Cost-effectiveness of Direct Oral Anticoagulant vs. Warfarin Among Atrial Fibrillation Patients With Intermediate Stroke Risk
- 1Department of Public Health, Graduate School, Yonsei University, Seoul, South Korea
- 2Department of Preventive Medicine, Yonsei University College of Medicine, Seoul, South Korea
- 3Division of Cardiology, Chung-Ang University Hospital, Seoul, South Korea
- 4Institute of Human Complexity and Systems Science, Yonsei University, Incheon, South Korea
- 5Division of Cardiology, Department of Internal Medicine, Kyung Hee University Hospital, Kyung Hee University, Seoul, South Korea
Background: Several studies have shown the cost-effectiveness of direct oral anticoagulants (DOACs), compared with warfarin, to prevent atrial fibrillation (AF) related complications. However, few have reported cost-effectiveness of DOACs in AF patients with intermediate stroke risk. Thus, we investigated the cost-effectiveness of DOACs vs. warfarin in non-valvular AF patients with intermediate stroke risk using national representative data.
Methods: We identified 7,954 newly diagnosed non-valvular AF patients (≥18 years) with intermediate stroke risk (CHA2DS2-VASc score: 1 for men and 2 for women) using the national healthcare utilization data from August 1, 2016, to July 31, 2019. Annual incidence rate of AF-related composite outcomes (heat failure, myocardial infarction, ischemic stroke, intracerebral hemorrhage, and gastrointestinal bleeding) was estimated. Cost-effectiveness was estimated using a Markov chain model with the transition probability of 1 year. The willingness-to-pay (WTP) was set at $32,000 per quality-adjusted life-year (QALY) gained.
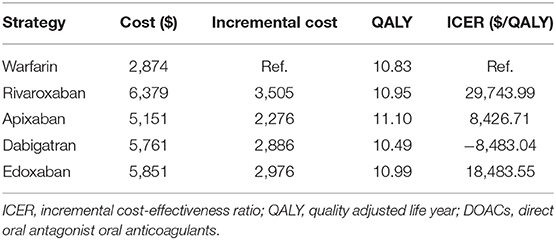
Results: The total cost of warfarin, rivaroxaban, apixaban, dabigatran and edoxaban was $2,874, $5,761, $5,151, $5,761 and $5,851, respectively. The QALYs gained were 10.83, 10.95, 11.10, 10.49 and 10.99 years, respectively. The incremental cost-effectiveness ratio of rivaroxaban, apixaban, dabigatran and edoxaban was $29,743.99, $8,426.71, -$8,483.04 and $18,483.55, respectively. The WTP was set at $32,000. DOACs (except dabigatran) were more cost-effective compared with warfarin because they did not exceed the WTP in the base-case analysis.
Conclusion: Our findings showed that DOACs were more cost-effective than warfarin in non-valvular AF patients with intermediate stroke risk.
Introduction
The global incidence and prevalence of atrial fibrillation (AF) have increased rapidly in elderly people (1). In the Republic of Korea, AF prevalence is estimated to be 2.1% among those aged more than 65 years, and it is expected to rise to 5.8% by 2060 due to rapid population aging (2). Accordingly, the risk of complications (including stroke) and the related cost of treating AF have increased steadily (3). In the United States, the estimated incremental cost for treating AF patients reached $26 billion during 2004–2006 (4). In Europe, AF-related costs increased up to €3,000 per patient-year from 1990 to 2009 (5).
Use of anticoagulants is of importance in preventing cardiovascular complications in patients with AF. AF increases the risk of developing a thrombus due to turbulent flow in the left atrium. The thrombus from the left atrium can cause embolization in a primary organ or tissue, leading to complications such as stroke or systemic embolism, myocardial infarction (MI), and heart failure (HF) (6–8). To prevent these complications, vitamin K antagonists or direct oral anticoagulants (DOACs; rivaroxaban, apixaban, dabigatran, and edoxaban) are prescribed to patients with AF.
Based on mounting evidence on the DOACs had more cost-effective than warfarin (9–12), guidelines in Europe and the USA recommend preferential use of DOACs over warfarin to prevent cardiovascular complications in patients with AF (13, 14). In line with this, use of DOACs to prevent stroke is subsidized in AF patients since 2015 in the Republic of Korea (15). However, this reimbursement scheme is limited to AF patients with high stroke risk (defined as a CHA2DS2-VASc score of 2 or higher), as most previous studies on the cost-effectiveness of DOACs included AF patients with high stroke risk or did not consider the risk of stroke (16). Some studies have investigated the cost-effectiveness of DOACs after a stratification on individuals regarding stroke risk (9, 17, 18). To the best of our knowledge, no purposely designed study has investigated the cost-effectiveness of DOACs by focusing on the intermediate stroke risk group.
