The prognostic value of admission D-dimer level in patients with cardiogenic shock after acute myocardial infarction
- Department of Cardiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: Shock is associated with the activation of the coagulation and fibrinolytic system, and D-dimer is the degradation product of cross-linked fibrin. However, the prognostic value of D-dimer in patients with cardiogenic shock (CS) after acute myocardial infarction (AMI) remains unclear.
Methods: We retrospectively analyzed the data of consecutive patients with CS complicating AMI. The primary endpoint was 30-day mortality and the secondary endpoint was the major adverse cardiovascular events (MACEs) including 30-day all-cause mortality, ventricular tachycardia/ventricular fibrillation, atrioventricular block, gastrointestinal hemorrhage, and non-fatal stroke. Restricted cubic spline (RCS) analyses were performed to assess the association between admission D-dimer and outcomes. A multivariable Cox regression model was performed to identify independent risk factors. The risk predictive potency with D-dimer added to the traditional risk scores was evaluated by C-statistics and the net reclassification index.
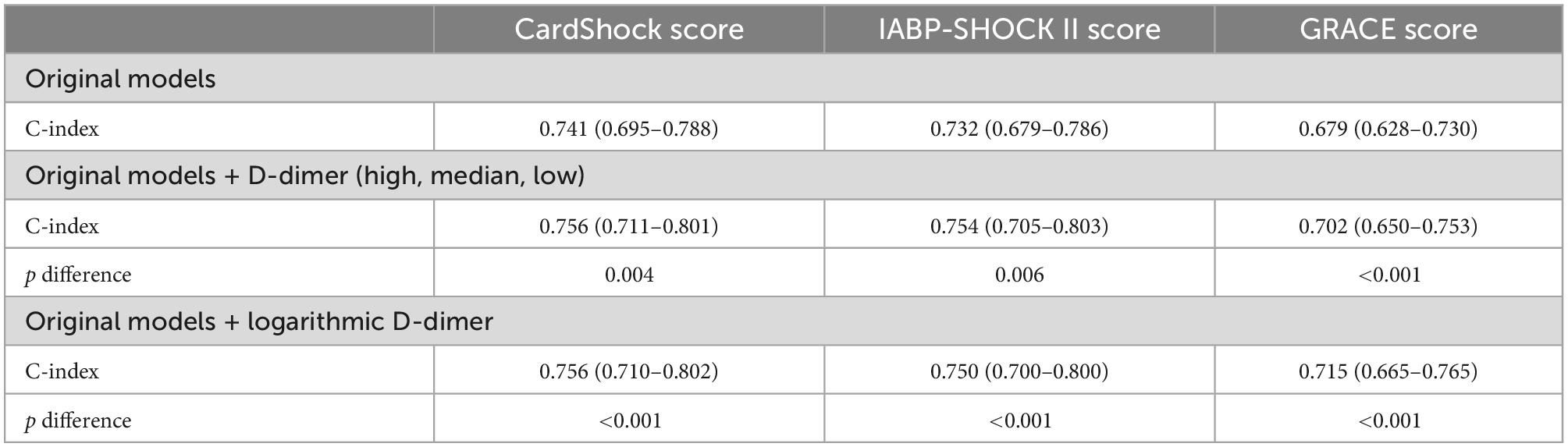
Results: Among 218 patients with CS complicating AMI, those who died during the 30-day follow-up presented with worse baseline characteristics and laboratory test results, including a higher level of D-dimer. According to the X-tile program result, the continuous plasma D-dimer level was divided into three gradients. The 30-day all-cause mortality in patients with low, medium, and high levels of D-dimer were 22.4, 53.3, and 86.2%, respectively (p < 0.001 for all). The 30-day incidence of MACEs was 46.3, 77.0, and 89.7%, respectively (p < 0.001). In the multivariable Cox regression model, the trilogy of D-dimer level was an independent risk predictor for 30-day mortality (median D-dimer cohort: HR 1.768, 95% CI 0.982–3.183, p = 0.057; high D-dimer cohort: HR 2.602, 95% CI 1.310–5.168, p = 0.006), a similar result was observed in secondary endpoint events (median D-dimer cohort: HR 2.012, 95% CI 1.329–3.044, p = 0.001; high D-dimer cohort: HR 2.543, 95% CI 1.452–4.453, p = 0.001). The RCS analyses suggested non-linear associations of D-dimer with 30-day mortality. The enrollment of D-dimer improved risk discrimination for all-cause death when combined with the traditional CardShock score (C-index: 0.741 vs. 0.756, pdifference = 0.004) and the IABP-SHOCK II score (C-index: 0.732 vs. 0.754, pdifference = 0.006), and the GRACE score (C-index: 0.679 vs. 0.715, pdifference < 0.001). Similar results were acquired after logarithmic transformed D-dimer was included in the risk score. The improvements in reclassification which were calculated as additional net reclassification index were 7.5, 8.6, and 12.8%, respectively.
Conclusion: Admission D-dimer level was independently associated with the short-term outcome in patients with CS complicating AMI and addition of D-dimer brought incremental risk prediction value to traditional risk prediction scores.
Introduction
Cardiogenic shock (CS) represents a critical hypoperfusion status resulting from cardiac output failing to meet the metabolic demands of multiple organs and the initial insult can be primarily attributed to cardiac dysfunction. Among the broad spectrum of etiologies, ventricular failure subsequent to acute myocardial infarction (AMI) remains the most frequent cause of CS (1).
Previous studies have shown that shock, regardless of the etiologies, is associated with the activation of coagulation and fibrinolysis (2). D-dimer is the degradation product of cross-linked fibrin, reflecting both thrombin production and the activation of fibrinolysis. Traditionally, atherothrombosis is regarded as the activation of platelets, while venous thromboses with coagulation dysfunction. However, an increasing body of evidence suggested that these two morbidities shared similar pathogenic pathways (3–5). Consequently, increased fibrin turnover is found during atherothrombosis (6).
Previous studies have shown that D-dimer provided risk stratification information for patients with AMI and the elevation of the D-dimer was associated with increased mortality in patients with AMI (7–10). However, the prognostic value of D-dimer has not been well-understood in patients with CS complicating AMI. In the present study, we aimed to evaluate the association of D-dimer with short-term prognosis in patients with CS complicating AMI and whether D-dimer could improve the risk prediction power based on the established risk score system.
Materials and methods
Study design
This retrospective observational study was performed in a single tertiary care institute (The first affiliated hospital of Chongqing medical university, Chongqing, China). Patients diagnosed with CS complicating AMI from January 2013 to September 2020 were enrolled and data included baseline characteristics, laboratory findings. The result of auxiliary examination were extracted from the electrical medical record system of our institution (the Classification of Diseases, 10th Revision, Clinical Modification were used to identify patients). The patients were followed by phone calls or clinical visits. To ensure data accuracy, the diagnosis of all events was reviewed by experienced cardiac physicians. The research protocol was approved by the ethics committee of the first affiliated hospital of Chongqing Medical University.
Definition
Diagnoses and classifications of AMI and CS were made in line with universal definitions up to date. The diagnosis of AMI was made according to the fourth universal definition of myocardial infarction (11). After reviewing and careful evaluation, the diagnosis of CS was made as sustained systolic blood pressure (SBP) < 90 mmHg and cardiac index < 2.2 L/min/m2 with adequate volume load, combined with clinical or laboratory signs of hypoperfusion, or the requirement for inotropic or vasopressor agents or mechanical circulation support to maintain blood pressure and cardiac index. Clinical signs of hypoperfusion include cold extremities, oliguria, mental confusion, dizziness, and narrow pulse pressure, and laboratory findings include metabolic acidosis, elevated serum lactate, and serum creatinine (12–15).
