The Association Between Metformin Treatment and Outcomes in Type 2 Diabetes Mellitus Patients With Heart Failure With Preserved Ejection Fraction: A Retrospective Study
- 1Department of Cardiology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Ningbo Medical Center Lihuili Hospital, Ningbo, China
- 3Department of Urology, Children's Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 4Jiaxing Key Laboratory of Cardiac Rehabilitation, Jiaxing, China
Background: Metformin is the first-line antidiabetic medication for type 2 diabetes mellitus (T2DM). However, the association between metformin and outcomes in T2DM patients with heart failure with preserved ejection fraction (HFpEF) is still unknown. We aimed to explore the association between metformin and adverse outcome in T2DM patients with HFpEF.
Methods: A total of 372 T2DM patients with HFpEF hospitalized from January 1, 2013, to December 31, 2017, were included in this retrospective cohort study. There were 113 and 259 subjects in metformin and non-metformin group, respectively. Subjects were followed up for all-cause mortality, cardiovascular death, all-cause hospitalization, and heart failure hospitalization.
Results: The median follow-up period was 47 months. Eleven patients (2.49% per patient-year) in the metformin group and 56 patients (5.52% per patient-year) in the non-metformin group deceased during follow-up (P = 0.031). However, a multivariable Cox regression failed to show that metformin was an independent factor of all-cause mortality [HR (95% CI) = 0.682 (0.346–1.345); P = 0.269]. A subgroup analysis revealed a significant association between metformin and all-cause mortality in patients with a higher hemoglobin A1c (HbA1c) level (HbA1c ≥7%) [HR (95% CI) = 0.339 (0.117–0.997); P = 0.045]. The 4-year estimated number needed to treat (NNT) with metformin compared with non-metformin for all-cause mortality was 12 in all populations and 8 in the HbA1c ≥7% subgroup.
Conclusions: Metformin was not independently associated with clinical outcomes in patients with T2DM and HFpEF, but was associated with lower all-cause mortality in the subgroup of patients with poor glycemic control. Prospective, randomized controlled trials are needed to further verify these findings.
Introduction
Heart failure with preserved ejection fraction (HFpEF) might be a heterogeneous syndrome of multiple discrete phenotypes and is prone to have multiple comorbidities, such as diabetes, hypertension, pulmonary disease, chronic kidney disease, and obesity (1), resulting in systemic and cardiac microvascular dysfunction (2, 3). Conventional therapies including angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARB), beta-blockers, mineralocorticoid receptor antagonists (MRA) can improve the long-term outcomes of heart failure with reduced ejection fraction (HFrEF) (4). However, these conventional medical therapies failed to reduce the risk of all-cause and cardiovascular death in HFpEF patients (5).
Type 2 diabetes mellitus (T2DM) is a common comorbidity in HFpEF and has a conspicuous negative impact on prognosis (6). As first-line antidiabetic therapy, metformin has cardiovascular protective effect through multiple mechanisms, including decreasing glucose, lowering weight, anti-inflammatory properties, and improving insulin resistance and endothelial function (7). Several observational studies indicated that metformin was associated with reduced mortality risk compared with other traditional antidiabetic drugs in T2DM patients with HF (patients with preserved or reduced left ventricular ejection fraction were included) (8). However, the impact of metformin on the outcome of HFpEF in T2DM patients has not been elucidated. Therefore, we performed a retrospective cohort study to investigate the association between metformin and this specific group of patients suffering from HFpEF with T2DM.
Materials and Methods
Study Population
This is a retrospective cohort study conducted among in-hospital HFpEF (4) with T2DM patients admitted in the Department of Cardiology, the Second Affiliated Hospital, Zhejiang University School of Medicine from January 1, 2013, to December 31, 2017. The main inclusion criteria were (1) ≥40years of age; (2) had a left ventricular ejection fraction (LVEF) ≥50% and New York Heart Association (NYHA) class II to IV symptoms; (3) elevated B-type natriuretic peptide (BNP) ≥35 pg/mL or N-terminal pro-B-type natriuretic peptide (NT-proBNP) ≥125 pg/mL; (4) echocardiographic evidence of relevant structural heart disease (left atrial enlargement or left ventricular hypertrophy) or diastolic dysfunction (meet at least three of the following criteria simultaneously: left atrial enlargement, tricuspid regurgitation peak velocity >2.8 m/s, septal e' <7 cm/s or lateral e' <10 cm/s, E/e' >14), or imaging findings of pulmonary congestion; (5) diagnosed as T2DM in medical records and remained antidiabetic drugs therapy regularly for at least 3 months.
Patients were excluded if they: (1) had cardiovascular disorders that may change their clinical course independently of heart failure (such as myocardial infarction, coronary artery bypass graft surgery, or other major cardiovascular surgery, stroke or transient ischemic attack in the past 90 days); (2) currently implanted left ventricular assist device, or cardiac resynchronization therapy; (3) had a history of acute decompensated heart failure within 1 week of screening; (4) had a specific heart failure etiologies including hypertrophic obstructive cardiomyopathy, amyloidosis, acute myocarditis, pericardial disease, primary valvular heart disease requiring surgery or intervention, or severe conduction disorders requiring pacemaker implantation; (5) were previously diagnosed of reduced left ventricular EF <40%; (6) significant impaired renal function (estimated glomerular filtration rate of <45 mL/min/1.73 m2 measured by the CKD-EPI equation or requiring dialysis at the time of screening) or hepatic function; (7) short life expectation; (8) loss of follow-up; (9) metformin users who failed to taking metformin continuously.