Therefore, this study investigated the cost-effectiveness of DOACs and warfarin among AF patients with intermediate stroke risk by using national representative data.
Materials and Methods
Data Source
In this study, we used data from the Health Insurance Review and Assessment (HIRA) on the demographics (age and sex), diagnosis [International Classification of Disease 10th Revision (ICD-10) codes and date of diagnosis], in-hospital mortality, information on prescriptions (date and chemical name), and cost. Because the HIRA database covers almost 98% of the total population (15), sampling or selection bias was minimized.
This study was approved by the Institutional Review Board of Yonsei University Health System (2021–1748–001), and informed consent was waived.
Study Population
Of the 781,583 individuals who visited hospitals with the AF code (ICD-10: I48) between August 1, 2016, and July 31, 2019, we further identified 7,954 non-valvular AF patients using the following inclusion criteria (Supplementary Figure 1):
Individuals who were not diagnosed with AF (or valvular AF) and AF-related complications from August 1, 2015, to July 31, 2016;
Individuals who visited outpatient clinics at least twice or were admitted to a hospital at least once for AF (ICD-10: I48, I48.0, and I48.1);
Adults (aged ≥ 18 years) who took warfarin or DOACs (rivaroxaban, apixaban, dabigatran and edoxaban) and we excluded AF patients with prescriptions of both DOACs and warfarin during the study period;
Individuals with intermediate stroke risk (defined as the CHA2DS2-VASc score of 1 in men or 2 in women).
CHA2DS2-VASc Score
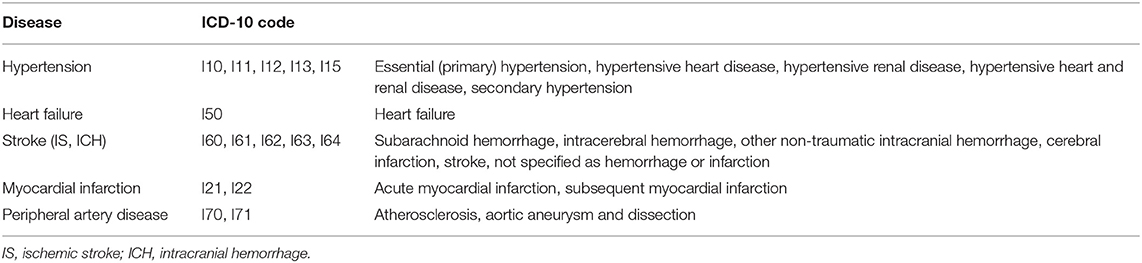
To calculate the CHA2DS2-VASc score (an indicator of stroke risk), this study set the index date for each participant. The index date is defined as the date of admission or of the second outpatient visit for AF, whichever came earlier. The CHA2DS2-VASc score was calculated based on the demographics on the index date and healthcare utilization 1 year prior to the index date. The CHA2DS2-VASc score is the sum of the scores of each component. One point was given to those who aged 65–75 years; those with a history of hypertension, diabetes, congestive HF, or vascular disease (MI or a peripheral vascular disease); and women. Two points were given to those aged 75 years or older and those with a history of stroke or systemic embolism. The ICD-10 codes used for calculating the CHA2DS2-VASc score are presented in Table 1.
