Gut microbiota remodeling: A promising therapeutic strategy to confront hyperuricemia and gout
- 1TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3College of Medical Technology, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4School of Traditional Chinese Medicine, Capital Medical University, Beijing, China
The incidence of hyperuricemia (HUA) and gout continuously increases and has become a major public health problem. The gut microbiota, which colonizes the human intestine, has a mutually beneficial and symbiotic relationship with the host and plays a vital role in the host’s metabolism and immune regulation. Structural changes or imbalance in the gut microbiota could cause metabolic disorders and participate in the synthesis of purine-metabolizing enzymes and the release of inflammatory cytokines, which is closely related to the occurrence and development of the metabolic immune disease HUA and gout. The gut microbiota as an entry point to explore the pathogenesis of HUA and gout has become a new research hotspot. This review summarizes the characteristics of the gut microbiota in patients with HUA and gout. Meanwhile, the influence of different dietary structures on the gut microbiota, the effect of the gut microbiota on purine and uric acid metabolism, and the internal relationship between the gut microbiota and metabolic endotoxemia/inflammatory factors are explored. Moreover, the intervention effects of probiotics, prebiotics, and fecal microbial transplantation on HUA and gout are also systematically reviewed to provide a gut flora solution for the prevention and treatment of related diseases.
1. Introduction
Uric acid (UA) is the terminal metabolite of human purine compounds, and excessive UA production and/or reduced UA excretion can lead to hyperuricemia (HUA, >6 mg/dl) (Bardin and Richette, 2014). Gout is a crystal-related joint disease caused by the deposition of monosodium urate (MSU) crystals in or around the joints. Gout is directly related to HUA caused by disorders of purine metabolism, decreased UA excretion, or increased UA production in the body (Figure 1) (Richette and Bardin, 2010; Dalbeth et al., 2016; Dalbeth et al., 2021). In recent years, the incidence of HUA and gout has continued to increase worldwide, which may be related to changes in lifestyle, such as the prevalence of a high-purine diet, fructose beverages, and alcohol consumption (Kolasinski, 2014; Jakše et al., 2019; Yokose et al., 2021). HUA and gout cause increased oxidative stress, endothelial dysfunction, inflammation, platelet adhesion and aggregation, and vasoconstriction (Macfarlane et al., 1983; Krishnan, 2010; Feig, 2014; So and Martinon, 2017; Zhao et al., 2021). HUA and gout are also independent risk factors for cardiovascular disease, metabolic syndrome, and acute kidney injury (Thottam et al., 2017; Bardin and Richette, 2017; Abeles and Pillinger, 2019). The prevalence of HUA and its complications has caused a heavy economic burden on society and families. With the trend of HUA and gout rising and occurring in younger individuals, patients have an urgent need for efficient and safe therapeutic methods or drugs. The scientific understanding of the pathogenesis of related diseases and the selection and optimization of therapeutic drugs have always been the focus of medical research and represent the great opportunities and challenges faced by HUA and gout treatment.
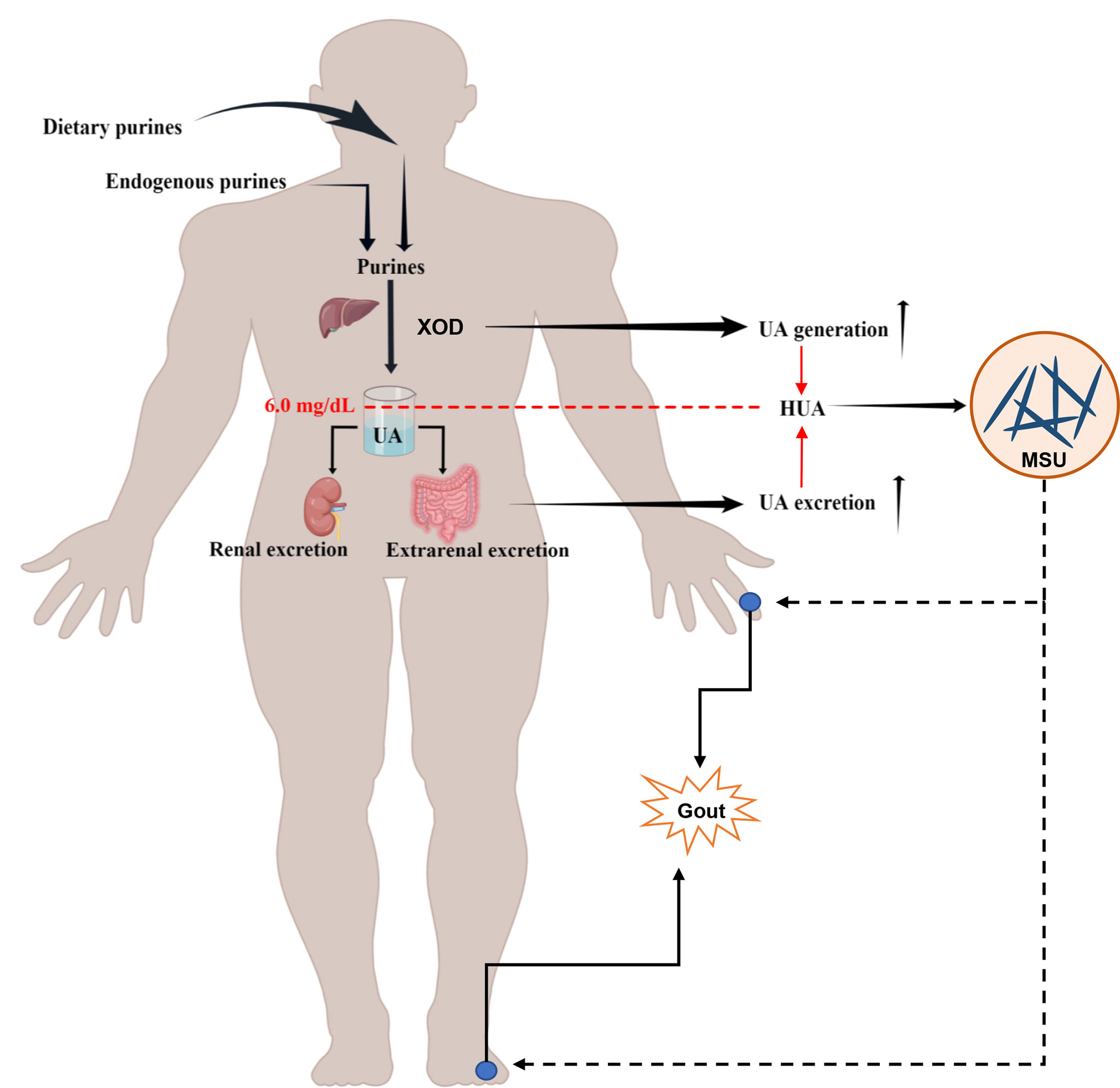
Figure 1 Schematic diagram of the pathophysiological model of HUA and gout formation. HUA (serum UA levels >6.0 mg/dl) is caused by an imbalance of UA metabolism, including an increase in UA production through endogenous purine and exogenous purine metabolism and a decrease in UA excretion owing to the reduction of renal excretion and extrarenal excretion. The deposition of MSU crystals in or around the joints induces gout.
Various microorganisms colonize the human gut and play an irreplaceable role in regulating human energy and metabolism. Structural changes or imbalances in the gut microbiota may cause organism metabolic disorders (Agus et al., 2021; Fan, 2021). Considering the gut microbiota as an entry point to explore the pathogenesis of diseases has become a new hotspot worldwide (Meng et al., 2018; Jackson et al., 2018; Angelucci et al., 2019; Bell et al., 2020), especially in research on investigating the pathogenesis of metabolic-related diseases, such as HUA and gout. Approximately 70% of UA is excreted through the kidneys, and the remainder is mainly excreted with the feces or further catabolized by the gut microbiota. Current studies have found that gut microbiota imbalances exist in patients with HUA and gout (Chu et al., 2021; Yin et al., 2022). In addition, the gut microbiota can be altered after UA reduction (Yu et al., 2018), and probiotics may have UA-lowering effects (Wu et al., 2021; Zhao and Lu, 2022). Therefore, an in-depth study of the relationship between gut microbes and HUA and gout is expected to make it a target for the prevention and treatment of related diseases.
2. Characteristics of the gut microbiota in patients with HUA and gout
There are many types of microorganisms in the human intestinal tract. The gut microbiota is composed of three different types of bacteria, namely, beneficial, harmful, and opportunistic pathogenic bacteria; among these, Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria are the most important components of the human gut microbiota (Lynch and Pedersen, 2016). In recent years, research has found that the gut microbiota of patients with HUA and gout has also undergone significant changes (Table 1).
Patients with HUA had reduced microbiota richness and diversity, an altered composition, and a lower relative abundance of Coprococcus than the normal controls (Wei and Zhang, 2022). The probiotics Faecalibacterium, Gemmiger, Bacteroides, Roseburia, Bifidobacterium, and Akkermansia were significantly increased in asymptomatic HUA patients compared to the controls (Yang et al., 2021). The increase in probiotics is presumed to be a compensatory phenomenon. Structural changes in the gut microbiota are an important cause of elevated UA levels. Highly metabolically active commensal bacteria, such as Faecalibacterium, play an important role in balancing gut immunity as a part of a healthy human microbiome (Miquel et al., 2013; Ferreira-Halder et al., 2017). As one of the first microbial colonizers of the neonatal gut, Bifidobacterium plays a key role in physiological development, including the maturation of the immune system and the use of dietary components (Hidalgo-Cantabrana et al., 2017). Through a comparative analysis of the gut microbiota of gout and HUA patients, the gut microbiota diversity in gout patients was lower than that in primary asymptomatic HUA patients (Kim and Yoon, 2022). It is speculated that different gut microbiota in asymptomatic HUA patients may prevent gout development, while differences in gout patients perhaps play a role in gout induction. Furthermore, an asymptomatic HUA group showed a higher Firmicutes-to-Bacteroidetes (F/B) ratio and a lower Prevotella-to-Bacteroides ratio (P/B) (Kim and Yoon, 2022). The F/B ratio is widely believed to have an important impact on the maintenance of normal intestinal homeostasis (Stojanov and Berlec, 2020). An increase or decrease in the F/B ratio is a manifestation of an organism’s dysbiosis.
The metagenomes of gout patients significantly differed from those of healthy controls, and the richness and diversity of the gut microbiota were reduced in gout patients (Shao et al., 2017; Chu et al., 2021). The relative abundances of Prevotella, Fusobacterium, and Bacteroides were increased in gout patients, while the relative abundances of Enterobacteriaceae and butyrate-producing species were decreased (Chu et al., 2021). Meanwhile, studies have also shown the upregulation of opportunistic pathogens, such as Bacteroides, Rhodococcus, Erysipelatoclostridium, and Anaerolineaceae, in gout patients (Shao et al., 2017). The gut microbes of gout patients were rich in Bacteroides caccae, Bacteroides xylanisolvens, Bacteroides ovatus, and Eubacterium rectale, but relatively deficient in Faecalibacterium prausnitzii and Bifidobacterium pseudocatenulatum (Guo et al., 2016). However, contrary findings showed that Fecalibacterium was the most dominant genus detected in untreated gout patients, followed by Clostridium and Cytophaga (Lin et al., 2021). An analysis of the gut microbiota in gout patients with and without tophi revealed that the genera Phascolarctobacterium, Bacteroides, Akkermansia, and Ruminococcus_gnavus_group had the lowest species diversity but a higher abundance in gout patients without tophi (Méndez-Salazar et al., 2021). Proteobacteria and Escherichia coli-Shigella were more abundant in tophi patients than controls (Méndez-Salazar et al., 2021). The differences of the gut microbiota in different studies are believed to be caused by the following reasons. On the one hand, differences may exist in purine metabolism, UA metabolism, and amino acid metabolism between HUA and gout patients, resulting in the transformation of gut microbiota. On the other hand, differences in sex and age affect the structure and composition of the gut microbiota (Kim and Jazwinski, 2018; Aron-Wisnewsky et al., 2020). In addition, although most clinical studies have ruled out the influence of underlying diseases and antibiotic treatment, other factors, such as diet, smoking, and alcohol consumption, are important factors in regulating changes in the gut microbiota (Engen et al., 2015; Huang and Shi, 2019; Zmora et al., 2019). In summary, alterations in the gut microbiota are clearly closely related to various factors. Therefore, an analysis of the gut microbiota in specific populations of HUA and gout patients could help improve the understanding of gut microbiota therapy.
