Disproportionate Mitral Regurgitation Determines Survival in Acute Heart Failure
- 1Department of Cardiology, St. Peter's Hospital, Surrey, United Kingdom
- 2Department of Research and Development, St. Peter's Hospital, Surrey, United Kingdom
- 3Institute of Cardiovascular Research, Royal Holloway University, University of London, Egham, United Kingdom
- 4JB Medical Ltd., Sudbury, United Kingdom
- 5Department of Cardiology, Royal Brompton and Harefield Hospital, London, United Kingdom
Objectives: To assess the prevalence and impact of mitral regurgitation (MR) on survival in patients presenting to hospital in acute heart failure (AHF) using traditional echocardiographic assessment alongside more novel indices of proportionality.
Background: It remains unclear if the severity of MR plays a significant role in determining outcomes in AHF. There is also uncertainty as to the clinical relevance of indexing MR to left ventricular volumes. This concept of disproportionality has not been assessed in AHF.
Methods: A total of 418 consecutive patients presenting in AHF over 12 months were recruited and followed up for 2 years. MR was quantitatively assessed within 24 h of recruitment. Standard proximal isovelocity surface area (PISA) and a novel proportionality index of effective regurgitant orifice/left ventricular end-diastolic volume (ERO/LVEDV) >0.14 mm2/ml were used to identify severe and disproportionate MR.
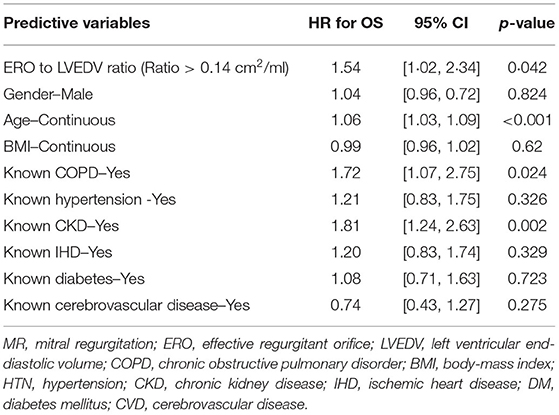
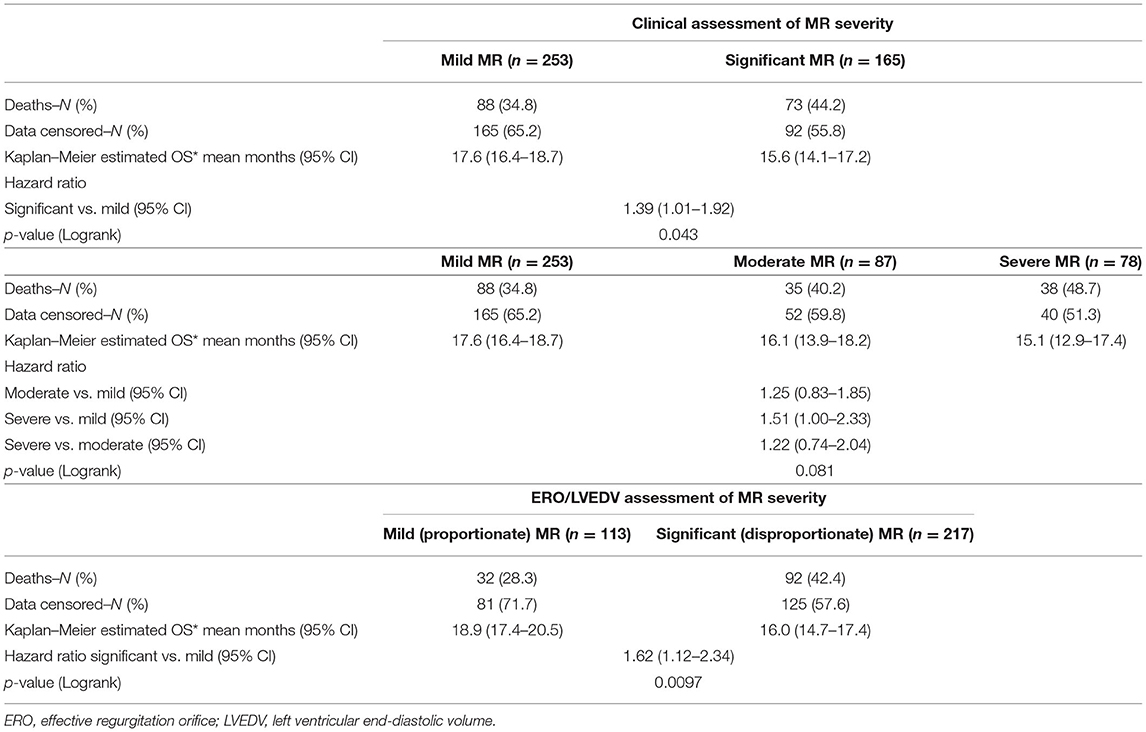
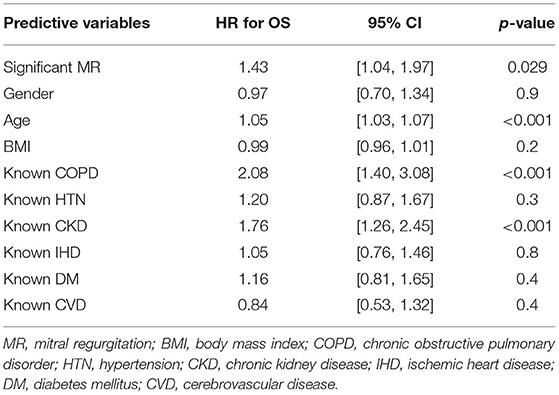
Results: Every patient had MR. About 331/418 (78.9%) patients were quantifiable by PISA. About 165/418 (39.5%) patients displayed significant MR. A larger cohort displayed disproportionate MR defined by either a proportionality index using ERO/LVEDV > 0.14 mm2/ml or regurgitant volumes/LVEDV > 0.2 [217/331 (65.6%) and 222/345 (64.3%), respectively]. The LVEDV was enlarged in significant MR−129.5 ± 58.95 vs. 100.0 ± 49.91 ml in mild, [p < 0.0001], but remained within the normal range. Significant MR was associated with a greater mortality at 2 years {44.2 vs. 34.8% in mild MR [hazard ratio (HR) 1.39; 95% CI: 1.01–1.92, p = 0.04]}, which persisted with adjustment for comorbid conditions (HR; 1.43; 95% CI: 1.04–1.97, p = 0.03). Disproportionate MR defined by ERO/LVEDV >0.14 mm2/ml was also associated with worse outcome [42.4 vs. 28.3% (HR 1.62; 95% CI 1.12–2.34, p = 0.01)].