Data Collection
The demographic characteristics, underlying diseases, laboratory reports, antidiabetic therapy, and nursing records of study patients were collected. Demographic characteristics included age, gender, body mass index (BMI), cigarette smoking, blood pressure, heart function classification, left ventricular ejection fraction. Main underlying diseases included hypertension (HTN), atrial fibrillation (AF), coronary heart disease (CHD), or cerebral infarction (CI). Main laboratory reports included BNP, NT-proBNP, hemoglobin A1c (HbA1c), hemoglobin, serum creatinine, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG). The CKD-EPI equation was adopted for estimating the glomerular filtration rate (eGFR) (9).
Outcomes
Clinical endpoints were obtained through a median follow-up of 47 months via the EMR database and telephone connection. The primary endpoint was all-cause mortality. Secondary endpoints were cardiovascular death, all-cause hospitalization, and hospitalization for heart failure.
Statistical Analysis
Baseline data were expressed as means ± standard deviation or median (25th and 75th percentiles). Student's t-test or Mann–Whitney-test was used to compare continuous variables between the two groups. Data were presented as the number (percentage), and the χ2-test was used to compare qualitative variables. The association between metformin and clinical outcomes was analyzed by Kaplan-Meier analysis. A multivariable Cox regression was performed using the stepwise regression, with a threshold of 0.1, to assess the independence of this association. Adjusted confounders included age, gender, body mass index, cigarette smoking, systemic blood pressure, diastolic blood pressure, left atrial diameter, left ventricular ejection fraction, NYHA class, duration of diabetes and heart failure, comorbidities including hypertension, atrial fibrillation, coronary heart disease, and cerebral infarction, laboratory findings including glycated hemoglobin, estimated glomerular filtration rate, hemoglobin, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglyceride, and usage of sulfonylureas, glinides, glucosidase inhibitors, and insulin.
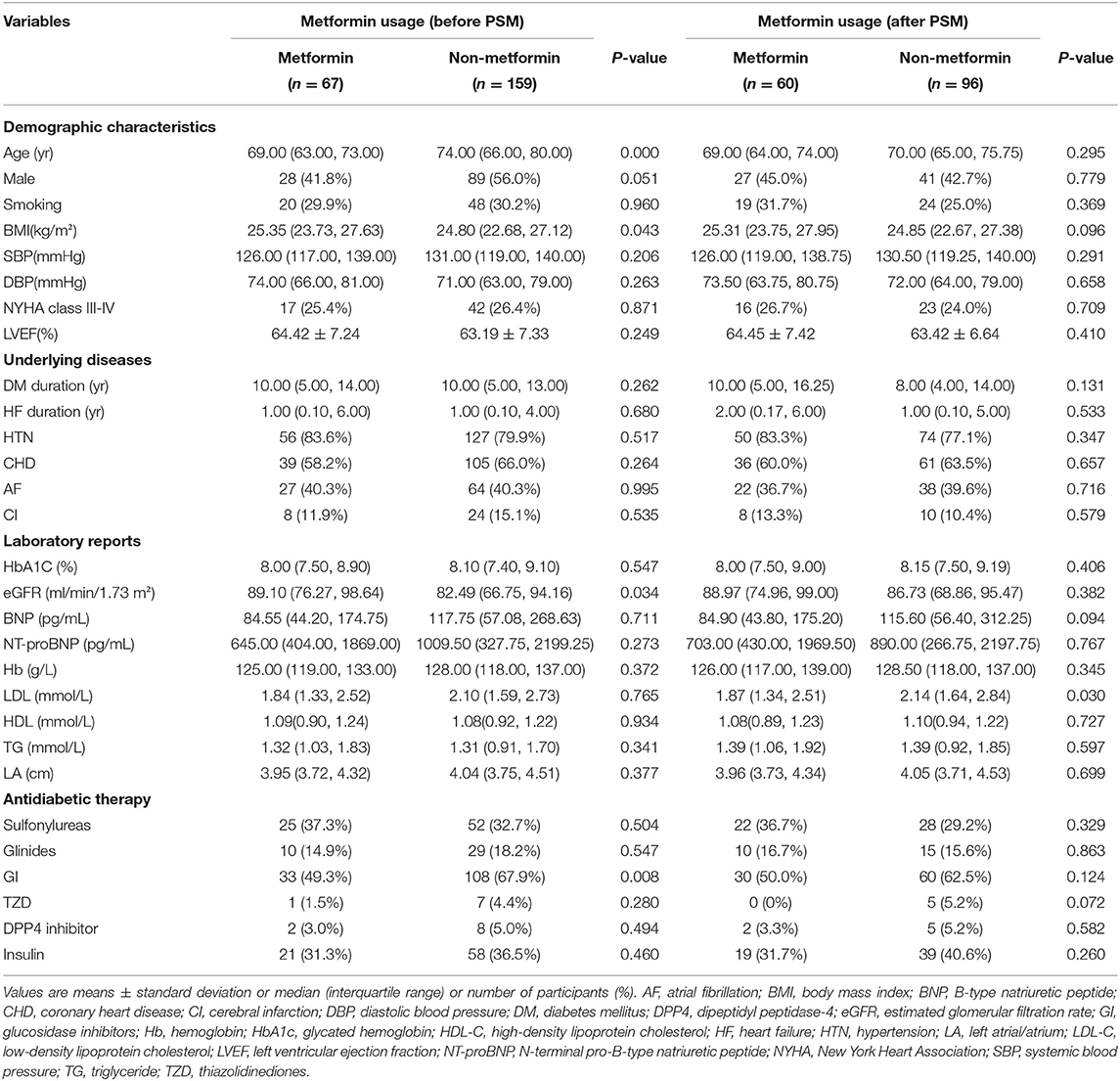
Further, we explored the association between metformin and the primary outcome in selected subgroups. Multivariable Cox regressions with the same way and adjustments as the above were used in the subgroup analysis. The best glycaemic targets are still unclear, but most agree on HbA1c thresholds <7.0% for the majority of adults with DM. We categorized the subjects as HbA1c <7% and HbA1c ≥7% and did a subgroup analysis among patients with higher HbA1c levels. Major imbalances were found in age, sex, eGFR, and GI treatment between metformin and non-metformin users in the HbA1c ≥7% subgroup. We performed propensity score matching (PSM) of age and sex for patients in two groups. Matching was performed using the nearest neighbor matching, with a default caliper of 0.1.