Definitions of events are as follows. All-cause mortality was defined as death from any cause. Arrhythmia was captured by means of electrocardiogram (ECG), Holter document, or electrocardiographic monitoring that was recorded in the medical records. In terms of ventricular tachycardia (VT), only sustained VT was included in our study, which was defined as a ventricular rhythm faster than 100 bpm lasting at least 30 s or requiring termination due to hemodynamic instability. Ventricular fibrillation (VF) was defined as loss of consciousness in patients without identifiable repetitive waveforms or intervals on ECG. Atrioventricular block (AVB) was defined as a delay or interruption in the transmission of an impulse from the atria to the ventricles. Both persistent and paroxysmal AVB were included in the present study. Non-fatal stroke was defined as focal neurologic signs thought to be of vascular origin that persisted for more than 24 hours, confirmed by computed tomographic scans or magnetic resonance imaging. Only symptomatic events were defined as events, and silent stroke was treated as an incidental finding. Gastrointestinal hemorrhage (GIH) was characterized as hematemesis, melena, or both with a hemoglobin decrease of at least 2 g/dL or leading to a transfusion of ≥2 units of blood.
Coronary angiography and percutaneous coronary intervention (PCI) procedure
After taking a loading dose of dual antiplatelet drugs (aspirin 100 mg and ticagrelor 180 mg/clopidogrel 300 mg), patients were immediately transferred to the catheterization laboratory for emergency coronary angiography. According to the angiography results, the revascularization strategy was individualized by the interventionists. Blood flow of the infarct-related artery was assessed according to Thrombolysis in Myocardial Infarction (TIMI) grading system. The usage of relevant devices such as intra-aortic balloon pump (IABP) was at the discretion of the experienced interventionists. After the procedure, all patients were transferred to the coronary care unit for close monitoring.
Laboratory and auxiliary tests
Blood samples for cardiac enzymes and arterial blood gas were collected as soon as patients were admitted to an emergency department and were analyzed at a central laboratory. D-dimer was measured in venous blood at hospital admission, using a kit device (Alere, Triage® Meter) with a transfer pipette for bedside measurement of D-dimer.
The clinical risk scores
The Intra-aortic Balloon Pump in Cardiogenic Shock (IABP-SHOCK II) score and the CardShock score have been described in previous reports (16, 17) and performed well in the validation analysis. The AUC of the CardShock score was 0.85 (95% CI: 0.79–0.90) and the AUC of the IABP-SHOCK II score was 0.79 (95% CI: 0.70–0.88), respectively. The GRACE score has excellent discrimination ability as reflected by the C statistic of 0.84 (18).
Clinical data required for calculating the risk scores were retrieved from medical records, including age, history of stroke, glucose, creatinine, arterial lactate, and TIMI flow grade after PCI.
Endpoints
Since a previous study revealed that most of the adverse events occurred in the early period after AMI (1), we set 30-day all-cause mortality as the primary endpoint. The secondary endpoint was the major adverse cardiovascular events (MACEs) including 30-day all-cause mortality, VT/VF, AVB, GIH, or non-fatal stroke.
Statistical analysis
Categorical variables were presented in numbers and percentages. Continuous variables which followed the normal distribution were presented in mean value and standard deviations, as median value, and interquartile range (25th and 75th) methods were employed for those who were not. Multiple imputations were performed for the missing values of lab test results.
Two independent sample t-test was used for comparisons of continuous variables, and the Mann-Whitney U test was used for those with a non-positive distribution. χ2 test or Fisher test was employed for categorical variables comparison. Survival analysis and cumulative incidence of endpoint events were assessed by Kaplan-Meier plot and log-rank test.
To visually assess the relationship between D-dimer and endpoints events, we used restricted cubic spline (RCS) with four knots at the 5th, 35th, 65th, and 95th centiles to flexibly model the relationship between D-dimer with all-cause mortality. D-dimer was transferred into logarithmic value to alleviate non-linearity. The optimal cut-off points were defined using the X-tile program (Rimm Lab, Yale School of Medicine).
The univariable Cox regression model was used to explore the relationship between D-dimer level and clinical outcomes. Based on the result of the univariable analysis, we took clinical relevance and model stability into consideration to decide which variables were selected for the multivariable Cox regression model.
The ability of risk discrimination was quantified by C-index, which was calculated before and after D-dimer was added to the risk score. The calibration curve was employed to visualize the agreement between model predictions and observation. Improvement in risk prediction was quantified by the reclassification index (NRI). Increases in predicted risk in cases and decreases in non-cases with a variation of more than 5% were regarded as improvements.
A two-tailed p-value of <0.05 was considered statistically significant in this study. All of the analyses were performed with the statistical software R V.3.6.1 (R Foundation for Statistical Computing, Vienna, Austria), and SPSS version 25.0 (IBM, USA).
Results
Baseline characteristics and outcomes
From January 2013 to September 2020, 245 patients were diagnosed with CS-complicating AMI in our institution, among which 27 patients were excluded due to incomplete data or did not undergo coronary angiography. Finally, 218 (89.0%) patients with complete data were analyzed in the present study.