Conclusions: MR was a universal feature in AHF and determines outcome in significant cases. Furthermore, disproportionate MR, defined either by effective regurgitant orifice (ERO) or volumetrically, is associated with a worse prognosis despite the absence of adverse left ventricular (LV) remodeling. These findings outline the importance of adjusting acute volume overload to LV volumes and call for a review of the current standards of MR assessment.
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT02728739, identifier NCT02728739.
Introduction
Acute heart failure (AHF) is associated with high mortality (1) and remains a substantial financial and healthcare burden (2). The recognition and prevention of precipitating factors, therefore, remain of the utmost importance (3). Acute and worsening of chronic degenerative mitral regurgitation (MR) (4, 5) is a recognized cause of AHF-related hospitalization (6) whereas the role of functional mitral regurgitation (FMR), secondary to cardiac remodeling and left ventricular (LV) dysfunction (7), is less established.
Functional mitral regurgitation (FMR) has a significant impact on morbidity and mortality (8). However, the complexity and heterogeneity of myocardial disease in heart failure (HF) and the subsequent alterations to the mitral valve apparatus have made the quantitative analysis of MR difficult. This has created disagreements between guidelines that suggest differing cut-offs for severe FMR (9, 10). Despite good prognostic value to these assessments (11), there has been no significant benefit from surgery and/or interventions based on these quantitative thresholds (12, 13).
It has become clear that the current standard of echocardiographic assessment, developed for primary MR, where the left heart has the advantage of intrinsic compliance (14) and time to compensate for volume overload (15), cannot be applied automatically to FMR without adjustments. There is emerging evidence that in this group of patients the volume loading from MR should be adjusted to the LV volume. A novel conceptual approach of using the ratio of MR effective regurgitant orifice (ERO) to left ventricular end-diastolic volume (LVEDV) has been suggested to explain differing outcomes in two recent, large, randomized, controlled trials of percutaneous mitral valve repair (16, 17) for patients with HF with significant FMR (18). There are ongoing calls for this approach to be validated in prospective studies (19). We have termed this value the proportionality index (PI).
The analysis of the implications of disproportionate MR has been investigated in individual retrospective assessments of both the MITRA-FR (20) and the COAPT (21) randomized-controlled trials alongside a combined appraisal (22). These assessments have provided conflicting results, with vigorous debate (23–25) and investigations as to the implications of disproportionality assessments based on either EROA/LVEDV > 0.14–0.15 or regurgitant volume (RV)/LVEDV > 0.2 (i.e., 20%) (26). This clearly calls for the assessment of this concept in a “real-world” clinical scenario faced by cardiologists and acute physicians.
Most previous studies have enrolled patients with chronic HF and optimized pharmacotherapy (17). Very little is known on the prevalence and significance of MR in patients presenting in acute HF. The handful of prospective studies investigating its role have not included either early or volumetric assessments and have mainly focused on stable patients (27, 28). Preliminary data from the European Heart Failure survey and US cohort studies suggest MR in hospitalized patients with HF is common (29, 30) but prognostic implications remain unclear. It is possible that MR is missed altogether in patients with AHF due to the dynamic nature of MR (31, 32), particularly if LV volumes remain within an accepted normal range. We, therefore, conducted a study to examine the prevalence and significance of MR in AHF and to determine whether proportionality indices would be effective at identifying patients who face adverse consequences of regurgitant mitral valves.
Methods
Patients and Trial Design
This was a prospective observational study to assess the prevalence of significant MR in consecutive patients admitted with an acute or exacerbation of chronic heart failure (A/ECHF) over 12 months following a 1 month rolling-in period in a single center [St Peter's Hospital (SPH), Chertsey, UK]. Enrolment, data collection, storage, and analysis occurred at this site. Hospital coding data from 2013 to 2016 was used to estimate a recruitment target of 500 patients.
Patients who displayed signs or symptoms of AHF were screened according to the pre-specified study protocol (Appendix 1 in Supplementary Material). Locations of assessment included the accident and emergency department, intensive care unit, high-dependency unit, acute medical unit, coronary care unit, respiratory ward, and care for the elderly ward. If A/ECHF was considered as the primary cause of admission following physician-led clinical examination, patients were consented and recruited into the study if bedside point-of-care brain natriuretic peptide (BNP) level was raised. They underwent transthoracic echocardiography (TTE) within 24 h of recruitment to assess cardiac and valvular function (Appendix 2 in Supplementary Material).
Patients with sepsis, respiratory failure secondary to pulmonary causes, stable chronic HF with an alternative diagnosis, and existing in-patients at the start of recruitment were not included. Patients in whom echocardiography was not possible (deceased, did not consent or discharged) were excluded from further analysis. All recruited patients were followed up for 2 years.
Trial Oversight
The trial was designed by the physician-led executive committee in conjunction with Ashford & St Peter's Hospital Trust Research and Development team. The research protocol was approved by relevant institutional review boards and ethics committees and all participants gave written informed consent. The study complied with the Declaration of Helsinki.
Data were stored electronically and were available for review by all authors. The first and last authors developed the manuscript for submission. The design and implementation of this project and the decision to submit for publication were by the last author. Statistical analysis was carried out by an independent organization with established expertise in the statistical analysis including government policy projects.
Study Data Collection
Diagnosis of AHF on admission was made by a dedicated study physician according to European Society of Cardiology (ESC) guidelines (10). BNP and TTE results were not disclosed to the emergency/acute clinical team. Demographic and past medical history data were identified from hospital records, while sex and ethnicity were self-reported by patients. Mortality data were recorded from the summary care record system used nationally by general practices in the United Kingdom and via the EvolveTM (Kainos, United Kingdom) online software for in-patient deaths recorded by SPH. If unavailable, general practices and family members were directly contacted.
Point-of-Care BNP
Point-of-care BNP measurement was performed using i-STAT Point of Care (POC) Serum BNP analyzer (Abbott, Illinois, USA) with cut-off value >100 pg/ml. This POC system has displayed good clinical agreement at lower BNP values (33). BNP cartridges were acquired and stored according to manufactures guidelines.