The number needed to treat (NNT) values for metformin therapy incremental to non-metformin treatment were estimated for years 1 to 4 for the primary endpoint. NNT values were estimated as the inverse of the difference in estimated absolute risk between the metformin and non-metformin groups at each time point. The absolute risk for the non-metformin group was calculated directly from Kaplan-Meier estimates.
A two-sided P < 0.05 was considered statistically significant. The statistical analysis was performed using IBM SPSS Statistics for Windows, version 22.0 (IBM, Armonk, New York).
Results
Baseline Characteristics
Among the 518 subjects, we excluded 146 subjects who did not meet our inclusion criteria. The remaining 372 subjects were divided into the metformin group (n = 113) and the non-metformin group (n = 259) (Figure 1). The median age was 71 years old (interquartile range, 65–79), and 52.4% were male gender. 26.6% of the subjects were categorized as NYHA class III-IV, and few patients have taken thiazolidinediones (TZD) or dipeptidyl peptidase-4 (DPP4) inhibitor. Major imbalances were spotted in age (P = 0.000), gender (P = 0.001), diastolic blood pressure (P = 0.001), LVEF (P = 0.021), eGFR (P = 0.000) and glucosidase inhibitor usage (P = 0.001) between metformin and non-metformin users (Table 1). The date of the last patient follow-up was August 2, 2020. The median duration of participation in the study was 47 months (interquartile range, 38–67).
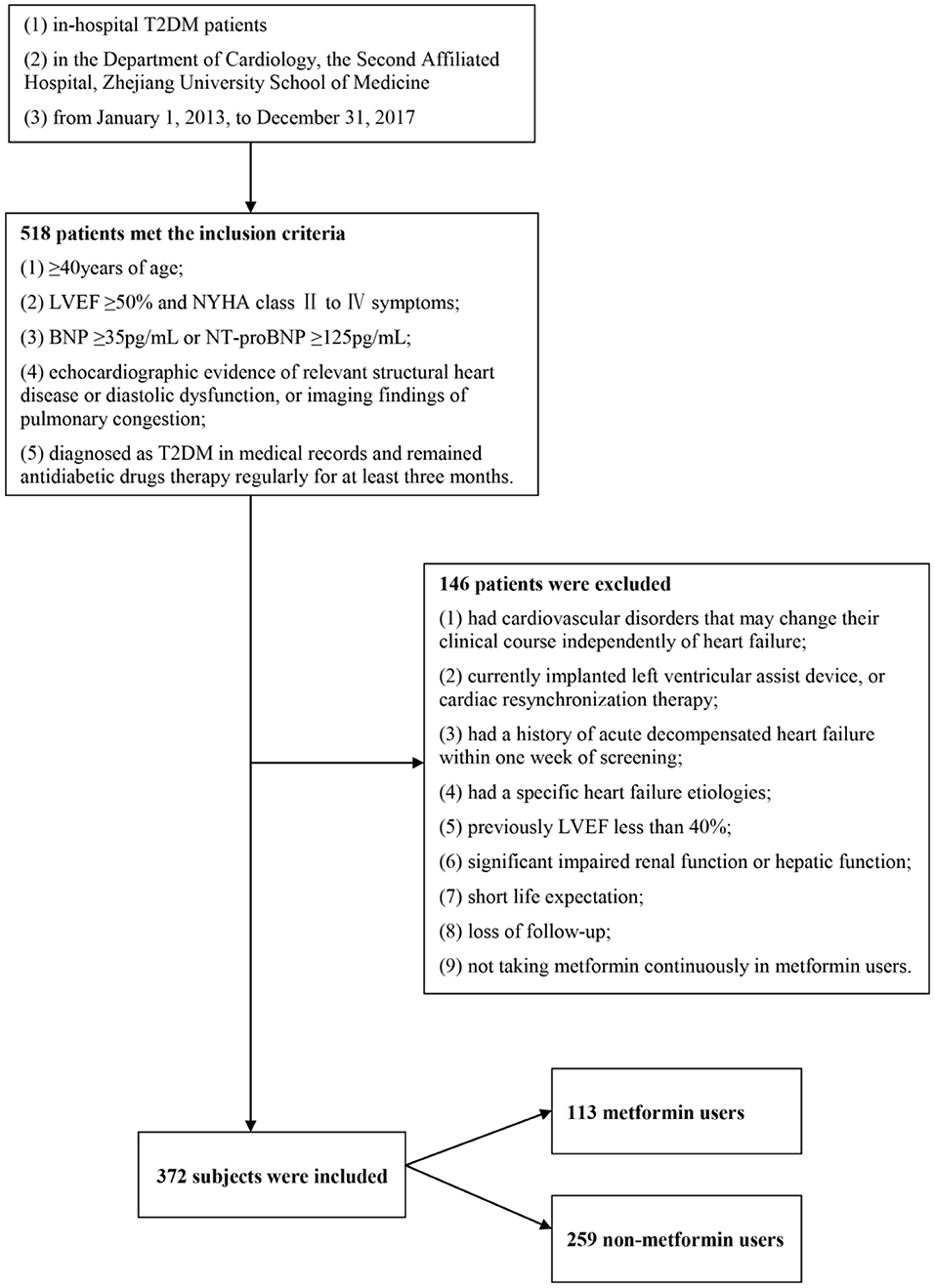
Figure 1. Study population. Among the 518 subjects, we excluded 146 subjects who did not meet our inclusion criteria. The remaining 372 subjects were divided into the metformin group (n = 113) and the non-metformin group (n = 259).
