First Study of Bacteremia Caused by Herbaspirillum huttiense in China: A Brief Research Report and Literature Review
- 1Department of Laboratory Medicine, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 2Department of Laboratory Medicine, Anhui Chest Hospital, Hefei, China
- 3Departmentof Laboratory Medicine, Anhui Medical University, Hefei, China
Bacteremia caused by Herbaspirillum huttiense (H. huttiense) is relatively rare in positive blood cultures. H. huttiense is an opportunistic bacterium in patients with cancer and cirrhosis and has also been described in immunocompromised hosts. In this study, H. huttiense was isolated from a patient with repeated chest tightness and chest pain. Smears were prepared, stained, and examined by microscopy. Single colonies were analyzed by Gram staining, matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS), 16S rRNA sequencing and Next-Generation Sequencing (NGS). Antibiotic sensitivity was assessed by agar dilution. Almost all publications on H. huttiense infections in the PubMed/ScienceDirect/EBSCO databases were reviewed and summarized. Blood sample culturing yielded white, gelatinous, and slightly raised colonies without hemolytic rings. The bacilli were found to be Gram-negative, and MS results showed 99.2% homology with H. huttiense. This was confirmed by 16S rRNA gene sequencing, phylogenetic tree analysis and NGS all of which were homologous with H. huttiense in GenBank. Antibiotic susceptibility tests were performed to determine the minimum inhibitory concentrations (MICs) of imipenem, meropenem, piperacillin-tazobactam, and levofloxacin. A comprehensive literature review revealed that H. huttiense was an emergent pathogen. After medical treatment, the patient’s body temperature returned to normal. This is the first report of bacteremia caused by H. huttiense in China. The findings could improve the awareness and attention of the rare pathogenic microorganisms in China.
Introduction
Herbaspirillum species are non-fermenting, strictly aerobic, Gram-negative curved or helical bacilli that do not have hemolytic rings. They are motile with polar flagella, and oxidase-, urease-, and catalase-positive. Herbaspirillum species were first reported by Baldani in 1996 (Baldani et al., 1996; Obradovic et al., 2007; Dobritsa et al., 2010) and widely distributed in the environment. As nitrogen-fixing bacteria, Herbaspirillum species inhabit the roots of plants in the rhizosphere and have been found in wells and other ground water (Tayeb et al., 2008;. Gulati et al., 2011; Souza et al., 2013). Herbaspirillum huttiense (H. huttiense) is a member of Herbaspirillum species and has the same properties. Pathologically, although a number of Herbaspirillum species have been identified and studied, only a few H. huttiense infections have been reported as human pathogens. For example, Regunath et al. (2015) reported the first case of severe community-acquired pneumonia and bacteremia caused by H. huttiense in an immunocompetent adult in the USA and Liu et al. (2019) reported the first case of septicemia caused by H. huttiense in Korea (Regunath et al., 2015; Liu et al., 2019). In the present study, we provided the first report of a bacteremia case caused by H. huttiense in China. We analyzed the H. huttiense clinical isolate by MALDI-TOF MS and 16S rRNA gene sequencing and summarized the prompt identification and results of antibiotic sensitivity.
Materials and Methods
Isolation and Characterization of H. huttiense
Only one strain of H. huttiense was successfully isolated from positive aerobic blood cultures between January 2018 and January 2022 in the Department of Laboratory Medicine in the First Affiliated Hospital of Anhui Medical University. One drop of blood from positive blood cultures was inoculated onto Columbia blood plate medium and the bacteria were cultured aerobically at 37°C and 5% CO2, followed by Gram staining and identification under microscopy.
MALDI-TOF MS Identification
MALDI-TOF MS identification was performed on a Vitek MS platform by the direct smear method in accordance with the instructions of the manufacturer. After acquiring the spectra, data were transferred to the analysis server which used software algorithms to compare the generated spectrum with the typical spectra in the scientific databases.
16S rRNA Sequencing and Phylogenetic Analysis
The original bacteria were purified and the genomic DNA was extracted. The forward and reverse primers used for PCR amplification of 16S rRNA gene were 27F(5’-AGAGTTTGATCATGGCTCAG-3’) and 1492R(5’-TACGGCTACCTTGTACGACTT-3’). The reaction procedure was 96°C for 3 min, 96°C for 30 s, 58°C for 30 s, 72°C for 1 min, 35 cycles, and 72°C for 10 min. The sequencing was compared with the 16S rRNA gene sequencing of known bacteria in the Genbank database. The phylogenetic tree was established using the MEGA7.0 software.