3. The gut microbiota in the pathogenesis of HUA and gout
3.1 Diet and intestinal microbiota homeostasis
Diet is an important factor affecting the microbial composition of the gastrointestinal tract. A high-fructose diet, high-fat diet, high-purine diet, and high-oxalate diet can lead to changes in the composition of the gut microbiota in animal models of HUA and gout (Figure 2).
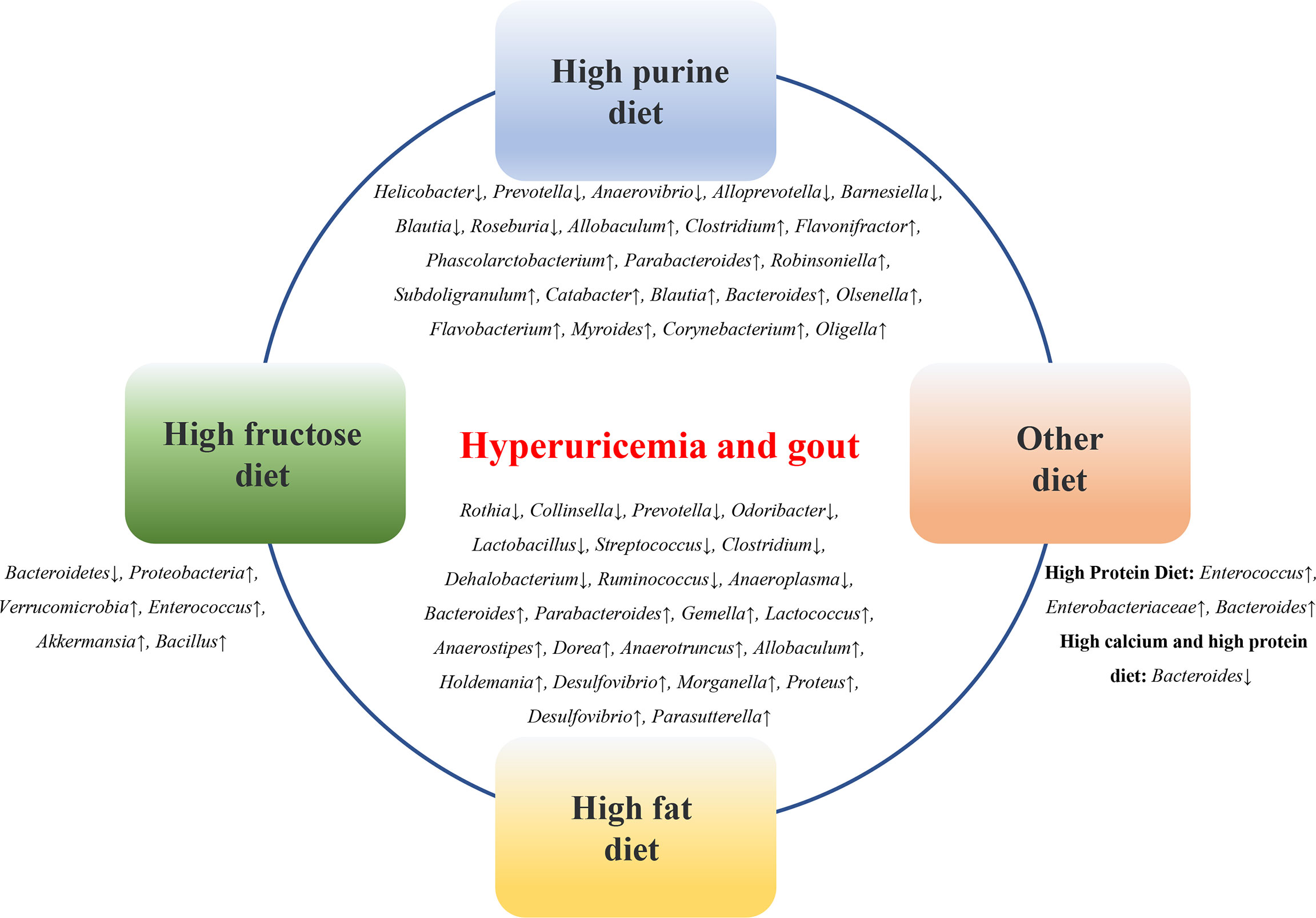
Figure 2 Diets affect the microbial composition of the gastrointestinal tract. High-fructose diet, high-fat diet, high-purine diet, etc. can lead to changes in the composition of intestinal flora in animal models of HUA and gout.
3.1.1 High-purine diet
Eighty percent of UA is derived from the degradation of endogenous purines, and 20% of UA is derived from exogenous purines, such as food. A high-purine diet, such as seafood, animal offal, and alcohol consumption, is a risk factor for HUA and gout and an important cause of gut microbiota imbalance in patients. Compared with the control group, the relative abundances of Firmicutes and Actinobacteria were significantly increased, and the relative abundances of Bacteroidetes and Proteobacteria were significantly reduced in a purine diet-induced HUA mouse model (Wan et al., 2020). In a quail HUA model induced by a high-purine diet, the proportion of Prevotellaceae was significantly higher than that in the normal group, while the abundance of Helicobacter was low (Bian et al., 2020). In a yeast and purine diet-induced HUA rat model, the diversity and abundance of the gut microbiota were altered, and the relative abundances of Prevotella, Anaerovibrio, Alloprevotella, and Barnesiella at the genus level were lower than those in the control rats, while Allobaculum, Clostridium_XlVa, Flavonifractor, Phascolarctobacterium, Clostridium_XVIII, Parabacteroides, Robinsoniella, Subdoligranulum, Catabacter, Blautia, Bacteroides, and Olsenella were relatively more abundant than those in the controls (Liu et al., 2020). In a purine diet-induced hyperuricemic nephropathy rat model, Flavobacterium, Myroides, Corynebacterium, Alcaligenaceae, Oligella, and other opportunistic pathogens were significantly increased, while the short-chain fatty acid (SCFA)-producing bacteria Blautia and Roseburia were significantly decreased (Pan et al., 2020). The changes in these microbial groups suggest that transformation in gut microbes may play a role in HUA and gout.
3.1.2 High-fructose diet
The risk of obesity, diabetes, cardiovascular disease, and metabolic syndrome is linked to the consumption of beverages containing sugar or high fructose corn syrup (Tappy and Lê, 2010; Bray, 2013; Jensen et al., 2018). Excessive fructose intake is an important reason for the increased prevalence of gout and HUA (Hak and Choi, 2008; Li et al., 2018; Nakagawa et al., 2019). Fructose metabolism activates adenosine monophosphate deaminase, promoting purine degradation and inosine production and resulting in elevated serum UA (Thottam et al., 2017). Research has shown that a large amount of fructose can activate nicotinamide adenine dinucleotide phosphate oxidase, which further prevents the excretion of UA through the ileum, resulting in an increase in the serum UA levels in the human body (Kaneko et al., 2017). A high-fructose diet can significantly reduce the diversity of gut microbes in the short term, with a significant increase in the ratio of Firmicutes to Bacteroidetes and a decrease in the proportion of probiotics such as Lactobacillus (Silva et al., 2018). The changes in the gut microbiota further reduce butyric acid and glutamate in the intestinal tract and increase the production of fructose, succinic acid, taurine, tyrosine, and xylose, leading to a disturbance in the intestinal microecology and further impairing the intestinal barrier function (Silva et al., 2018). Studies have found the following two main types of flora in mice after high-fructose feeding: increases in Proteobacteria, and significant decreases in Bacteroidetes (Do et al., 2018). In a mouse model of HUA induced by high fructose, the abundance of Bacteroidetes was significantly decreased, and the abundance of Verrucomicrobia, Enterococcus, Akkermansia, and Bacillus was increased (Wang et al., 2019). In addition, a high consumption of fructose can promote intestinal malnutrition and lipopolysaccharide (LPS)-related inflammation while accelerating the degradation of purines (Wang and Qi, 2020). Therefore, lowering the intake of fructose and adopting a healthier diet may help better manage HUA and gout and reduce related diseases. Meanwhile, further observations and evaluations of the actual efficacy of specific dietary measures in HUA and gout patients in clinical practice are needed.
3.1.3 High-fat diet
As a public health problem, a high-fat diet has been shown to be associated with various digestive system diseases, cardiovascular diseases, urinary system diseases, tumors, etc. and can accelerate the occurrence of diseases due to inflammation and metabolic changes (Yin et al., 2018; Tong et al., 2021). A high-fat diet is closely associated with changes in the gut microbiota. Studies have shown that at the genus level, 12 genera (Bacteroides, Parabacteroides, Gemella, Lactococcus, Anaerostipes, Dorea, Anaerotruncus, Allobaculum, Holdemania, Desulfovibrio, Morganella, and Proteus) were enriched in HUA rats induced by a high-fat diet containing 10% yeast extract, but 10 other genera (Rothia, Collinsella, Prevotella, Odoribacter, Lactobacillus, Streptococcus, Clostridium, Dehalobacterium, Ruminococcus, and Anaeroplasma) were less abundant (Yu et al., 2018). Another study showed that the relative abundances of Muribaculaceae, Bacteroides, and Lachnospiraceae were significantly decreased in a gout model induced by MSU crystals combined with high-fat diet feeding compared with the normal control group (Feng et al., 2022). In a gout model induced by a combination of MSU crystals and high-fat diet feeding, changes in the gut microbiota were induced, including increased levels of Desulfovibrio and Parabacteria (Lin et al., 2020).
3.1.4 Other diets
Studies have found that the expression of adenosine triphosphate-binding cassette superfamily G member 2 (ABCG2) was significantly increased in an oxonic acid diet-induced HUA rat model, supplementation with probiotics could reduce this expression, and significant differences in the microbiota were observed between the treated and untreated fecal samples (García-Arroyo et al., 2018). Enterococcus, Enterobacteriaceae, and Bacteroidetes were enriched in the cecum of high-protein diet-induced gout goslings (Xi et al., 2020). A high-calcium and high-protein diet-induced gout model exhibited liver and kidney damage in geese, an impaired intestinal barrier, and a significantly decreased abundance of Bacteroides (Ma et al., 2021). Implementing dietary solutions for the closely linked diet-gut microbiota-HUA/gout is a new challenge and opportunity, which is of great significance for the prevention and treatment of HUA and gout.
3.2 Gut microbiota and metabolism of purine, UA, and amino acids
3.2.1 Gut microbiota and purine metabolism
UA is the final product of purine metabolism in the human body. Purine nucleotides are hydrolyzed into adenine and guanine, deaminated to form xanthine, and then oxidized to form UA. The gut microbiota can affect the metabolism of purine. Lactobacillus gasseri PA-3 in the intestine can absorb and utilize purine, thereby reducing the intestinal absorption of purine in the diet and reducing the serum UA levels (Yamada et al., 2016). Xanthine dehydrogenase (XDH), which is responsible for purine oxidative metabolism, can be secreted by bacteria of the genus Escherichia, such as E. coli (Wang et al., 2017). Lactobacillus DM9218 can effectively reduce the serum UA levels in HUA rats by inhibiting xanthine oxidase (XOD) activity (Li et al., 2014). Shiga-toxigenic E. coli (STEC) and enteropathogenic E. coli (EPEC) can promote the release of XOD in intestinal epithelial cells, accelerate the decomposition of hypoxanthine and xanthine, and convert more purines for UA (Crane et al., 2013). Lactobacillus reuteri TSR332 and Lactobacillus fermentum TSF331 can control the development of HUA by degrading purine (Kuo et al., 2021). Intestinal microbes play an important role in the process of purine metabolism (Figure 3). How to use these beneficial intestinal bacteria to regulate purine metabolism and then affect the production of UA is a problem that should be considered in current research.