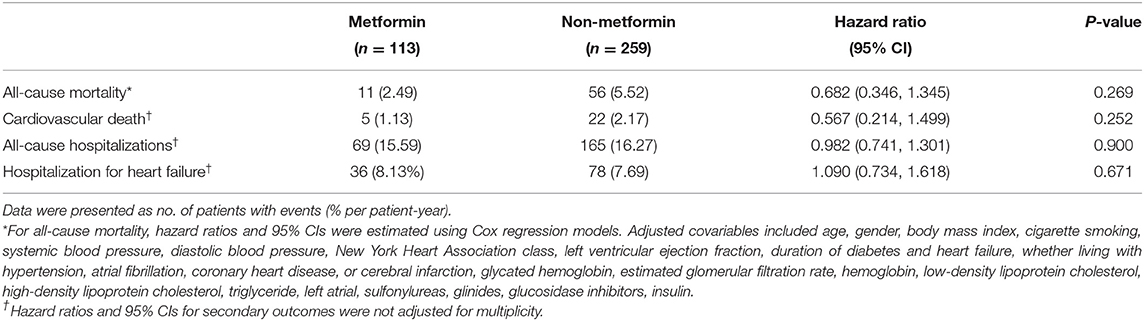
Association Between Metformin and Clinical Outcomes
In current study, 11 patients (2.49% per patient-year) in metformin group and 56 patients (5.52% per patient-year) in non-metformin group deceased during follow-up. The 1-, 2-, 3-, and 4-year survival rates of the metformin group were 100, 97.3, 92.7, and 88.7%, and the non-metformin group were 97.7, 94.2, 87.1, and 80.5%, respectively, with statistically significant differences (P = 0.031) (Figure 2A). There was no statistically significant association between metformin and cardiovascular death (P = 0.252), all-cause hospitalizations (P = 0.900), and hospitalization for heart failure (P = 0.671) (Figures 2B–D and Table 2). However, a multivariable Cox regression failed to show that metformin was an independent factor of all-cause mortality [HR (95% CI) = 0.682 (0.346–1.345); P = 0.269] (Table 2).
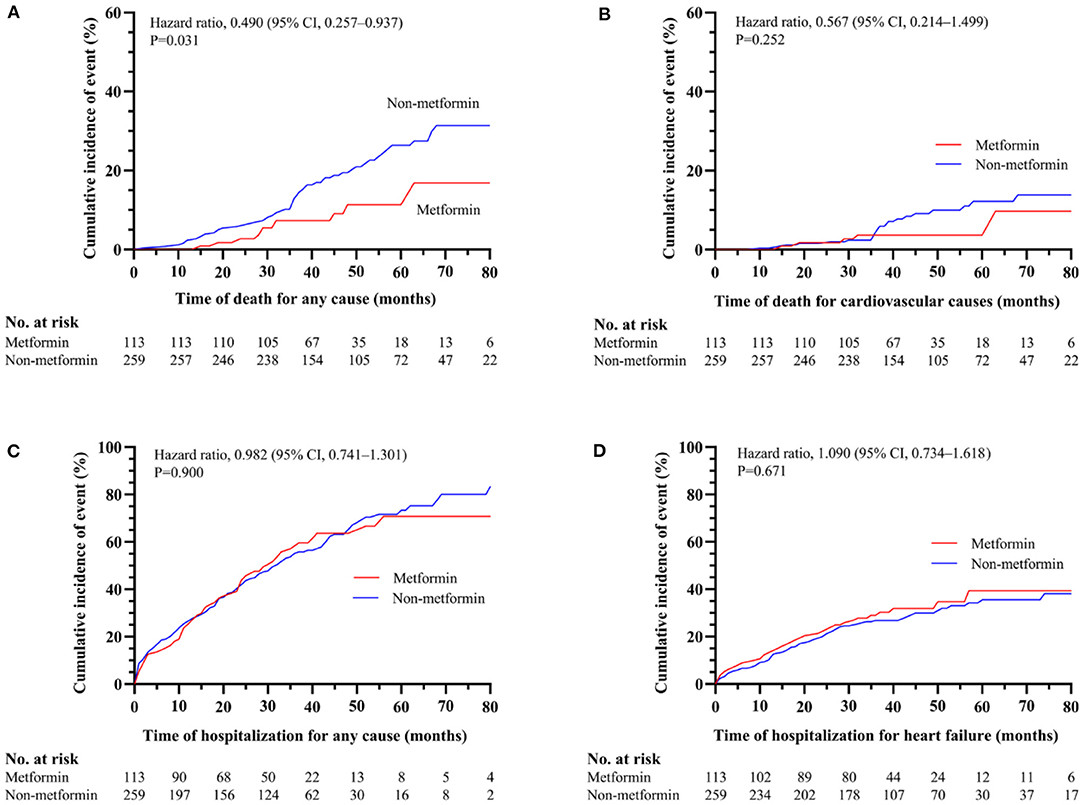
Figure 2. The association between metformin and non-metformin and clinical outcomes in the overall cohort. Metformin was positively related to all-cause mortality (P = 0.031) (A). However, metformin has no effect on cardiovascular death (P = 0.252) (B), all-cause hospitalizations (P = 0.900) (C) or hospitalization for heart failure (P = 0.671) (D).
