.
MaR1 and Abdominal Aortic Aneurysm
Abdominal aortic aneurysm (AAA) is related to the degradation of the atheromatous aorta elastic media. It is commonly believed that inflammation is associated with AAA development and contributes to elastin breakdown, smooth muscle cell (SMC) phenotypic switching, and extracellular matrix degradation (34, 35). Most recently, Elder et al. reported that MaR1 treatment significantly suppressed murine aneurysm formation in a topical elastase AAA model (36). Mechanistically, MaR1 promotes macrophage-dependent efferocytosis of apoptotic/necrotic SMCs and reduced vascular remodeling by increased TGF-β2 secretion. In SMC-TGFβ2r−/− mice, MaR1-dependent alleviation of AAA formation and increase of efferocytosis of SMCs is abolished. Furthermore, blockade of LGR6 receptor obliterated the protective effects of MaR1 on AAA formation and vascular remodeling (36).
MaR1 and Other Inflammatory Vascular Diseases
Inflammation is also important pathology in neointimal formation and restenosis after angioplasty. In a mouse model of carotid ligation arterial injury, MaR1 significant reduced early proliferation of VSMCs in the arterial wall and inhibited vascular remodeling through reducing neutrophil and monocyte/macrophage recruitment, as well as increasing M2 polarization in the arteries (37). Upon thrombogenesis, MaR1 has been reported to regulate platelet hemostatic function by enhancing platelet aggregation and spreading, while suppressing release of pro-inflammatory and prothrombotic mediators (38). All these observations point to the therapeutic rationale for MaR1 in inflammatory vascular diseases, modulation of inflammation resolution by MaR1 may provide new directions to limit the vascular injury response and maintain vascular homeostasis.
MaR1 and Myocardial Infarction and Cardiac Remodeling
Inflammation is critical to the onset and progression of cardiac remodeling and heart failure. While acute inflammatory response usually coincides with active resolving phase, SPMs may act as a guide for resolution of inflammation in the injured myocardium following acute myocardial infarction. Halade et al. illustrated that the abundance of MaR1 and other members of SPMs was significantly enhanced in the infarcted left ventricle within 24 h after myocardial infarction in mice. Moreover, they proved that the splenic macrophages recruited to the infarcted left ventricle represent the major source of these SPMs, highlighting the importance of macrophage-derived SPMs, including MaR1, in retarding heart failure (39). In addition to its dominant anti-inflammatory actions in cardiac macrophages, Wahyuni et al. illustrated that MaR1 can also induce physiological cardiomyocyte hypertrophy through RORα/IGF-1/PI3K/Akt pathway (40). However, the direct action and mechanism of MaR1 on pathological cardiac remodeling and ischemic cardiac injury has not been explored.
MaR1 and Sepsis-Associated Cardiac Injury
Sepsis is an inflammatory lethal syndrome and about half of patients with sepsis develop cardiac dysfunction. By detecting the peripheral blood lipid mediator profiles in sepsis patients, Dalli et al. recently reported that patients with sepsis had differentially expressed SPMs, this was correlated with survival and clinical outcomes (41). Similarly, in cecal ligation and puncture induced sepsis mouse model, Chen et al. observed significant reduction of multiple SPMs production in the heart tissues, whereas the levels of MaR1 had a ~6-fold reduction (42). Moreover, Li et al. demonstrated that MaR1 treatment remarkedly decreased the serum lactate dehydrogenase and creatine kinase levels and improved LPS-induced cardiac dysfunction in mice via promoting M2 macrophage differentiation, alleviating inflammatory response, and reducing oxidative stress (43). Taken together, administration of MaR1 may be beneficial in reducing sepsis-induced cardiac injury.
MaR1 and Hypertensive Cardiovascular Disease
Inflammation has been demonstrated as a major component of hypertension and hypertension related organ damage, including peripheral vascular resistance and heart dysfunction. Although the direct relationship between MaR1 and hypertension has not been experimentally tested, by comparing the serum levels of lipoxin and Resolvins in hypertension and normotension patients, Yücel et al. found that the lipoxin A4 (LXA4), RvD1 and Resolvin E1 (RvE1) levels were negatively correlated with systolic blood pressure and total cholesterol levels, implying that reduced SPMs may serve as indicators of the development of hypertension (44). Indeed, RvD1, which is also derived from DHA, had shown protective potential in angiotensin II induced hypertension and cardiac remodeling (45). Resolvins also showed potential to combat pulmonary arterial hypertension and inhibit pulmonary vessel constriction (46, 47). Therefore, it is reasonable for us to expect that MaR1 exhibits beneficial effects on hypertension and hypertensive cardiovascular disease.