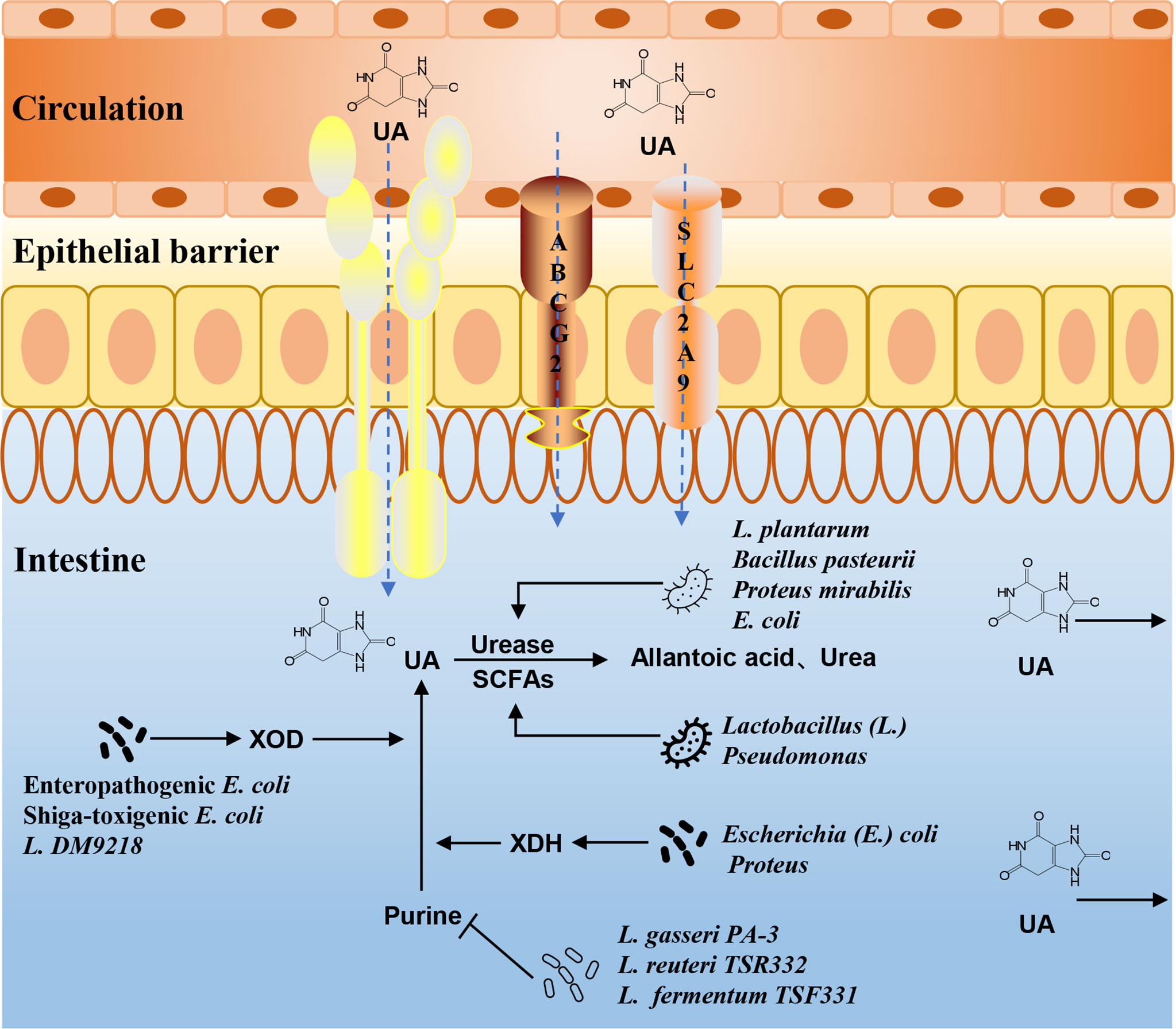
Figure 3 The mechanism of gut microbiota in UA and purine metabolism. The intestinal flora can affect purine metabolism, XOD, XDH, and urease activities, or the composition of SCFAs, and then alter the production and excretion of UA.
3.2.2 Gut microbiota and UA metabolism
The gut is a main pathway of UA excretion, and the microbial environment in the gut is the basis for this function. There are two main ways to excrete UA in healthy people. Two-thirds are excreted from the urethra through renal tubular secretion, and the remaining one-third is excreted through the intestines. UA transporters in intestinal epithelial cells are responsible for transporting UA from the blood to the intestinal lumen, where it is directly excreted or broken down by the gut microbiota. Lactobacillus and Pseudomonas promote the decomposition and excretion of UA in the intestine by producing SCFAs (Wrigley et al., 2020). The gut microbiota can also regulate intestinal epithelial ABCG2, urate transporter soluble carrier protein 2 family member 9 (SLC2A9), and other UA transporters, which, in turn, affect UA metabolism (Hosomi et al., 2012; DeBosch et al., 2014). The activities of UA metabolism-related enzymes are also closely related to the gut microbiota. Uricase, which converts UA into allantoin and urea, is widely found in Bacillus pasteurii, Proteus mirabilis, E. coli, etc. (Christians and Kaltwasser, 1986; Rando et al., 1990; Nakagawa et al., 1996). Lactobacillus sp. OL-5, Lactobacillus plantarum Mut-7, and L. plantarum Dad-13 had higher intracellular uricase activity (Handayani and Hidayat, 0000). Experimental studies have shown that the abundance of Lactobacillus, Streptococcus, and Clostridium with purine absorption and UA decomposition decreased in the gut microbiota of HUA rats, while the abundance of Proteus, which secretes XDH, increased (Yu et al., 2018). The changes in the microbiota may play an important role in the regulation and metabolism of the UA levels (Figure 3).
3.2.3 Gut microbiota and amino acid metabolism
Amino acid metabolism also plays an important role in the development of HUA and gout (Mahbub et al., 2017; Pan et al., 2020; Huang et al., 2020). A reduced species abundance of Enterobacteriaceae was associated with amino acid metabolism and environmental perception, which together resulted in increased serum UA and C-reactive protein levels in gout patients (Chu et al., 2021). After metagenomics and cluster analysis of feces from gout patients and healthy people, the samples were divided into high gout clusters dominated by Bacteroides and low gout clusters with increased Faecalibacterium (Henson, 2021). The high gout cluster exhibited increased synthesis of the amino acids D-alanine and L-alanine and by-products of branched-chain amino acid catabolism, while the low gout cluster exhibited increased the production of butyrate, the sulfur-containing amino acids L-cysteine and L-methionine, and the L-cysteine catabolite H2S (Henson, 2021). Using a urate oxidase (Uox)-KO mouse model that spontaneously developed overt HUA and urate nephropathy, the gut microbiota composition and function were altered, and a distinct metabolome existed. Among them, amino acid metabolism plays a key role, and characteristic metabolites are strongly influenced by differential bacterial genera. Furthermore, an impaired gut integrity and profound alterations in the solute carrier family lead to dysregulated amino acid transport, which, in turn, affects the serum UA levels and CD4+ Th17-driven inflammation (Song et al., 2022). Of course, research investigating the effect of gut microbiota on amino acid metabolism needs to be further conducted.
In summary, the current research mainly focuses on the influence of the gut microbiota on purine metabolism, UA metabolism, and amino acid metabolism. Whether these metabolic processes can affect changes in the gut microbiota and thereby alter HUA and gout symptoms has not been discussed. Therefore, the next step should be to further explore the interaction between the gut microbiota and these metabolic processes to provide a theoretical research basis for the treatment of HUA and gout.
3.3 Gut microbiota and the secretion of LPS
LPS is a component of the cytoderm of Gram-negative bacteria in the gut microbiota. Disturbances in the intestinal microenvironment can significantly suppress the activity of Gram-negative bacteria. Furthermore, the increased secretion of LPS can prompt the body to produce a large amount of cytokines, increase the permeability of the intestinal wall, and induce low-grade chronic inflammation, which is called “metabolic endotoxemia” (Fuke and Nagata, 2019; Ota et al., 2020). Recent studies have found that LPS is closely related to metabolic syndrome, obesity, and other diseases (Ghosh et al., 0000; Miller et al., 2005; Qin et al., 2007; Hersoug et al., 2018). The loss of the gut barrier resulting from gut dysbiosis leads to gut-derived LPS translocation, which plays an important role in the development of goose gout by interfering with renal function (Xi et al., 2019). Changes in the gut microbiota can increase intestinal permeability, leading to changes in LPS entering the circulatory system, thereby controlling metabolic endotoxemia, inflammation, and related diseases (Cani et al., 2008). XOD is a key enzyme in the production of UA, and an increase in XOD activity leads to an increase in UA production. Studies have shown that chronic inflammation caused by elevated serum LPS levels is often accompanied by elevated XOD activity (Ramos et al., 2018). The levels of inflammatory cytokines, LPS, and XOD activity in Uox-KO mice were significantly higher than those in wild-type mice (Guo et al., 2021). The abundance of Bifidobacterium and Lactobacillus in feces from HUA mice was significantly reduced, and the levels of UA, XOD activity, and LPS were significantly increased compared with those in normal mice (Cao et al., 2017). After a certain period of probiotic treatment, the number of Bifidobacterium and Lactobacillus in the gut microbiota increased, and the activities of serum UA, LPS, and XOD decreased (Cao et al., 2017). The above studies suggest that the mechanism of HUA may be related to LPS-activated XOD activity caused by a gut microbiota imbalance (Figure 4).
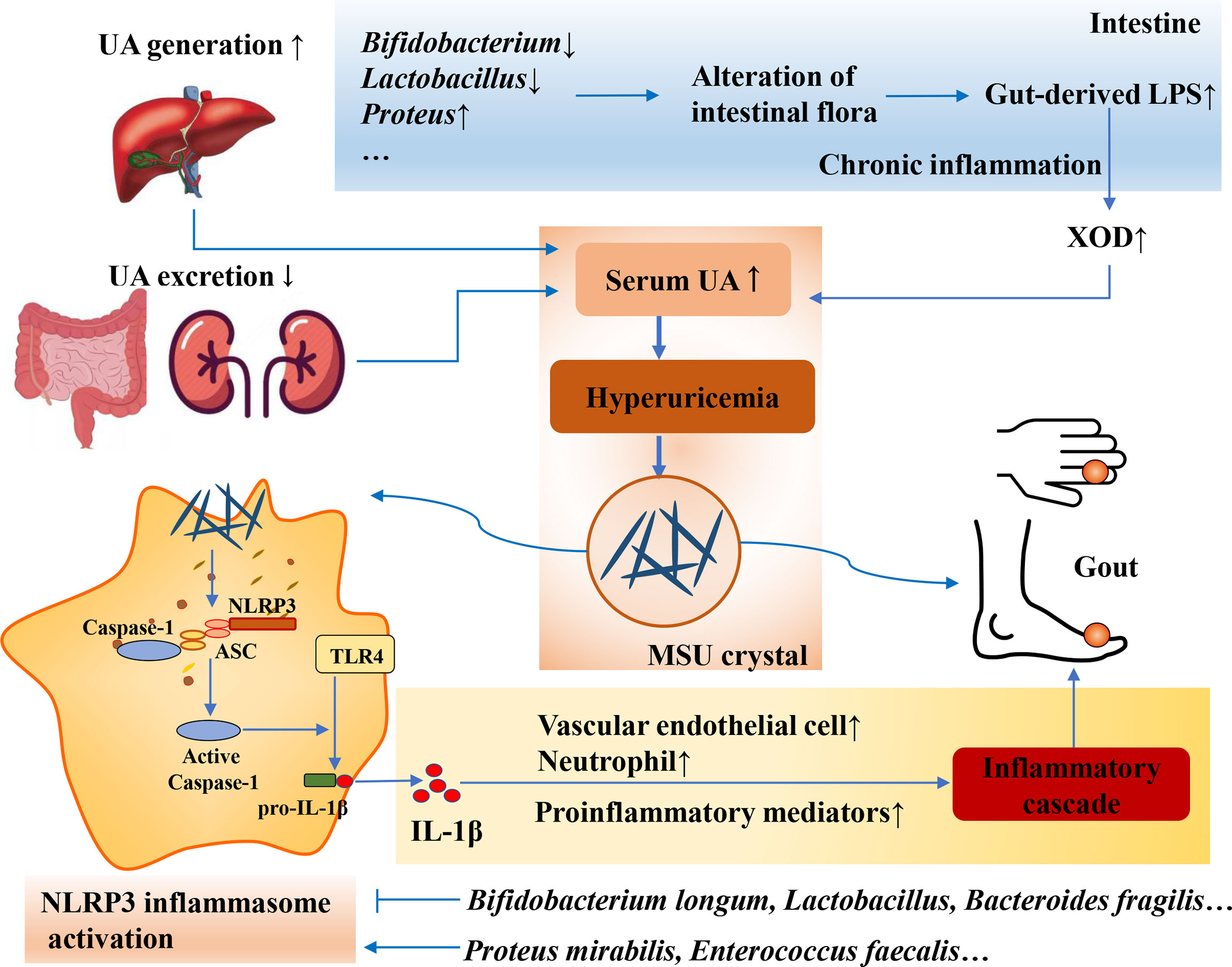
Figure 4 Intestinal flora affects the secretion of LPS and inflammatory response. Loss of gut barrier resulting from gut dysbiosis leads to gut-derived LPS translocation and chronic inflammation, resulting in the increase of SOD activity and serum UA. Moreover, gut microbiota can also modulate NLRP3 inflammasome activation, and the commensal microbiota shapes the ability of the host to respond to acute inflammatory stimuli that are dependent on the extra-intestinal inflammasome.