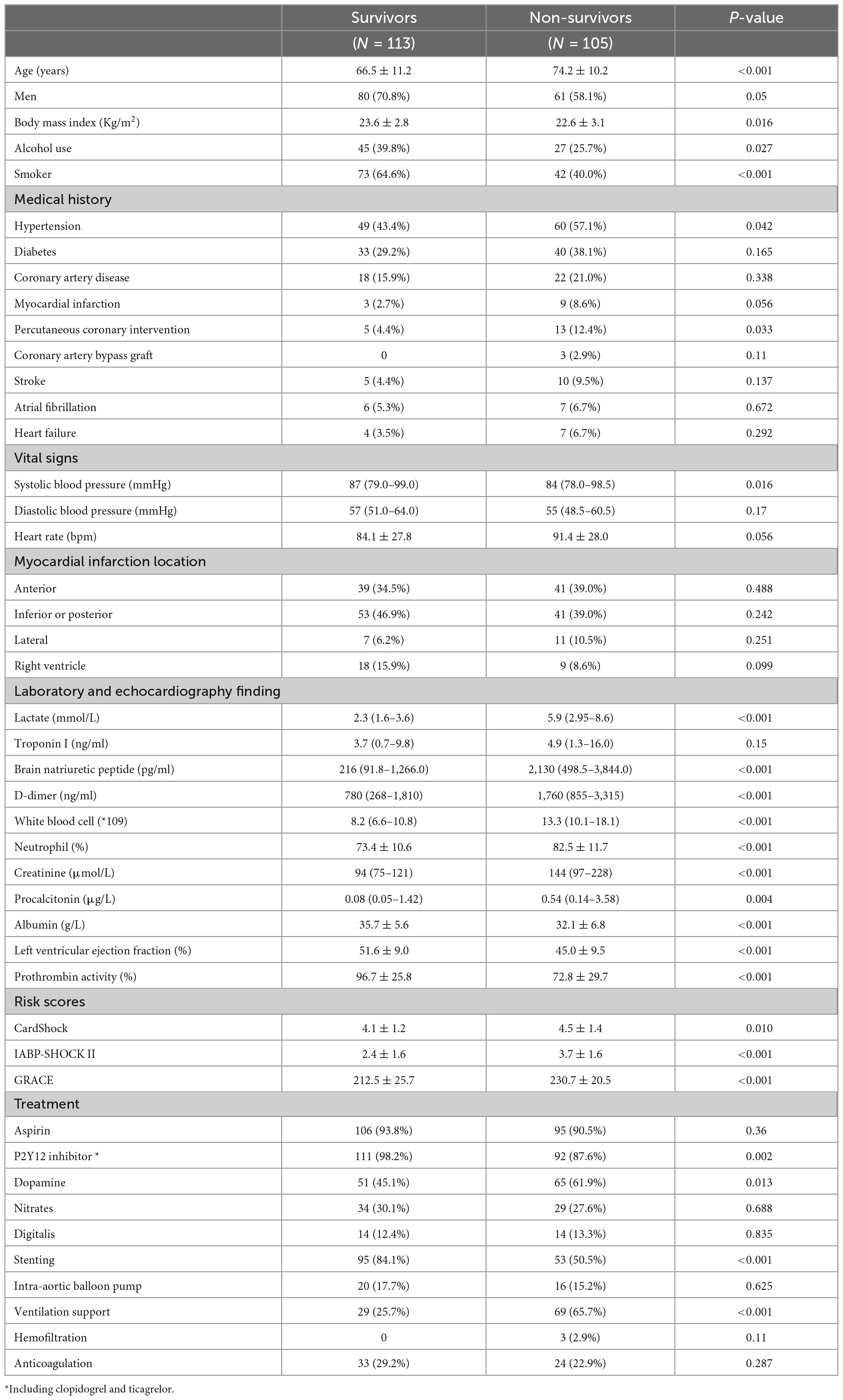
The baseline characteristics were displayed in Table 1. Compared with survivors, the non-survivors tended to be older and female, but less likely to be a drinker or smoker. The non-survivors were more likely to have a history of PCI and hypertension. On admission, the non-survivors presented with lower systolic blood pressure and a higher level of lactate, B-type natriuretic peptide, white blood cell count, creatinine, procalcitonin, and D-dimer (all p < 0.05). The latter group also had relatively lower albumin, prothrombin activity, and left ventricular ejection fraction (all p < 0.05). There was no significant difference in terms of myocardial infarction location between survivors and non-survivors. The risk scores (CardShock, IABP-SHOCK II, GRACE) in non-survivors were significantly higher than in survivors. Stent implantation was performed more frequently in the survivors while the non-survivors were more likely to receive ventilation support (all p < 0.001). As for medication use, except for dopamine and P2Y12 inhibitors, other medications were well-balanced between survival and non-survival patients.
According to the cut-off values of D-dimer derived from the X-tile program, patients were divided into three groups, low D-dimer group (<720 ng/ml), median D-dimer group (720–3,600 ng/ml), and high D-dimer group (>3,600 ng/ml). The baseline characteristics and comparisons among the three groups were displayed in Supplementary Table 1. Generally, patients with high D-dimer levels tended to be older and presented with higher risk scores, lactate, B-type natriuretic peptide, and white blood cell count. Moreover, there was a lower rate of stent implantation in patients with high D-dimer levels.
Associations between D-dimer and outcomes
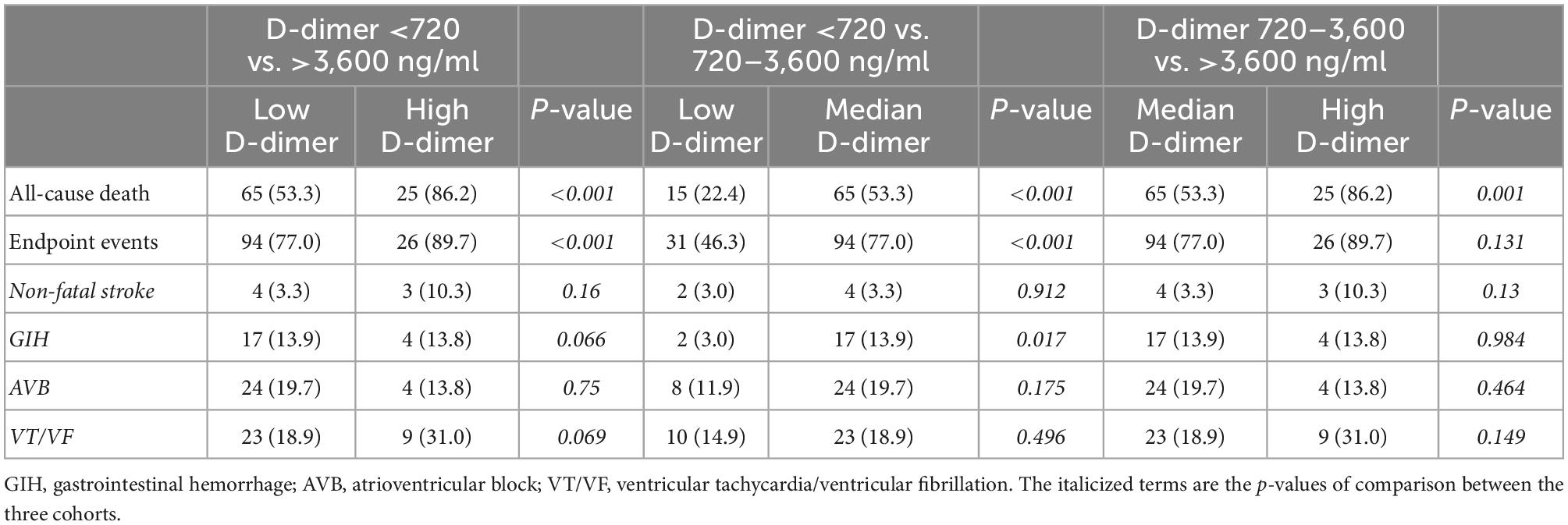
The primary and secondary endpoints are shown in Table 2. The 30-day all-cause mortality and MACEs incidence increased with D-dimer increase (all p < 0.001). The Kaplan-Meier curves are shown in Figure 1. As shown, compared with patients with low D-dimer, patients with median and high D-dimer had a significantly increased risk of 30-day mortality (Figure 1A). Also, as D-dimer increased, the rate of free from MACEs significantly decreased (Figure 1B).
