Micro-CT-Based Quantification of Extracted Thrombus Burden Characteristics and Association With Angiographic Outcomes in Patients With ST-Elevation Myocardial Infarction: The QUEST-STEMI Study
- 1First Department of Cardiology, AHEPA University Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 2Hellenic Centre for Marine Research, Institute of Marine Biology, Biotechnology, and Aquaculture, Heraklion, Greece
- 3Biology Department, University of Crete, Heraklion, Greece
- 4Laboratory of Forensic Medicine and Toxicology, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 5Blood Centre, AHEPA University Hospital, Thessaloniki, Greece
- 6Lab of Computing, Medical Informatics, and Biomedical Imaging Technologies, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 7School of Mathematics, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 8LifeWatch ERIC, Sector II-II, Seville, Spain
- 9Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
Background: Angiographic detection of thrombus in STEMI is associated with adverse outcomes. However, routine thrombus aspiration failed to demonstrate the anticipated benefit. Hence, management of high coronary thrombus burden remains challenging. We sought to assess for the first time extracted thrombotic material characteristics utilizing micro-computed tomography (micro-CT).
Methods: One hundred thirteen STEMI patients undergoing thrombus aspiration were enrolled. Micro-CT was undertaken to quantify retrieved thrombus volume, surface, and density. Correlation of these indices with angiographic and electrocardiographic outcomes was performed.
Results: Mean aspirated thrombus volume, surface, and density (±standard deviation) were 15.71 ± 20.10 mm3, 302.89 ± 692.54 mm2, and 3139.04 ± 901.88 Hounsfield units, respectively. Aspirated volume and surface were significantly higher (p < 0.001) in patients with higher angiographic thrombus burden. After multivariable analysis, independent predictors for thrombus volume were reference vessel diameter (RVD) (p = 0.011), right coronary artery (RCA) (p = 0.039), and smoking (p = 0.027), whereas RVD (p = 0.018) and RCA (p = 0.019) were predictive for thrombus surface. Thrombus volume and surface were independently associated with distal embolization (p = 0.007 and p = 0.028, respectively), no-reflow phenomenon (p = 0.002 and p = 0.006, respectively), and angiographically evident residual thrombus (p = 0.007 and p = 0.002, respectively). Higher thrombus density was correlated with worse pre-procedural TIMI flow (p < 0.001). Patients with higher aspirated volume and surface developed less ST resolution (p = 0.042 and p = 0.023, respectively).
Conclusions: Angiographic outcomes linked with worse prognosis were more frequent among patients with larger extracted thrombus. Despite retrieving larger thrombus load in these patients, current thrombectomy devices fail to deal with thrombotic material adequately. Further studies of novel thrombus aspiration technologies are warranted to improve patient outcomes.
Clinical Trial Registration: QUEST-STEMI trial ClinicalTrials.gov number: NCT03429608 Date of registration: February 12, 2018. The study was prospectively registered.
Introduction
Despite the tremendous progress in cardiovascular medicine over the last decades, ST-segment elevation myocardial infarction (STEMI) still remains one of the leading causes of mortality worldwide (1). Intracoronary thrombosis developed after plaque erosion or rupture, causing partial or total occlusion of coronary vessels which is the most common underlying pathophysiologic mechanism in STEMI. Thrombus burden (TB) is an important prognostic determinant (2), as it has been associated with an increase in the rate of major adverse cardiac and cerebrovascular events (MACCE) (3).
The optimal therapy for STEMI is timely performed primary percutaneous coronary intervention (pPCI) (4). However, myocardial recovery and restoration of epicardial coronary blood flow are often suboptimal due to thrombus embolization (5), leading to perturbed microvascular perfusion and obstruction of the microvasculature.
Manual aspiration thrombectomy (MATh) was first described in 1980 as a useful adjunctive therapy to conventional PCI with the potential to remove the thrombotic component of the culprit lesion (6, 7). However, large randomized controlled trials (RCTs) and meta-analyses failed to demonstrate the theoretically anticipated benefit for routine MATh, suggesting lack of synergy between MATh and pPCI with a subsequent increase in the risk of stroke (8–11). Therefore, current ESC guidelines do not recommend routine thrombus aspiration (class of recommendation IIIA) (12).
Several hypotheses might serve to explain why MATh did not succeed in the majority of recent trials, such as the presence of small amount of thrombus at the culprit lesion before thrombectomy or of large amount of residual thrombus after thrombectomy (13). Indeed, it was shown that the elective application of MATh in certain cases with large thrombus burden (9, 14, 15) as a bail-out therapy showed some cardiovascular (CV) benefit (reduced CV death), which was counterbalanced by an increased risk of stroke. Previous studies utilizing optical coherence tomography (OCT) have shown that there is substantial amount of residual thrombus even after thrombectomy (13, 14, 16). Interestingly, STEMI patients with greater residual TB after MATh had microvascular dysfunction and more significant myocardial damage than those with smaller residual TB (14).
Thus, the prognostic significance of initial angiographic TB and post-aspiration residual TB has already been investigated. However, evidence on the association of extracted thrombotic material characteristics with post-pPCI angiographic outcomes is lacking.
Micro-computed tomography (micro-CT) is an emerging technology with high spatial resolution in the submicrometer range, which is increasingly employed in medicinal studies (17–20). Despite being initially used for skeletal imaging, the development of contrast agents, which amplify the low intrinsic contrast of soft-tissues in X-ray absorption, facilitates detailed micro-CT imaging of soft tissues (21). Since micro-CT allows non-destructive 3D imaging of both the internal and external structures of samples, exceeding the capabilities of histomorphometric analysis (21), it can be employed to accurately quantify extracted thrombotic material characteristics, which have been subjective to date. These novel imaging parameters could be used to improve patient risk stratification, enabling individualized treatment of patients with STEMI.
Materials and Methods
The design of the QUEST-STEMI study has been previously described (22).
Study Population and PCI Procedures
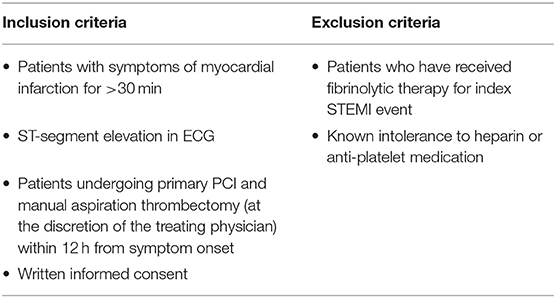
QUEST-STEMI (ClinicalTrials.gov Identifier: NCT03429608) is a prospective cohort trial including patients, who presented to AHEPA University Hospital with STEMI and underwent primary PCI and MATh at the discretion of the treating physician within 12 h of symptom onset. The eligibility criteria are depicted in Table 1. The Scientific Committee of AHEPA Hospital approved the study protocol, and all trial procedures comply with the principles set by the Declaration of Helsinki (23). Each participant provided written informed consent before being enrolled in the study.