MaR1 and Cardiovascular Metabolic Syndrome
Mounting evidence indicates that patients with metabolic disorders, including obesity, diabetes mellitus, and non-alcoholic fatty liver disease (NAFLD), are at increased risk of cardiovascular events and have a higher cardiovascular morbidity and mortality (48, 49). Metabolic inflammation has been considered as a key process that contributes to multiorgan morbidity (50). Most recently, Fang et al. explored the association between serum MaR1 levels and NAFLD, they found the circulating MaR1 levels were decreased in patients with NAFLD and a negative correlation between NAFLD and serum MaR1 concentrations was identified, indicating that MaR1 might play an important role in the development of NAFLD (51). Similarly, plasma MaR1 concentration was decreased in type 2 diabetic patients and was closely associated with obesity, impaired glucose and lipid metabolism, and enhanced insulin resistance (52). In ob/ob and diet-induced obese mice, MaR1 treatment could inhibit macrophage recruitment, reduce the ratio of M1:M2 macrophages, increase anti-inflammatory adipokine expression, and eventually improve insulin sensitivity and reverse adipose tissue dysfunction (53). MaR1 ameliorated obesity-associated liver steatosis by decreasing lipogenic enzymes, inducing fatty acid oxidation, and activating autophagy through AMPK signaling pathway (54). Moreover, Jung et al. illustrated that MaR1 ameliorated NAFLD via AMPK/SERCA2b-mediated suppression of endoplasmic reticulum stress (55).
Non-alcoholic steatohepatitis (NASH) is a potential progressive liver diseases as a subtype of NAFLD (56). Chronic activation of the hepatic pro-inflammatory immune responses play a vital role in the pathological process of NASH (57). Liver macrophage is a key regulator of hepatic homeostasis by releasing cytokines and modulating immune response in NASH (58). As an endogenous ligand of nuclear receptor RORα, MaR1 was able to increase the expression and transcriptional activity of RORα and thus promoted the M2 polarity of liver macrophages, thus protected mice from high fat diet-induced NASH. More interestingly, activation of RORα can in feedback increase the level of MaR1 through transcriptional induction of 12-LOX, the key enzyme in MaR1 biosynthesis, thus reestablishing the MaR1/RORα/12-LOX autoregulatory circuit may provide a potential therapeutic strategy for treating NASH (20).
MaR2, MCTRs, And MaR-Ls in Cardiovascular Diseases
MaR2 is identified in Maresins metabolome in macrophages via conversion by human 12-LOX followed by sEH, displayed potent anti-inflammatory and pro-resolving actions (13). In the zymosan injection induced peritonitis mice, MaR2 revealed the potential anti-inflammatory action, although MaR2 is equivalent to MaR1 in restricting neutrophil infiltration, MaR1 is more effective in enhancing macrophage phagocytosis than MaR2 (13). However, the anti-inflammatory effect of MaR2 in cardiovascular diseases has never been confirmed, this warrants further investigation.
Inflammation resolution is an active process which can lead to tissue regeneration. MCTRs were identified as evolutionarily conserved chemical signals that are peptide-lipid conjugated mediators in the Maresins biosynthesis and functionally communicate during resolution of inflammation to activate tissue regeneration (59, 60). With regard to MCTRs and cardiovascular disorders, Yang et al. found that post-treatment with MCTR1 can significantly improve sepsis-induced cardiac dysfunction. Mechanistically, MCTR1 reduced neutrophil chemotaxis and infiltration in LPS-stimulated hearts via attenuating IL-17A production from γδT cells (61). Moreover, Chiang et al. demonstrated that MCTRs can functionally block LTD4-stimulated negative inotropic action on tunicate hearts and antagonize LTD4-initiated vascular leakage (21). Despite these findings, the role of MCTRs in cardiovascular pathogenesis are still largely unknown. Also, we are still far from understanding the role of MaR-Ls in cardiovascular diseases.