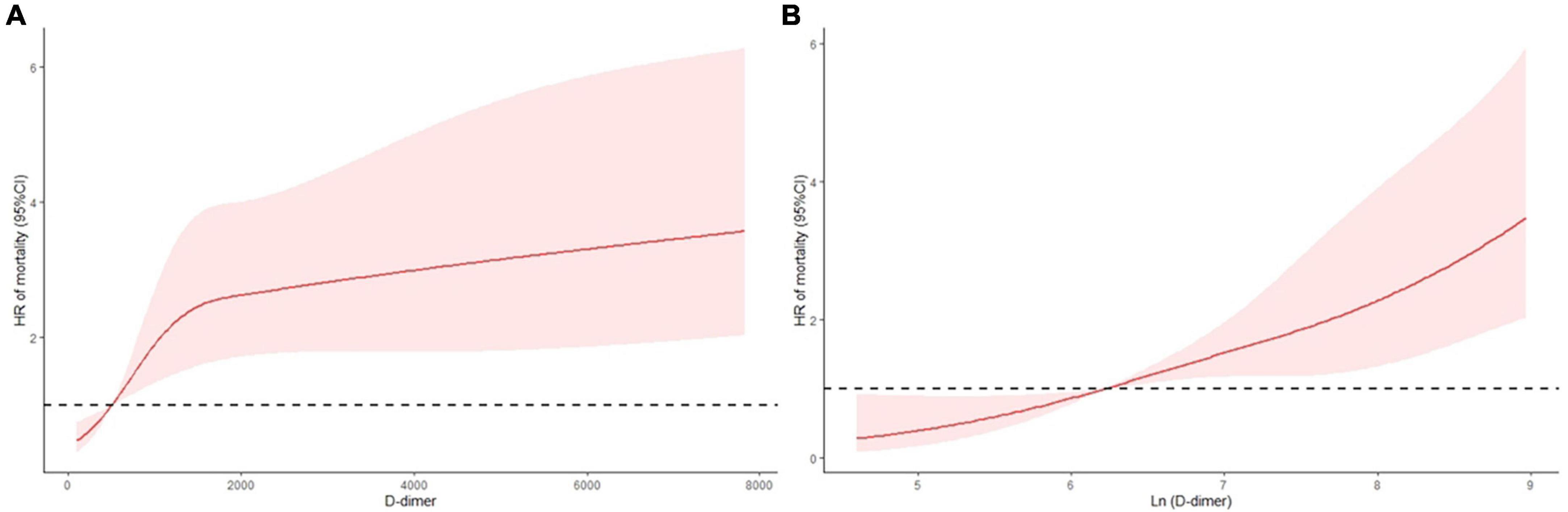
Figure 1. K-M curves for 30-day all-cause mortality and secondary endpoint events according to low (<720 ng/ml), median (720–3,600 ng/ml), and high (>3,600 ng/ml) levels of D-dimer. (A) All-cause mortality, (B) secondary endpoint events.
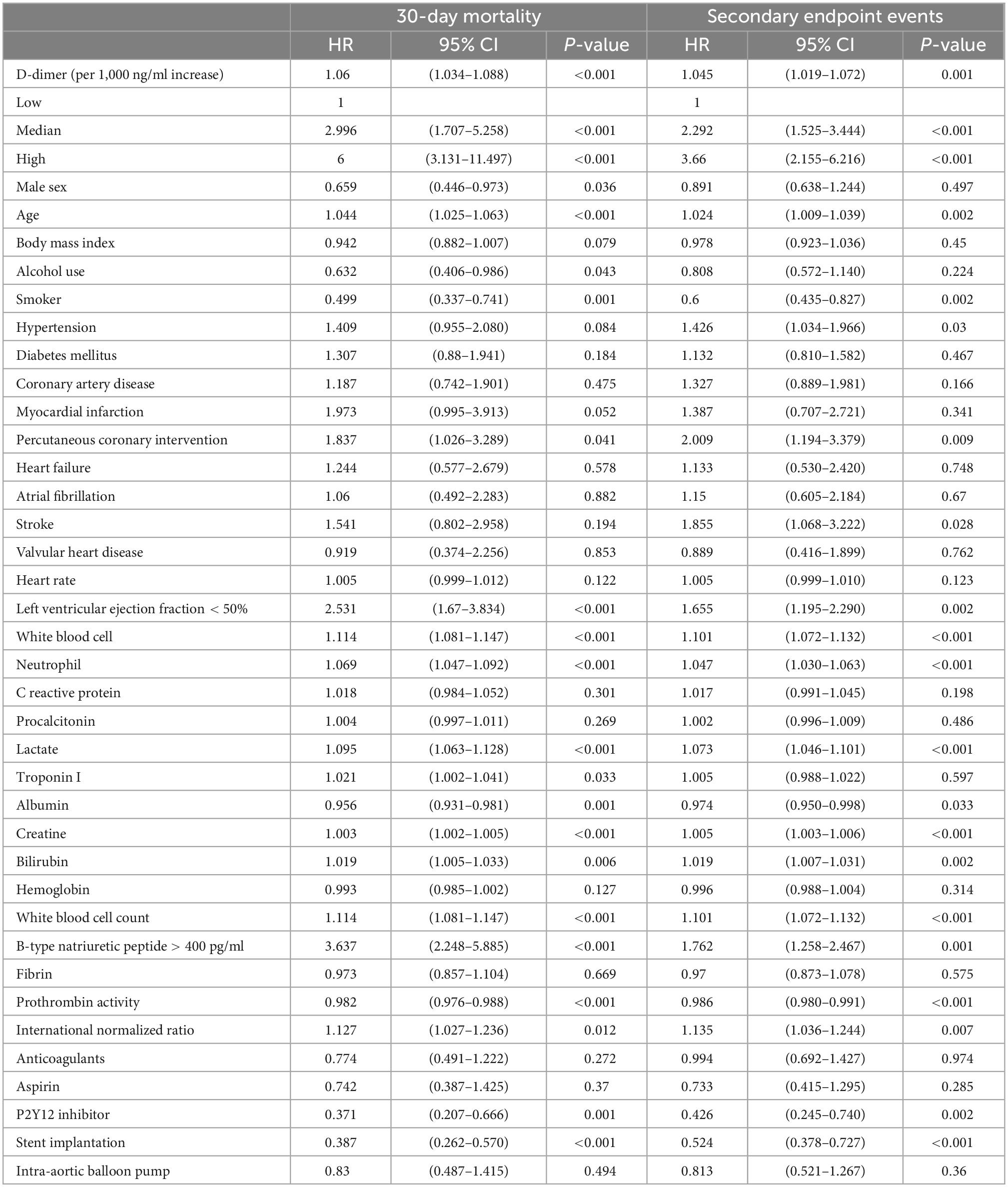
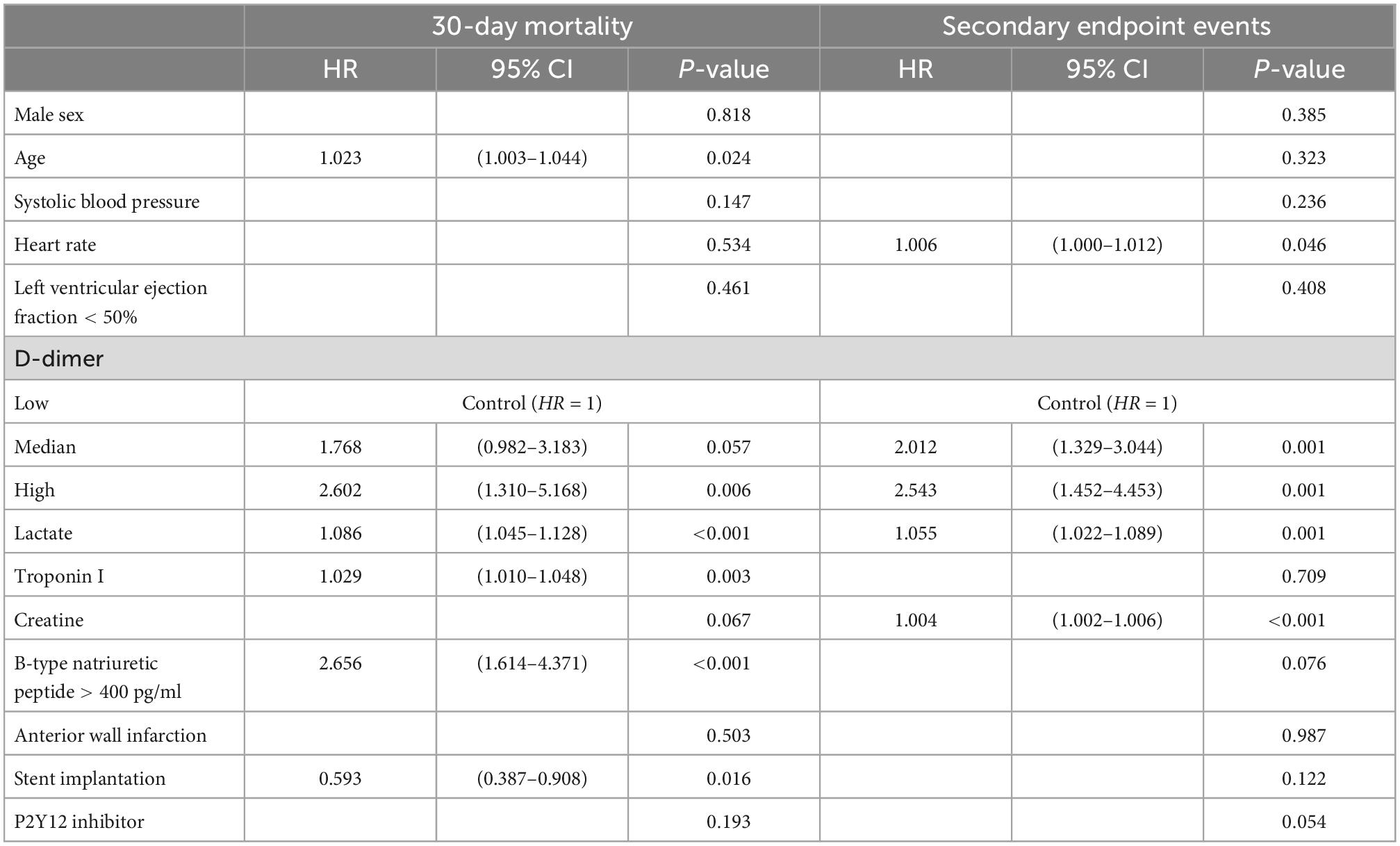
In univariable analysis, a higher D-dimer level (per 1,000 ng/ml increase) was associated with an increased incidence of endpoint events (HR = 1.060, 95% CI 1.034–1.088, p < 0.001 and HR = 1.045, 95% CI 1.019–1.072, p < 0.001 for 30-day mortality and MACEs, respectively) (Table 3). After adjustments for confounding variates, D-dimer remained an independent risk factor in multivariable analysis. Compared with the low D-dimer level group, patients with medium and high levels of D-dimer showed a significantly higher risk of 30-day mortality (HR = 1.768, 95% CI 0.982–3.183, p = 0.057 and HR = 2.602, 95% CI 1.310–5.168, p = 0.006, respectively). A similar relationship was observed for MACEs. Compared with the low D-dimer level group, patients with medium and high levels of D-dimer also had a significantly higher risk of MACEs (HR = 2.012, 95% CI 1.329–3.044, p = 0.001 and HR = 2.543, 95% CI (1.452–4.453), p = 0.001, respectively) (Table 4).
In Figure 2, we employed RCS to flexibly model and visualize the relationship of D-dimer level with all-cause mortality in the studied cohort. The RCS analysis demonstrated that the increase in D-dimer was constantly associated with higher all-cause mortality at relatively lower D-dimer levels (Figure 2A). However, the implications of extremely high D-dimer levels were discordant with those of low D-dimer levels. Nevertheless, after logarithmic transformation, the RCS curve inclined to be linear-like (Figure 2B).
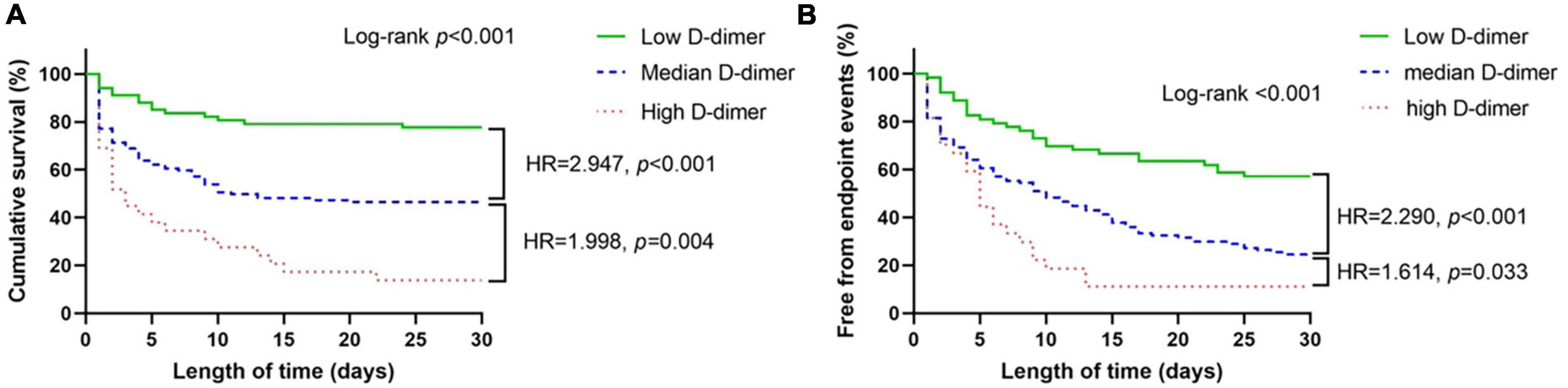
Figure 2. Continuous hazard ratio across D-dimer (A) and logarithmic D-dimer (B) according to restricted cubic spline analysis. HR = hazard ratio, line = predicted HR, dashed area = 95% confidence interval.