Angiographic Analysis, ECG, and Thrombus Aspiration Procedure
Before primary PCI, each patient received guideline-directed pharmaceutical therapy (unfractionated heparin (100 IU/kg) and a loading dose of aspirin (325 mg) and either ticagrelor (180 mg) or clopidogrel (600 mg) (12). Intravenous GP IIb/IIIa inhibitors were administered at the interventionalist's discretion. Thrombus aspiration was undertaken according to standard practices, as described (12, 22). Briefly, after crossing the lesion with a wire, the thrombectomy catheter was advanced proximal to the occluded segment. Continuous manual suction was recommended via a proximal-to-distal approach, so that active aspiration was initiated before the catheter crossed the thrombotic occlusion (8, 10). The thrombectomy catheter was slowly passed through the lesion multiple times (at least two), so that a minimum of 40 cc of blood and material were extracted.
A 12-lead ECG was obtained at presentation and 90 min post-intervention. ST-segment deviation was assessed, as previously described (24). Based on the degree of resolution of ST-segment elevation, patients were classified into three groups: (1) complete ST resolution (>70%); (2) partial ST resolution (30–70%); and (3) absent ST resolution (<30%).
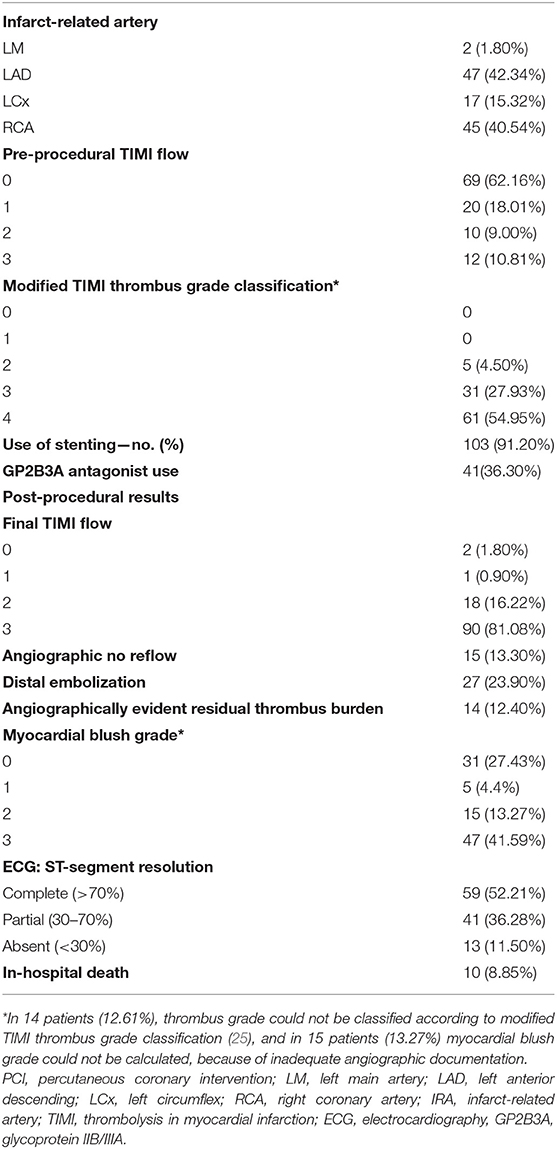
Coronary angiograms were analyzed by two experienced interventional cardiologists (GSi, GSo). Angiographic thrombus burden was assessed based on the modified TIMI (thrombolysis in myocardial infarction) thrombus classification scale by Sianos et al. (25). According to this classification, patients with TIMI Grade 5 thrombus are classified to another thrombus category (G0–G4) post-flow achievement with either guidewire crossing or a small balloon. Furthermore, baseline, post-MATh, and post-procedural antegrade coronary flow was evaluated based on TIMI classification (26). Reference vessel diameter (RVD), minimum luminal diameter, percentage of diameter stenosis, and lesion length were also calculated using quantitative coronary angiography. Presence of distal embolization (21) and angiographically evident residual thrombus were also recorded. For the present analysis, a patient was regarded to have angiographically evident residual thrombus, if modified TIMI thrombus grades 2–4 were present (27).
Micro-CT Analysis
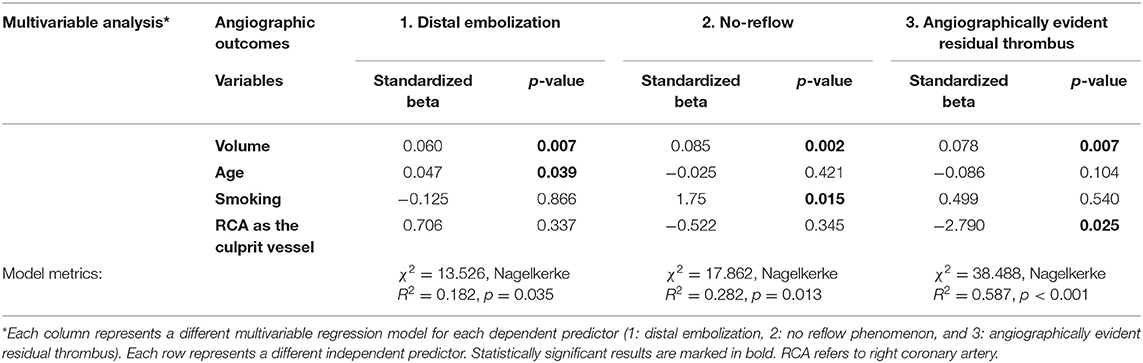
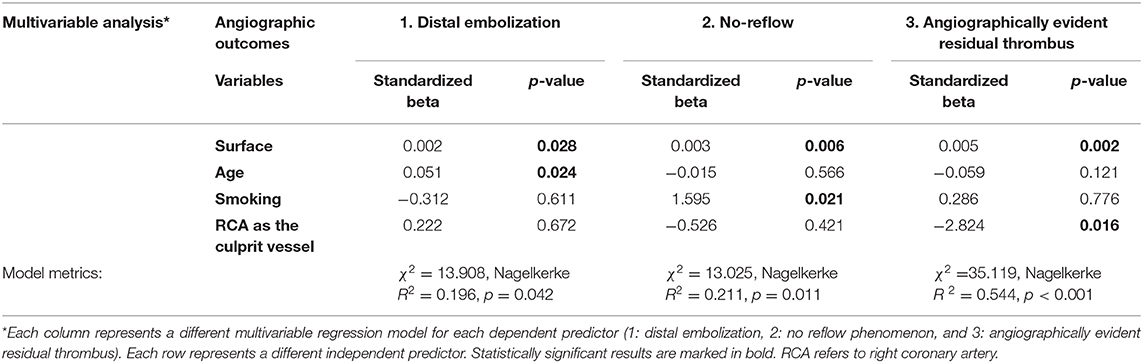
The detailed protocol for the micro-CT scanning and analysis of aspirated thrombi has been previously described (22). Briefly, extracted thrombotic material was initially preserved in 10% formalin for 24 h and then successively dehydrated in ethanol solutions up to 70% and stained using 0.3% phosphotungstic acid (PTA) in 70% ethanol according to Metscher's protocol (28). All scans were performed with a SkyScan 1172 micro-tomograph (Bruker, Kontich, Belgium, Figure 1A) at the Hellenic Center for Marine Research (HCMR) (48 kV, 204 μA, no filter, 360° rotation). Projection images were reconstructed into cross sections using SkyScan's NRecon software (Bruker, Kontich, Belgium). The cross-section images were loaded into the software CT Analyser v.1.14.4.1 (CTAn, Bruker, Kontich, Belgium) to extract measurements for the volume and density of thrombi (Figures 1B,C) as mean grayscale values (±Standard Deviation), which were also converted to Hounsfield units (HU). The presence of different cell types within a thrombus was also quantified by comparing their density with in-vitro produced thrombi with known homogeneous composition, as described (22). The range of grayscale values used for the full thrombus specimen was 25–255. Red (erythrocyte-rich) thrombi showed densities 80–255 grayscale values, whereas clots with the highest platelet content (white thrombi) had densities in the range 25–80 (Figure 2). Analysis of the thrombi was undertaken by two independent blinded assessors.