Genome Sequencing and Data Assembly
The draft genome sequence of H. huttiense was analyzed by NGS. Genome sequencing was performed using the Illumina NovaSeq platform by generating paired-end libraries. Genomic DNA libraries for each isolate were prepared using the TruSeq DNA Sample Preparation Kit (Illumina). Adapter contamination was removed by AdapterRemoval v2 and the reads were filtered by SOAPec v2. The filtered reads were assembled into contigs and scaffolds using A5-miseq v20160825.
In Vitro Antibiotic Sensitivity Test
All antibiotic sensitivity was tested using the agar dilution method.
Literature Review
An electronic search was conducted in the PubMed/ScienceDirect/EBSCO databases using the key words “Herbaspirillum huttiense” to systematically search for almost all published literatures.
Case Description
A 72-year-old man was admitted to our hospital and was diagnosed with coronary atherosclerotic cardiopathy, mitral and tricuspid insufficiency and lacunar infarction. The patient was hospitalized for a total of 58 days, from June 15th to August 12th, 2020. During hospitalization, the patient developed a high fever with an axillary temperature reaching 39.2°C. On laboratory investigations, the patient had a procalcitonin (PCT) level of 86.73 ng/mL, a CRP level of 88.17mg/L, a WBC count of 8.89×109/L, and a neutrophil percentage of 84.50%. The blood was cultured using a BacT/Alert three dimensional automated blood culture system. Each blood culture consisted