Conclusions and Perspectives
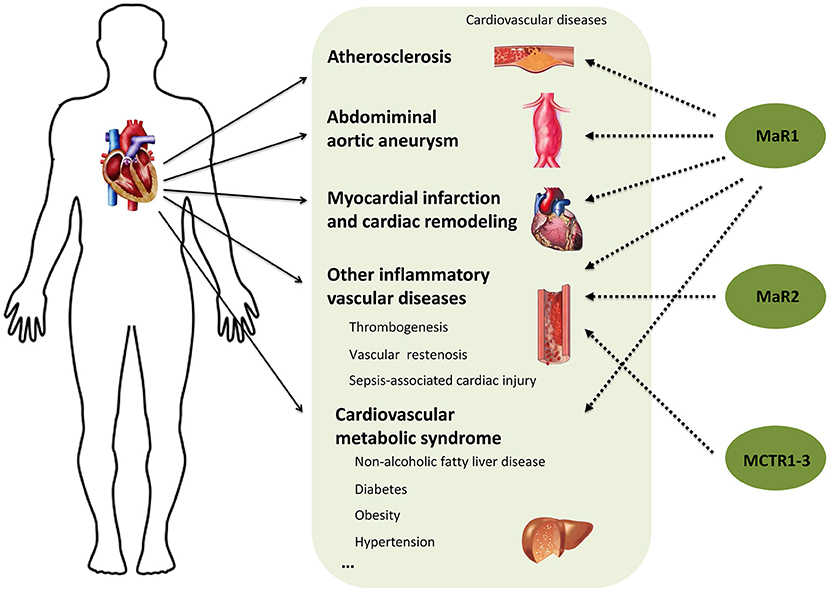
A large number of studies have shown that inflammation and its resolution are central to most clinically relevant cardiovascular diseases. Although targeting inflammation has been shown to effectively reduce mortality, it has confirmed that anti-inflammatory therapies also enhance the risk of impairing host defense. Thus, boosting inflammation resolution without being overtly immunosuppressive via supplementation of pro-resolving mediators has become new strategy for the treatment of cardiovascular diseases. Maresins are a new family of bioactive lipid mediators synthesized from an ω-3 polyunsaturated fatty acid DHA and have displayed strong anti-inflammatory and pro-resolving abilities and broad beneficial actions in many inflammatory diseases (62, 63). In the current review, by overviewing the studies of Maresins, MaR1 in particular, in the common inflammation associated cardiovascular diseases (Figure 2), we can conclude that administration of MaR1 may serve as a promising therapeutic option and it will be of great significance to synthesize drugs based on endogenous Maresins for the treatment of cardiovascular disease. However, many important questions are still largely unanswered and require in-depth investigation. For example, (1) What is the specific receptor and downstream signaling pathway for MaR1 in a particular cardiovascular disease model? (2) In addition to its anti-inflammatory and pro-resolving activities on monocytes/macrophages and neutrophils, does MaR1 exhibit direct actions on lymphocytes and the mediated immune responses in cardiovascular tissues? (3) What is the effect of MaR1 on cardiomyocytes? fibroblasts? and/or other resident cardiovascular cell types? (4) Do other Maresins exhibit similar protective effects and mechanisms as MaR1 in the anti-inflammatory therapy for cardiovascular diseases? Finally, most of the current evidence are from animal studies, more clinical studies are definitely required to provide better understanding in patients.
Author Contributions
ML prepared the manuscript draft. HH carefully checked the manuscript and the references. LC strictly commented and amended the paper. All authors contributed to the final version of this review.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 32071157).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alfaddagh A, Martin SS, Leucker TM, Michos ED, Blaha MJ, Lowenstein CJ, et al. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am J Prev Cardiol. (2020) 4:100130. doi: 10.1016/j.ajpc.2020.100130
2. Halade GV, Lee DH. Inflammation and resolution signaling in cardiac repair and heart failure. EBioMedicine. (2022) 79:103992. doi: 10.1016/j.ebiom.2022.103992
3. Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. (2017) 31:1273–88. doi: 10.1096/fj.201601222R
4. Chiang N, Libreros S, Norris PC, de la Rosa X, Serhan CN. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J Clin Invest. (2019) 129:5294–311. doi: 10.1172/JCI129448
5. Abdolmaleki F, Kovanen PT. Resolvins: emerging players in autoimmune and inflammatory diseases. Clin Rev Allergy Immunol. (2020) 58:82–91. doi: 10.1007/s12016-019-08754-9
6. Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. (2012) 26:1755–65. doi: 10.1096/fj.11-201442
7. Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. (2011) 111:5922–43. : doi: 10.1021/cr100396c
8. Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. (2009) 206:15–23. doi: 10.1084/jem.20081880
9. Tang S, Wan M, Huang W. Maresins: specialized proresolving lipid mediators and their potential role in inflammatory-related diseases. Mediators Inflamm. (2018) 2018:2380319. doi: 10.1155/2018/2380319