Composite Outcome Measure
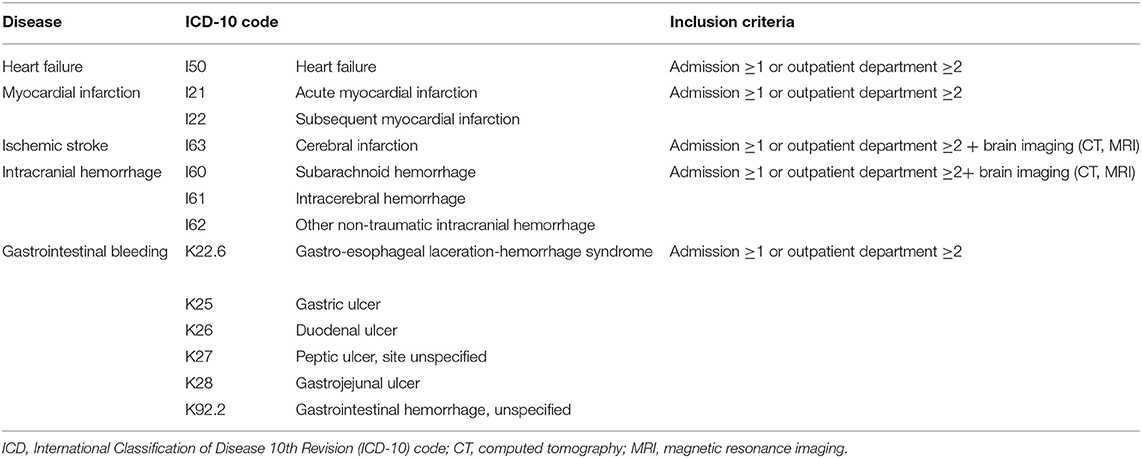
In this study, we calculated annual rates of the composite outcome of the following AF-related complications: HF, MI, ischemic stroke (IS), intracerebral hemorrhage (ICH), and gastrointestinal (GI) bleeding. For each composite outcome, there were records of outpatient visits at least twice or admission at least once. If the definition is satisfied no matter how many times an event occurs within a year, the analysis was conducted with one event occurrence. The ICD-10 codes used for calculating the composite outcome are presented in Table 2.
Cost-Effectiveness Analysis
A Markov chain decision-analysis model was constructed to evaluate the cost-effectiveness of DOACs compared with warfarin in intermediate stroke risk patients. We categorized health status into healthy with AF, post-health event (post-HF, post-IS, post-ICH, post-GI bleeding, or post-MI), and death. All health events, except for GI bleeding, were assumed to remain in the post-disease state or to transition to the death state. GI bleeding was assumed to transition to the healthy with AF state or death state (Supplementary Figure 2).
This study assumed the transition of health status in a 1-year cycle. Transient probabilities were determined by the annual incidence rates of five diseases (HF, MI, IS, ICH, and GI bleeding). The Markov chain decision-analysis model was repeated for 20 cycles. The discount rate was set as 4.5% (19).
This study took the perspective of a Korea healthcare system. All cost incurred from the point of view of Korean payers (medicine cost, cost due to health event) were included the cost consisted of medication cost, health event-related cost, and post-health event cost. Annual costs of medication were defined as 365 times the daily medication price. Health event-related cost was defined as the average admission cost for each health event. Post-event cost was estimated by subtracting the medication cost and event-related cost from the total costs. The willingness-to-pay (WTP) was set at $32,000 per quality-adjusted life-year (QALY) to reflect the South Korean gross domestic product ($31,494 in 2020). All costs were converted to USD (1 USD=1,000 KRW). We used the QALYs provided by previous studies (20–25).
Sensitivity Analysis
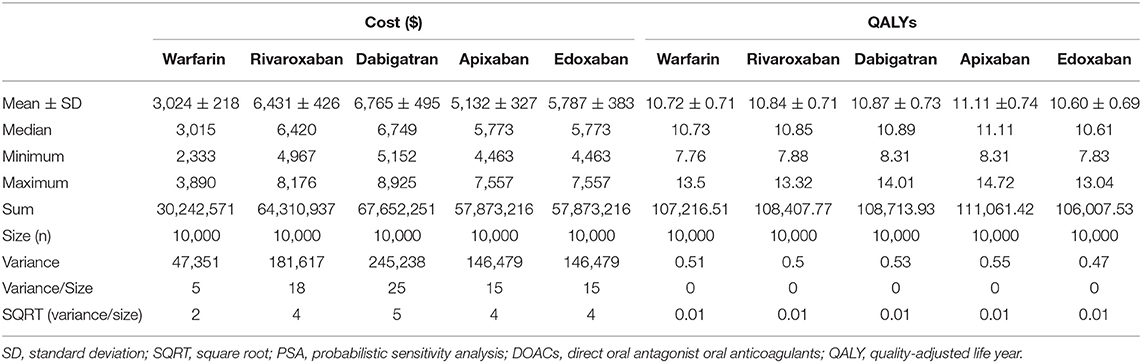
Deterministic sensitivity analysis (DSA) was performed to examine which input parameter among those used in the above cost-effectiveness analysis most affects the model. In addition, probabilistic sensitivity analysis (PSA) was performed using a Monte Carlo simulation (10,000 times). The parameter used in sensitivity analysis shown in Supplementary Table 2.
All analyses were performed in TreeAge Pro 2020 (TreeAge Software, Inc., Williamstown, MA, USA) and SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
The input parameter used to this analysis is shown in Supplementary Table 1.