Echocardiography
Echocardiography was performed using a dedicated G.E. Vivid S70 (GE Healthcare, Illinois, USA) machine. Images were stored and analyzed offline using EchoPac software version 201 (GE Healthcare, USA). Most of the TTE studies were performed by a single accredited operator according to study protocol (Appendix 3 in Supplementary Material). Every study was analyzed by the primary operator and cross-checked by an expert in echocardiography. Standard echo parameters of left heart geometry: (LVEDV and left ventricular end-systolic volumes (LVESV), LA area (LAA) were measured. MR quantitative analysis was performed using the PISA method to derive MR ERO area and regurgitant volume (RV) (34). Significant MR was defined as MR greater than mild severity, with grading categorized according to ESC guidelines (34). Systolic pulmonary artery pressure (sPAP) was estimated from tricuspid regurgitant jet and jugular vein respiratory fluctuations.
Statistical Analysis
Data analysis was primarily carried out JB. Receiver Operator Curve analyses were carried out for the ERO and the PI (ERO/LVEDV). The optimum cut-off for the prediction of 24-month mortality was estimated by identifying the sensitivity and specificity associated with the maximum Youden Index. These cut-offs were then used as a binary determinant of proportionate vs. disproportionate MR. To evaluate volumetric assessments of proportionality in MR we also included the regurgitant volume/LVEDV and defined proportionate vs. disproportionate MR as < or > 0.2, as outlined in Namazi et al. (26).
Sociodemographic and baseline characteristics were summarized by severity group and overall for the complete analysis set. Categorical variables were reported as numbers and percentages and between-groups comparisons were compared using the chi-squared test or Fisher's exact test, as appropriate. Continuous variables were reported as means and standard deviations or as medians and interquartile ranges and compared using Student's t-test or the Mann–Whitney U-test.
For the primary analysis of 24-month mortality, unstratified Kaplan–Meier curves were constructed. Hazard ratios were estimated using an unadjusted Cox-regression model, with statistical significance being assessed using the log rank test. Secondary analyses were carried out using Cox-regression analyses adjusted for significant covariates. The selection of covariates to be included was based on initial multiple univariate regression analyses, modified according to clinical opinion from the research team. These were gender, age, body-mass index, and pre-existing diagnoses of chronic obstructive pulmonary disorder, hypertension, chronic kidney disease, ischemic heart disease, diabetes mellitus, and cerebrovascular disease. For all comparisons, the threshold of statistical significance was set at a two-sided α value of 0.05.
Data Storage
Enrolled patients had an objective, echocardiographic and clinical characteristics collected via a standardized collection form which was stored online in a password-protected database specifically devised for study by Metanoic Health Ltd., United Kingdom.
Data were entered by primary operators and double-checked by independent specialists. Histograms were performed on all continuous data to screen for statistical outliers using Statistical Package for the Social Sciences (SPSS) version 24 (IBM, New York, USA). Any outlying data points were then rechecked to screen for input errors or errors of measurement. The echocardiography data was retained on two separate hard drives to allow for off-site analysis and to reduce the risk of data loss in accordance with Good Clinical Practice research protocols.
Results
With a 1-month run-in period, 616 consecutive patients presenting with symptoms of A/ECHF were assessed for eligibility for the MRAHF study from July 2016 to August 2017. About 447 (72.6%) participants were recruited. About 418 individuals were included in the final analysis after excluding the data from rehospitalization and three individuals lost to follow-up.
All patients were found to have MR (100%) and 434/447 (97.1%) patients had functional MR as their underlying etiology. Based on clinical interpretation of MR on echocardiography patients were divided into two groups: all patients with moderate and above severity of MR were included in group 1 (significant MR) whereas all other patients in group 2 had mild MR. There was a high prevalence of ESC guideline-defined significant MR in our cohort. 165 (39.5%) of enrolled patients had significant MR, 253 (60.5%) had mild MR.
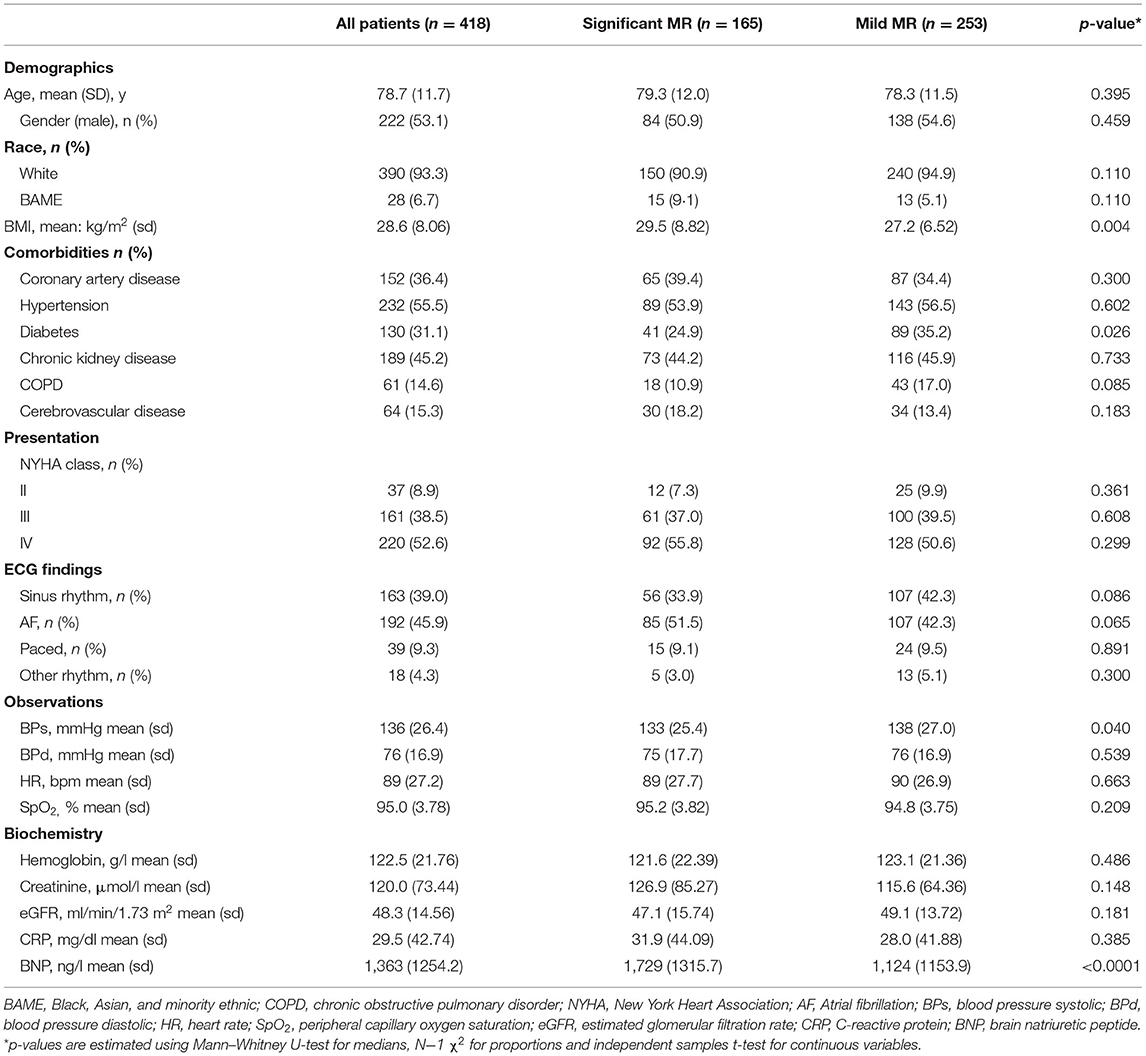
There were broad similarities in demographics, comorbidities, and presenting features between patients with significant and mild MR (Table 1). The mean age across both groups was 78.7; 53.1% were males and 93.3% self-identified as “white” ethnicity. Patients were highly symptomatic −361 (91.1%) with NYHA class III/IV presentation but not in cardiogenic shock [mean blood pressure (BP) 136/76 mmHg]. Patients did not have features of severe anemia or infection. The overall BNP averaged 1,363 ng/l and this was higher in patients with severe MR (1,729 ng/l) compared to mild MR (1,124 ng/l) (p < 0.0001).