10. Li QF, Hao H, Tu WS, Guo N, Zhou XY. Maresins: anti-inflammatory pro-resolving mediators with therapeutic potential. Eur Rev Med Pharmacol Sci. (2020) 24:7442–53. doi: 10.26355/eurrev_202007_21913
11. Perica MM, Delas I. Essential fatty acids and psychiatric disorders. Nutr Clin Pract. (2011) 26:409–25. doi: 10.1177/0884533611411306
12. Dalli J, Serhan C. Macrophage proresolving mediators-the when and where. Microbiol Spectr. (2016) 4:2014. doi: 10.1128/microbiolspec.MCHD-0001-2014
13. Deng B, Wang CW, Arnardottir HH, Li Y, Cheng CY, Dalli J, et al. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS ONE. (2014) 9:e102362. doi: 10.1371/journal.pone.0102362
14. Dalli J, Vlasakov I, Riley IR, Rodriguez AR, Spur BW, Petasis NA, et al. Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc Natl Acad Sci USA. (2016) 113:12232–7. doi: 10.1073/pnas.1607003113
15. Hong S, Lu Y, Tian H, Alapure BV, Wang Q, Bunnell BA, et al. Maresin-like lipid mediators are produced by leukocytes and platelets and rescue reparative function of diabetes-impaired macrophages. Chem Biol. (2014) 21:1318–29. doi: 10.1016/j.chembiol.2014.06.010
16. Lee CH. Role of specialized pro-resolving lipid mediators and their receptors in virus infection: a promising therapeutic strategy for SARS-CoV-2 cytokine storm. Arch Pharm Res. (2021) 44:84–98. doi: 10.1007/s12272-020-01299-y
17. Park J, Langmead CJ, Riddy DM. New advances in targeting the resolution of inflammation: implications for specialized pro-resolving mediator GPCR drug discovery. ACS Pharmacol Transl Sci. (2020) 3:88–106. doi: 10.1021/acsptsci.9b00075
18. Gu J, Luo L, Wang Q, Yan S, Lin J, Li D, et al. Maresin 1 attenuates mitochondrial dysfunction through the ALX/cAMP/ROS pathway in the cecal ligation and puncture mouse model and sepsis patients. Lab Invest. (2018) 98:715–33. doi: 10.1038/s41374-018-0031-x
19. Tang D, Fu G, Li W, Sun P, Loughran PA, Deng M, et al. Maresin 1 protects the liver against ischemia/reperfusion injury via the ALXR/Akt signaling pathway. Mol Med. (2021) 27:18. doi: 10.1186/s10020-021-00280-9
20. Han YH, Shin KO, Kim JY, Khadka DB, Kim HJ, Lee YM, et al. A maresin 1/RORalpha/12-lipoxygenase autoregulatory circuit prevents inflammation and progression of nonalcoholic steatohepatitis. J Clin Invest. (2019) 129:1684–98. doi: 10.1172/JCI124219
21. Chiang N, Riley IR, Dalli J, Rodriguez AR, Spur BW, Serhan CN. New maresin conjugates in tissue regeneration pathway counters leukotriene D(4)-stimulated vascular responses. FASEB J. (2018) 32:4043–52. doi: 10.1096/fj.201701493R
22. Libby P. Inflammation in atherosclerosis-no longer a theory. Clin Chem. (2021) 67:131–42. doi: 10.1093/clinchem/hvaa275
23. Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. (2006) 86:515–81. doi: 10.1152/physrev.00024.2005
24. Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. (2010) 10:36–46. doi: 10.1038/nri2675
25. Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, et al. Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ Res. (2016) 119:1030–8. doi: 10.1161/CIRCRESAHA.116.309492
26. Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. (2016) 7:12859. doi: 10.1038/ncomms12859
27. Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, et al. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. (2013) 27:2573–83. doi: 10.1096/fj.13-227728
28. Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. (2014) 40:315–27. doi: 10.1016/j.immuni.2014.02.009
29. Jung TW, Park HS, Choi GH, Kim D, Ahn SH, Kim DS, et al. Maresin 1 attenuates pro-inflammatory reactions and ER stress in HUVECs via PPARalpha-mediated pathway. Mol Cell Biochem. (2018) 448:335–47. doi: 10.1007/s11010-018-3392-y