Improvement for risk discrimination by D-dimer
The discriminating abilities of external risk scores in our studied cohort were evaluated by C-index, the CardShock score (C-index 0.741, 95% CI 0.695–0.788), the IABP-SHOCK II score (C-index 0.732, 95% CI 0.679–0.786), and the GRACE score (C-index 0.679, 95% CI 0.628–0.730). After addition of D-dimer, the discriminating ability was improved with statistical significance (C-index: 0.756, 95% CI 0.711–0.801 p = 0.0.04, C-index: 0.754, 95% CI 0.705–0.803 p = 0.006, and C-index: 0.702, 95% CI 0.650–0.753 p < 0.001, respectively). Similar results were acquired when logarithmic D-dimer was included (C-index 0.756, 95% CI 0.710–0.802 p < 0.001, C-index 0.750, 95% CI 0.700–0.800 p < 0.001, and C-index 0.715, 95% CI 0.665–0.765 p < 0.001, respectively) (Table 5). The calibration curve showed good agreement between predicted and observed 30-day mortality in all of the abovementioned models (Supplementary Figures 1–4).
The increment of risk reclassification by D-dimer
Using the risk prediction models combined with established risk factors and stratified D-dimer level, we calculated the predicted 30-day mortality and compared it with the actual observations (Figure 3). The average predicted mortality of the high D-dimer level was well-matched with the observed mortality. A similar result was observed for the median and low D-dimer cohorts, and the rates of concordance were 91.0, 77.0, and 86.2% for patients with high, medium, and low D-dimer, respectively. The net reclassification index was calculated for improvements in risk predictions after addition of plasma D-dimer, and the inclusion of D-dimer allowed more patients to be reclassified into more appropriate risk profiles (8.6% in IABP-SHOCK II score, 7.5% in CardShock score, and 12.8% in GRACE score. Detailed information is available in Supplementary Table 2.