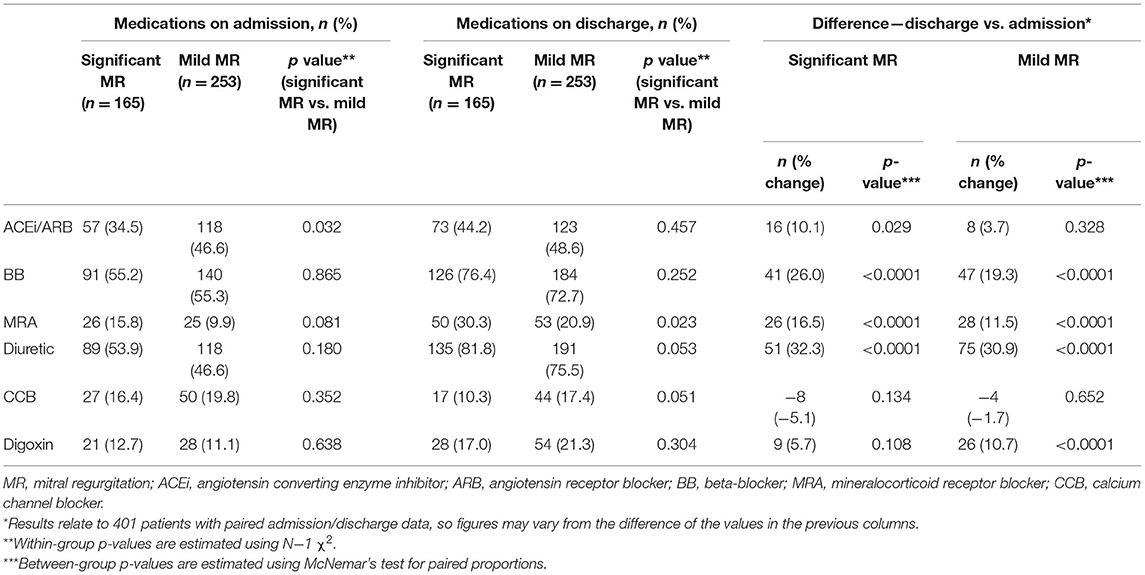
The medical therapy at index admission was similar between both groups (Table 2) except for angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEi/ARBs) which were less common in the group with significant MR [34.5 vs. 46.6% (p = 0.032)]. Both groups had an increase in the intensity of HF therapy at discharge. There was a higher rate of prescription of mineralocorticoid receptor antagonists in the significant MR group [30.3 vs. 20.9% (p = 0.023)]. Based on this, the clinical team, blind to study findings, provided better optimization of medications for patients with significant MR.
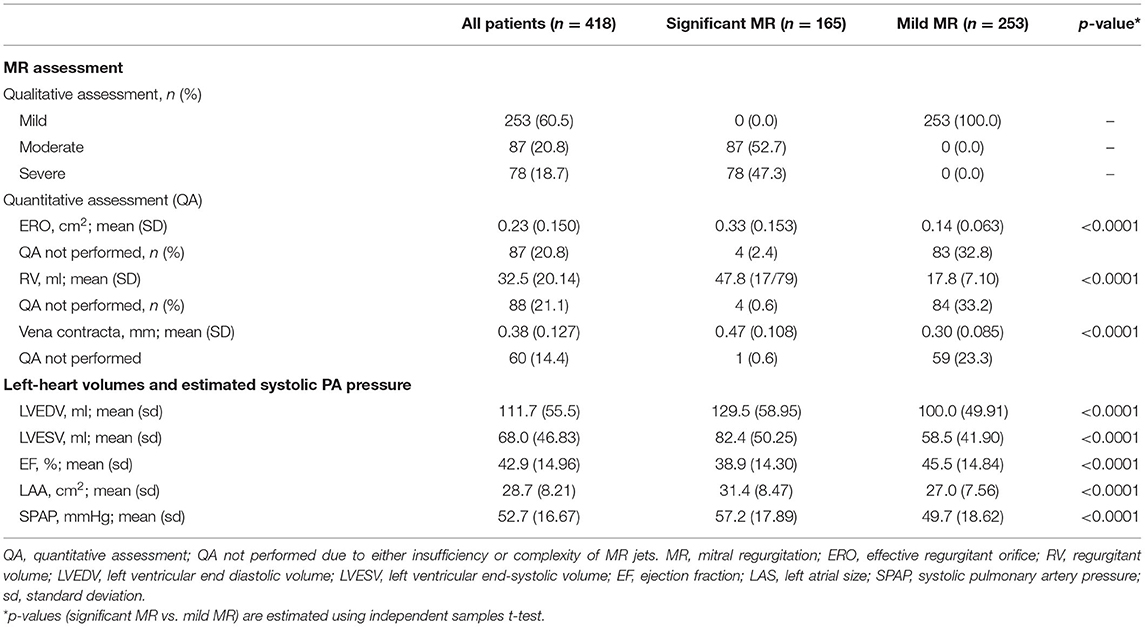
Quantitative assessment of MR on echocardiography indicated significantly higher EROA and regurgitant volume (RV) in significant MR (Table 3). LV volumes remained within the normal range in both groups, however LVEDV [129.5 vs. 100.0 ml (p < 0.0001)] and LVESV [82.4 vs. 58.5 ml (p < 0.0001)] were greater in significant MR. The left atrium was also significantly larger [LAA 31.4 vs. 27.0 cm2 (p < 0.0001)]. LV ejection fraction (LVEF) differed between the groups [38.9 vs. 45.5% (p < 0.0001)] but remained above cut-off level for HF with reduced EF. The estimated sPAP was 57.2 mmHg in significant MR vs. 49.7 mmHg in mild MR [p < 0.0001]. Quantitative assessment was not performed in a minority of mild MR individuals due to insufficiency of jets. Trivial (<4%) numbers of LV/sPAP measurements were not obtained.