Figure 1. Thrombus analysis via micro-computed tomography. (A) Thrombi are mounted on a specific head inside SkyScan 1172; (B,C) Representative computer generated renderings of thrombi. Clots were stained using 0.3% phosphotungstic acid and scanned via SkyScan 1172. NRecon (Bruker, Kontich, Belgium) software was used for the reconstruction of the projections.
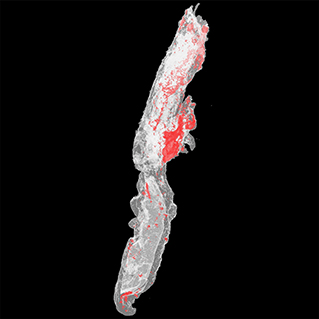
Figure 2. 3D color visualization of a thrombus sample. A 3D model of a thrombus sample was created using CTAn software (Bruker, Kontich, Belgium), and color visualization was performed via CTVol software (Bruker, Kontich, Belgium). Erythrocyte-rich regions were rendered in red, whereas platelet-rich regions were rendered in white.
Statistical Analysis
Statistical analysis was performed using SPSS v26 (SPSS software, Chicago, IL, USA) software. Continuous data are presented as mean and standard deviation, whereas categorical variables are expressed as counts and percentages. The inter- and intraobserver reproducibility of thrombus measurements were assessed based on data obtained from a subset of 34 subjects (30% of study population), performing Spearman's correlations, and the intraclass correlation coefficient (ICC) (29, 30).
The data were analyzed by non-parametric tests as indicated by the Shapiro–Wilk-test for normality. Group differences were tested using the Wilcoxon–Mann–Whitney-test for continuous measures. The Kruskal–Wallis H-test was used to compare between two or more groups of an independent variable on a continuous or ordinal dependent variable. Univariable analysis was initially carried out to clarify the association of demographic characteristics, history of smoking, medical history, statin, antiplatelet or anticoagulant use, pain-to-balloon time, pre-procedural TIMI flow, RVD, and culprit vessel with extracted thrombus characteristics (volume, surface, and density) and with angiographic and electrocardiographic outcomes. The variables with statistical significance (p-value <0.05) were included in the multivariable regression models.
Linear and logistic regression using stratified bootstrapping to account for the non-parametric nature of the data was used to identify independent predictors of thrombus characteristics and of angiographic outcomes. Correlation coefficients were investigated to address potential multicollinearity among the predicting variables in the created regression models (31). Multivariable logistic regression models, including both volume and surface as independent variables, demonstrated potential multicollinearity (correlation coefficients higher than 0.89). Therefore, thrombus volume and surface were not included in the same model as independent predictors. R, R2, Durbin–Watson, and Nagelkerke R2 metrics along with p-values are reported for the linear and logistic models, respectively.
Results
Study Population
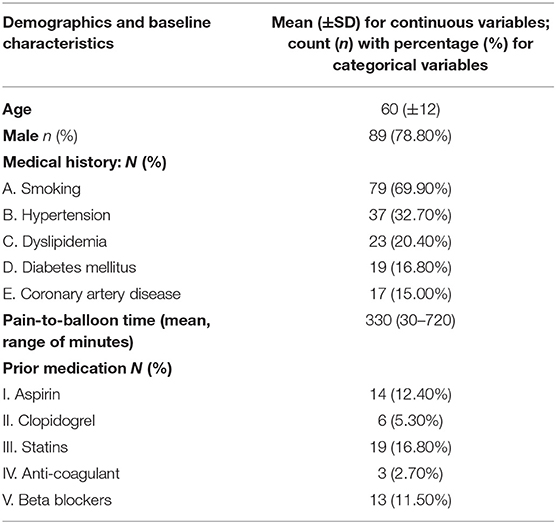
During the study period, 113 consecutive patients were enrolled based on the study eligibility criteria. The baseline clinical and angiographic characteristics are shown in Tables 2, 3. Mean age was 60.05 (±12.12) years, and 89 patients (78.8%) were male. Smoking was reported by 69.9% of participants, and reperfusion was achieved on an average 330.2 (±245.74) min from symptom onset. A no-reflow phenomenon was observed in 13.3% of patients and distal embolization in 23.9% of the participants. Residual thrombus was angiographically evident in 14 patients (12.4%), whereas MBG was equal to 0 or 1 in 36 (31.86%) of patients.
Micro-CT Findings on Extracted Thrombus Burden
Micro-tomography effectively quantified the volume, surface, and density of all aspirated thrombi. No sample disintegration was observed, and hence all thrombi were suitable for micro-CT scanning. The mean extracted thrombus volume, surface, and density were 15.71 (± 20.10) mm3, 302.89 (± 692.54) mm2, and 3139.04 (± 901.88) HU, respectively.
Intraobserver and interobserver reliabilities were high for all thrombus volume (interobserver: 0.995; intraobserver: 1.000), thrombus surface (interobserver: 0.999; intraobserver: 0.999), and thrombus density (interobserver: 0.982; intraobserver: 0.982). Interclass correlation coefficients for thrombus volume, surface, and density were equal to 0.995 (95% C.I.: 0.981–0.998), 0.995 (95% C.I.: 0.991–0.996), and 0.987 (95% C.I.: 0.966–0.993), respectively.
Association of Extracted Thrombus Burden Characteristics With Intracoronary Thrombus Classification
Aspirated thrombus volume and surface were significantly higher (p-value <0.001) in patients with higher intracoronary angiographic thrombus burden according to modified TIMI thrombus grade classification (Figure 3A). Similarly, aspirated thrombus density analysis revealed that higher values of density (indicating a higher proportion of erythrocytes within the clot) were significantly correlated with larger intracoronary angiographic thrombus burden according to modified TIMI thrombus grade classification (p-value = 0.037).