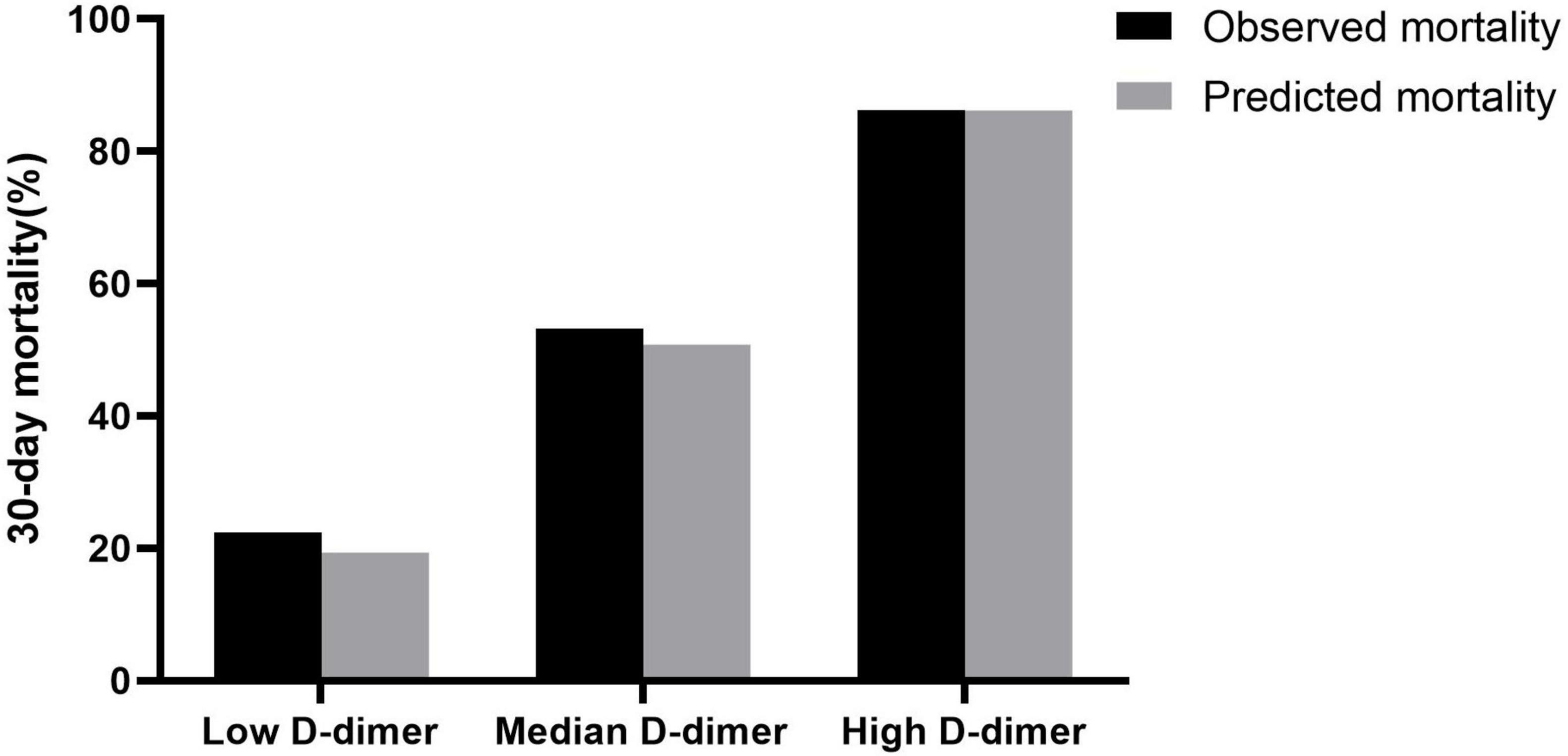
Figure 3. Predicted and observed mortality according to low (<720 ng/ml), median (720–3,600 ng/ml), and high (>3,600 ng/ml) levels of D-dimer at 30 days.
Discussion
The main findings from our present study are as follows. First, admission D-dimer level was independently associated with short-term outcomes in patients with CS complicating AMI. Second, adding D-dimer to the traditional risk models could improve the predictive power. Third, the predictive improvements in risk models were consistent with the data in the real world. To the best of our knowledge, this is the first study to evaluate the association of admission D-dimer with short-term outcomes in patients with CS complicating AMI.
Sepsis, trauma, and myocardial injury are three major causes contributing to shock. Regardless of the initial insult, shock always involves tissue dysoxia, slow capillary flow, and endothelial damage. Damage-associated molecular patterns (DAMPs) released by injured cells, such as histones, mitochondrial DNA, and cell-free DNA, combined with pattern recognition receptors (toll-like receptors, nucleotide-binding oligomerization domain-like receptors, etc.) trigger the signal transduction pathway, leading to expression of inflammatory cytokines. Among all those inflammatory factors, tumor necrosis factor-α (TNF-α) and Interleukin-6 (IL-6) are recognized as major contributors to thrombin generation (19). Inflammatory response with the release of inflammatory factors induced expression of tissue factor on monocytic cells and endothelial cells (20), leading to thrombin generation.
In patients with AMI, unstable plaque ruptures and exposes subendothelial components to blood flow. Highly prothrombotic proteins, including von Willebrand factor and tissue factor, trigger platelet activation and coagulation cascade, further inducing local thrombus formation and homologous myocardial ischemia. Severe cardiac ischemia and subsequent myocardial necrosis lead to profound depression of cardiac contractility and deterioration of cardiac compensation, resulting in a deleterious spiral of reduced cardiac output, low blood pressure, and further coronary ischemia, followed by an additional reduction in contractility (21). In the setting of AMI complicated by CS, systemic activation of inflammation and coagulation occur, resulting in microcirculatory deterioration. Moreover, inflammation and coagulation interact with each other, resulting in a vicious circle. In addition, inflammatory mediators such as Interleukin-6 (IL-1), IL-6, and TNF-α, are released, aggravating coagulation and endothelial damage. The fibrinolytic system is also activated, followed by plasmin digestion of fibrin which results in the generation of D-dimer. Therefore, an elevated D-dimer level indicates procoagulant activity and ongoing fibrinolysis.
The association of D-dimer with the outcome in patients with coronary artery disease has been widely established. The LIPID trial which enrolled patients with stable coronary heart disease demonstrated that higher D-dimer levels were associated with an increased risk of death after a median of 6 years of follow-up (10). Furthermore, the D-dimer level was found to be higher in patients with ST-elevation myocardial infarction (STEMI) than in those with chronic coronary disease or healthy individuals (22). A biomarker sub-study of HORIZONS-AMI also found that D-dimer ≥0.71 μg/ml measured at admission was associated with higher mortality within 3 years follow-up in patients with AMI (23), indicating an ongoing thrombotic and fibrinolytic process during atherogenesis. Therefore, D-dimer is a reliable marker to predict the outcome in patients with coronary heart disease.