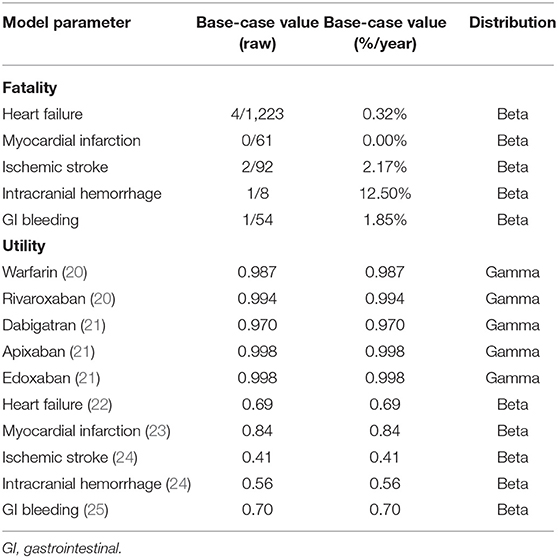
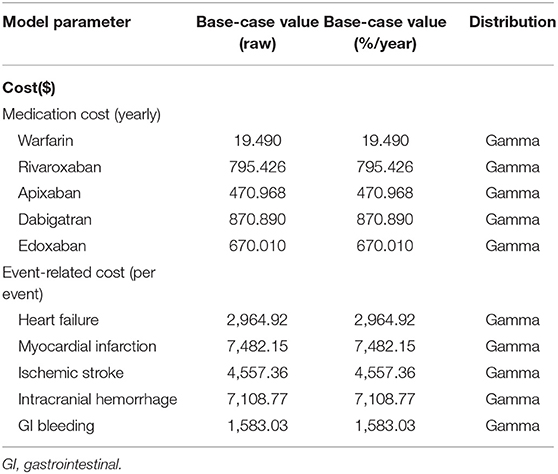
We derived the annual incidence rate from a nationwide database. The annual incidence rate of IS was 0.75, 1.07, 1.50, 4.55, and 1.33% for warfarin, rivaroxaban, apixaban, dabigatran and edoxaban, respectively, with a CHA2DS2-VASc score = 1. Patients with CHA2DS2-VASc score = 2 had annual incidence rates of IS of 1.52, 0.64, 0.62, 0.53 and 0.64% for warfarin, rivaroxaban, apixaban, dabigatran and edoxaban, respectively. The utilities of warfarin, rivaroxaban, apixaban, dabigatran and edoxaban were 0.987, 0.994, 0.998, 0.970 and 0.998, respectively (Table 3). Their annual medication costs were $19.490, $795.420, $470.968, $870.890 and $670.010, respectively (Table 4).
In the base-case analysis, the total cost of warfarin, rivaroxaban, apixaban, dabigatran and edoxaban in non-valvular AF patients was $2,874, $6,379, $5,151, $5,761 and $5,851, respectively (Table 5). The QALYs gained were 10.83, 10.95, 11.10, 10.49 and 10.99 years, respectively. The incremental cost-effectiveness ratios (ICERs) of rivaroxaban, apixaban, dabigatran and edoxaban (compared with warfarin) were $29,743.99, $8,426.71, –$8,483.04 and $18,483.55 each, respectively, and these estimates did not exceed the WTP threshold ($32,000 per QALY). Rivaroxaban, apixaban, and edoxaban were thus cost-effective compared with warfarin.
In the deterministic sensitivity analysis, the input parameter most affecting the ICER was the drug cost in all analyses of DOACs compared with warfarin (Supplementary Figures 3–6). In a probabilistic sensitivity analysis, rivaroxaban, apixaban, dabigatran and edoxaban were cost-effective at 21.19, 49.73, 22.3, and 9.97% compared with warfarin, respectively (Table 6). In the acceptability curves for warfarin and DOACs, the ICERs of apixaban and rivaroxaban were lower than that of warfarin at the WTP ($32,000; Figure 1). The cost-effectiveness scatter plot is presented in Supplementary Figure 7.