3.4 Gut microbiota and inflammatory response
The gut microbiome and SCFAs play critical roles in regulating the body’s inflammatory response to MSU crystals. SCFAs, including acetate, butyrate, and propionate, are the main metabolites of dietary fiber fermented by gut microbes (Sivaprakasam et al., 2016). It is also the key to regulating dietary fiber and the intestinal microbiota, and has important physiological functions, such as reducing the intestinal inflammatory response, improving intestinal epithelial barrier function, and maintaining fluid balance and energy metabolism (Tan et al., 2014). Studies have shown that the inflammatory response to an intra-articular injection of MSU crystals is dependent on SCFAs produced by microbes in the gut and that adding acetate to the drinking water of germ-free mice can restore this inflammatory response (Vieira et al., 2015). After comparing the differences in the gut microbiome and serum SCFAs in 20 patients with acute gouty arthritis between acute and convalescent states, the results showed that the gut bacterial composition significantly changed and that the acetate levels were significantly elevated in a convalescent state (Park and Lee, 2022). In addition, a study showed that several metabolites in feces from gout patients that play a role in regulating inflammation differ from those in normal people, such as increased succinic acid (Shao et al., 2017).
Inflammasome activation can be induced by various microbial pathogens, often mediating host defense by activating inflammatory responses and limiting pathogen replication. Among them, the nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome is involved in the development of gout as a sensor of metabolic stress (Martinon et al., 2006; So and Martinon, 2017; Jhang et al., 2018). In addition to its role in defense against pathogens, recent studies have shown that the gut microbiota can modulate NLRP3 inflammasome activation (Figure 4). In the absence of microbiota, the production of SCFAs, which are required for inflammasome assembly and interleukin (IL)-1β production, is reduced, and macrophages from germ-free animals produce limited oxygen and inflammasome assembly (Vieira et al., 2015). These results clearly demonstrate that the commensal microbiota shapes the ability of the host to respond to acute inflammatory stimuli that are dependent on the extraintestinal inflammasome. Enterobacteriaceae, especially the pathogen Proteus mirabilis, induce the activation of NLRP3 inflammasome (Seo et al., 2015). Enterococcus faecalis activates the NLRP3 inflammasome, leading to the increased secretion of IL-1β and the generation of pyroptosis (Ran et al., 2019; Yin et al., 2020). Lactobacillus reduces reactive oxygen species production by restoring the abnormal mitochondrial membrane potential, thereby inhibiting the activation of NLRP3 (Saber and Abd El-Fattah, 2021). Bifidobacterium longum may downregulate the expression of IL-18 and IL-1β by inhibiting the NLRP3 inflammasome (Gu et al., 2016). Bacteroides fragilis negatively regulates the NLRP3-mediated inflammatory signaling pathway, inhibits the activation of macrophages and the secretion of proinflammatory mediators, such as IL-18 and IL-1β, and reduces the level of intestinal inflammation (Shao et al., 2021). Currently, the regulation of these florae is mainly concentrated in diseases such as enteritis, and the therapeutic effect on HUA and gout needs to be further developed and utilized. Probiotic intervention is promising for modulating the NLRP3 inflammasome signaling pathway to improve HUA and gout.
4. Gut microbiota as a potential therapeutic target in HUA and gout
4.1 Probiotics
Currently, the main drugs used to lower UA are XOD inhibitors and uricosuric drugs, but these drugs have certain side effects. Relevant progress has been achieved in the use of probiotics for the treatment of HUA and gout. Lactobacillus, Bifidobacterium, and Saccharomyces have a long history of safe and effective use as probiotics (Sanders et al., 2019).
Regarding probiotic treatment, a study found that L. gasseri PA-3 can improve the UA levels in healthy people, HUA patients, and gout patients (Yamanaka et al., 2019). Experiments in an in vitro colon model found that L. gasseri PA3 decreased gut microbiota diversity, increased the relative abundance of Lactobacillus and Escherichia, and decreased the relative abundance of Bacteroides and Phascolarctobacterium (Xiang et al., 2019). L. rhamnosus R31, L. rhamnosus R28-1, and L. reuteri L20M3 promote the production of SCFAs in a purine-independent manner and alleviate the serum and urine UA concentrations in HUA mice (Ni et al., 2021). These strains also reversed the elevated LPS concentrations, liver inflammation, and kidney damage associated with HUA (Ni et al., 2021). The serum UA levels in mice fed Limosilactobacillus fermentum JL-3 were lower than those in the control group (Wu et al., 2021). The JL-3 strain also restored some inflammatory markers and oxidative stress indicators [IL-1β, malonaldehyde, creatinine (CRE), and blood urea nitrogen (BUN)] associated with HUA, while gut microbial diversity results showed that JL-3 can regulate the gut microbiota imbalance caused by HUA (Wu et al., 2021). Lactobacillus brevis DM9218 reduces the serum UA levels and hepatic XOD activity in fructose-fed mice (Wang et al., 2019). It can prevent liver damage caused by high fructose and delay the accumulation of UA by degrading inosine, regulating intestinal dysbiosis, enhancing intestinal barrier function, and reducing hepatic LPS (Wang et al., 2019). In a model of HUA induced by potassium oxonate and a high-purine diet, L. reuteri TSR332 and L. fermentum TSF331 stabilized the serum UA levels in rats, and no obvious side effects were observed (Kuo et al., 2021). There are many types of probiotics, and their mechanisms of action and their ability to colonize the gut are different. Therefore, rigorous and reliable data are still needed to further prove the effect of probiotics on HUA and gout.
4.2 Prebiotics
Published in 2017, prebiotics are defined as “substrates selectively utilized by host microorganisms with health benefits”, which expands the concept of prebiotics to potentially include noncarbohydrate substances, applications to body parts other than the gastrointestinal tract, and many categories other than food (Gibson et al., 2017). Prebiotics can play a role in the treatment of HUA and gout by altering the structure of the gut microbiota (Table 2).
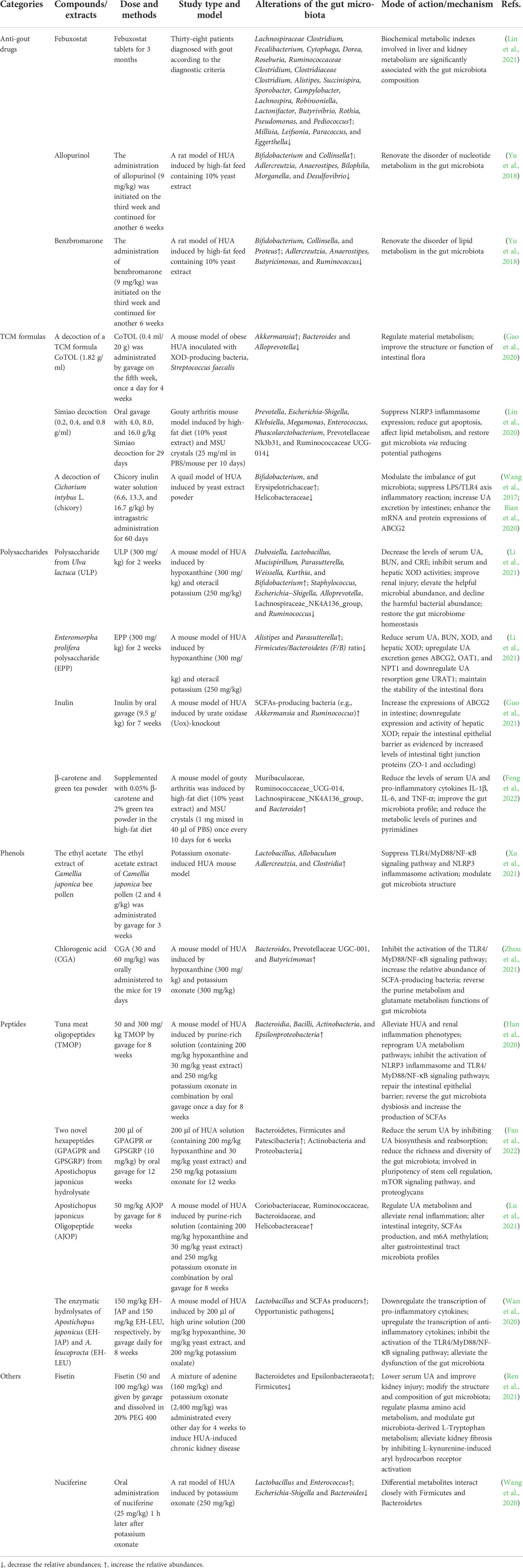
Table 2 Experiment and mechanism of prebiotics in prevention of HUA and gout targeting gut microbiota.
4.2.1 Anti-gout drugs
In addition to lifestyle improvements, drug therapy is an important approach in the treatment of HUA and gout and includes allopurinol and febuxostat, which inhibit UA production; benzbromarone, which promotes uricosuric excretion; and febuxostat, a xanthine oxidoreductase inhibitor. Recent studies have shown that in addition to improving UA symptoms, these drugs have positive effects in regulating the gut microbiota. A restriction of gut microbiota biodiversity was detected in untreated gout patients, and febuxostat partially restored this alteration (Lin et al., 2021). Biochemical metabolic indices involved in liver and kidney metabolism were significantly correlated with the composition of the gut microbiota in patients with gout. After the treatment of HUA with allopurinol and benzbromarone, the intestinal microbiota of rats changed. Both drugs could lead to an increase in Bifidobacterium and Collinsella and a decrease in Adlercreutzia and Anaerostipes after the reduction in UA (Lin et al., 2021). In addition, Bilophila, Morganella, and Desulfovibrio decreased after allopurinol treatment, while Butyricimonas and Ruminococcus decreased and Proteus increased after benzbromarone treatment (Lin et al., 2021). The above studies suggest that anti-gout drugs may exert a UA-lowering effect by regulating the gut microbiota, but their correlation needs to be further studied.