Association Between Metformin and Primary Outcome in Selected Subgroups for Multiplicity
We explored the association between metformin and the primary outcome in selected subgroups (stratified according to age, gender, anemia, atrial fibrillation, level of HbA1c, and BMI), using a Cox proportional-hazards model to adjust the hazard ratio and widths of the confidence intervals, and the results were listed in Figure 3. Notably, metformin usage was associated with lower all-cause mortality only in patients with HbA1c ≥7% [HR (95% CI) = 0.339 (0.117–0.977); P = 0.045].
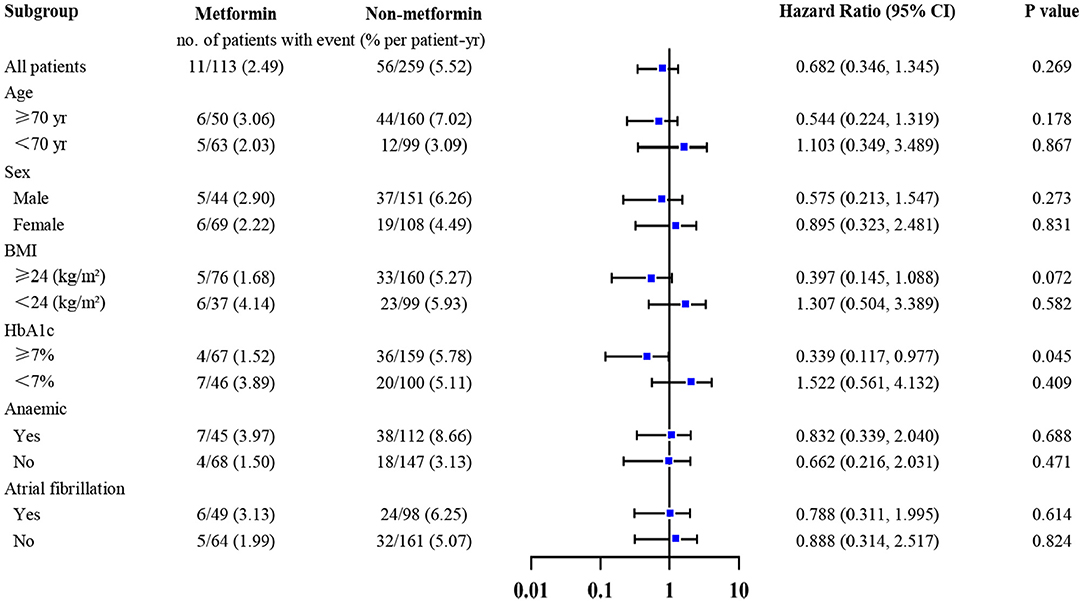
Figure 3. Association between metformin and all-cause mortality in selected subgroups adjusted for multiplicity. Metformin was only associated with lower all-cause mortality in patients whose HbA1c ≥7% [HR (95% CI) = 0.339 (0.117–0.977); P = 0.045]. The widths of the confidence intervals were adjusted for multiplicity, using the stepwise regression, with a threshold of 0.1. Adjusted covariables included age, gender, body mass index, cigarette smoking, systemic blood pressure, diastolic blood pressure, New York Heart Association class, left ventricular ejection fraction, duration of diabetes and heart failure, whether living with hypertension, atrial fibrillation, coronary heart disease, or cerebral infarction, glycated hemoglobin, estimated glomerular filtration rate, hemoglobin, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, left atrial, sulfonylureas, glinides, glucosidase inhibitors, insulin.