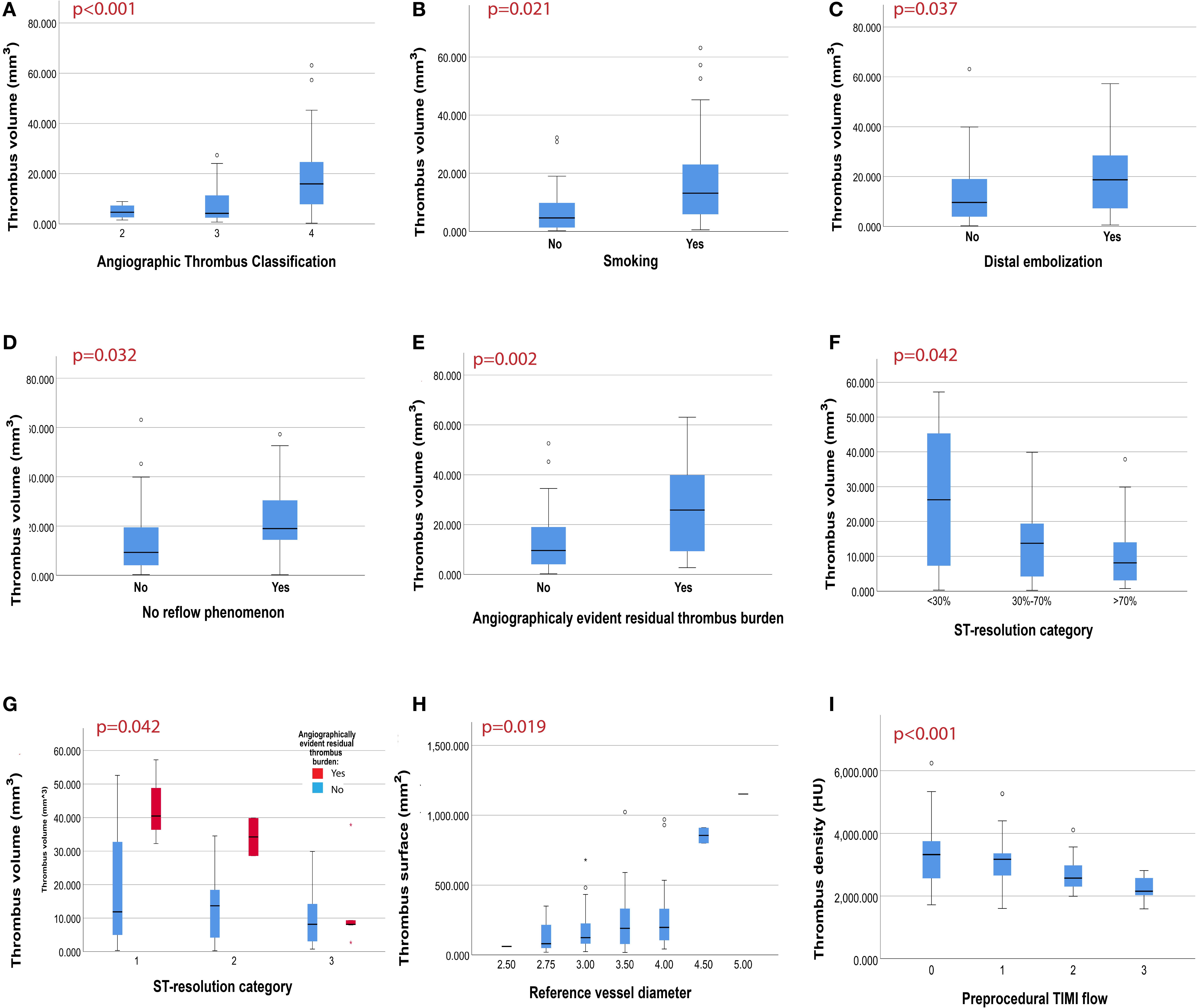
Figure 3. Main findings of the QUEST STEMI study. (A) Association of thrombus volume with angiographic thrombus classification by modified TIMI thrombus grade classification [Grade 2: 4.64 (2.66–8.92) mm3, Grade 3: 4.21 (3.72–8.80) mm3, Grade 4: 15.94 (10.60–19.51) mm3]. (B) Association of thrombus volume with smoking history [yes: 13.14 (9.31–16.35) mm3, no: 4.64 (2.66–8.69) mm3]. (C–E) Association of thrombus volume with angiographic outcomes {(C) distal embolization [yes: 18.70 (9.27–24.52) mm3, no: 9.61 (6.61–13.82) mm3], (D) no-reflow phenomenon: [yes: 18.98 (15.79–32.26) mm3, no: 9.31 (6.61–13.65) mm3], and (E) angiographically evident residual thrombus [yes: 25.81 (9.31–39.87) mm3, no: 9.61 (6.61–13.65) mm3]}. (F,G) Association of thrombus volume with electrocardiographic outcomes [complete ST resolution; 8.12 (5.93–10.31) mm3, partial ST resolution; 13.74 (6.42–16.97) mm3 and absent ST resolution: 26.26 (7.31–45.26) mm3]. (H) Association of thrombus surface with reference vessel diameter [RVD: 2.5 mm: 150.30 (±175.22) mm2, 3 mm: 187.54 (±152.22) mm2, 3.5 mm: 233.21 (±198.23) mm2, 4 mm: 539.17 (±1388.82) mm2, 4.5 mm: 855.73 (±78.26) mm2]. (I) Association of thrombus density with pre-procedural TIMI flow [TIMI 0: 3322 (3023–53523) HU, TIMI I: 3171 (2863–3331) HU, TIMI II: 2574 (2307–3564) HU, TIMI III: 2152 (2019–2594) HU].