4.2.2 TCM formulas
Although the effect of Western medicine in the treatment of gout is currently obvious, there are problems, such as easy recurrence and some adverse reactions after drug withdrawal. Traditional Chinese medicines (TCMs) have a long history in the treatment of gout and have the advantages of multichannel, multitarget, and multilevel symptomatic treatment. TCMs exert therapeutic effects by lowering UA, exerting anti-inflammatory, antioxidation effects, and protecting the kidneys. CoTOL (consisting of Glabrous Greenbrier Rhizome, Dioscorea septemloba Thunb, Curcuma Longa, Parasitic loranthus, Herba Siegesbeckiae, Maydis stigma, Semen Coicis, and Corydalis Rhizoma) is a TCM formulation used clinically for the treatment of gout and HUA. An analysis of CoTOL targeted bacteria in a mouse model of obesity HUA inoculated with the XDH-producing bacterium Streptococcus faecalis. The results showed that the S. faecalis inoculation resulted in elevated UA and altered the gut microbiota structure, while the CoTOL treatment increased the abundance of Akkermansia and decreased the abundance of Bacteroides and Alloprevotella (Gao et al., 2020). In gouty arthritis model mice, Simiao Decoction can effectively reduce the serum UA levels, reduce myeloperoxidase (MPO), XOD, and adenosine deaminase (ADA) activities, and relieve gout-related symptoms such as foot swelling and pain (Lin et al., 2020). In addition, Simiao Decoction reduced some serum proinflammatory cytokines, including IL-1β, IL-9, IFN (interferon)-γ, macrophage inflammatory protein (MIP)-1α, and MIP-1β (Lin et al., 2020). By reducing potential pathogens and restoring gut microbiota, the gut ecosystem may be a potential anti-inflammatory target of Simiao Decoction. The serum UA levels were significantly decreased, and the fecal UA levels were significantly increased after an intervention with a TCM chicory (Cichorium intybus L.) (Bian et al., 2020). In addition, chicory can repair intestinal mucosal damage and improve the permeability of the intestinal barrier. A sequencing analysis showed that chicory restores the gut microbiota and alleviates HUA by increasing probiotic flora (Bifidobacterium, Erysipelotrichaceae) and reducing pathogenic flora (Helicobacteraceae) (Bian et al., 2020). Further studies showed that this may be related to the stimulation of intestinal uric acid excretion by regulating the expression of ABCG2 (Wang et al., 2017). The gut microbiota has become a new way to understand TCM and has great potential in the exploration of TCM waste, add-on therapy and TCM individualized precision medicine (Yue et al., 2019). In particular, the role of traditional Chinese medicine in regulating the gut microbiota should be considered to deepen our understanding of the functions of gut microbiota metabolites and the mechanisms of specific diseases (Cheng et al., 2021; Liu et al., 2022). Studying the effects of interventions with TCM formulas on the gut microbiota is beneficial for explaining the multichannel and multitarget characteristics acting on the body, thereby contributing to giving full play to the advantages of TCMs in fighting gout.
4.2.3 Polysaccharides
Plant polysaccharides can improve the intestinal tissue morphology, maintain the integrity of the intestinal barrier, enhance the immune response, and regulate the gut microbiota, such that the internal environment of the intestinal tract is in a good state (Lovegrove et al., 2017). Polysaccharides can act as a unique carbon source for specific gut microbiota during fermentation and may be an active component in regulating the gut microbiota. A polysaccharide from Ulva lactuca (ULP) exerts a UA-lowering effect by regulating the gut microbiota, which is characterized by increasing the abundance of beneficial microorganisms while reducing the abundance of harmful bacteria to restore the homeostasis of the gut microbiota (Li et al., 2021). A polysaccharide from Enteromorpha prolifera (EPP) significantly reduced serum UA, BUN, serum XOD, and hepatic XOD in HUA mice. In addition, EPP maintained the stability of the gut microbiota, confirming that Parasutterella is closely related to the regulation of HUA (Li et al., 2021).
In recent years, studies have shown that dietary fiber, as one of the seven essential nutrients for the human body, can play an important role in immunity and metabolism by regulating the structure of the gut microbiota (Holscher, 2017; Gill et al., 2021). Dietary fiber cannot be digested and absorbed by the gastrointestinal tract and cannot produce energy. Adding dietary fiber to the diet can significantly change the diversity of gut microbes and increase the abundance of SCFA-producing flora (So et al., 2018; Zhao and Zhang, 2018; Dalile et al., 2019). Inulin supplementation can effectively relieve HUA, increase the expression of ABCG2 in the intestine, and downregulate the expression and activity of XOD in the liver of Uox-knockout mice (Guo et al., 2021). Further studies showed that inulin supplementation enhanced the microbial diversity and increased the relative abundance of beneficial bacteria, including SCFA-producing bacteria, such as Akkermansia and Ruminococcus. Furthermore, inulin treatment increased the production of SCFAs (acetate, propionate, and butyrate concentrations) derived from the gut microbiota in Uox-knockout mice (Guo et al., 2021), which was positively correlated with efficacy in relieving HUA. Adding dietary fiber-rich β-carotene and green tea powder to the diet reduced joint swelling and pain in mice with gouty arthritis; decreased serum UA and three proinflammatory cytokines, namely, IL-1β, IL-6, and tumor necrosis factor (TNF-α); improved the gut microbiota profile; and decreased purine and pyrimidine metabolism (Feng et al., 2022).
4.2.4 Phenols
Phenolic compounds are widely present in plants and are secondary metabolites synthesized by plants during normal development. Phenols are not only a type of natural antioxidant, but also a type of important biologically active substance in food (Veiga and Costa, 2020). Camellia japonica bee pollen polyphenols changed the gut microbiota structure of HUA model mice and increased the abundance of beneficial bacteria such as Lactobacillus, and the content of SCFAs was also increased accordingly (Xu et al., 2021). Chlorogenic acid (CGA) reduces the UA, BUN, CRE, aspartate transaminase (AST), and alanine transaminase (ALT) levels and inhibits XOD activity (Zhou et al., 2021). CGA inhibited the activation of the TLR4/MyD88/NF-κB signaling pathway in the kidney, thereby reducing inflammation in HUA mice. Furthermore, CGA treatment increased fecal SCFA production and increased the relative abundance of SCFA-producing bacteria, including Bacteroidetes, Prevotaceae UGC-001, and Butyricimonas, and reversed purine and glutamate metabolism in the gut microbiota (Zhou et al., 2021).
4.2.5 Peptides
Bioactive peptides have a positive effect on the life activities of living organisms. In recent years, such peptides have gradually become one of the research hotspots in the fields of food, health food, and special medical food. Studies have shown that proteases produced by microorganisms in the gut act on proteins in food to produce bioactive peptides. Furthermore, bioactive peptides have obvious regulatory effects on the structure of the gut microbiota, and changes in the structure of the gut microbiota have an important impact on the health of the host. Tuna meat oligopeptide (TMOP) attenuates HUA and renal inflammatory phenotypes, reprograms uric acid metabolic pathways, inhibits the activation of NLRP3 inflammasome and TLR4/MyD88/NF-κB signaling pathway, and inhibits p65-NF-κB phosphorylation (Han et al., 2020). Furthermore, TMOP treatment repaired the intestinal epithelial barrier, reversed gut dysbiosis, and increased SCFA production (Han et al., 2020). In a HUA mouse model, hexapeptides (GPAGPR and GPSGRP) reduced serum UA by inhibiting UA biosynthesis and reabsorption, and attenuated renal inflammation by inhibiting NLRP3 inflammasome activation. Both peptides, as potential microbiota modulators, reduced gut microbiota richness and diversity, altering the composition at the phylum and genus levels (Fan et al., 2022). Apostichopus japonicus oligopeptide (AJOP) can significantly alleviate HUA, regulate UA metabolism, inhibit the activation of NLRP3 inflammasome and NF-kB-related signaling pathways, and restore the m6A methylation levels (Lu et al., 2021). Furthermore, fecal microbiota transplantation (FMT) effectively alleviated HUA in mice by selectively modulating the corresponding pathways associated with AJOP treatment (Lu et al., 2021), suggesting that the underlying mechanism of AJOP’s protective effect is partially dependent on the microbiota. In a mouse model of diet-induced HUA, both EH-JAP and EH-LEU inhibited UA biosynthesis and promoted UA excretion (Wan et al., 2020), thereby alleviating the hyperuricemic phenotype. Furthermore, EH-JAP and EH-LEU treatments alleviate gut microbiota dysfunction by increasing the abundance of beneficial lactobacilli and SCFA producers and reducing the abundance of opportunistic pathogens (Wan et al., 2020).
4.2.6 Other prebiotics
In HUA-induced chronic kidney disease (CKD), the flavonoid component fisetin improves renal function, renal fibrosis, and intestinal dysbiosis (Ren et al., 2021). The alkaloid component nuciferine can significantly relieve HUA. In addition, a 16S rRNA analysis revealed that diverse gut microbes were closely associated with changes in differential metabolites, especially bacteria from the phyla Firmicutes and Bacteroidetes, and suggested that urinary indoxyl sulfate and N-acetylglutamate may be potential biomarkers in addition to UA for the early diagnosis and prevention of HUA (Wang et al., 2020). Various components can play a prebiotic role by regulating the gut microbiota, protecting the intestinal mucosal barrier, and increasing the content of SCFAs, maintaining human health, or helping treat diseases. In the future, the development and utilization of related prebiotics should be strengthened.
4.3 FMT
FMT is a treatment method that transplants the intestinal microbiota of healthy hosts into patients through the digestive tract to restore the diversity of intestinal microbes. It is a current clinical research hotspot. The serum UA concentration is closely related to the balance of the intestinal microecology. Using fecal microbial transplantation to maintain intestinal microecology in patients with HUA and gout may be a new treatment method. Washing microbiota transplantation reduces the serum UA levels in gout patients, is associated with a reduction in the frequency and duration of acute gout flares, reduces the diamine oxidase and endotoxin levels, and helps improve their damaged gut barrier function (Xie et al., 2021). The antihyperuricemic effects of TMOP and AJOP can be transmitted by transplanting fecal microbiota from TMOP/AJOP-treated mice (Han et al., 2020; Lu et al., 2021). Anserine treatment reversed gut dysbiosis, repaired the intestinal epithelial barrier, and increased SCFA production, and an anti-HUA effect was demonstrated by the transplantation of fecal microbiota from anserine-treated mice (Han et al., 2021). Although the current evidence concerning the beneficial effects of fecal microbial transplantation on human health is insufficient, fecal microbial transplantation currently provides more treatment modes for patients with HUA and gout and has important implications for human health.
5. Discussion
Intestinal dysbiosis in patients with gout mainly manifests as an increase in opportunistic pathogens and a decrease in bacteria that promote the production of anti-inflammatory cytokines. The gut microbiota is involved in the metabolism of purines and UA. An imbalance in the gut microbiota could increase the concentration of UA, and the long-term deposition of UA in the joints can cause gout. Intestinal dysbiosis may lead to the occurrence and development of gout by inducing the body to produce endotoxin, triggering chronic inflammation, or altering the metabolism of SCFAs. Thus far, some progress has been achieved in research investigating the relationship between the gut microbiota and HUA and gout (Chu et al., 2021; Yin et al., 2022), which not only helps elucidate its pathogenesis but also may provide a new direction for the diagnosis and treatment of HUA and gout, which may provide a new target for the treatment of gout.
In the prevention and treatment of HUA and gout, maintaining intestinal microecological balance plays an important role in the regulation of UA, which may provide new hope for the treatment of gout patients. At present, through microecological therapy, such as probiotics, prebiotics, and fecal microbial transplantation, adjusting the intestinal microecological balance to prevent HUA and gout is a clinical research hotspot. At the same time, however, many questions remain to be resolved. On the one hand, it is easy to breed other microorganisms during the microbial culture. Therefore, it is very important to ensure the safety of microorganisms and prevent contamination by miscellaneous bacteria. In addition, there are differences in UA and purine metabolism between humans and experimental animals. Therefore, more clinical trials are needed to elucidate the mechanism of gut microbiota in HUA and gout patients. Another problem is that a patient’s condition needs to be considered, and an accurate diagnosis and proper examination are necessary. The use of gut microbiota in HUA and gout treatment is still in its infancy, and its efficacy and safety in vitro, in vivo, and in clinical trials need to be gradually verified before widespread application. The complexity of the gut microbiota, the lack of evidence-based medicine for new treatment methods, and the lack of clinical data all require further exploration in future research.