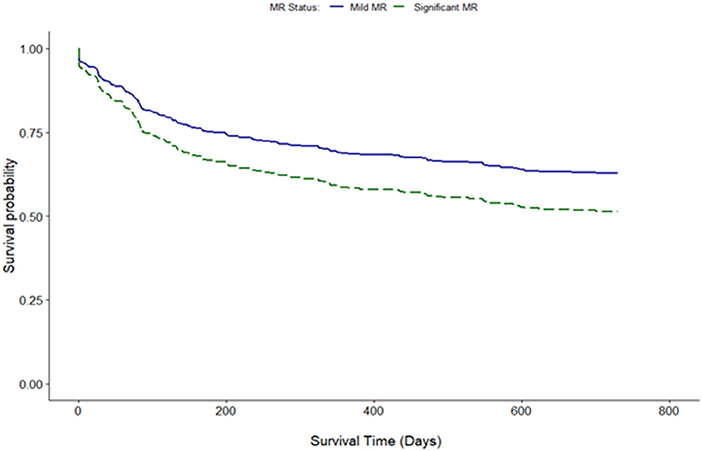
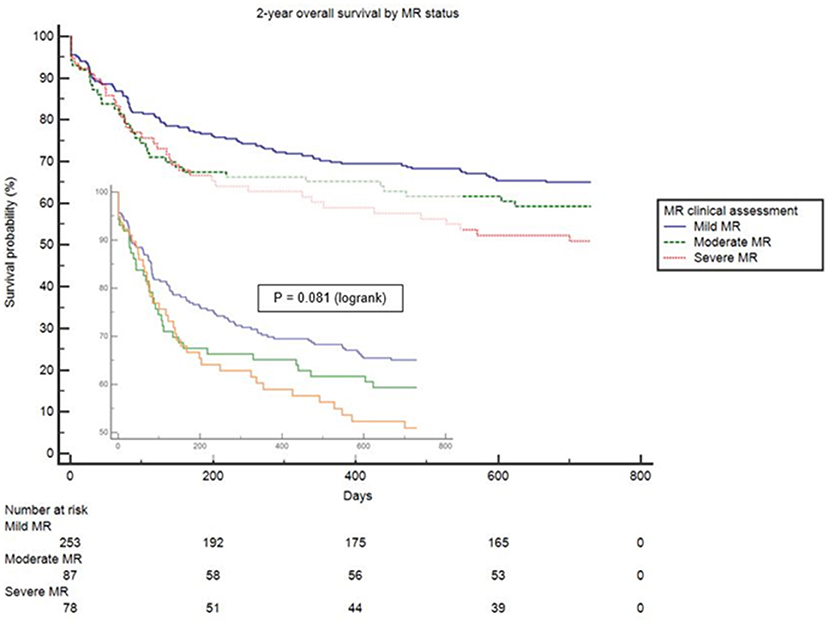
Clinical interpretation of significant MR was an important differentiator in the long-term outcome. At 2 years, those with significant MR had 73 (44.2%) deaths compared with 88 (34.8%) in the mild MR group (hazard ratio 1.39 [CI 1.01–1.92], p = 0.043) (Figure 1). Cox-regression analyses adjusted for multiple covariates confirmed that significant MR is associated with a greater risk of mortality at 2-years [hazard ratio 1.43 (1.04–1.97), p = 0.029] (Figure 2 and Table 4). Traditional echocardiographic grading of the severity of MR displayed a clear trend in survival but was not able to predict significant differences between the three severity grades (p = 0.081) (Figure 3 and Table 5).
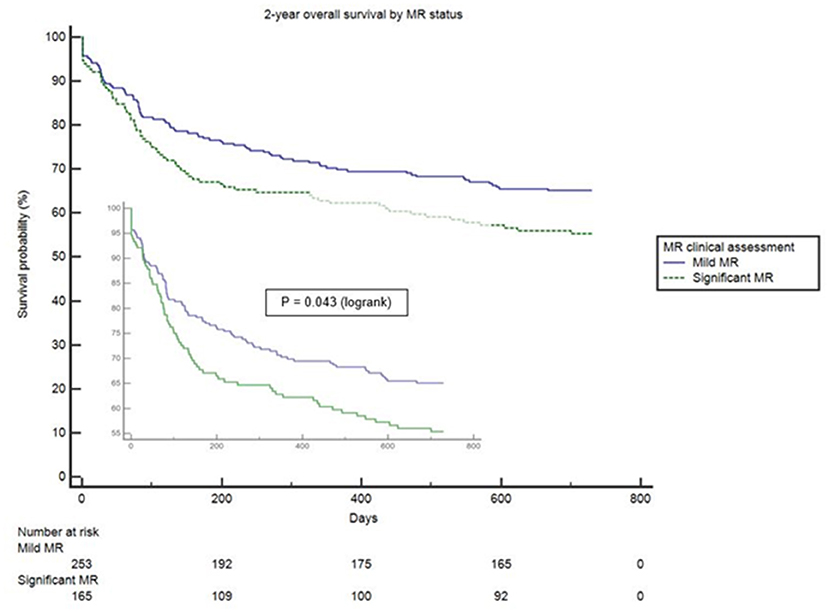
Figure 1. Unadjusted survival curve of 2-year all-cause mortality comparing mild and significant MR.
Proportionality index (PI) cut-off was defined at 0.14 mm2/ml by ROC analysis. Disproportionate MR was discovered in 217/331 individuals (65.6%). Regardless of the magnitude of volume overload, the presence of disproportionate MR was an important predictor of outcome from index event; there were 92 (42.4%) deaths compared with 32 (28.3%) in patients with and without proportionate MR [hazard ratio (HR) 1.62 (CI 1.12–2.34), p = 0.010] (Figure 4 and Table 5). Cox-regression analyses adjusted for multiple covariates also confirmed that disproportionate MR is associated with a greater risk of mortality at 2 years [HR 1.54 (1.02–2.34), p = 0.042] (Figure 5 and Table 6). Volumetric disproportionate MR (defined by RV/LVEDV > 0.2) was discovered similarly in 222/345 (64.3%) patients. There were 95 (42.8%) deaths in patients with disproportionate MR defined by regurgitant volumes, significantly more than the 39 (31.7%) with proportionate MR (p = 0.045).