Determinants of Extracted Thrombus Burden Characteristics
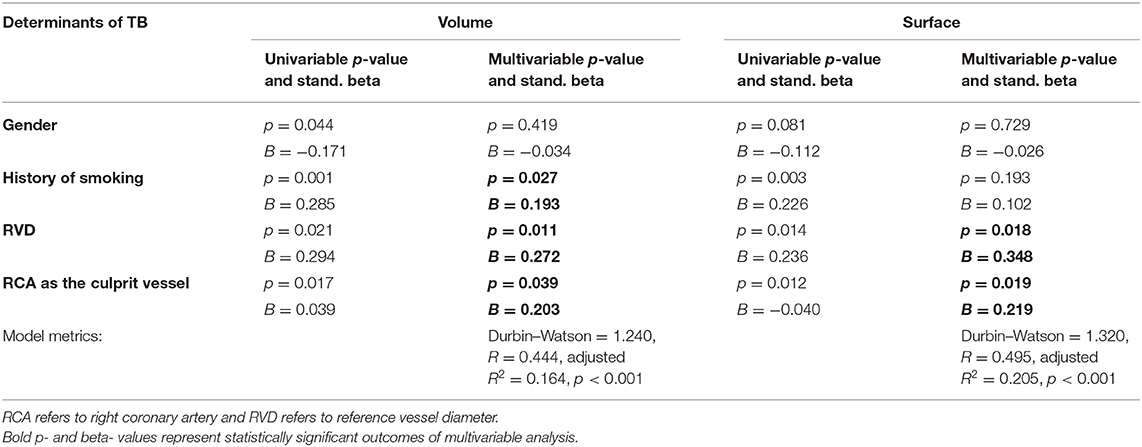
Univariable analysis revealed that male gender, history of smoking, RVD, and right coronary artery (RCA) as the culprit vessel are significantly associated with higher extracted thrombus volume (p-values = 0.044, 0.001, 0.021, 0.017, respectively). Preprocedural TIMI flow was not associated with aspirated volume (p-value = 0.794). On bootstrapped multivariable linear regression analysis (Table 4), RVD (p-value = 0.011), RCA as the culprit vessel (p-value = 0.039), and history of smoking (p-value = 0.027) were independent predictors of higher extracted thrombus volume (Figure 3B).
Higher extracted thrombus surface was—both univariably and multivariably (Table 4)—significantly linked with larger RVD (p-value = 0.018) and RCA as the culprit vessel (p-value = 0.019; Figure 3H), whereas the association of surface with preprocedural TIMI flow was not significant (p-value = 0.860).
It is worth mentioning that the Durbin–Watson values of our regression models (for volume: 1.240 and for surface: 1.320) indicate that there is no significant autocorrelation. Despite, R2-values of these models propose a significant, but weak, predictive value (R2 < 0.300).
As for thrombus density, univariable analysis demonstrated that higher density values (erythrocyte-rich clots) were significantly correlated with worse pre-procedural TIMI flow (p-value < 0.001; Figure 3I), but no other statistically significant predictor of density was revealed and hence no multivariable regression model was performed.
Association of Extracted Thrombus Burden Characteristics With Angiographic Outcomes
Higher extracted thrombus volume was significantly linked with angiographic outcomes suggestive of poor patient prognosis, including distal embolization (p-value = 0.037), and no reflow phenomenon (p-value = 0.032; Figures 3C,D). Additionally, angiographic evidence of residual thrombus was more frequent in patients with larger aspirated thrombus (p-value = 0.002; Figure 3E). Furthermore, a non-significant trend toward worse MBG in patients with larger extracted thrombus volume was observed (p = 0.073), hence multivariable regression analysis on MBG was not performed.
Bootstrapped multivariable logistic regression analyses (Table 5) showed that aspirated thrombus volume remained an independent predictor for (i) distal embolization (p-value = 0.007), (ii) no-reflow phenomenon (p-value = 0.002) together with smoking (p = 0.015), and (iii) angiographically evident residual thrombus (p-value = 0.007).
Similarly, bootstrapped multivariable logistic regression analysis on thrombus surface (Table 6) showed that the higher surface of aspirated thrombus was independently associated with (i) distal embolization (p-value = 0.028) along with age (p-value = 0.024), (ii) no-reflow phenomenon (p-value = 0.006) along with smoking (p-value = 0.021), and (iii) angiographically evident residual thrombus (p-value = 0.002) along with RCA (p-value = 0.016). On the other hand, multivariable regression analysis on MBG was not performed, since univariable analysis did not yield statistically significant results (p-value = 0.226).
Of note, multivariable model metrics indicate that the predictive models for angiographically evident residual thrombus explain the most of the variation compared with the other models (volume, smoking, age, RCA: Nagelkerke R2 = 0.587 and surface, age, smoking, RCA: Nagelkerke R2 = 0.544). However, the other multivariable models created for the prediction of distal embolization and no-reflow phenomenon have weak, but statistically significant, predictive value (Nagelkerke R2 < 0.300).
Additionally, thrombus density was not associated with distal embolization (p-value = 0.246), no-reflow phenomenon (p-value = 0.859), angiographically evident residual thrombus (p-value = 0.549), or MBG (p-value = 0.155) in univariable analysis and therefore no multivariable regression model was executed.
Association of Extracted Thrombus Burden Characteristics With Electrocardiographic Outcomes
ECG analysis revealed that patients with higher aspirated thrombus volume and surface developed significantly less ST resolution (p-value = 0.042 and 0.023, respectively). After classifying patients by the presence of angiographically evident residual thrombus, ST-segment resolution was less in patients with angiographically evident residual thrombus and particularly in those with higher aspirated thrombus volume (Figures 3F,G). On the other hand, no significant correlation between thrombus density and ST-segment resolution (p-value = 0.451) was observed.
Discussion
To our knowledge, this is the first study to comprehensively evaluate extracted thrombotic material in patients with STEMI using micro-CT. The main findings of our study (Figure 4) were as follows: (1) thrombus analysis by micro-CT is feasible, reliable, and reproducible; (2) larger thrombus (higher volume and surface) was extracted in patients with high intracoronary TB under angiographic imaging; (3) higher aspirated thrombus volume and surface were associated with adverse angiographic outcomes, including distal embolization and no-reflow phenomenon; (4) angiographically evident residual thrombus was more frequent among patients with larger retrieved thrombus; (5) a non-significant trend (p = 0.073) toward worse myocardial blush grade in patients with larger extracted thrombus volume was observed; (6) aspirated thrombus volume and surface were significantly higher in smokers; and (7) worse pre-procedural TIMI flow was observed in thrombi with higher density.
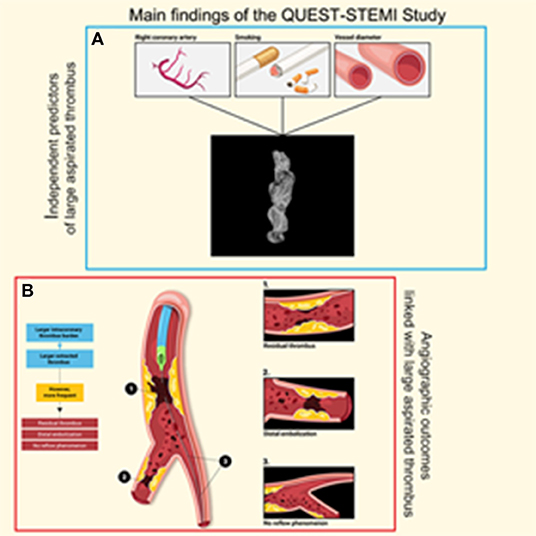
Figure 4. Visual overview of the main findings of the QUEST STEMI study. (A) Independent predictors of large aspirated thrombus and (B) Angiographic outcomes linked with large aspirated thrombus.
Thrombus is a typical histopathologic characteristic of patients suffering from STEMI, which has been linked with worse patient prognosis (32). Qualitative and quantitative assessment of coronary thrombi has been a challenge. Recently, OCT has been employed to effectively quantify and characterize intracoronary thrombus before (15) and after (14, 16) MATh.