Currently, research investigating the mechanism of gut microecology leading to gout mainly focuses on biochemical aspects, such as UA metabolism and the release of inflammatory factors. High levels of UA and joint pain are caused by problems with the body’s immune response (Chen et al., 2017; Cabău et al., 2020; Joosten, 2020). Therefore, it is reasonable to believe that the intestinal microecology is closely related to the occurrence of gout immune mechanisms. Accelerating research investigating the relationship between gut microbiota and gout immunity could help elucidate the mechanism of gout. Furthermore, attention should be given to dynamic research concerning the gut microecology in gout to study the dynamic development process of the microecology in different stages and different living environments in gout and identify the key microorganisms and their functional genes related to the occurrence and development of gout. In addition, attention should be given to the influence of age, course of disease, symptoms, and other factors on the intestinal microecology in gout and the process and method of reconstruction after the destruction of the intestinal microecology in gout. On this basis, we focused on translational medical research developing gout gut microecological regulators, and standardized treatment methods.
Author contributions
Conceptualization, ZW, JT; methodology, YL, JH, YL; software, WL, ZL; validation, ZW, JT; investigation, XX, YL; resources, ZB; data curation, WL; writing—original draft preparation, ZW; writing—revised manuscript, ZW, YL, WL; writing—review and editing, JT and JH; visualization, ZB; supervision, XX; project administration, ZW; funding acquisition, ZW and JT All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by Macao Science and Technology Development Fund (SKL-QRCM(UM)), Key R&D project of Sichuan Province (2022YFG0145), “Xinglin Scholar” Scientific Research Promotion Plan of Chengdu University of Traditional Chinese Medicine (BSH2021017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abeles, A. M., Pillinger, M. H. (2019). Gout and cardiovascular disease: crystallized confusion. Curr. Opin. Rheumatol 31 (2), 118–124. doi: 10.1097/BOR.0000000000000585
Agus, A., Clément, K., Sokol, H. (2021). Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 70, 6, 1174–1182. doi: 10.1136/gutjnl-2020-323071
Angelucci, F., Cechova, K., Amlerova, J., Hort, J. (2019). Antibiotics, gut microbiota, and alzheimer's disease. J. Neuroinflamm. 16 (1), 108. doi: 10.1186/s12974-019-1494-4
Aron-Wisnewsky, J., Vigliotti, C., Witjes, J., Le, P., Holleboom, A. G., Verheij, J., et al. (2020). Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 17, 5, 279–297. doi: 10.1038/s41575-020-0269-9
Bardin, T., Richette, P. (2014). Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol 26 (2), 186–191. doi: 10.1097/BOR.0000000000000028
Bardin, T., Richette, P. (2017). Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 15 (1), 123. doi: 10.1186/s12916-017-0890-9
Bian, M., Wang, J., Wang, Y., Nie, A., Zhu, C., Sun, Z., et al. (2020). Chicory ameliorates hyperuricemia via modulating gut microbiota and alleviating LPS/TLR4 axis in quail. BioMed. Pharmacother. 131, 110719. doi: 10.1016/j.biopha.2020.110719
Bray, G. A. (2013). Energy and fructose from beverages sweetened with sugar or high-fructose corn syrup pose a health risk for some people. Adv. Nutr. (Bethesda Md) 4 (2), 220–225. doi: 10.3945/an.112.002816
Cabău, G., Crișan, T. O., Klück, V., Popp, R. A., Joosten, L. A. B. (2020). Urate-induced immune programming: Consequences for gouty arthritis and hyperuricemia. Immunol. Rev. 294, 1, 92–105. doi: 10.1111/imr.12833
Cani, P. D., Bibiloni, R., Knauf, C., Waget, A., Neyrinck, A. M., Delzenne, N. M., et al. (2008). Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57 (6), 1470–1481. doi: 10.2337/db07-1403
Cao, T., Li, X., Mao, T., Liu, H., Tian, Z. (2017). Probiotic therapy alleviates hyperuricemia in C57BL/6 mouse model. Biomedical Research 28(5):2244–2249.
Cheng, H., Liu, J., Tan, Y., Feng, W., Peng, C. (2021). Interactions between gut microbiota and berberine, a necessary procedure to understand the mechanisms of berberine. J. Pharm. Anal. 12, 542–556. doi: 10.1016/j.jpha.2021.10.003
Chen, J., Wu, M., Yang, J., Wang, J., Qiao, Y., Li, X. (2017). The immunological basis in the pathogenesis of gout. Iran J. Immunol. 14 (2), 90–98.
Christians, S., Kaltwasser, H. (1986). Nickel-content of urease from bacillus pasteurii. Arch. Microbiol. 145 (1), 51–55. doi: 10.1007/BF00413026
Chu, Y., Sun, S., Huang, Y. (2021). Metagenomic analysis revealed the potential role of gut microbiome in gout. NPJ Biofilms Microbiomes 7, 1, 66. doi: 10.1038/s41522-021-00235-2
Crane, J. K., Naeher, T. M., Broome, J. E., Boedeker, E. C. (2013). Role of host xanthine oxidase in infection due to enteropathogenic and shiga-toxigenic escherichia coli. Infect. Immun. 81 (4), 1129–1139. doi: 10.1128/IAI.01124-12
Dalbeth, N., Gosling, A. L., Gaffo, A., Abhishek, A. (2021). Gout. Lancet 397 (10287), 1843–1855. doi: 10.1016/S0140-6736(21)00569-9
Dalbeth, N., Merriman, T. R., Stamp, L. K. (2016). Gout. Lancet 388 (10055), 2039–2052. doi: 10.1016/S0140-6736(16)00346-9
Dalile, B., Van Oudenhove, L., Vervliet, B., Verbeke, K. (2019). The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 8, 461–478. doi: 10.1038/s41575-019-0157-3
DeBosch, B. J., Kluth, O., Fujiwara, H., Schürmann, A., Moley, K. (2014). Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat. Commun. 5, 4642. doi: 10.1038/ncomms5642
Do, M. H., Lee, E., Oh, M. J., Kim, Y., Park, H. Y. (2018). High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 10 (6), 761. doi: 10.3390/nu10060761
Engen, P. A., Green, S. J., Voigt, R. M., Forsyth, C. B., Keshavarzian, A. (2015). The gastrointestinal microbiome: Alcohol effects on the composition of intestinal microbiota. Alcohol Res. Curr. Rev. 37 (2), 223–236.
Fan, Y. (2021). Gut microbiota in human metabolic health and disease. Gut 19 (1), 55–71. doi: 10.1038/s41579-020-0433-9
Fan, S., Huang, Y., Lu, G., Sun, N., Wang, R., Lu, C. (2022). Novel anti-hyperuricemic hexapeptides derived from apostichopus japonicus hydrolysate and their modulation effects on the gut microbiota and host microRNA profile. Food Funct. 13, 7, 3865–3878. doi: 10.1039/D1FO03981D
Feig, D. I. (2014). Serum uric acid and the risk of hypertension and chronic kidney disease. Curr. Opin. Rheumatol 26 (2), 176–185. doi: 10.1097/BOR.0000000000000033
Feng, Y., Yu, Y., Chen, Z., Wang, L., Ma, J., Bai, X., et al. (2022). Effects of β-carotin and green tea powder diets on alleviating the symptoms of gouty arthritis and improving gut microbiota in C57BL/6 mice. Front. Microbiol. 13, 837182. doi: 10.3389/fmicb.2022.837182
Ferreira-Halder, C. V., Faria, A. V. S., Andrade, S. S. (2017). Action and function of faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 31 (6), 643–648. doi: 10.1016/j.bpg.2017.09.011
Fuke, N., Nagata, N. (2019). Regulation of gut microbiota and metabolic endotoxemia with dietary factors. Nutrients 11 (10), 2277. doi: 10.3390/nu11102277
Gao, Y., Sun, J., Zhang, Y., Shao, T., Li, H., Wang, M., et al. (2020). Effect of a traditional Chinese medicine formula (CoTOL) on serum uric acid and intestinal flora in obese hyperuricemic mice inoculated with intestinal bacteria. Evid. Based Complement. Alternat. Med. 2020, 8831937. doi: 10.1155/2020/8831937
García-Arroyo, F. E., Gonzaga, G., Muñoz-Jiménez, I., Blas-Marron, M. G., Silverio, O., Tapia, E., et al. (2018). Probiotic supplements prevented oxonic acid-induced hyperuricemia and renal damage. PLoS One 13, 8. doi: 10.1371/journal.pone.0202901
Ghosh, S. S., Wang, J., Yannie, P. J., Ghosh, S. (2020). Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocr. Soc. 4(2), bvz039. doi: 10.1210/jendso/bvz039.
Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., et al. (2017). Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14 (8), 491–502. doi: 10.1038/nrgastro.2017.75
Gill, S. K., Rossi, M., Bajka, B., Whelan, K. (2021). Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 18 (2), 101–116. doi: 10.1038/s41575-020-00375-4
Guo, Y., Yu, Y., Li, H., Ding, X., Li, X., Jing, X., et al. (2021). Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in uox-knockout mice. Eur. J. Nutr. 60, 4, 2217–2230. doi: 10.1007/s00394-020-02414-x
Guo, Z., Zhang, J., Wang, Z., Ang, K. Y., Huang, S., Hou, Q., et al. (2016). Intestinal microbiota distinguish gout patients from healthy humans. Sci. Rep. 6, 20602. doi: 10.1038/srep20602
Gu, Q. Y., Zhang, J., Feng, Y. C. (2016). Role of NLRP3 inflammasome in bifidobacterium longum-regulated visceral hypersensitivity of postinfectious irritable bowel syndrome. Artif. cells Nanomed. Biotechnol. 44 (8), 1933–1937. doi: 10.3109/21691401.2015.1111238
Hak, A. E., Choi, H. K. (2008). Lifestyle and gout. Curr. Opin. Rheumatol. 20 (2), 179–186. doi: 10.1097/BOR.0b013e3282f524a2
Handayani, U., Hidayat, R. (2018). Screening of lactic acid bacteria producing uricase and stability assessment in simulated gastrointestinal conditions. Int. Food Res. Res. J., 25 (4), 1661–1667.
Han, J., Wang, Z., Lu, C. (2021). The gut microbiota mediates the protective effects of anserine supplementation on hyperuricaemia and associated renal inflammation. Food Funct. 12, 19, 9030–9042. doi: 10.1039/D1FO01884A
Han, J., Wang, X., Tang, S., Lu, C., Wan, H., Zhou, J., et al. (2020). Protective effects of tuna meat oligopeptides (TMOP) supplementation on hyperuricemia and associated renal inflammation mediated by gut microbiota. FASEB J. 34 (4), 5061–5076. doi: 10.1096/fj.201902597RR
Henson, M. A. (2021). Interrogation of the perturbed gut microbiota in gouty arthritis patients through in silico metabolic modeling. Eng. Life Sci. 21 (7), 489–501. doi: 10.1002/elsc.202100003
Hersoug, L. G., Møller, P., Loft, S. (2018). Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 31 (2), 153–163. doi: 10.1017/S0954422417000269
Hidalgo-Cantabrana, C., Delgado, S., Ruiz, L., Ruas-Madiedo, P., Sánchez, B., Margolles, A. (2017). Bifidobacteria and their health-promoting effects. Microbiol. Spectr. 5 (3), BAD-0010–2016. doi: 10.1128/microbiolspec.BAD-0010-2016
Holscher, H. D. (2017). Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8 (2), 172–184. doi: 10.1080/19490976.2017.1290756
Hosomi, A., Nakanishi, T., Fujita, T., Tamai, I. (2012). Extra-renal elimination of uric acid via intestinal efflux transporter BCRP/ABCG2. PloS One 7 (2), e30456. doi: 10.1371/journal.pone.0030456
Huang, C., Shi, G. (2019). Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 17 (1), 225. doi: 10.1186/s12967-019-1971-7
Huang, Y., Xiao, M., Ou, J., Lv, Q., Wei, Q., Chen, Z., et al. (2020). Identification of the urine and serum metabolomics signature of gout. Rheumatol. (Oxford) 59 (10), 2960–2969. doi: 10.1093/rheumatology/keaa018
Illiano, P., Brambilla, R., Parolini, C. (2020). The mutual interplay of gut microbiota, diet and human disease. Nat. Commun. 287 (5), 833–855. doi: 10.1111/febs.15217
Jackson, M. A., Verdi, S., Maxan, M. E., Shin, C. M., Zierer, J. (2018). Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat. Commun. 9, 1, 2655. doi: 10.1038/s41467-018-05184-7
Jakše, B., Jakše, B., Pajek, M., Pajek, J. (2019). Uric acid and plant-based nutrition. Nutrients 11 (8), 1736. doi: 10.3390/nu11081736.