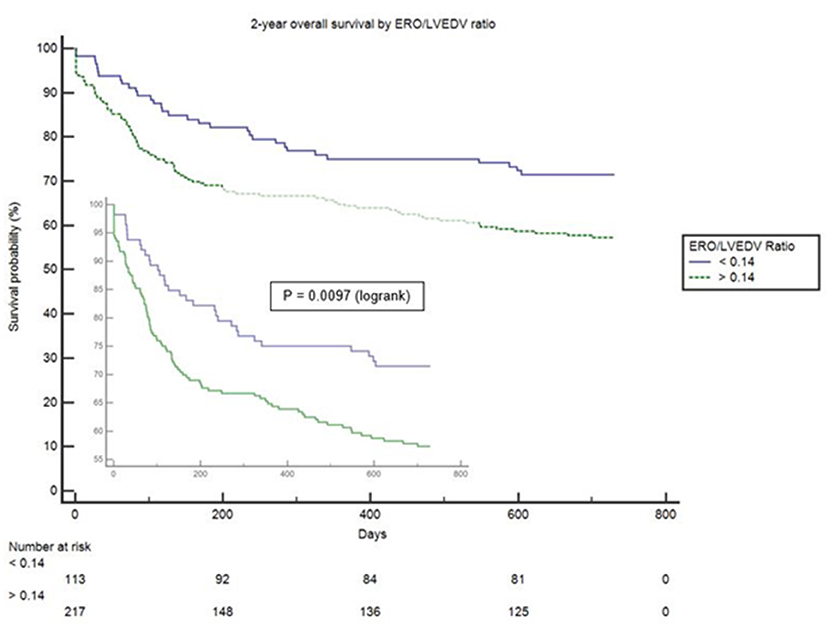
Figure 4. Unadjusted survival curve of 2-year all-cause mortality comparing proportionate and disproportionate MR, defined by an ERO/LVEDV ratio.
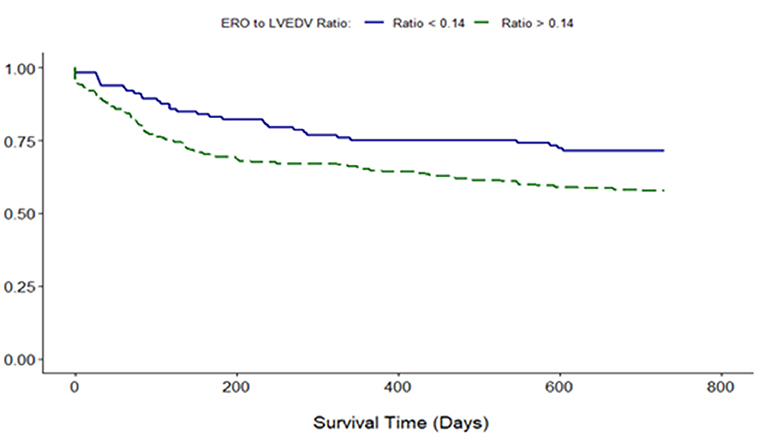
Figure 5. Adjusted survival curve of 2-year all-cause mortality comparing proportionate and disproportionate MR, defined by an ERO/LVEDV ratio.
Discussion
This is the first “real-world” prospective study to assess the prevalence of MR in patients presenting with acute HF to an emergency department before the effect of intensive diuresis. In contrast to previous studies (30), patients with HF presenting with sepsis and other medical emergencies were excluded. Our study revealed that all patients requiring admission had some degree of MR. There was a high prevalence of traditionally defined clinically significant MR of moderate to a severe degree (39.5%), and disproportionate, MR defined by an index of proportionality defined by the ERO/LVEDV > 0.14 (65.6%).
Demographic and other clinical characteristics remained broadly similar between those presenting with significant and mild MR. BNP, a well-established biomarker of ventricular disease severity in degenerative and functional MR (35), was the only distinguishing clinical parameter between patients with and without significant MR. However, there is no clear cut-off level for its use in AHF due to the heterogeneous nature of the myocardial injury. We, therefore, used portable, bedside echocardiography to identify and quantify MR. This was particularly important given that functional MR tends to be dynamic in nature and will likely settle with aggressive diuresis. Dynamic MR has been proven to have a prognostic impact in AHF (28) and we have expanded early hemodynamic assessment further by using volume-indexed parameters of MR.
Functional mitral regurgitation (FMR) is a distinct entity in terms of pathophysiology and prognostic implications (36) and to our knowledge, there is no consensus as to the timing of hemodynamic assessment of MR in AHF. Prior studies investigating MR in HF often require optimization of medical therapy before enrolment (17). Moreover, the severity and stage of underlying left heart geometric change due to ischemia, progressive myocardial disease, and/or LA enlargement makes it difficult to have a uniform approach for the assessment of left heart geometry (37, 38). MR is considered to be severe when the volume of chronic MR expands LV size beyond a given threshold—which has previously been determined to have a prognostic impact (10, 11). In our study, LV volumes were larger in patients with significant MR but remained within the normal range and significantly lower than in patients enrolled in percutaneous intervention trials for MR (16, 17).
The observed 2-year mortality in our cohort (38.5%) did not substantially differ from other AHF studies (1). The differences in short- and long-term mortality remained significant even after multivariate adjustments for comorbid conditions and demographics. Standard echo assessment (32) was useful but did not provide a clear separation point in survival between moderate and severe MR beyond clinical evaluation. Despite the absence of severe LV remodeling, patients with significant MR had higher mortality rates compared to the mild MR group despite similar (if not better) optimization of pharmacotherapy on index admission. At our center discharge medications suggested more intensive HF therapy in patients with significant MR.
When MR EROA was adjusted to LV volumes using a PI > 0.14 mm2/ml we observed a rapid separation in survival from index admission for patients with disproportionate MR. We did not observe differences in either the prevalence or prognostic implications of using the EROA indexed to LVEDV as compared to the MR regurgitant volume.