In this study, using a novel technology (micro-CT) we aimed to develop a methodology for the visualization and the quantitative 3D analysis of extracted thrombotic material in patients with STEMI undergoing MATh during pPCI. Micro-CT is an established imaging modality, which facilitates high-resolution, non-destructive visualization of 3D structures along with quantitative volumetric measurements and tissue characterizations. Our results indicate that this method shows high reliability and repeatability for measuring the volume, surface, and density of extracted clots. Interestingly, micro-CT-quantified thrombus burden was strongly correlated with the intracoronary TB under angiographic imaging according to modified TIMI thrombus grade classification (25), suggesting that higher amount of thrombus is retrieved in patients with larger thrombotic load as seen in angiography.
In cases of large TB, fully optimizing stent expansion, while simultaneously preventing distal embolization and protecting the distal coronary vasculature, remains a clinical challenge, given the fact that routine MATh has not been proven effective (12). The inability of MATh to demonstrate a clinical benefit (8, 33, 34) might be the result of current MATh technology limitations, including inadequate retrieval of thrombus, thrombus embolization downstream prior to aspiration, and limited ability to deal with and not to dislodge large organized thrombotic material to other vascular territories during removal of the aspiration catheter (13). Interestingly, several trials using OCT demonstrated large residual thrombus after aspiration with current thrombus aspiration devices (16, 35). Hence, effective thrombus removal is crucial.
Our study is the first to show that angiographic outcomes, linked with worse prognosis, including distal embolization (5), no-reflow phenomenon (36), and angiographically evident residual thrombus, are more frequent among patients with larger retrieved thrombus, as assessed quantitatively. Similarly, we observed a trend toward worse MBG in patients with larger aspirated thrombus volume. Moreover, in these patients a lower extent of ST-segment resolution was observed, which could be potentially attributed to a higher prevalence of residual thrombus. These findings suggest that, despite retrieving higher thrombus load, current MATh technology fails to adequately deal with thrombotic material in patients with large TB, who are at higher risk for adverse outcomes and who would theoretically benefit most from effective thrombus removal. Therefore, given the fact that residual thrombus due to ineffective thrombectomy has been associated with impaired microcirculatory perfusion and more significant myocardial injury (14), further studies are warranted to explore novel more effective thrombus aspiration technologies with the potential to improve patient outcomes (37).
Regarding variables correlated with larger extracted thrombus, we observed that aspirated thrombus volume was higher among smokers. This finding is in line with previous studies showing a greater thrombus burden in smokers (38). Smoking may induce a hypercoagulable state increasing blood viscosity (39) and promoting platelet aggregation and thrombogenesis. These mechanisms could also explain our finding that smoking constitutes an independent risk factor for no-reflow phenomenon, although this relationship has not been yet confirmed (40). Apart from smoking, our data suggest that lumen diameter and RCA are predictive factors for larger retrieved thrombi. Larger coronary vessels can accommodate greater amounts of thrombotic material. Another potential explanation could be lower shear stress observed in vessels with greater diameter, as shear stress has been shown to influence the mechanisms supporting platelet aggregation and adhesion to the thrombogenic area through different pathways (41). Current literature also supports that thrombi in RCA tend to be larger possibly due to proximal propagation of thrombotic material related to fewer branch points (42, 43).
Moreover, micro-CT analysis of thrombus density demonstrated that patients with clots of high density (erythrocyte-rich thrombi) experienced worse pre-procedural TIMI flow. This finding is in line with previous histopathologic reports (44) showing that TIMI flow was worse in patients with red clots, compared to patients with white clots. A similar OCT-based study by Higuma et al. (35) showed that thrombi with greater erythrocyte-positive area were associated with worse angiographic outcomes.
Limitations
Our study has several limitations. Firstly, all patients were enrolled from a single center, which could limit the generalizability of our results. Secondly, the study lacks intracoronary imaging data, thus the presence of residual thrombus was based on visual evaluation. Moreover, MATh was performed at the discretion of the interventionalist executing the pPCI and therefore the possibility of selection bias cannot be excluded. It is also possible that periprocedural IIBIIIA inhibitor administration and operators' technique could have affected the outcomes of our study. However, all operators were strongly encouraged to follow the same thrombectomy procedure. Thus, derived outcomes should not have been substantially affected, by inconsistent aspiration techniques. Additionally, formalin used to preserve extracted clots may have caused thrombus shrinkage and subsequent underestimation of thrombus measurements. Similarly, sample staining with PTA has altered the measured density of the thrombotic material. However, both formalin fixation and PTA staining affected equally all specimens, since the same process was applied to all of them. Hence, we expect that these processes did not influence the comparability of our measurements. Last, this trial was not designed to investigate potential associations between micro-CT findings and hard clinical outcomes.
In conclusion, novel imaging techniques, such as micro-CT, can be employed for accurate and reproducible assessment of extracted thrombotic material, paving the way for more extensive research in this field. Our results indicate that the inadequacy of MATh to provide the anticipated benefit to patients with STEMI and large thrombotic load could be attributed to the limitations of current aspiration thrombectomy devices. A major question to be addressed by future studies is whether the development and optimization of more efficient MATh devices could facilitate effective thrombus removal and minimal residual thrombus burden and subsequently improve short- and long-term outcomes of coronary procedures in this challenging setting.
Data Availability Statement
The datasets presented in this article are not readily available because data are available from corresponding author upon reasonable request and with permission of AHEPA University Hospital. Requests to access the datasets should be directed to Georgios Sianos, e-mail: gsianos@auth.gr.
Ethics Statement
The studies involving human participants were reviewed and approved by Scientific Committee, University General Hospital of Thessaloniki, AHEPA and Medical Ethics Committee, Medical School, Aristotle University of Thessaloniki. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GSi, HK, JM, and GG developed the concept and the trial protocol. EK, AP, and GSi wrote the manuscript. AK, GSo, and SH were responsible for coronary angiography execution. EP, KKo, and LS contributed to the research data management and statistical considerations, whereas DM, NS, and TZ were responsible for patient recruitment and follow-up execution. EC, KKe, and CA were responsible for micro-CT imaging execution, while FG contributed to the study with artificial clots creation. The principal investigator and supervisor of the whole study was GSi. All authors have read and approved the final manuscript.
Funding
This study was cofinanced by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH–CREATE–INNOVATE (Project Code: T1EDK-04005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ECG, Electrocardiogram; MACCE, Major Adverse Cardiac and Cerebrovascular events; MATh, Manual Aspiration Thrombectomy; MBG, Myocardial Blush Grade; Micro-CT, Micro-Computed Tomography; OCT, Optical Coherence Tomography; PCI, Percutaneous Coronary Intervention; RVD, Reference Vessel Diameter; STEMI, ST-Elevated Myocardial Infarction; TIMI, Thrombolysis In Myocardial Infarction; TB, Thrombus Burden.