Jensen, T., Abdelmalek, M. F., Sullivan, S., Nadeau, K. J., Green, M., Roncal, C., et al. (2018). Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 68 (5), 1063–1075. doi: 10.1016/j.jhep.2018.01.019
Jhang, J. J., Lin, J. H., Yen, G. C. (2018). Beneficial properties of phytochemicals on NLRP3 inflammasome-mediated gout and complication. J. Agric. Food Chem. 66, 4, 765–772. doi: 10.1021/acs.jafc.7b05113
Joosten, L. A. B. (2020). Asymptomatic hyperuricaemia: a silent activator of the innate immune system. Immunol. Rev. 16 (2), 75–86. doi: 10.1038/s41584-019-0334-3
Kaneko, C., Ogura, J., Sasaki, S., Okamoto, K., Kobayashi, M., Kuwayama, K., et al. (2017). Fructose suppresses uric acid excretion to the intestinal lumen as a result of the induction of oxidative stress by NADPH oxidase activation. Biochim. Biophys. Acta Gen. Subj. 1861 (3), 559–566. doi: 10.1016/j.bbagen.2016.11.042
Kim, S., Jazwinski, S. M. (2018). The gut microbiota and healthy aging: A mini-review. Gerontology 64 (6), 513–520. doi: 10.1159/000490615
Kim, H. W., Yoon, E. J. (2022). Distinct gut microbiota in patients with asymptomatic hyperuricemia: A potential protector against gout development. Yonsei Med. J. 63, 3, 241–251. doi: 10.3349/ymj.2022.63.3.241
Kolasinski, S. L. (2014). Food, drink, and herbs: alternative therapies and gout. Curr. Rheumatol. Rep. 16 (4), 409. doi: 10.1007/s11926-014-0409-8
Krishnan, E. (2010). Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatol. (Oxford) 49 (7), 1229–1238. doi: 10.1093/rheumatology/keq037
Kuo, Y. W., Hsieh, S. H., Chen, J. F., Liu, C. R., Chen, C. W., Huang, Y. F., et al. (2021). Lactobacillus reuteri TSR332 and lactobacillus fermentum TSF331 stabilize serum uric acid levels and prevent hyperuricemia in rats. PeerJ 9, e11209. doi: 10.7717/peerj.11209
Li, X., Chen, Y., Gao, X., Wu, Y., El-Seedi, H. R. (2021). Antihyperuricemic effect of green alga ulva lactuca ulvan through regulating urate transporters. J. Agric. Food Chem. 69, 38, 11225–11235. doi: 10.1021/acs.jafc.1c03607
Li, X., Gao, X., Zhang, H., Liu, Y., Sarker, M. M. R., Wu, Y., et al. (2021). The anti-hyperuricemic effects of green alga enteromorpha prolifera polysaccharide via regulation of the uric acid transporters in vivo. Food Chem. Toxicol. 158, 112630. doi: 10.1016/j.fct.2021.112630
Lin, X., Shao, T., Huang, L., Wen, X., Wang, M., Wen, C., et al. (2020). Simiao decoction alleviates gouty arthritis by modulating proinflammatory cytokines and the gut ecosystem. Front. Pharmacol. 11, 955. doi: 10.3389/fphar.2020.00955
Lin, X., Shao, T., Wen, X., Wang, M., Wen, C., He, Z. (2020). Combined effects of MSU crystals injection and high fat-diet feeding on the establishment of a gout model in C57BL/6 mice. Adv. Rheumatol. 60, 1, 52. doi: 10.1186/s42358-020-00155-3
Lin, S., Zhang, T., Zhu, L., Pang, K., Lu, S., Liao, X., et al. (2021). Characteristic dysbiosis in gout and the impact of a uric acid-lowering treatment, febuxostat on the gut microbiota. J. Genet. Genomics = Yi Chuan xue bao 48 (9), 781–791. doi: 10.1016/j.jgg.2021.06.009
Liu, X., Lv, Q., Ren, H., Gao, L., Zhao, P., Yang, X., et al. (2020). The altered gut microbiota of high-purine-induced hyperuricemia rats and its correlation with hyperuricemia. PeerJ 8, e8664. doi: 10.7717/peerj.8664
Liu, J., Tan, Y., Cheng, H., Zhang, D., Feng, W., Peng, C. (2022). Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 13(4), 1106–1126. doi: 10.14336/AD.2022.0104
Li, M., Yang, D., Mei, L., Yuan, L., Xie, A., Yuan, J. (2014). Screening and characterization of purine nucleoside degrading lactic acid bacteria isolated from Chinese sauerkraut and evaluation of the serum uric acid lowering effect in hyperuricemic rats. PloS One 9 (9), e105577. doi: 10.1371/journal.pone.0105577
Li, R., Yu, K., Li, C. (2018). Dietary factors and risk of gout and hyperuricemia: a meta-analysis and systematic review. Asia Pac J. Clin. Nutr. 27 (6), 1344–1356. doi: 10.6133/apjcn.201811_27(6).0022
Lovegrove, A., Edwards, C. H., De Noni, I., Patel, H., El, S. N., Grassby, T., et al. (2017). Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 57 (2), 237–253. doi: 10.1080/10408398.2014.939263
Lu, C., Tang, S., Han, J., Fan, S., Huang, Y., Zhang, Z., et al. (2021). Apostichopus japonicus oligopeptide induced heterogeneity in the gastrointestinal tract microbiota and alleviated hyperuricemia in a microbiota-dependent manner. Mol. Nutr. Food Res. 65 (14), e2100147. doi: 10.1002/mnfr.202100147
Lynch, S. V., Pedersen, O. (2016). The human intestinal microbiome in health and disease. N. Engl. J. Med. 375 (24), 2369–2379. doi: 10.1056/NEJMra1600266
Macfarlane, D. G., Slade, R., Hopes, P. A., Hartog, M. H. (1983). A study of platelet aggregation and adhesion in gout. Clin. Exp. Rheumatol 1 (1), 63–66.
Mahbub, M. H., Yamaguchi, N., Takahashi, H., Hase, R., Amano, H., Kobayashi-Miura, M., et al. (2017). Alteration in plasma free amino acid levels and its association with gout. Environ. Health Prev. Med. 22, 1, 7. doi: 10.1186/s12199-017-0609-8
Martinon, F., Pétrilli, V., Mayor, A., Tardivel, A., Tschopp, J. (2006). Gout-associated uric acid crystals activate the NALP3 inflammasome. J. Agric. Food Chem. 440 (7081), 237–241. doi: 10.1038/nature04516
Ma, W., Zhou, L., Li, Y., Xia, D., Chen, J., Chen, J., et al. (2021). Persistent purine metabolic abnormality induces the aggravation of visceral inflammation and intestinal microbiota dysbiosis in magang goose. Front. Vet. Sci. 8, 737160. doi: 10.3389/fvets.2021.737160
Méndez-Salazar, E. O., Vázquez-Mellado, J., Casimiro-Soriguer, C. S., Dopazo, J., Çubuk, C., Zamudio-Cuevas, Y., et al. (2021). Taxonomic variations in the gut microbiome of gout patients with and without tophi might have a functional impact on urate metabolism. Mol. Med. 27, 1, 50. doi: 10.1186/s10020-021-00311-5
Meng, C., Bai, C., Brown, T. D., Hood, L. E., Tian, Q. (2018). Human gut microbiota and gastrointestinal cancer. Genomics Proteomics Bioinf. 16 (1), 33–49. doi: 10.1016/j.gpb.2017.06.002
Miller, S. I., Ernst, R. K., Bader, MW. LPS (2005). TLR4 and infectious disease diversity. J. Endocr Soc. 3 (1), 36–46. doi: 10.1038/nrmicro1068
Miquel, S., Martín, R., Rossi, O., Bermúdez-Humarán, L. G., Chatel, J. M., Sokol, H., et al. (2013). Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 16 (3), 255–261. doi: 10.1016/j.mib.2013.06.003
Mohammad, S, Thiemermann, C. (2021). Role of metabolic endotoxemia in systemic inflammation and potential interventions. Nutrients 11, 594150. doi: 10.3389/fimmu.2020.594150
Nakagawa, S., Ishino, S., Teshiba, S. (1996). Construction of catalase deficient escherichia coli strains for the production of uricase. Biosci. Biotechnol. Biochem. 60 (3), 415–420. doi: 10.1271/bbb.60.415
Nakagawa, T., Lanaspa, M. A., Johnson, R. J. (2019). The effects of fruit consumption in patients with hyperuricaemia or gout. Rheumatol. (Oxford) 58 (7), 1133–1141. doi: 10.1093/rheumatology/kez128
Ni, C., Li, X., Wang, L., Li, X., Zhao, J., Zhang, H., et al. (2021). Lactic acid bacteria strains relieve hyperuricaemia by suppressing xanthine oxidase activity via a short-chain fatty acid-dependent mechanism. Food Funct. 12 (15), 7054–7067. doi: 10.1039/D1FO00198A
Pan, L., Han, P., Ma, S., Peng, R., Wang, C., Kong, W., et al. (2020). Abnormal metabolism of gut microbiota reveals the possible molecular mechanism of nephropathy induced by hyperuricemia. Acta Pharm. Sin. B 10 (2), 249–261. doi: 10.1016/j.apsb.2019.10.007
Park, H. K., Lee, S. J. (2022). Treatment of gouty arthritis is associated with restoring the gut microbiota and promoting the production of short-chain fatty acids. Arthritis Res. Ther. 24 (1), 51. doi: 10.1186/s13075-022-02742-9
Qin, L., Wu, X., Block, M. L., Liu, Y., Breese, G. R., Hong, J. S., et al. (2007). Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55 (5), 453–462. doi: 10.1002/glia.20467
Ramos, M. F. P., Monteiro de Barros, A., Razvickas, C. V., Borges, F. T., Schor, N. (2018). Xanthine oxidase inhibitors and sepsis. Int. J. Immunopathol. Pharmacol. 32, 2058738418772210. doi: 10.1177/2058738418772210
Ran, S., Chu, M., Gu, S., Wang, J., Liang, J. (2019). Enterococcus faecalis induces apoptosis and pyroptosis of human osteoblastic MG63 cells via the NLRP3 inflammasome. Int. Endod. J. 52, 1, 44–53. doi: 10.1111/iej.12965
Rando, D., Steglitz, U., Mörsdorf, G., Kaltwasser, H. (1990). Nickel availability and urease expression in Proteus mirabilis. Arch. Microbiol. 154 (5), 428–432. doi: 10.1007/BF00245222
Ren, Q., Cheng, L., Guo, F., Tao, S., Zhang, C., Ma, L. (2021). Fisetin improves hyperuricemia-induced chronic kidney disease via regulating gut microbiota-mediated tryptophan metabolism and aryl hydrocarbon receptor activation. J. Agric. Food Chem. 69, 37, 10932–10942. doi: 10.1021/acs.jafc.1c03449
Richette, P., Bardin, T. (2010). Gout. Lancet 375 (9711), 318–328. doi: 10.1016/S0140-6736(09)60883-7
Saber, S., Abd El-Fattah, E. E. (2021). A novel combination therapy using rosuvastatin and lactobacillus combats dextran sodium sulfate-induced colitis in high-fat diet-fed rats by targeting the TXNIP/NLRP3 interaction and influencing gut microbiome composition. Pharmaceuticals (Basel) 14 (4), 341. doi: 10.3390/ph14040341
Sanders, M. E., Merenstein, D. J., Reid, G. (2019). Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 16, 10, 605–616. doi: 10.1038/s41575-019-0173-3
Seo, S. U., Kamada, N., Muñoz-Planillo, R., Kim, Y. G., Kim, D., Koizumi, Y., et al. (2015). Distinct commensals induce interleukin-1β via NLRP3 inflammasome in inflammatory monocytes to promote intestinal inflammation in response to injury. Immunity 42 (4), 744–755. doi: 10.1016/j.immuni.2015.03.004
Shao, T., Shao, L., Li, H., Xie, Z., He, Z., Wen, C. (2017). Combined signature of the fecal microbiome and metabolome in patients with gout. Front. Microbiol. 8, 268. doi: 10.3389/fmicb.2017.00268