Several mechanisms may explain the predictive value of D-dimer for endpoint events in patients with CS complicating AMI. Firstly, elevated admission D-dimer reflected the severity of activated inflammation, coagulation, and fibrinolysis. Secondly, the restoration of patency in coronary arteries by primary PCI may fail to achieve the restoration of tissue perfusion, known as the no-reflow phenomenon, which is an independent predictor of worse outcomes. Ayhan et al. (24) demonstrated that the D-dimer level on admission independently predicted the occurrence of no-reflow after PCI. Among multiple factors involved in no-reflow, a high thrombus burden was well-accepted as one of the strongest factors. Recently, a meta-analysis written by Biccirè et al. (25) demonstrated that in patients with acute coronary syndrome (ACS), D-dimer level was not only positively associated with higher in-hospital and short/long-term complications, but also positively correlated with the no-reflow phenomenon, indicating that D-dimer was a useful marker to identify patients with residual thrombotic risk after ACS. Our present study extended previous findings and demonstrated the prognostic value of D-dimer in patients with CS-complicating AMI. Moreover, previous studies have shown that D-dimer levels reflect clot degradability (26). A higher D-dimer level might indicate a relatively unstable thrombus structure and susceptibility to lysis (26, 27). During interventions on coronary arteries, thrombotic particles occur due to the fragmentation of materials in the culprit lesion (28, 29). Mobilization of thrombotic material and plaque debris could cause distal embolism. A previous study analyzed the components of thrombus aspirated from patients undergoing PCI for STEMI and found that in patients with distal embolization, the clots contained more erythrocyte components, along with a bigger size of clots (30, 31). Clots rich in erythrocytes, known as “red clots,” were characterized by unstable features and worse clinical outcomes (32, 33). Thus, it is feasible that patients with higher D-dimer levels are inclined to be those with “red clots.” Therefore, elevated D-dimer levels may indirectly reflect the thrombus size and components.
In our present study, the non-survivors were older and presented with lower systolic blood pressure and a higher level of lactate, B-type natriuretic peptide, white blood cell count, creatinine, risk scores (CardShock, IABP-SHOCK II, GRACE), but had lower left ventricular ejection fraction and stenting rate, which are all risk factors associated with poor outcome. Notably, nutritional indices are also important prognostic factors. Bicciré et al. (34) recently demonstrated that a low albumin level was associated with worse in-hospital adverse events including CS, resuscitated cardiac arrest, and death in patients with STEMI. Although the albumin level was associated with outcome in the univariable analysis, it was not found to be an independent risk factor in the present study following multivariable analysis. The inconsistency between our study and the study referred to may possibly be due to different inclusion criteria between the two studies. Our present study focused on the CS complicating AMI, while Bicciré et al. enrolled STEMI patients. However, the sample size in both studies was relatively small, and more studies are still warranted to clarify the prognostic value of albumin levels in patients with ACS complicated by CS.
Several models have been established to evaluate the outcome in patients with CS-complicating AMI, such as the IABP-SHOCK II score and CardShock score (17, 18); however, these models had only modest discrimination power, whereas the addition of D-dimer to these traditional models further improved the discrimination power in our study. Moreover, the predictive models were consistent with real practice, underscoring the improved utility of adding D-dimer to the traditional predictive models. Indeed, previous studies have shown that the D-dimer level could predict both the development of heart failure and the outcome in patients with AMI (7, 8). According to our present findings, D-dimer was not only a risk factor but also a predictor for the outcome in patients with CS complicating AMI.
Our present study has some strength in daily practice. First, as a simple testing, D-dimer could provide useful information for risk stratification in patients with CS-complicating AMI. Moreover, the addition of D-dimer to traditional risk models further improved the predictive power. Therefore, D-dimer should be regarded as a factor taking into current risk models. Second, previous studies have shown that the administration of anticoagulation therapy could reduce the D-dimer level (35). Whether reduction of D-dimer by anticoagulation therapy is associated with reduced risk in CS, will require further investigation.
Limitation
As a single-center, small sample size, and retrospective observational study, our present study has some inherent limitations. First, the significant difference in revascularization rate between survivors and non-survivors could cause potential bias, as revascularization is strongly recommended for patients with AMI complicated with CS according to current guidelines (36). Due to the complex lesions or a serious critical condition some patients could not endure a revascularization procedure. The low revascularization rate also indicated a more severe status in non-survivors. Although we adjusted for this confounding factor, it may still cause potential bias. Second, the D-dimer level can be influenced by many clinical conditions such as active infections or pro-thrombotic states. Although the enrolled patients had no tumors or autoimmune diseases, other potential co-existing diseases may cause elevated D-dime. Moreover, whether an active infection and AMI coexisted cannot be completely excluded, because in the setting of AMI or AMI complicated by CS, inflammatory indicators, such as neutrophil and C-reactive protein, are usually elevated and may cause potential confounding effects. In addition, we only collected the D-dimer data at admission and did not perform a series of testing, which may provide more information for the association of D-dimer with the outcome. Therefore, more studies are still warranted to confirm our findings.
Conclusion
Admission D-dimer was an independent risk factor associated with short-term outcome in patients with CS complicating AMI and the addition of D-dimer brought incremental risk prediction value to traditional risk prediction scores.
Data availability statement
The datasets presented in this article are not readily available because based on the privacy policy of our institution, application for the dataset should not be granted at this time. Requests to access the datasets should be directed to BH, huangbi120@163.com.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YJ completed all the analyses, writing, and figure/table development. SL and BH audited all the analyses, writing, and figure/table development. BS, GM, SC, YW, YZ, and ZX contributed advice and expertise on programming, data collection, and editorial support. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank Department of Cardiology, the First Affiliated Hospital of Chongqing Medical University, Chongqing, China for completing the data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1083881/full#supplementary-material
References
1. Chioncel O, Parissis J, Mebazaa A, Thiele H, Desch S, Bauersachs J, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock – a position statement from the heart failure association of the European society of cardiology. Eur J Heart Fail. (2020) 22:1315–41.
2. Vervloet M, Thijs L, Hack C. Derangements of coagulation and fibrinolysis in critically ill patients with sepsis and septic shock. Semin Thromb Hemost. (1998) 24:33–44. doi: 10.1055/s-2007-995821
3. Franchini M, Mannucci P. Association between venous and arterial thrombosis: clinical implications. Eur J Intern Med. (2012) 23:333–7.
4. Lowe G. Coagulation factors, activation markers and risk of coronary heart disease: the Northwick park heart studies. J Thromb Haemost. (2008) 6:256–8.
6. Figueras J, Monasterio Y, Lidón R, Nieto E, Soler-Soler J. Thrombin formation and fibrinolytic activity in patients with acute myocardial infarction or unstable angina: in-hospital course and relationship with recurrent angina at rest. J Am Coll Cardiol. (2000) 36:2036–43.
7. Akgul O, Uyarel H, Pusuroglu H, Gul M, Isiksacan N, Turen S, et al. Predictive value of elevated D-dimer in patients undergoing primary angioplasty for ST elevation myocardial infarction. Blood Coagul Fibrinolysis. (2013) 24:704–10.
8. Zhang X, Wang S, Liu J, Wang Y, Cai H, Wang D, et al. D-dimer and the incidence of heart failure and mortality after acute myocardial infarction. Heart. (2021) 107:237–44.
9. Zhang X, Wang S, Sun L, Fang S, Yu B. Prognostic value of D-dimer in acute myocardial infarction complicated by heart failure with preserved ejection fraction. ESC Heart Fail. (2020) 7:4118–25. doi: 10.1002/ehf2.13027
10. Simes J, Robledo K, White H, Espinoza D, Stewart R, Sullivan D, et al. D-dimer predicts long-term cause-specific mortality, cardiovascular events, and cancer in patients with stable coronary heart disease: LIPID study. Circulation. (2018) 138:712–23. doi: 10.1161/CIRCULATIONAHA.117.029901
11. Thygesen K, Alpert J, Jaffe A, Chaitman B, Bax J, Morrow D, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72:2231–64.