References
1. Benjamin EJ, Paul M, Alvaro A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. (2019) 139:e56–528. doi: 10.1161/CIR.0000000000000659
2. Tanboga IH, Topcu S, Aksakal E, Kalkan K, Sevimli S, Acikel M. Determinants of angiographic thrombus burden in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb. (2014) 20:716–22. doi: 10.1177/1076029613483169
3. Singh M, Berger PB, Ting HH, Rihal CS, Wilson SH, Lennon RJ, et al. Influence of coronary thrombus on outcome of percutaneous coronary angioplasty in the current era (the Mayo Clinic experience). Am J Cardiol. (2001) 88:1091–6. doi: 10.1016/S0002-9149(01)02040-9
4. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
5. Henriques JPS, Zijlstra F, Ottervanger JP, de Boer M-J, van 't Hof AWJ, Hoorntje JCA, et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J. (2002) 23:1112–7. doi: 10.1053/euhj.2001.3035
6. DeWood MA, Spores J, Notske R, Mouser LT, Burroughs R, Golden MS, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. (1980) 303:897–902. doi: 10.1056/NEJM198010163031601
7. Angerås O, Haraldsson I, Redfors B, Fröbert O, Petursson P, Albertsson P, et al. Impact of thrombus aspiration on mortality, stent thrombosis, and stroke in patients with ST-segment-elevation myocardial infarction: a report from the Swedish coronary angiography and angioplasty registry. J Am Heart Assoc. (2018) 7:7680. doi: 10.1161/JAHA.117.007680
8. Jolly SS, Cairns JA, Yusuf S, Meeks B, Pogue J, Rokoss MJ, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. (2015) 372:1389–98. doi: 10.1056/NEJMoa1415098
9. Jolly SS, James SK, DŽavík V, Cairns JA, Mahmoud KD, Zijlstra F, et al. Thrombus aspiration in ST elevation myocardial infarction: an individual patient meta-analysis. Circulation. (2016) 135:143–52. doi: 10.1161/CIRCULATIONAHA.116.025371
10. Lagerqvist B, Fröbert O, Olivecrona GK, Gudnason T, Maeng M, Alström P, et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. (2014) 371:1111–20. doi: 10.1056/NEJMoa1405707
11. Vlaar PJ, Svilaas T, van der Horst IC, Diercks GFH, Fokkema ML, de Smet BJGL, et al. Cardiac death and reinfarction after 1 year in the thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction study (TAPAS): a 1-year follow-up study. Lancet (London, England). (2008) 371:1915–20. doi: 10.1016/S0140-6736(08)60833-8
12. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
13. Powers ER. Aspiration thrombectomy: the possible importance of effective thrombus removal and minimal residual thrombus burden. JACC Cardiovasc Interv. (2016) 9:2012–3. doi: 10.1016/j.jcin.2016.07.037
14. Higuma T, Soeda T, Yamada M, Yokota T, Yokoyama H, Izumiyama K, et al. Does residual thrombus after aspiration thrombectomy affect the outcome of primary PCI in patients with ST-segment elevation myocardial infarction? An optical coherence tomography study. JACC Cardiovasc Interv. (2016) 9:2002–11. doi: 10.1016/j.jcin.2016.06.050
15. Amabile N, Hammas S, Fradi S, Souteyrand G, Veugeois A, Belle L, et al. Intra-coronary thrombus evolution during acute coronary syndrome: regression assessment by serial optical coherence tomography analyses. Eur Heart J Cardiovasc Imaging. (2015) 16:433–40. doi: 10.1093/ehjci/jeu228
16. Bhindi R, Kajander OA, Jolly SS, Kassam S, Lavi S, Niemelä K, et al. Culprit lesion thrombus burden after manual thrombectomy or percutaneous coronary intervention-alone in ST-segment elevation myocardial infarction: the optical coherence tomography sub-study of the TOTAL (ThrOmbecTomy versus PCI ALone) trial. Eur Heart J. (2015) 36:1892–900. doi: 10.1093/eurheartj/ehv176
17. Jinnouchi H, Torii S, Kutyna M, Sakamoto A, Kolodgie FD, Finn AV, et al. Micro-computed tomography demonstration of multiple plaque ruptures in a single individual presenting with sudden cardiac death. Circ Cardiovasc Imaging. (2018) 11:e008331. doi: 10.1161/CIRCIMAGING.118.008331
18. Kojonazarov B, Belenkov A, Shinomiya S, Wilchelm J, Kampschulte M, Mizuno S, et al. Evaluating systolic and diastolic cardiac function in rodents using microscopic computed tomography. Circ Cardiovasc Imaging. (2018) 11:e007653. doi: 10.1161/CIRCIMAGING.118.007653
19. Zelt JGE, Mielniczuk LM. Micro computed tomography in experimental pulmonary arterial hypertension. Circ Cardiovasc Imaging. (2018) 11:e008631. doi: 10.1161/CIRCIMAGING.118.008631
20. DiCorpo D, Tiwari A, Tang R, Griffin M, Aftreth O, Bautista P, et al. The role of micro-CT in imaging breast cancer specimens. Breast Cancer Res Treat. (2020) 180:343–57. doi: 10.1007/s10549-020-05547-z
21. Sangaralingham SJ, Ritman EL, McKie PM, Ichiki T, Lerman A, Scott CG, et al. Cardiac micro-computed tomography imaging of the aging coronary vasculature. Circ Cardiovasc Imaging. (2012) 5:518–24. doi: 10.1161/CIRCIMAGING.112.973057
22. Karagiannidis E, Konstantinidis NV, Sofidis G, Chatzinikolaou E, Sianos G. Rationale and design of a prospective, observational study for the quantitative estimation of thrombus burden in patients with ST-elevation myocardial infarction using micro-computed tomography: the QUEST-STEMI trial. BMC Cardiovasc Disord. (2020) 20:1–8. doi: 10.1186/s12872-020-01393-5
23. World Medical Association. World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
24. Harkness JR, Sabatine MS, Braunwald E, Morrow DA, Sloan S, Wiviott SD, et al. Extent of ST-segment resolution after fibrinolysis adds improved risk stratification to clinical risk score for ST-segment elevation myocardial infarction. Am Heart J. (2010) 159:55–62. doi: 10.1016/j.ahj.2009.10.033
25. Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol. (2010) 22(Suppl. B):6B–14B. Available online at: https://pubmed.ncbi.nlm.nih.gov/20947930/
26. Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, et al. Thrombolysis in myocardial infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. (1987) 76:142–54. doi: 10.1161/01.CIR.76.1.142