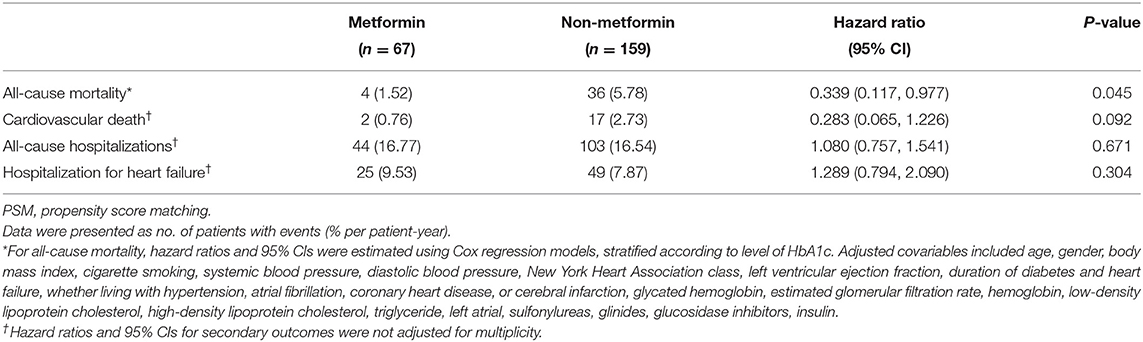
Association Between Metformin Usage and Clinical Outcomes in Subjects With HbA1c ≥7%
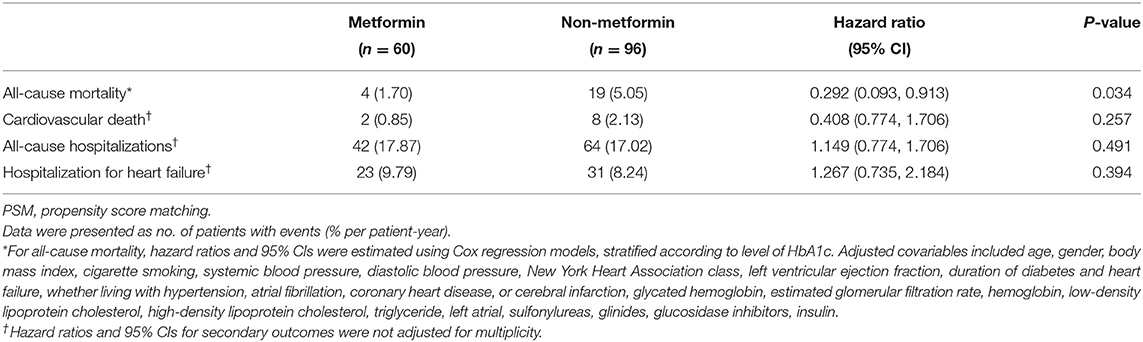
The demographic and clinical characteristics of subjects with HbA1c ≥7% are described in Table 3. Among the 226 subjects in the HbA1C>7% subgroup, 156 subjects were included for further analysis after PSM. The significant differences between the metformin and non-metformin groups (i.e., differences in age, sex, eGFR, and GI treatment) were adjusted after match. Multivariable Cox regression analysis demonstrated that metformin usage was significantly associated with lower all-cause mortality before match [HR (95% CI) = 0.339 (0.117–0.977); P = 0.045] (Table 4). The associations remained unchanged after PSM [HR (95% CI) = 0.292 (0.093–0.913); P = 0.034] (Table 5). Same as the results in the full cohort, there was no independent association between metformin use and secondary outcomes in the HbA1c ≥7% subgroup (Figure 4 and Tables 4, 5).
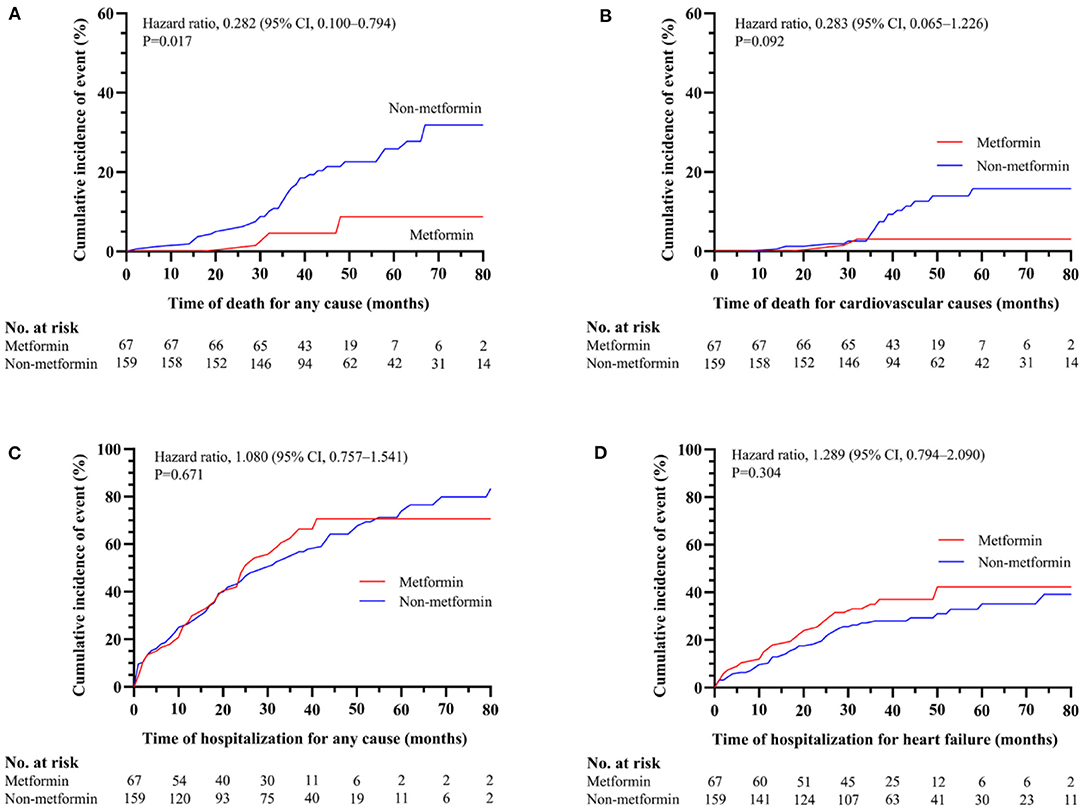
Figure 4. The association between metformin and non-metformin and clinical outcomes in HbA1c ≥7% subgroup. Metformin was positively related to the all-cause mortality (P = 0.017) (A) but has no effect on cardiovascular death (P = 0.092) (B), all-cause hospitalizations (P = 0.671) (C), or hospitalization for heart failure (P = 0.304) (D).