30. Chatterjee A, Sharma A, Chen M, Toy R, Mottola G, Conte MS. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS ONE. (2014) 9:e113480. doi: 10.1371/journal.pone.0113480
31. Chen L, Liu H, Wang Y, Xia H, Gong J, Li B, et al. Maresin 1 maintains the permeability of lung epithelial cells in vitro and in vivo. Inflammation. (2016) 39:1981–9. doi: 10.1007/s10753-016-0433-0
32. Jin S, Chen H, Li Y, Zhong H, Sun W, Wang J, et al. Maresin 1 improves the Treg/Th17 imbalance in rheumatoid arthritis through miR-21. Ann Rheum Dis. (2018) 77:1644–52. doi: 10.1136/annrheumdis-2018-213511
33. Saito-Sasaki N, Sawada Y, Mashima E, Yamaguchi T, Ohmori S, Yoshioka H, et al. Maresin-1 suppresses imiquimod-induced skin inflammation by regulating IL-23 receptor expression. Sci Rep. (2018) 8:5522. doi: 10.1038/s41598-018-23623-9
34. Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. (2005) 365:1577–89. doi: 10.1016/S0140-6736(05)66459-8
35. Anagnostakos J, Lal BK. Abdominal aortic aneurysms. Prog Cardiovasc Dis. (2021) 65:34–43. doi: 10.1016/j.pcad.2021.03.009
36. Elder CT, Filiberto AC, Su G, Ladd Z, Leroy V, Pruitt EY, et al. Maresin 1 activates LGR6 signaling to inhibit smooth muscle cell activation and attenuate murine abdominal aortic aneurysm formation. FASEB J. (2021) 35:e21780. doi: 10.1096/fj.202100484R
37. Akagi D, Chen M, Toy R, Chatterjee A, Conte MS. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. (2015) 29:2504–13. doi: 10.1096/fj.14-265363
38. Lannan KL, Spinelli SL, Blumberg N, Phipps RP. Maresin 1 induces a novel pro-resolving phenotype in human platelets. J Thromb Haemost. (2017) 15:802–13. doi: 10.1111/jth.13620
39. Halade GV, Norris PC. Splenic leukocytes define the resolution of inflammation in heart failure. Sci Signal. (2018) 11:1818. doi: 10.1126/scisignal.aao1818
40. Wahyuni T, Kobayashi A, Tanaka S, Miyake Y, Yamamoto A, Bahtiar A, et al. Maresin-1 induces cardiomyocyte hypertrophy through IGF-1 paracrine pathway. Am J Physiol Cell Physiol. (2021) 321:C82–93. doi: 10.1152/ajpcell.00568.2020
41. Dalli J, Colas RA, Quintana C, Barragan-Bradford D, Hurwitz S, Levy BD, et al. Human sepsis eicosanoid and proresolving lipid mediator temporal profiles: correlations with survival and clinical outcomes. Crit Care Med. (2017) 45:58–68. doi: 10.1097/CCM.0000000000002014
42. Chen J, Purvis GSD, Collotta D, Al Zoubi S, Sugimoto MA, Cacace A, et al. RvE1 attenuates polymicrobial sepsis-induced cardiac dysfunction and enhances bacterial clearance. Front Immunol. (2020) 11:2080. doi: 10.3389/fimmu.2020.02080
43. Li D, Wang M, Ye J, Zhang J, Xu Y, Wang Z, et al. Maresin 1 alleviates the inflammatory response, reduces oxidative stress and protects against cardiac injury in LPS-induced mice. Life Sci. (2021) 277:119467. doi: 10.1016/j.lfs.2021.119467
44. Yücel H, Özdemir AT. Low LXA4, RvD1 and RvE1 levels may be an indicator of the development of hypertension. Prostaglandins Leukot Essent Fatty Acids. (2021) 174:102365. doi: 10.1016/j.plefa.2021.102365
45. Olivares-Silva F, De Gregorio N, Espitia-Corredor J, Espinoza C, Vivar R, Silva D, et al. Resolvin-D1 attenuation of angiotensin II-induced cardiac inflammation in mice is associated with prevention of cardiac remodeling and hypertension. Biochim Biophys Acta Mol Basis Dis. (2021) 1867:166241. doi: 10.1016/j.bbadis.2021.166241
46. Jannaway M, Torrens C, Warner JA, Sampson AP. Resolvin E1, resolvin D1 and resolvin D2 inhibit constriction of rat thoracic aorta and human pulmonary artery induced by the thromboxane mimetic U46619. Br J Pharmacol. (2018) 175:1100–8. doi: 10.1111/bph.14151