27. Gibson CM, De Lemos JA, Murphy SA, Marble SJ, McCabe CH, Cannon CP, et al. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction a TIMI 14 substudy. Circulation. (2001) 103:2550–4. doi: 10.1161/01.CIR.103.21.2550
28. Metscher BD. Micro CT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. (2009) 9:11. doi: 10.1186/1472-6793-9-11
29. Popovic ZB, Thomas JD. Assessing observer variability: a user's guide. Cardiovasc Diagn Ther. (2017) 7:317–24. doi: 10.21037/cdt.2017.03.12
30. McHugh ML. Interrater reliability: the kappa statistic. Biochem Medica. (2012) 22:276–82. doi: 10.11613/BM.2012.031
31. Midi H, Sarkar SK, Rana S. Collinearity diagnostics of binary logistic regression model. J Interdiscip Math. (2010) 13:253–67. doi: 10.1080/09720502.2010.10700699
32. Sianos G, Papafaklis MI, Daemen J, Vaina S, van Mieghem CA, van Domburg RT, et al. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction. The importance of thrombus burden. J Am Coll Cardiol. (2007) 50:573–83. doi: 10.1016/j.jacc.2007.04.059
33. Ge J, Schäfer A, Ertl G, Nordbeck P. Thrombus aspiration for ST-segment-elevation myocardial infarction in modern era: still an issue of debate? Circ Cardiovasc Interv. (2017) 10:1–12. doi: 10.1161/CIRCINTERVENTIONS.117.005739
34. Stone GW, Maehara A, Witzenbichler B, Godlewski J, Parise H, Dambrink JHE, et al. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA. (2012) 307:1817–26. doi: 10.1001/jama.2012.421
35. Higuma T, Soeda T, Abe N, Yamada M, Yokoyama H, Shibutani S, et al. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after per. JACC Cardiovasc Interv. (2015) 8:1166–76. doi: 10.1016/j.jcin.2015.02.026
36. Iijima R, Shinji H, Ikeda N, Itaya H, Makino K, Funatsu A, et al. Comparison of coronary arterial finding by intravascular ultrasound in patients with “transient no-reflow” versus “reflow” during percutaneous coronary intervention in acute coronary syndrome. Am J Cardiol. (2006) 97:29–33. doi: 10.1016/j.amjcard.2005.07.104
37. Karagiannidis E, Sofidis G, Stalikas N, Koletsa T, Kartas A, Keklikoglou K, et al. Rationale and design of a prospective, single-arm trial for the evaluation of safety and feasibility of large thrombus burden aspiration in the context of ST elevation myocardial infarction. Hell J Cardiol. (2020) 61:450–2. doi: 10.1016/j.hjc.2020.04.002
38. Gupta T, Kolte D, Khera S, Harikrishnan P, Mujib M, Aronow WS, et al. Smoker's paradox in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Am Heart Assoc. (2016) 5:1–11. doi: 10.1161/JAHA.116.003370
39. Inoue T. Cigarette smoking as a risk factor of coronary artery disease and its effects on platelet function. Tob Induc Dis. (2004) 2:27. doi: 10.1186/1617-9625-2-1-27
40. Ndrepepa G, Tiroch K, Keta D, Fusaro M, Seyfarth M, Pache J, et al. Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv. (2010) 3:27–33. doi: 10.1161/CIRCINTERVENTIONS.109.896225
41. Ruggeri ZM, Loredana MG. Adhesion mechanisms in platelet function. Circ Res. (2007) 100:1673–85. doi: 10.1161/01.RES.0000267878.97021.ab
42. Srikanth S, Ambrose JA. Pathophysiology of coronary thrombus formation and adverse consequences of thrombus during PCI. Curr Cardiol Rev. (2012) 8:168–76. doi: 10.2174/157340312803217247
43. Ren H, Zheng Y, Hu X, Yang Y, Zhang Y, Sun Y, et al. High thrombus burden: a review of mechanisms and treatments. Int J Clin Exp Med. (2019) 12:13068–78. Available online at: http://www.ijcem.com/files/ijcem0096007.pdf
44. Yunoki K, Naruko T, Inoue T, Sugioka K, Inaba M, Iwasa Y, et al. Relationship of thrombus characteristics to the incidence of angiographically visible distal embolization in patients with ST-segment elevation myocardial infarction treated with thrombus aspiration. JACC Cardiovasc Interv. (2013) 6:377–85. doi: 10.1016/j.jcin.2012.11.011
Keywords: micro-computed tomography, thrombus aspiration, thrombus, ST-elevation myocardial infarction, interventional cardiology
Citation: Karagiannidis E, Papazoglou AS, Sofidis G, Chatzinikolaou E, Keklikoglou K, Panteris E, Kartas A, Stalikas N, Zegkos T, Girtovitis F, Moysidis DV, Stefanopoulos L, Koupidis K, Hadjimiltiades S, Giannakoulas G, Arvanitidis C, Michaelson JS, Karvounis H and Sianos G (2021) Micro-CT-Based Quantification of Extracted Thrombus Burden Characteristics and Association With Angiographic Outcomes in Patients With ST-Elevation Myocardial Infarction: The QUEST-STEMI Study. Front. Cardiovasc. Med. 8:646064. doi: 10.3389/fcvm.2021.646064
Received: 24 December 2020; Accepted: 22 February 2021;
Published: 21 April 2021.
Edited by:
Takashi Muramatsu, Fujita Health University Hospital, JapanReviewed by:
Rob Krams, Queen Mary University of London, United KingdomChristos Bourantas, University College London, United Kingdom
Copyright © 2021 Karagiannidis, Papazoglou, Sofidis, Chatzinikolaou, Keklikoglou, Panteris, Kartas, Stalikas, Zegkos, Girtovitis, Moysidis, Stefanopoulos, Koupidis, Hadjimiltiades, Giannakoulas, Arvanitidis, Michaelson, Karvounis and Sianos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgios Sianos, gsianos@auth.gr
†ORCID: Georgios Sofidis orcid.org/0000-0001-5802-0379
Efstratios Karagiannidis orcid.org/0000-0001-8328-5942
Andreas S Papazoglou orcid.org/0000-0003-4981-8121
Dimitrios V. Moysidis orcid.org/0000-0001-9083-0267
Anastasios Kartas orcid.org/0000-0002-1170-9133
Nikolaos Stalikas orcid.org/0000-0002-0190-5392
Eleftherios Panteris orcid.org/0000-0002-5846-388X
Kleoniki Keklikoglou orcid.org/0000-0002-6693-2033
Evangelia Chatzinikolaou orcid.org/0000-0002-7171-5105
Georgios Sianos orcid.org/0000-0002-3042-0757
 Efstratios Karagiannidis
Efstratios Karagiannidis Andreas S Papazoglou1†
Andreas S Papazoglou1†  Georgios Sofidis
Georgios Sofidis Evangelia Chatzinikolaou
Evangelia Chatzinikolaou Kleoniki Keklikoglou
Kleoniki Keklikoglou Thomas Zegkos
Thomas Zegkos Leandros Stefanopoulos
Leandros Stefanopoulos Kleanthis Koupidis
Kleanthis Koupidis Christos Arvanitidis
Christos Arvanitidis Georgios Sianos
Georgios Sianos