Our study indicates that hearts that are disproportionately affected by MR carry a greater risk of mortality, suggesting MR is an active driver of poor outcomes. Our study suggests either the EROA or RV is a clinically useful indexing parameter in the context of AHF. Subject to further confirmation by other outcome studies, our data asserts that functional MR should be assessed and managed completely differently to primary MR—using adjustments, namely, ratio/indexed parameters, rather than absolute volumetric analysis, to define thresholds for intervention in FMR patients.
The differences in the pathophysiology between primary and secondary MR (including the rate of change of atrial compliance) should therefore predicate adjustment of echocardiographic evaluation of regurgitant jets, transvalvular flow, the subvalvular apparatus, and the ventricle itself. We suggest that the current standards of cardiac assessment in HF should be updated to reflect the findings from this study and to lower the threshold of LV volumes for prognostically significant MR. This approach to the assessment of functional MR might become an important additional predictive tool to the current biomarkers such as BNP and cardiac troponin (39). This would be of particular benefit to individuals who could undergo surgical/catheter-based interventions to correct FMR.
The strengths of our study include the long-term follow-up, the consecutive enrolment of AHF presentations, and the small number of patients lost to follow-up. A limitation of our study is that it was undertaken at a single center where a majority of our patients self-identified as “White” ethnicity. However, interoperator variability in TTE is a well-characterized limitation of echocardiography and the single-center design of our study facilitated the use of a single operator in most echo assessments in our study, mitigating this limitation. We also did not adjust for differences between treatments in our groups because both admission pharmacotherapy and optimization at discharge occurred similarly between groups according to local and national guidelines. We assume that the difference in mortality would have been broader given more intensive HF therapy in patients with significant MR.
In conclusion, our prospective study demonstrated the high mortality of patients presenting in AHF, particularly those complicated by disproportionate MR. This approach of rapid MR evaluation might help identify those patients likely to benefit from interventions beyond pharmacological optimization. We consider these findings a significant “real-world” addition = to the ongoing debate on the management of disproportionate MR which has direct relevance to both acute physicians and cardiologists. Subject to further confirmatory studies, MR, particularly disproportionate, should not be ignored as a reflection of underlying poor LV performance but viewed as an active driver of poor outcome.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Anonymised/deidentified data will be made available for a period of 6 months from the publication of this article and made available at request with a signed data access agreement. Study protocol and statistical analysis plan will be made available from publication for a period of 6 months.
Ethics Statement
The studies involving human participants were reviewed and approved by Ashford and Saint Peter's NHS Foundation Trust. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MB and AB developed the manuscript for publication. AB is a principal investigator and designed the concept. IB, DF, IJ, PS, and AB planned the study protocol. OL and EA were primarily responsible for echocardiographic data collection and analysis, supervised by AB. JS, JB, IJ, and MB were responsible for patient recruitment, database curation, and verifying the data along with the main statistical analysis which was implemented and designed by JB with clinical input from MB and AB. All authors had access to the database and all authors reviewed the manuscript before publication.
Funding
This study was funded by ASPH R&D and Abbott Laboratories. They had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. AB had final responsibility for the decision to submit for publication.
Conflict of Interest
JB is owner of the company JB Medical Ltd, an independent statistical company which was not involved in the funding of this study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.742224/full#supplementary-material
References
1. Lassus JP, Siirilä-Waris K, Nieminen MS, Tolonen J, Tarvasmäki T, Peuhkurinen K, et al. Long-term survival after hospitalization for acute heart failure—differences in prognosis of acutely decompensated chronic and new-onset acute heart failure. Int J Cardiol. (2013) 168:458–62. doi: 10.1016/j.ijcard.2012.09.128
2. De Lissovoy G, Fraeman K, Teerlink JR, Mullahy J, Salon J, Sterz R, et al. Hospital costs for treatment of acute heart failure: economic analysis of the REVIVE II study. The European Journal of Health Economics. (2010) 11:185–93. doi: 10.1007/s10198-009-0165-2
3. Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. (2009) 53:557–73. doi: 10.1016/j.jacc.2008.10.041
4. Calvo FE, Figueras J, Cortadellas J, Soler-Soler J. Severe mitral regurgitation complicating acute myocardial infarction: Clinical and angiographic differences between patients with and without papillary muscle rupture. Eur Heart J. (1997) 18:1606–10. doi: 10.1093/oxfordjournals.eurheartj.a015140
5. Antoine C, Benfari G, Michelena HI, Maalouf JF, Nkomo VT, Thapa P, et al. Clinical outcome of degenerative mitral regurgitation: critical importance of echocardiographic quantitative assessment in routine practice. Circulation. (2018) 138:1317–26. doi: 10.1161/CIRCULATIONAHA.117.033173
6. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. (2006) 368:1005–11. doi: 10.1016/S0140-6736(06)69208-8
7. Trichon BH, Felker GM, Shaw LK, Cabell CH, O'Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. (2003) 91:538–43. doi: 10.1016/S0002-9149(02)03301-5
8. Lancellotti P, Gérard PL, Piérard LA. Long-term outcome of patients with heart failure and dynamic functional mitral regurgitation. Eur Heart J. (2005) 26:1528–32. doi: 10.1093/eurheartj/ehi189
9. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. (2017) 70:252–89. doi: 10.1016/j.jacc.2017.03.011
10. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91. doi: 10.1016/j.rec.2017.12.013
11. Rossi A, Dini FL, Faggiano P, Cicoira M, Frattini S, Simioniuc A, Gullace M, Ghio S, Enriquez-Sarano M, Temporelli PL. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. (2011) 97:1675–80. doi: 10.1136/hrt.2011.225789