12. Ponikowski P, Voors A, Anker S, Bueno H, Cleland J, Coats A, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. (2016) 18:891–975.
13. Wayangankar S, Bangalore S, McCoy L, Jneid H, Latif F, Karrowni W, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the CathPCI registry. JACC Cardiovasc Interv. (2016) 9:341–51. doi: 10.1016/j.jcin.2015.10.039
14. Mebazaa A, Tolppanen H, Mueller C, Lassus J, DiSomma S, Baksyte G, et al. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med. (2016) 42:147–63. doi: 10.1007/s00134-015-4041-5
15. Furer A, Wessler J, Burkhoff D. Hemodynamics of cardiogenic shock. Interv Cardiol Clin. (2017) 6:359–71.
16. Poss J, Koster J, Fuernau G, Eitel I, de Waha S, Ouarrak T, et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. (2017) 69:1913–20.
17. Harjola V, Lassus J, Sionis A, Kober L, Tarvasmaki T, Spinar J, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. (2015) 17:501–9.
18. Granger C, Goldberg R, Dabbous O, Pieper K, Eagle K, Cannon C, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. (2003) 163:2345–53.
19. Levi M, van der Poll T, ten Cate H, van Deventer S. The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest. (1997) 27:3–9. doi: 10.1046/j.1365-2362.1997.570614.x
20. Alhamdi Y, Toh C. Recent advances in pathophysiology of disseminated intravascular coagulation: the role of circulating histones and neutrophil extracellular traps. F1000Res. (2017) 6:2143. doi: 10.12688/f1000research.12498.1
21. van Diepen S, Katz J, Albert N, Henry T, Jacobs A, Kapur N, et al. Contemporary management of cardiogenic shock: a scientific statement from the American heart association. Circulation. (2017) 136:e232–68.
22. Francis C, Connaghan D, Scott W, Marder V. Increased plasma concentration of cross-linked fibrin polymers in acute myocardial infarction. Circulation. (1987) 75:1170–7.
23. Kikkert W, Claessen B, Stone G, Mehran R, Witzenbichler B, Brodie B, et al. D-dimer levels predict ischemic and hemorrhagic outcomes after acute myocardial infarction: a HORIZONS-AMI biomarker substudy. J Thromb Thrombolysis. (2014) 37:155–64. doi: 10.1007/s11239-013-0953-5
24. Erkol A, Oduncu V, Turan B, Kilicgedik A, Sirma D, Gozubuyuk G, et al. The value of plasma D-dimer level on admission in predicting no-reflow after primary percutaneous coronary intervention and long-term prognosis in patients with acute ST segment elevation myocardial infarction. J Thromb Thrombolysis. (2014) 38:339–47. doi: 10.1007/s11239-013-1044-3
25. Biccirè F, Farcomeni A, Gaudio C, Pignatelli P, Tanzilli G, Pastori D. D-dimer for risk stratification and antithrombotic treatment management in acute coronary syndrome patients: asystematic review and metanalysis. Thromb J. (2021) 19:102. doi: 10.1186/s12959-021-00354-y
26. Undas A, Szuldrzynski K, Stepien E, Zalewski J, Godlewski J, Tracz W, et al. Reduced clot permeability and susceptibility to lysis in patients with acute coronary syndrome: effects of inflammation and oxidative stress. Atherosclerosis. (2008) 196:551–7. doi: 10.1016/j.atherosclerosis.2007.05.028
27. Varin R, Mirshahi S, Mirshahi P, Klein C, Jamshedov J, Chidiac J, et al. Whole blood clots are more resistant to lysis than plasma clots–greater efficacy of rivaroxaban. Thromb Res. (2013) 131:e100–9. doi: 10.1016/j.thromres.2012.11.029
28. Limbruno U, De Carlo M, Pistolesi S, Micheli A, Petronio A, Camacci T, et al. Distal embolization during primary angioplasty: histopathologic features and predictability. Am Heart J. (2005) 150:102–8. doi: 10.1016/j.ahj.2005.01.016
29. Fokkema M, Vlaar P, Svilaas T, Vogelzang M, Amo D, Diercks G, et al. Incidence and clinical consequences of distal embolization on the coronary angiogram after percutaneous coronary intervention for ST-elevation myocardial infarction. Eur Heart J. (2009) 30:908–15. doi: 10.1093/eurheartj/ehp033
30. Napodano M, Ramondo A, Tarantini G, Peluso D, Compagno S, Fraccaro C, et al. Predictors and time-related impact of distal embolization during primary angioplasty. Eur Heart J. (2009) 30:305–13. doi: 10.1093/eurheartj/ehn594
31. Izgi A, Kirma C, Tanalp A, Dundar C, Oduncu V, Aung S, et al. Predictors and clinical significance of angiographically detected distal embolization after primary percutaneous coronary interventions. Coron Artery Dis. (2007) 18:443–9. doi: 10.1097/MCA.0b013e3282a3064e
32. Tosun H, Kamisli S, Tecellioglu M, Alan S, Tecellioglu F, Oztanir M, et al. Red and white thrombus characteristics in patients undergoing carotid endarterectomy. J Stroke Cerebrovasc Dis. (2021) 30:105451. doi: 10.1016/j.jstrokecerebrovasdis.2020.105451
33. Quadros A, Cambruzzi E, Sebben J, David R, Abelin A, Welter D, et al. Red versus white thrombi in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: clinical and angiographic outcomes. Am Heart J. (2012) 164:553–60. doi: 10.1016/j.ahj.2012.07.022
34. Bicciré F, Pastori D, Tanzilli A, Pignatelli P, Viceconte N, Barillà F, et al. Low serum albumin levels and in-hospital outcomes in patients with ST segment elevation myocardial infarction. Nutr Metab Cardiovasc Dis. (2021) 31:2904–11.
35. AlKhalfan F, Kerneis M, Nafee T, Yee M, Chi G, Plotnikov A, et al. D-dimer levels and effect of rivaroxaban on those levels and outcomes in patients with acute coronary syndrome (an ATLAS ACS-TIMI 46 trial substudy). Am J Cardiol. (2018) 122:1459–64. doi: 10.1016/j.amjcard.2018.07.032
Keywords: cardiogenic shock, acute myocardial infarction, D-dimer, risk score, short-term outcome
Citation: Jiang Y, Zhu Y, Xiang Z, Sasmita BR, Wang Y, Ming G, Chen S, Luo S and Huang B (2023) The prognostic value of admission D-dimer level in patients with cardiogenic shock after acute myocardial infarction. Front. Cardiovasc. Med. 9:1083881. doi: 10.3389/fcvm.2022.1083881
Received: 29 October 2022; Accepted: 19 December 2022;
Published: 09 January 2023.
Edited by:
Dennis W. T. Nilsen, Stavanger University Hospital, NorwayReviewed by:
Flavio Giuseppe Biccirè, Sapienza University of Rome, ItalyFrancisco J. Noriega, Hospital Clínico San Carlos, Spain
Copyright © 2023 Jiang, Zhu, Xiang, Sasmita, Wang, Ming, Chen, Luo and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suxin Luo,  luosuxin_0204@163.com; Bi Huang,
luosuxin_0204@163.com; Bi Huang,  huangbi120@163.com
huangbi120@163.com
 Yi Jiang
Yi Jiang Yuansong Zhu
Yuansong Zhu Zhenxian Xiang
Zhenxian Xiang Bryan Richard Sasmita
Bryan Richard Sasmita Yaxin Wang
Yaxin Wang  Gong Ming
Gong Ming Bi Huang
Bi Huang