Shao, X., Sun, S., Zhou, Y., Wang, H., Yu, Y., Hu, T., et al. (2021). Bacteroides fragilis restricts colitis-associated cancer via negative regulation of the NLRP3 axis. Cancer Lett. 523, 170–181. doi: 10.1016/j.canlet.2021.10.002
Silva, J. C. P., Mota, M., Martins, F. O., Nogueira, C., Gonçalves, T., Carneiro, T., et al. (2018). Intestinal microbial and metabolic profiling of mice fed with high-glucose and high-fructose diets. J. Proteome Res. 17, 8, 2880–2891. doi: 10.1021/acs.jproteome.8b00354
Sivaprakasam, S., Prasad, P. D., Singh, N. (2016). Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 164, 144–151. doi: 10.1016/j.pharmthera.2016.04.007
So, A. K., Martinon, F. (2017). Inflammation in gout: mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 13 (11), 639–647. doi: 10.1038/nrrheum.2017.155
Song, S., Lou, Y., Mao, Y., Wen, X., Fan, M., He, Z., et al. (2022). Alteration of gut microbiome and correlated amino acid metabolism contribute to hyperuricemia and Th17-driven inflammation in uox-KO mice. Front. Immunol. 13, 804306. doi: 10.3389/fimmu.2022.804306
So, D., Whelan, K., Rossi, M., Morrison, M., Holtmann, G., Kelly, J. T., et al. (2018). Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Science 107 (6), 965–983. doi: 10.1093/ajcn/nqy041
Stojanov, S., Berlec, A. (2020). The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 8 (11), 1715. doi: 10.3390/microorganisms8111715
Tan, J., McKenzie, C., Potamitis, M., Thorburn, A. N., Mackay, C. R., Macia, L. (2014). The role of short-chain fatty acids in health and disease. Adv. Immunol. 121, 91–119. doi: 10.1016/B978-0-12-800100-4.00003-9
Tappy, L., Lê, K. A. (2010). Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 90 (1), 23–46. doi: 10.1152/physrev.00019.2009
Thottam, G. E., Krasnokutsky, S., Pillinger, M. H. (2017). Gout and metabolic syndrome: a tangled web. Curr. Rheumatol. Rep. 19 (10), 60. doi: 10.1007/s11926-017-0688-y
Tong, Y., Gao, H., Qi, Q., Liu, X., Li, J., Gao, J., et al. (2021). High fat diet, gut microbiome and gastrointestinal cancer. Theranostics 11 (12), 5889–5910. doi: 10.7150/thno.56157
Veiga, M., Costa, E. M. (2020). Impact of plant extracts upon human health: A review. Crit. Rev. Food Sci. Nutr. 60 (5) 873–886. doi: 10.1080/10408398.2018.1540969
Vieira, A. T., Macia, L., Galvão, I., Martins, F. S., Canesso, M. C., Amaral, F. A., et al. (2015). A role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of gout. Arthritis Rheumatol. (Hoboken NJ) 67 (6), 1646–1656. doi: 10.1002/art.39107
Wang, Y., Lin, Z., Zhang, B., Nie, A., Bian, M. (2017). Cichorium intybus l. promotes intestinal uric acid excretion by modulating ABCG2 in experimental hyperuricemia. Nutr. Metab. (Lond.) 14, 38. doi: 10.1186/s12986-017-0190-6
Wang, H., Mei, L., Deng, Y., Liu, Y., Wei, X., Liu, M., et al. (2019). Lactobacillus brevis DM9218 ameliorates fructose-induced hyperuricemia through inosine degradation and manipulation of intestinal dysbiosis. Nutrition 62, 63–73. doi: 10.1016/j.nut.2018.11.018
Wang, Y., Qi, W. (2020). High-fructose diet increases inflammatory cytokines and alters gut microbiota composition in rats. Mediators Inflamm. 2020, 6672636. doi: 10.1155/2020/6672636
Wang, L. M., Wang, P., Teka, T., Zhang, Y. C., Yang, W. Z., Zhang, Y., et al. (2020). (1)H NMR and UHPLC/Q-Orbitrap-MS-Based metabolomics combined with 16S rRNA gut microbiota analysis revealed the potential regulation mechanism of nuciferine in hyperuricemia rats. J Agric. Food Chem. 68, 47, 14059–14070. doi: 10.1021/acs.jafc.0c04985
Wang, C. H., Zhang, C., Xing, X. H. (2017). Metabolic engineering of escherichia coli cell factory for highly active xanthine dehydrogenase production. Bioresource Technol. 245 (Pt B), 1782–1789. doi: 10.1016/j.biortech.2017.05.144
Wan, H., Han, J., Tang, S., Bao, W., Lu, C., Zhou, J., et al. (2020). Comparisons of protective effects between two sea cucumber hydrolysates against diet induced hyperuricemia and renal inflammation in mice. Food Funct. 11 (1), 1074–1086. doi: 10.1039/C9FO02425E
Wei, J., Zhang, Y. (2022). Association between gut microbiota and elevated serum urate in two independent cohorts. Arthritis Rheumatol. 74, 4, 682–691. doi: 10.1002/art.42009
Wrigley, R., Phipps-Green, A. J., Topless, R. K., Major, T. J., Cadzow, M., Riches, P., et al. (2020). Pleiotropic effect of the ABCG2 gene in gout: involvement in serum urate levels and progression from hyperuricemia to gout. Arthritis Res. Ther. 22, 1, 45. doi: 10.1186/s13075-020-2136-z
Wu, Y., Ye, Z., Feng, P., Li, R., Chen, X., Tian, X., et al. (2021). Limosilactobacillus fermentum JL-3 isolated from "Jiangshui" ameliorates hyperuricemia by degrading uric acid. Gut Microbes 13 (1), 1–18. doi: 10.1080/19490976.2021.1897211
Xiang, S., Fu, J., Ye, K., Zheng, Y. (2019). Effect of lactobacillus gasseri PA3 on gut microbiota in an in vitro colonic simulation. Food Sci. Nutr. 7, 12, 3883–3891. doi: 10.1002/fsn3.1236
Xie, W. R., Yang, X. Y., Deng, Z. H., Zheng, Y. M., Zhang, R., Wu, L. H., et al. (2021). Effects of washed microbiota transplantation on serum uric acid levels, symptoms and intestinal barrier function in patients with acute and recurrent gout: a pilot study. Dig Dis. doi: 10.1159/000521273
Xi, Y., Huang, Y., Li, Y., Yan, J., Shi, Z. (2020). Fermented feed supplement relieves caecal microbiota dysbiosis and kidney injury caused by high-protein diet in the development of gosling gout. Anim. an Open Access J. MDPI 10 (11), 2139. doi: 10.3390/ani10112139
Xi, Y., Yan, J., Li, M., Ying, S., Shi, Z. (2019). Gut microbiota dysbiosis increases the risk of visceral gout in goslings through translocation of gut-derived lipopolysaccharide. Poult Sci. 98 (11), 5361–5373. doi: 10.3382/ps/pez357
Xu, Y., Cao, X., Zhao, H. (2021). Impact of camellia japonica bee pollen polyphenols on hyperuricemia and gut microbiota in potassium oxonate-induced mice. Nutrients 13 (8), 2665. doi: 10.3390/nu13082665
Yamada, N., Iwamoto, C., Kano, H., Yamaoka, N., Fukuuchi, T., Kaneko, K., et al. (2016). Evaluation of purine utilization by lactobacillus gasseri strains with potential to decrease the absorption of food-derived purines in the human intestine. Nucleosides nucleotides Nucleic Acids 35 (10-12), 670–676. doi: 10.1080/15257770.2015.1125000
Yamanaka, H., Taniguchi, A., Tsuboi, H., Kano, H., Asami, Y. (2019). Hypouricaemic effects of yoghurt containing lactobacillus gasseri PA-3 in patients with hyperuricaemia and/or gout: A randomised, double-blind, placebo-controlled study. Modern Rheumatol. 29 (1), 146–150. doi: 10.1080/14397595.2018.1442183
Yang, H. T., Xiu, W. J., Liu, J. K., Yang, Y., Hou, X. G., Zheng, Y. Y., et al. (2021). Gut microbiota characterization in patients with asymptomatic hyperuricemia: probiotics increased. Bioengineered 12 (1), 7263–7275. doi: 10.1080/21655979.2021.1976897
Yin, J., Li, Y., Han, H., Chen, S., Gao, J., Liu, G., et al. (2018). Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J. Pineal. Res. 65, 4, e12524. doi: 10.1111/jpi.12524
Yin, H., Liu, N., Chen, J. (2022). The role of the intestine in the development of hyperuricemia. NPJ Biofilms Microbiomes 13, 845684. doi: 10.3389/fimmu.2022.845684
Yin, W., Liu, S., Dong, M., Liu, Q., Shi, C., Bai, H., et al. (2020). A new NLRP3 inflammasome inhibitor, dioscin, promotes osteogenesis. Small (Weinheim an der Bergstrasse Germany) 16 (1), e1905977. doi: 10.1002/smll.201905977
Yokose, C., McCormick, N., Choi, H. K. (2021). Dietary and lifestyle-centered approach in gout care and prevention. Curr. Rheumatol. Rep. 23 (7), 51. doi: 10.1007/s11926-021-01020-y
Yue, S. J., Wang, W. X., Yu, J. G., Chen, Y. Y., Shi, X. Q., Yan, D., et al. (2019). Gut microbiota modulation with traditional Chinese medicine: A system biology-driven approach. Pharmacol. Res. 148, 104453. doi: 10.1016/j.phrs.2019.104453
Yu, Y., Liu, Q., Li, H., Wen, C., He, Z. (2018). Alterations of the gut microbiome associated with the treatment of hyperuricaemia in Male rats. Front. Microbiol. 9, 2233. doi: 10.3389/fmicb.2018.02233
Zhao, H., Lu, Z. (2022). The potential of probiotics in the amelioration of hyperuricemia. Food Funct. 13, 5, 2394–2414. doi: 10.1039/D1FO03206B
Zhao, L., Zhang, F. (2018). Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359, 6380, 1151–1156. doi: 10.1126/science.aao5774
Zhao, Z., Zhao, Y., Zhang, Y., Shi, W., Li, X., Shyy, J. Y., et al. (2021). Gout-induced endothelial impairment: The role of SREBP2 transactivation of YAP. FASEB. J. 35, 6, e21613. doi: 10.1096/fj.202100337R
Zhou, X., Zhang, B., Zhao, X., Lin, Y., Wang, J., Wang, X., et al. (2021). Chlorogenic acid supplementation ameliorates hyperuricemia, relieves renal inflammation, and modulates intestinal homeostasis. Food Funct. 12 (12), 5637–5649. doi: 10.1039/D0FO03199B
Keywords: gout, hyperuricemia, gut microbiota, uric acid, probiotics, prebiotics
Citation: Wang Z, Li Y, Liao W, Huang J, Liu Y, Li Z and Tang J (2022) Gut microbiota remodeling: A promising therapeutic strategy to confront hyperuricemia and gout. Front. Cell. Infect. Microbiol. 12:935723. doi: 10.3389/fcimb.2022.935723
Received: 06 May 2022; Accepted: 15 July 2022;
Published: 10 August 2022.
Edited by:
Jaime Garcia-Mena, Centro de Investigaciones y Estudios Avanzados (CINVESTAV) (IPN), Mexico City, MexicoReviewed by:
Yue Zhang, Beijing University of Chinese Medicine, ChinaShi-Jun Yue, Shaanxi University of Chinese Medicine, China
Copyright © 2022 Wang, Li, Liao, Huang, Liu, Li and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhilei Wang, wangzl1993@outlook.com; Jianyuan Tang, tangjianyuan163@163.com
†These authors share first authorship
 Zhilei Wang
Zhilei Wang Yuchen Li3†
Yuchen Li3†  Ju Huang
Ju Huang