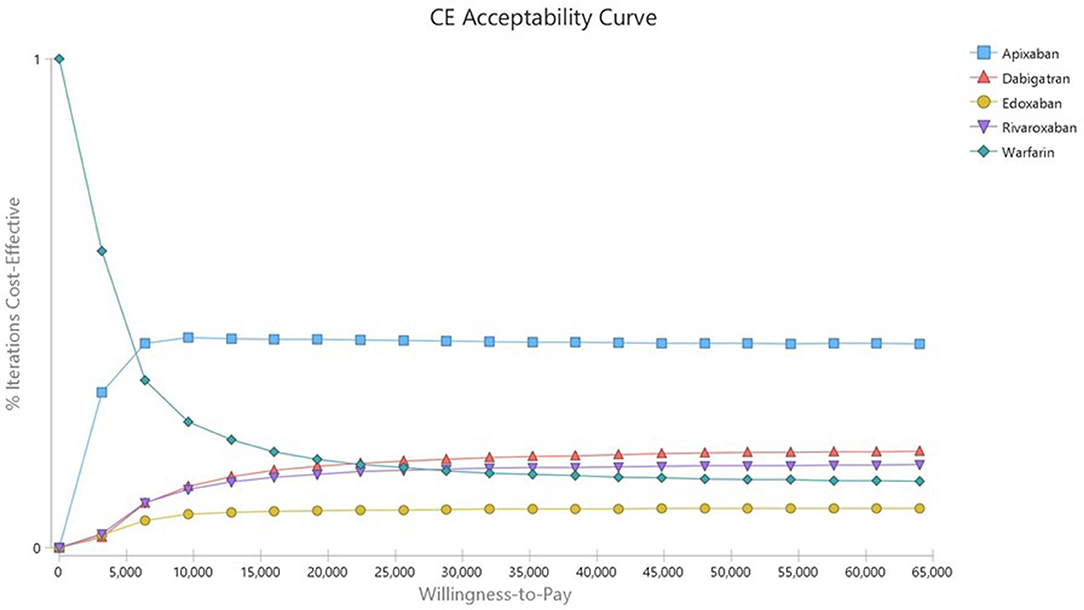
Figure 1. Cost-effectiveness acceptability curve of Warfarin vs. DOACs in AF patients with intermediate stroke risk.
Discussion
This study is the first to investigate the cost-effectiveness of DOACs compared with warfarin in South Korean non-valvular AF patients with intermediate stroke risk with fully independent from pharmaceutical company. In the base-case analysis, apixaban was the best alternative treatment to warfarin (with an ICER of $8,426.71). Rivaroxaban was also cost-effective compared with warfarin (with an ICER of $29,743.99). However, Dabigatran was not cost effective than warfarin (With an ICER of -$8,483.04). Although edoxaban exhibited an ICER of $18,483.55, it was not cost-effective compared with warfarin in the sensitivity analysis.
It is well known based on previous studies that the cost-effectiveness of DOACs is higher than that of warfarin (26, 27), which is consistent with our findings. Furthermore, the present study showed that apixaban and rivaroxaban were cost-effective compared with warfarin in both the base-case and sensitivity analyses (28, 29), which is consistent with previous studies (28–33). By contrast, our sensitivity analysis showed dabigatran and edoxaban to be less cost-effective than warfarin among patients with AF, which is not consistent with previous studies (33, 34). This discrepancy may stem from the difference in study population: the previous studies did not consider stroke risk (26, 27), while the present study focused on the intermediate stroke risk group.
There is little evidence in previous literature on the cost-effectiveness of DOACs in AF patients with intermediate stroke risk, and only a few investigated the cost-effectiveness of DOACs compared with warfarin while considering stroke risk (9, 17, 18). A UK study, which included 1,000 AF patients, showed that apixaban was more cost-effective than aspirin among AF patients with intermediate stroke risk, both having an ICER of $26,852 for a CHADS score of 1 and $14,001 for a CHA2DS2-VASc score of 1, respectively (17). However, our study included 7,954 AF patients with intermediate stroke risk, and the ICER of apixaban was $8,426.71 per QALY. A recent study from the Republic of Korea on AF patients with a CHA2DS2-VASc score of 1 (N=805) found that rivaroxaban was more cost-effective than warfarin (with an ICER of $98,051 per QALY) (9). The present study (N=7,954) included AF patients with intermediate stroke risk (having a CHA2DS2-VASc score of 1 in men 2 in women) and showed that the ICERs of apixaban and rivaroxaban were $8,426.71 and $29,743.99 per QALY, respectively.
In accordance with the previous studies, our findings clearly showed that DOACs were more cost-effective than warfarin. The higher cost-effectiveness of DOACs (vs. warfarin) in AF patients with intermediate stroke risk aligns with the current recommendations from Europe and the USA (13, 14). Nonetheless, DOACs are not subsidized in AF patients with intermediate stroke risk in many countries including the UK, USA, and China. In Korea, National Health Insurance program covers almost 100% of the Korean population (15). Thus, present study may provide evidence on the need for including AF patients with intermediate stroke risk as the beneficiary group of the DOACs' subsidy scheme.