Absolute Risk Reduction of All-Cause Mortality With Metformin in Comparison With Non-metformin
Cumulative incidence of event, incident rates, relative risk reduction, and number needed to treat (NNT) values for the overall cohort and the HbA1c ≥7% subgroup by year are displayed in Table 6. The 4-year estimated NNT with metformin compared with non-metformin for all-cause mortality was 12 in all populations and 8 in the HbA1c ≥7% subgroup.
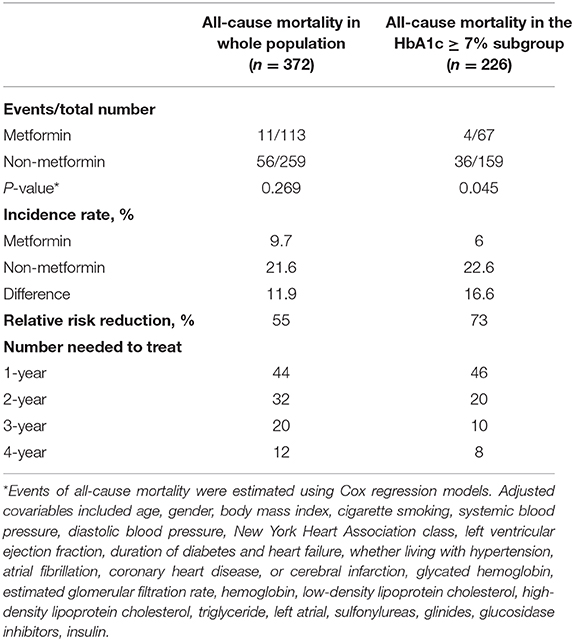
Table 6. Event rates, incidence rates, and number needed to treat for all-cause mortality for comparison of metformin with non-metformin.
Discussion
In the present study, we investigated the association between metformin and adverse outcome on HFpEF with T2DM populations. The major finding was that metformin was linked to a lower incidence of death from any causes in HFpEF and T2DM patients with poor glucose control.
Tremendous advancements have been made in the treatment of HFrEF employing neurohumoral activation. Nevertheless, no therapy has been shown to reduce morbidity or mortality in HFpEF patients (4). Furthermore, the pathophysiology underlying HFpEF is heterogeneous and is associated with multiple phenotypes, including cardiovascular and non-cardiovascular comorbidities (2). The cause of death and hospitalization among HFpEF patients is more likely to be non-cardiovascular than patients with HFrEF (10). Thus, management of comorbidity is an essential task for HFpEF patients.
Compared with the general population, diabetes doubles and quintuples HF's risk in males and females, respectively (11). About 45% of HFpEF patients suffer from diabetes (1). Diabetic patients tended to combine with structural and functional echocardiographic abnormalities (6). Clinical trial data suggest that among individuals with HFpEF, those with diabetes were associated with worse health-related quality of life and increased risk of hospitalization, cardiovascular mortality, and all-cause mortality (6, 12).
The most critical finding in the present study is that metformin reduced all-cause mortality in HFpEF and T2DM patients with poor glucose control. Metformin treatment improved glycaemic and reduced cardiovascular mortality, without the risk of hypoglycemia or bodyweight gains associated with the use of other antidiabetic drugs (13–15). Therefore, it is currently the preferred oral antidiabetics in T2DM and heart failure patients (16). A recent study showed that long-term prescription of metformin could improve left ventricular diastolic function and delay the progression of HFpEF in T2DM and hypertension population (17). Slater et al. reported that metformin improves diastolic function in a mouse model with HFpEF-like symptoms by lowering titin-based passive stiffness (18). The mechanisms by which metformin exerts favorable effects of metformin on HF progression are still not fully understood. Adenosine monophosphate-activated protein kinase (AMPK) is correlated with cardiac fatty acids uptake, autophagy, mitochondrial biogenesis, and energy regulation. Changes in the adenosine AMPK pathway play a major role in developing myocardial impairment (19, 20). In addition to glycaemic control, metformin appears pleiotropic effects. In a diabetic heart, metformin regulates lipid and glucose metabolism via AMPK activation and further improves cardiac energy metabolism (20). What's more, metformin can improve mitochondria function, increasing nitric oxide bioavailability, inhibit the interstitial accumulation of collagen and cardiomyocyte apoptosis through AMPK-dependent or AMPK-independent pathways, and thereby reduce cardiac remodeling and hypertrophy, and preserve cardiac function (7, 20).
It is worth noting that metformin was linked to a lower incidence of all-cause mortality only in patients with poor glycemic control. Prolonged exposure to pronounced hyperglycemia may have a more sustainable myocardial damage than the lower glucose status, therefore increasing the relative adverse influence of hyperglycemia in HF patients. This might explain why metformin showed a more significant cardioprotective effect in those with poor glycemic control. Finally, a higher HbA1c level means a higher glucose status, a sign of insufficient insulin effect or the underlying insulin resistance. Nevertheless, the specific underlying mechanism needs to be clarified.
Historically, metformin should not be used in patients with heart failure due to lactic acidosis risk (21). Nowadays, clinical observations and experimental studies have provided increasing evidence of the safety and benefits of metformin in patients with diabetes and heart failure. A systematic review of observational studies indicates that metformin can be safely used in patients with diabetes mellitus and HF, even in heart failure with reduced left ventricular ejection fraction or chronic kidney failure. Meanwhile, none of the trials demonstrate that metformin was associated with an increased risk of lactic acidosis than other hypoglycemic agents (8).