12. Wu AH, Aaronson KD, Bolling SF, Pagani FD, Welch K, Koelling TM. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol. (2005) 45:381–7. doi: 10.1016/j.jacc.2004.09.073
13. Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. (2014) 370:23–32. doi: 10.1056/NEJMoa1312808
14. Reed D, Abbott RD, Smucker ML, Kaul S. Prediction of outcome after mitral valve replacement in patients with symptomatic chronic mitral regurgitation. The importance of left atrial size. Circulation. (1991) 84:23–34. doi: 10.1161/01.CIR.84.1.23
15. Enriquez-Sarano M, Nkomo VT, Michelena HI. Mitral regurgitation. In: Valvular Heart Disease Humana Press (2009). p. 221–46. doi: 10.1007/978-1-59745-411-7_10
16. Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. (2018) 379:2297–306. doi: 10.1056/NEJMoa1805374
17. Stone GW, Lindenfeld J, Abraha WT, Kar S, Lim DS, Mishell JM, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. (2018) 379:2307–18. doi: 10.1056/NEJMoa1806640
18. Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: a new conceptual framework that reconciles the results of the MITRA-FR and COAPT trials. Cardiovasc Imag. (2019) 12:353–62. doi: 10.1016/j.jcmg.2018.11.006
19. O'Gara PT, Mack MJ. Secondary Mitral Regurgitation. N Engl J Med. (2020) 383:1458–67. doi: 10.1056/NEJMcp1903331
20. Messika-Zeitoun D, Iung B, Armoiry X, Trochu JN, Donal E, Habib G, et al. Impact of mitral regurgitation severity and left ventricular remodeling on outcome after MitraClip implantation: results from the MITRA-FR trial. Cardiovasc Imag. (2021) 14:742–52. doi: 10.1016/j.jcmg.2020.07.021
21. Lindenfeld J, Abraham WT, Grayburn PA, Kar S, Asch FM, Lim DS, et al. Association of effective regurgitation orifice area to left ventricular end-diastolic volume ratio with transcatheter mitral valve repair outcomes: a secondary analysis of the COAPT trial. JAMA Cardiol. (2021) 6:427–36. doi: 10.1001/jamacardio.2020.7200
22. Gaasch WH, Aurigemma GP, Meyer TE. An appraisal of the association of clinical outcomes with the severity of regurgitant volume relative to end-diastolic volume in patients with secondary mitral regurgitation. JAMA Cardiol. (2020) 5:476–81. doi: 10.1001/jamacardio.2019.5980
23. Goliasch G, Bartko PE. The paradox of secondary mitral regurgitation: why less is more. JACC: Cardiovasc Imaging. (2021) 14:740–1 doi: 10.1016/j.jcmg.2020.08.016
24. Namazi, Delgado & Bax. The author's reply. JACC: Cardiovascular Imaging. (2021) 14:880–1. doi: 10.1016/j.jcmg.2021.01.039
25. Gillam L. D. (2021). Reconciling COAPT and mitra-FR results based on mitral regurgitation severity and left ventricular size: it's not so simple. JAMA Cardiol. (2021) 6:376–8. doi: 10.1001/jamacardio.2020.7214
26. Namazi F, van der Bijl P, Fortuni F, Mertens BJ, Kamperidis V, van Wijngaarden SE, et al. Regurgitant volume/left ventricular end-diastolic volume ratio: prognostic value in patients with secondary mitral regurgitation. JACC: Cardiovasc Imaging. (2021) 14:730–9. doi: 10.1016/j.jcmg.2020.06.032
27. De la Espriella R, Santas E, Miñana G, Bodí V, Valero E, Payá R, et al. Functional mitral regurgitation predicts short-term adverse events in patients with acute heart failure and reduced left ventricular ejection fraction. Am J Cardiol. (2017) 120:1344–8. doi: 10.1016/j.amjcard.2017.07.023
28. Kubo S, Kawase Y, Hata R, Maruo T, Tada T, Kadota K. Dynamic severe mitral regurgitation on hospital arrival as prognostic predictor in patients hospitalized for acute decompensated heart failure. Int J Cardiol. (2018) 273:177–82. doi: 10.1016/j.ijcard.2018.09.093
29. Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, et al. The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe: Part 1: patient characteristics and diagnosis. Eur Heart J. (2003) 24:442–63. doi: 10.1016/S0195-668X(02)00823-0
30. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Euro Heart J. (2006) 27:2725–36. doi: 10.1093/eurheartj/ehl193
31. Hung J, Otsuji Y, Handschumacher MD, Schwammenthal E, Levine RA. Mechanism of dynamic regurgitant orifice area variation in functional mitral regurgitation: physiologic insights from the proximal flow convergence technique. J Am Coll Cardiol. (1999) 33:538–45. doi: 10.1016/S0735-1097(98)00570-1
32. Khabbaz KR, Mahmood F, Shakil O, Warraich HJ, Gorman JH III, Gorman RC, et al. Dynamic 3-dimensional echocardiographic assessment of mitral annular geometry in patients with functional mitral regurgitation. Ann Thoracic Surg. (2013) 95:105–10. doi: 10.1016/j.athoracsur.2012.08.078
33. Shah K, Terracciano GJ, Jiang K, Maisel AS, Fitzgerald RL. Comparability of results between point-of-care and automated instruments to measure B-type natriuretic peptide. West J Emerg Med. (2010) 11:44.
34. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Euro Heart J. (2013) 14:611–44. doi: 10.1093/ehjci/jet105
35. Detaint D, Messika-Zeitoun D, Chen HH, Rossi A, Avierinos JF, Scott C, et al. Association of B-type natriuretic peptide activation to left ventricular end-systolic remodeling in organic and functional mitral regurgitation. Am J Cardiol. (2006) 97:1029–34. doi: 10.1016/j.amjcard.2005.10.061
36. Pierard LA, Carabello BA. Ischaemic mitral regurgitation: pathophysiology, outcomes and the conundrum of treatment. Eur Heart J. (2010) 31:2996–3005. doi: 10.1093/eurheartj/ehq411
37. Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence or absence of asynergy. Am J Cardiol. (1976) 37:7–11. doi: 10.1016/0002-9149(76)90491-4
38. Anwar AM, Soliman OI, Geleijnse ML, Nemes A, Vletter WB, Folkert J. Assessment of left atrial volume and function by real-time three-dimensional echocardiography. Int J Cardiol. (2008) 123:155–61. doi: 10.1016/j.ijcard.2006.12.017
Keywords: acute heart failure (AHF), mitral regurgitation, disproportionate mitral regurgitation, heart failure, disproportionate MR, disproportionate
Citation: Berrill M, Beeton I, Fluck D, John I, Lazariashvili O, Stewart J, Ashcroft E, Belsey J, Sharma P and Baltabaeva A (2021) Disproportionate Mitral Regurgitation Determines Survival in Acute Heart Failure. Front. Cardiovasc. Med. 8:742224. doi: 10.3389/fcvm.2021.742224
Received: 21 July 2021; Accepted: 25 October 2021;
Published: 02 December 2021.
Edited by:
Gaetano Ruocco, Regina Montis Regalis Hospital, ItalyReviewed by:
Eisuke Amiya, The University of Tokyo Hospital, JapanGuido Pastorini, Regina Montis Regalis Hospital, Italy
Copyright © 2021 Berrill, Beeton, Fluck, John, Lazariashvili, Stewart, Ashcroft, Belsey, Sharma and Baltabaeva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aigul Baltabaeva, a.baltabaeva@rbht.nhs.uk
 Max Berrill
Max Berrill Ian Beeton1
Ian Beeton1  Aigul Baltabaeva
Aigul Baltabaeva