Although our study included all AF patients in Korea during the study period and used real-world hospital utilization data to estimate cost-effectiveness, there are some limitations to be noted. First, the generalizability of the findings to other countries is limited since this study only included Koreans. Although many studies have been conducted in the West, they reported results similar to this study. Second, as an inherent issue of cost-effectiveness research, our findings are based on several assumptions. We assumed that the cost only included medication and health-related costs. Another assumption was that the transition probability evaluated the incidence of health events within 1 year. The other assumption was that we didn't consider health losses related to aging. To examine uncertainty, we performed a sensitivity analysis using Monte Carlo simulation. Third, in this study, the patients who had switched to the counter group had been excluded. The cost-effectiveness of warfarin depends on the time in therapeutic range (TTR), which was not evaluated in the present study (35). Warfarin users with poor TTR maintenance or minor bleeding episodes which were not included in clinical outcome, might underestimate cost of warfarin group. Fourth, because health events usually proceeded by systemic embolism and limitations of diagnosis existed in claims data. We could not consider systemic embolism as a final health event. Thus, considering the preventative effect of DOACs on systemic embolism (36, 37), our estimates might have been underestimated the cost-effectiveness of DOACs.
Lastly, only direct costs related to clinical events were considered in this study. As warfarin has a narrow therapeutic window and interactions with food or drugs, there may be indirect costs related to monitoring of the international normalized ratio, clinic visits, time spent during traveling and waiting, and consultation. This issue may have led to the underestimation of the cost-effectiveness of DOACs vs. warfarin.
In summary, we evaluated the cost-effectiveness of DOACs and warfarin using real-world hospital utilization data. The data of the study might be affected by reimbursement criteria of DOAC, not included the intermediate stroke risk patients in Korea. However, in this group of patients, the incidence of clinical events was substantial and DOAC has shown to be cost effective.
However, because it has been only a few years since DOACs were subsidized in South Korea, the long-term follow-up data are not sufficient to evaluate cost-effectiveness of DOACs and warfarin. Considering the result, the reimbursement scope of DOAC should be extended to the intermediate stroke risk patients who would benefit from DOAC. Therefore, future research on this topic might be necessary.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
The health economic model was designed by J-BK and CK and performed by JChoi and WK. JChoi reviewed literature and drafted the manuscript, which was reviewed and revised by CK, J-BK, SS, and JCho. YK supported statistical method. Results were reviewed and interpreted by CK and J-BK. All authors contributed directly or indirectly to this study and agreed to manuscript submission.
Funding
This study was supported by a Research Grant from the Korean Healthcare Technology R&D Project funded by the Ministry of Health and Welfare (HC19C0130) and Grant (2020-ER6301-00) from the Korea Disease Control and Prevention Agency in the Republic of Korea.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.849474/full#supplementary-material
References
1. Fang MC, Chen J, Rich MW. Atrial fibrillation in the elderly. Am J Med. (2007) 120:481–7. doi: 10.1016/j.amjmed.2007.01.026
2. Joung B, Lee JM, Lee KH, Kim TH, Choi EK, Lim WH, et al. 2018 Korean guideline of atrial fibrillation management. Korean Circ J. (2018) 48:1033–80. doi: 10.4070/kcj.2018.0339
3. Sheikh A, Patel NJ, Nalluri N, Agnihotri K, Spagnola J, Patel A, et al. Trends in hospitalization for atrial fibrillation: epidemiology, cost, and implications for the future. Prog Cardiovasc Dis. (2015) 58:105–16. doi: 10.1016/j.pcad.2015.07.002