47. Liu G, Wan N, Liu Q, Chen Y. Resolvin E1 attenuates pulmonary hypertension by suppressing Wnt7a/β-catenin signaling. Hypertension. (2021) 78:1914–26. doi: 10.1161/HYPERTENSIONAHA.121.17809
48. Roos CJ, Quax PH, Jukema JW. Cardiovascular metabolic syndrome: mediators involved in the pathophysiology from obesity to coronary heart disease. Biomark Med. (2012) 6:35–52. doi: 10.2217/bmm.11.105
49. Tana C, Ballestri S, Ricci F, Di Vincenzo A. Cardiovascular risk in non-alcoholic fatty liver disease: mechanisms and therapeutic implications. Int J Environ Res Public Health. (2019) 16:73104. doi: 10.3390/ijerph16173104
50. Gehrke N, Schattenberg JM. Metabolic inflammation-a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology. (2020) 158:1929–47.e6. doi: 10.1053/j.gastro.2020.02.020
51. Fang X, Wang H, Ye T, Fu X, Tan X, Zeng Y, et al. Low serum Maresin-1 levels are associated with non-alcoholic fatty liver disease: a cross-sectional study. Lipids Health Dis. (2021) 20:96. doi: 10.1186/s12944-021-01518-5
52. Miao T, Huang B, He N, Sun L, Du G, Gong X, et al. Decreased plasma maresin 1 concentration is associated with diabetic foot ulcer. Mediators Inflamm. (2020) 2020:4539035. doi: 10.1155/2020/4539035
53. Martinez-Fernandez L, Gonzalez-Muniesa P, Laiglesia LM, Sainz N, Prieto-Hontoria PL, Escote X, et al. Maresin 1 improves insulin sensitivity and attenuates adipose tissue inflammation in ob/ob and diet-induced obese mice. FASEB J. (2017) 31:2135–45. doi: 10.1096/fj.201600859R
54. Laiglesia LM, Lorente-Cebrian S, Martinez-Fernandez L, Sainz N, Prieto-Hontoria PL, Burrell MA, et al. Maresin 1 mitigates liver steatosis in ob/ob and diet-induced obese mice. Int J Obes. (2018) 42:572–9. doi: 10.1038/ijo.2017.226
55. Jung TW, Kim HC, Abd El-Aty AM, Jeong JH. Maresin 1 attenuates NAFLD by suppression of endoplasmic reticulum stress via AMPK-SERCA2b pathway. J Biol Chem. (2018) 293:3981–8. doi: 10.1074/jbc.RA117.000885
56. Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. (2019) 70:531–44. doi: 10.1016/j.jhep.2018.10.033
57. Farrell GC, van Rooyen D, Gan L, Chitturi S. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver. (2012) 6:149–71. doi: 10.5009/gnl.2012.6.2.149
58. Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. (2017) 17:306–21. doi: 10.1038/nri.2017.11
59. Dalli J, Chiang N, Serhan CN. Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc Natl Acad Sci USA. (2014) 111:E4753–61. doi: 10.1073/pnas.1415006111
60. Serhan CN, Chiang N, Dalli J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol Aspects Med. (2018) 64:1–17. doi: 10.1016/j.mam.2017.08.002
61. Yang Y, Li XY, Li LC, Xiao J, Zhu YM, Tian Y, et al. γδ T/interleukin-17A contributes to the effect of maresin conjugates in tissue regeneration 1 on lipopolysaccharide-induced cardiac injury. Front Immunol. (2021) 12:674542. doi: 10.3389/fimmu.2021.674542
62. Saito-Sasaki N, Sawada Y, Nakamura M. Maresin-1 and inflammatory disease. Int J Mol Sci. (2022) 23:31367. doi: 10.3390/ijms23031367
Keywords: Maresins, pro-resolving lipid mediator, inflammation, cardiovascular disease, macrophage
Citation: Liu M, He H and Chen L (2022) Protective Potential of Maresins in Cardiovascular Diseases. Front. Cardiovasc. Med. 9:923413. doi: 10.3389/fcvm.2022.923413
Received: 19 April 2022; Accepted: 02 June 2022;
Published: 04 July 2022.
Edited by:
Wilfried Le Goff, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Gabrielle Fredman, Albany Medical College, United StatesCopyright © 2022 Liu, He and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihong Chen, lihong@dmu.edu.cn
 Min Liu
Min Liu Huixiang He
Huixiang He  Lihong Chen
Lihong Chen