Several limitations of our study should be acknowledged. First, our study was retrospective rather than randomized prospectively planned, and causality cannot be inferred from these retrospective findings. Second, clinical data were obtained in a single-center instead of multi-centers. Third, the amount of the subject was relatively small. Finally, since our study was conducted before the general introduction of novel antidiabetics, including sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists, they were not included in our analysis.
In conclusion, there was no independent association between metformin use and outcome in the cohort of T2DM with HFpEF. However, metformin was associated with lower all-cause mortality in the subgroup of patients with poor glycemic control. Prospective, large sample studies are necessary to determine the optimal treatment for HFpEF patients with T2DM.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Board of the Second Affiliated Hospital, Zhejiang University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZC and YL: designed the study. JWa, TY, and XM: contributed data. JWa, YL, and JWe: drafted the manuscript. All authors: were involved in critically revising the manuscript.
Funding
This work was supported by funding from the National Natural Science Foundation of China (81970396 and 81900416), the Zhejiang Provincial Natural Science Foundation for Distinguished Young Scholars (LR20H020002), and the Zhejiang Provincial Natural Science Foundation of China (LQ19H020006 and LY19H040012).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, et al. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circ Heart Fail. (2018) 11:e004962. doi: 10.1161/CIRCHEARTFAILURE.118.004962
2. Parikh KS, Sharma K, Fiuzat M, Surks HK, George JT, Honarpour N, et al. Heart failure with preserved ejection fraction expert panel report: current controversies and implications for clinical trials. JACC Heart Fail. (2018) 6:619–32. doi: 10.1016/j.jchf.2018.06.008
3. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. (2013) 62:263–71. doi: 10.1016/j.jacc.2013.02.092
4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2016) 18:891–975. doi: 10.1093/eurheartj/ehw128
5. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. (2017) 135:e146–603. doi: 10.1161/CIR.0000000000000491
6. Kristensen SL, Mogensen UM, Jhund PS, Petrie MC, Preiss D, Win S, et al. Clinical and echocardiographic characteristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction: a report from the I-preserve trial (Irbesartan in heart failure with preserved ejection fraction). Circulation. (2017) 135:724–35. doi: 10.1161/CIRCULATIONAHA.116.024593
7. Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol. (2019) 15:569–89. doi: 10.1038/s41574-019-0242-2
8. Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail. (2013) 6:395–402. doi: 10.1161/CIRCHEARTFAILURE.112.000162
9. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
10. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2017) 14:591–602. doi: 10.1038/nrcardio.2017.65
11. Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. (1979) 241:2035–8. doi: 10.1001/jama.241.19.2035
12. MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. (2008) 29:1377–85. doi: 10.1093/eurheartj/ehn153
13. United Kingdom Prospective Diabetes Study 24: a 6-year randomized controlled trial comparing sulfonylurea insulin and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. United Kingdom Prospective Diabetes Study Group. Ann Intern Med. (1998) 128:165–75. doi: 10.7326/0003-4819-128-3-199802010-00001
14. Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. (2016) 164:740–51. doi: 10.7326/M15-2650
15. Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA. (2016) 316:313–24. doi: 10.1001/jama.2016.9400
16. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Ceriello A, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. (2020) 41:255–323. doi: 10.1093/eurheartj/ehz486
17. Gu J, Yin ZF, Zhang JF, Wang CQ. Association between long-term prescription of metformin and the progression of heart failure with preserved ejection fraction in patients with type 2 diabetes mellitus and hypertension. Int J Cardiol. (2020) 306:140–5. doi: 10.1016/j.ijcard.2019.11.087
18. Slater RE, Strom JG, Methawasin M, Liss M, Gotthardt M, Sweitzer N, et al. Metformin improves diastolic function in an HFpEF-like mouse model by increasing titin compliance. J Gen Physiol. (2019) 151:42–52. doi: 10.1085/jgp.201812259
19. Yao F, Zhang M, Chen L. 5′-Monophosphate-activated protein kinase (AMPK) improves autophagic activity in diabetes and diabetic complications. Acta Pharm Sin B. (2016) 6:20–5. doi: 10.1016/j.apsb.2015.07.009
20. Dziubak A, Wójcicka G, Wojtak A, Bełtowski J. Metabolic effects of metformin in the failing heart. Int J Mol Sci. (2018) 19:2869. doi: 10.3390/ijms19102869
Keywords: metformin, heart failure with preserved ejection fraction, type 2 diabetes mellitus—exenatide, survival analysis (source: MeSH NLM), mortality
Citation: Wang J, Lu Y, Min X, Yuan T, Wei J and Cai Z (2021) The Association Between Metformin Treatment and Outcomes in Type 2 Diabetes Mellitus Patients With Heart Failure With Preserved Ejection Fraction: A Retrospective Study. Front. Cardiovasc. Med. 8:648212. doi: 10.3389/fcvm.2021.648212
Received: 31 December 2020; Accepted: 22 February 2021;
Published: 12 March 2021.
Edited by:
Junjie Xiao, Shanghai University, ChinaReviewed by:
Yue Wu, The First Affiliated Hospital of Xi'an Jiaotong University, ChinaNicholas Cauwenberghs, KU Leuven, Belgium
Copyright © 2021 Wang, Lu, Min, Yuan, Wei and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhejun Cai, caizhejun@zju.edu.cn
†These authors have contributed equally to this work
 Jianfang Wang1,2†
Jianfang Wang1,2†  Yi Lu
Yi Lu Tan Yuan
Tan Yuan Zhejun Cai
Zhejun Cai