4. Kim MH, Johnston SS, Chu B-C, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. (2011) 4:313–20. doi: 10.1161/CIRCOUTCOMES.110.958165
5. Wolowacz SE, Samuel M, Brennan VK, Jasso-Mosqueda J-G, Van Gelder IC. The cost of illness of atrial fibrillation: a systematic review of the recent literature. Europace. (2011) 13:1375–85. doi: 10.1093/europace/eur194
6. Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. (2009) 30:1038–45. doi: 10.1093/eurheartj/ehn579
7. Perino AC, Kaiser DW, Lee RJ, Fan J, Askari M, Schmitt SK, et al. Incidence and outcomes of patients with atrial fibrillation and major bleeding complications: from the TREAT-AF study. J Interv Card Electrophysiol. (2021) 62:133–42. doi: 10.1007/s10840-020-00873-0
8. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. (2014) 114:1453–68. doi: 10.1161/CIRCRESAHA.114.303211
9. Kim H, Kim H, Cho SK, Kim JB, Joung B, Kim C. Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Korean Circ J. (2019) 49:252–63. doi: 10.4070/kcj.2018.0220
10. Wei H, Cui C, Cui X, Liu Y, Li D. Cost-effectiveness analysis of dabigatran, rivaroxaban and warfarin in the prevention of stroke in patients with atrial fibrillation in China. BMC Health Serv Res. (2021) 21:96. doi: 10.1186/s12913-021-06084-1
11. Athanasakis K, Boubouchairopoulou N, Karampli E, Tarantilis F, Savvari P, Bilitou A, et al. Cost effectiveness of apixaban versus warfarin or aspirin for stroke prevention in patients with atrial fibrillation: a Greek perspective. Am J Cardiovasc Drugs. (2017) 17:123–33. doi: 10.1007/s40256-016-0204-1
12. Lopes RD, Berger SE, Di Fusco M, Kang A, Russ C, Afriyie A, et al. A review of global health technology assessments of non-VKA oral anticoagulants in non-valvular atrial fibrillation. Int J Cardiol. (2020) 319:85–93. doi: 10.1016/j.ijcard.2020.06.061
13. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. (2019) 74:104–32. doi: 10.1161/CIR.0000000000000719
14. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. (2021) 42:373-498. doi: 10.1093/eurheartj/ehaa798
15. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea health Insurance review and assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. (2017) 32:718–28. doi: 10.3346/jkms.2017.32.5.718
16. Shen NN, Wu Y, Wang N, Kong LC, Zhang C, Wang JL, et al. Direct oral anticoagulants vs. vitamin-K antagonists in the elderly with atrial fibrillation: a systematic review comparing benefits and harms between observational studies and randomized controlled trials. Front Cardiovasc Med. (2020) 7:132. doi: 10.3389/fcvm.2020.00132
17. Lip GY, Lanitis T, Mardekian J, Kongnakorn T, Phatak H, Dorian P. Clinical and economic implications of apixaban versus aspirin in the low-risk nonvalvular atrial fibrillation patients. Stroke. (2015) 46:2830–7. doi: 10.1161/STROKEAHA.115.009995
18. Wu B, Kun L, Liu X, He B. Cost–effectiveness of different strategies for stroke prevention in patients with atrial fibrillation in a health resource-limited setting. Cardiovasc Drugs Ther. (2014) 28:87–98. doi: 10.1007/s10557-013-6490-9
19. Health Insurance Review and Assessment Service (HIRA). Guidelines for Economic Evaluation of Pharmaceuticals in Korea. (2021). HIRA
20. Gage BF, Cardinalli AB, Owens D. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. (1996) 156:1829–36. doi: 10.1001/archinte.1996.00440150083009
21. Zhao YJ, Lin L, Zhou HJ, Tan KT, Chew AP, Foo CG, et al. Cost-effectiveness modelling of novel oral anticoagulants incorporating real-world elderly patients with atrial fibrillation. Int J Cardiol. (2016) 220:794–801. doi: 10.1016/j.ijcard.2016.06.087
22. Wouters OJ, Naci H, Samani NJ. QALYs in cost-effectiveness analysis: an overview for cardiologists. Heart. (2015) 101:1868–73. doi: 10.1136/heartjnl-2015-308255
23. Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. (2006) 26:410–20. doi: 10.1177/0272989X06290495
24. Luengo-Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM, et al. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology. (2013) 81:1588–95. doi: 10.1212/wnl.0b013e3182a9f45f
25. Kongnakorn T, Lanitis T, Annemans L, Thijs V, Goethals M, Marbaix S, et al. Stroke and systemic embolism prevention in patients with atrial fibrillation in Belgium: comparative cost effectiveness of new oral anticoagulants and warfarin. Clin Drug Investig. (2015) 35:109–19. doi: 10.1007/s40261-014-0253-7
26. Kasmeridis C, Apostolakis S, Ehlers L, Rasmussen LH, Boriani G, Lip GY. Cost effectiveness of treatments for stroke prevention in atrial fibrillation: focus on the novel oral anticoagulants. Pharmacoeconomics. (2013) 31:971–80. doi: 10.1007/s40273-013-0090-1
27. Ferreira J, Mirco A. Systematic review of cost-effectiveness analyses of novel oral anticoagulants for stroke prevention in atrial fibrillation. Rev Port Cardiol. (2015) 34:179–91. doi: 10.1016/j.repce.2014.08.016
28. Lanitis T, Cotté F, Gaudin A, Kachaner I, Kongnakorn T, Durand-Zaleski I. Stroke prevention in patients with atrial fibrillation in France: comparative cost-effectiveness of new oral anticoagulants (apixaban, dabigatran, and rivaroxaban), warfarin, and aspirin. J Med Econ. (2014) 17:587–98. doi: 10.3111/13696998.2014.923891
29. Athanasakis K, Karampli E, Tsounis D, Bilitou A, Kyriopoulos J. Cost-effectiveness of apixaban vs. other new oral anticoagulants for the prevention of stroke: an analysis on patients with non-valvular atrial fibrillation in the Greek healthcare setting. Clin Drug Investig. (2015) 35:693–705. doi: 10.1007/s40261-015-0321-7
30. Hersi AS, Osenenko KM, Kherraf SA, Aziz AA, Sambrook RJ. Cost-effectiveness of apixaban for stroke prevention in non-valvular atrial fibrillation in Saudi Arabia. Ann Saudi Med. (2019) 39:265–78. doi: 10.5144/0256-4947.2019.265
31. Lee S, Mullin R, Blazawski J, Coleman CI. Cost-effectiveness of apixaban compared with warfarin for stroke prevention in atrial fibrillation. PLoS ONE. (2012) 7:e47473. doi: 10.1371/journal.pone.0047473
32. Pinyol C, Cepeda JM, Roldan I, Roldan V, Jimenez S, Gonzalez P, et al. A systematic literature review on the cost-effectiveness of apixaban for stroke prevention in non-valvular atrial fibrillation. Cardiol Ther. (2016) 5:171–86. doi: 10.1007/s40119-016-0066-2
33. Magnuson EA, Vilain K, Wang K, Li H, Kwong WJ, Antman EM, et al. Cost-effectiveness of edoxaban vs warfarin in patients with atrial fibrillation based on results of the ENGAGE AF–TIMI 48 trial. Am Heart J. (2015) 170:1140–50. doi: 10.1016/j.ahj.2015.09.011
34. Dilokthornsakul P, Nathisuwan S, Krittayaphong R, Chutinet A, Permsuwan U. Cost-effectiveness analysis of non-vitamin K antagonist oral anticoagulants versus warfarin in Thai patients with non-valvular atrial fibrillation. Heart Lung Circ. (2020) 29:390–400. doi: 10.1016/j.hlc.2019.02.187
35. Hospodar AR, Smith KJ, Zhang Y, Hernandez I. Comparing the cost effectiveness of non-vitamin k antagonist oral anticoagulants with well-managed warfarin for stroke prevention in atrial fibrillation patients at high risk of bleeding. Am J Cardiovasc Drugs. (2018) 18:317–25. doi: 10.1007/s40256-018-0279-y
36. Martha JW, Pranata R, Raffaelo WM, Wibowo A, Akbar MR. Direct acting oral anticoagulant vs. warfarin in the prevention of thromboembolism in patients with non-valvular atrial fibrillation with valvular heart disease-a systematic review and meta-analysis. Front Cardiovasc Med. (2021) 8:764356. doi: 10.3389/fcvm.2021.764356
Keywords: atrial fibrillation, cost-effectiveness, anticoagulants, warfarin, intermediate stroke risk
Citation: Choi JH, Kim W, Kim YT, Cho J, Shin SY, Kim C and Kim J-B (2022) Cost-effectiveness of Direct Oral Anticoagulant vs. Warfarin Among Atrial Fibrillation Patients With Intermediate Stroke Risk. Front. Cardiovasc. Med. 9:849474. doi: 10.3389/fcvm.2022.849474
Received: 06 January 2022; Accepted: 21 March 2022;
Published: 11 April 2022.
Edited by:
Gen-Min Lin, Hualien Armed Forces General Hospital, TaiwanReviewed by:
Zhi-Chun Gu, Shanghai JiaoTong University, ChinaKalin Clifford, Texas Tech University Health Sciences Center Jerry H. Hodge School of Pharmacy, United States
Francisco C. C. Darrieux, University of São Paulo, Brazil
Copyright © 2022 Choi, Kim, Kim, Cho, Shin, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-Bae Kim, jinbbai@khu.ac.kr; Changsoo Kim, PREMAN@yuhs.ac
 Ju Hee Choi
Ju Hee Choi Woojin Kim
Woojin Kim Yun Tae Kim1
Yun Tae Kim1  Jaelim Cho
Jaelim Cho Seung Yong Shin
Seung Yong Shin Changsoo Kim
Changsoo Kim Jin-Bae Kim
Jin-Bae Kim