of a set of two (aerobic and anaerobic) bottles. Two sets of blood samples were collected from the patient. On July 3rd, two aerobic blood cultures were found positive after 20.8 h. The anaerobic blood cultures remained negative. Subsequent blood cultures were redone, and positive aerobic bacteria were still confirmed as H. huttiense. The patient was treated with meropenram and tigecycline for anti-infection. Moxifloxacin and piperacillin-tazobactam were changed when the condition of the patient improved. Finally the patient’s body temperature returned to normal and discharged from hospital when cured.
Results
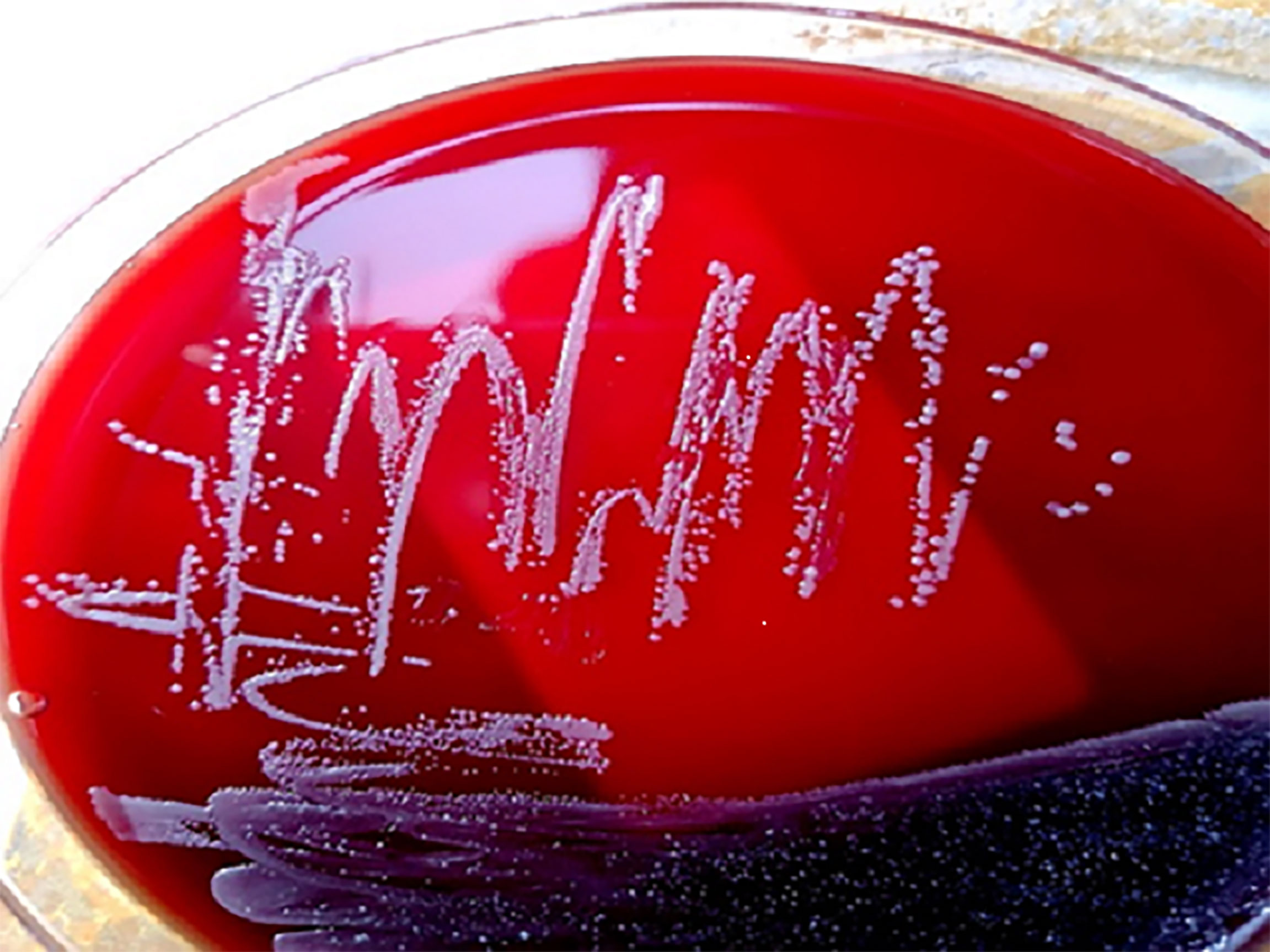
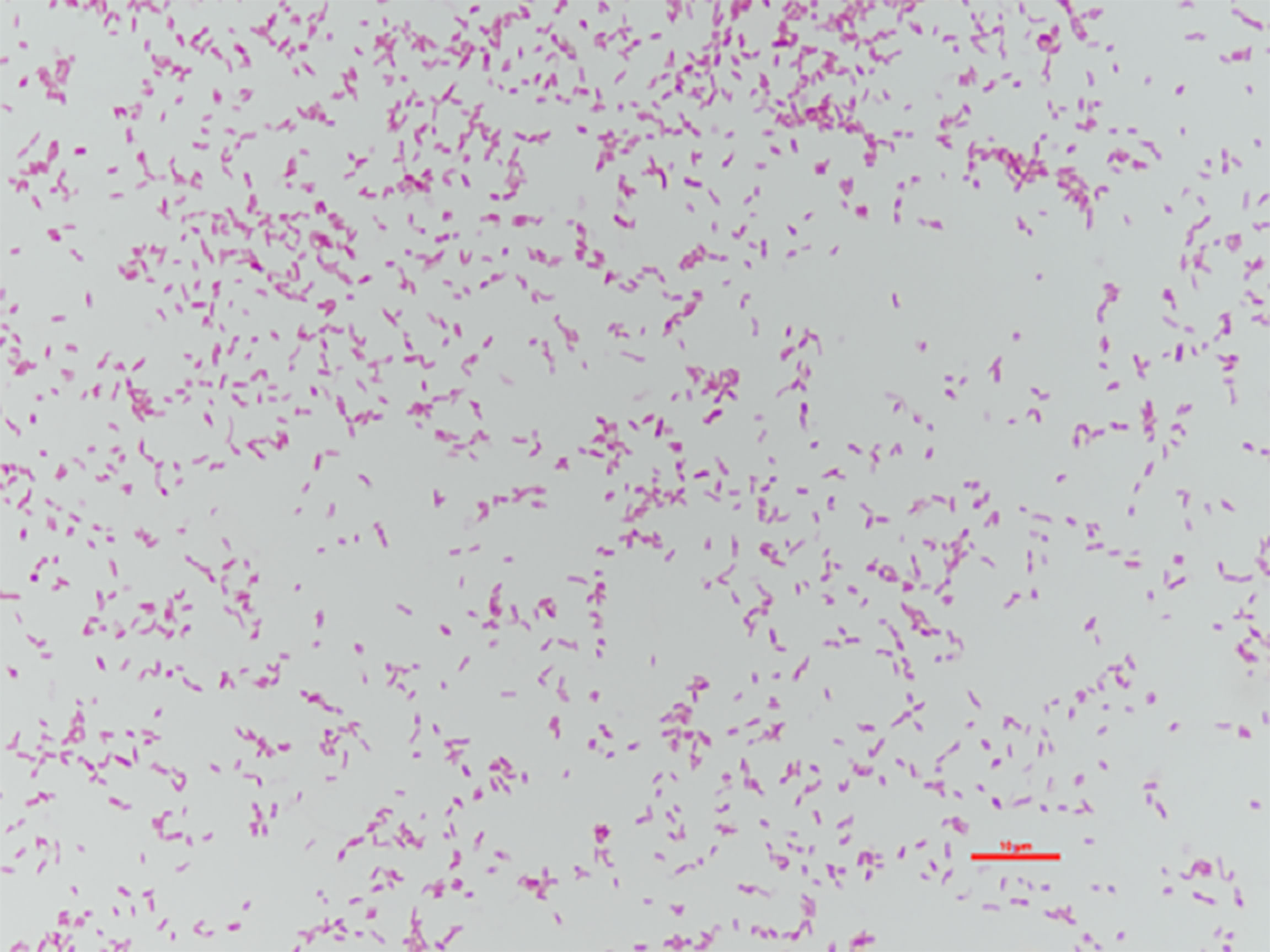
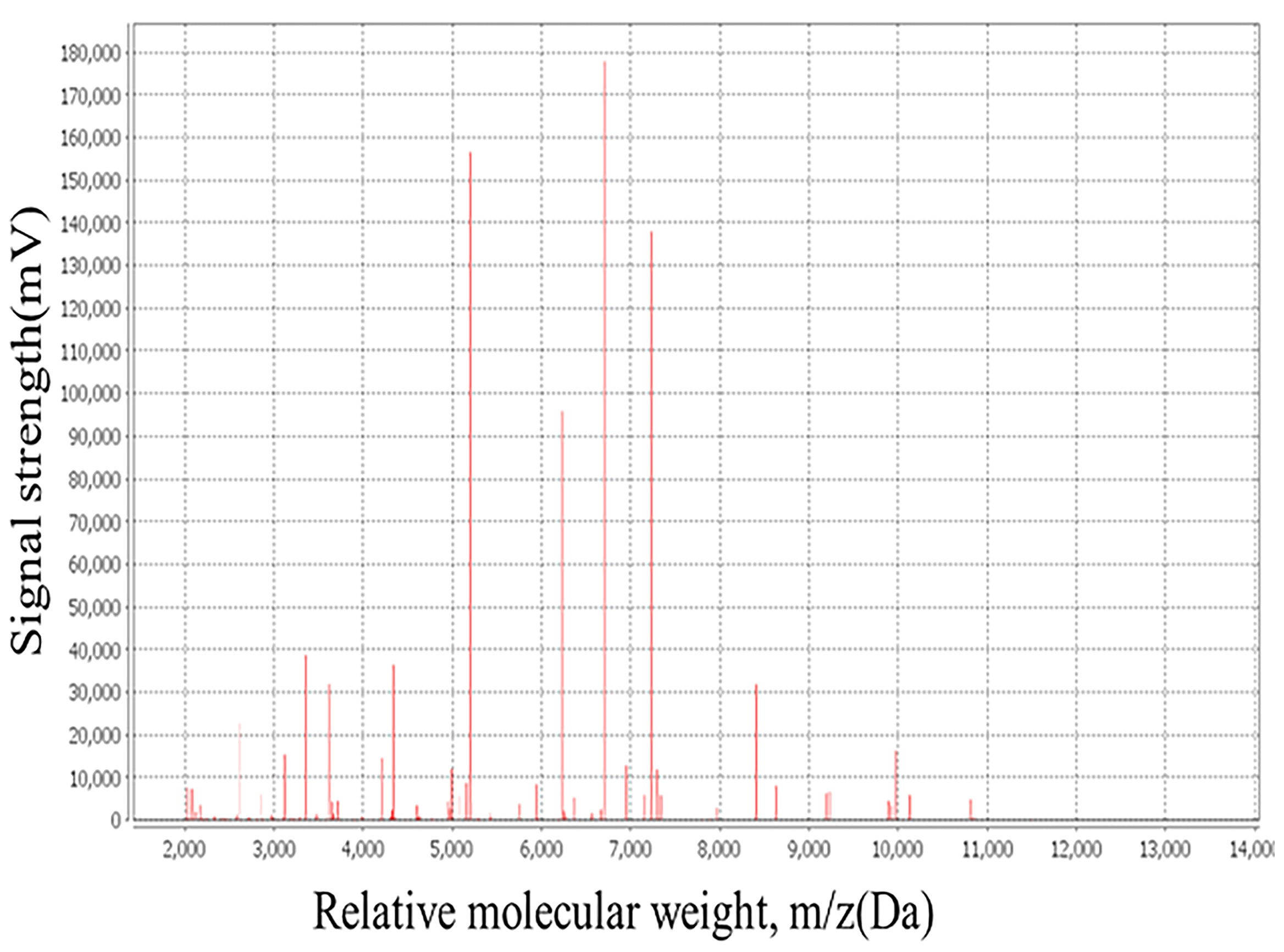
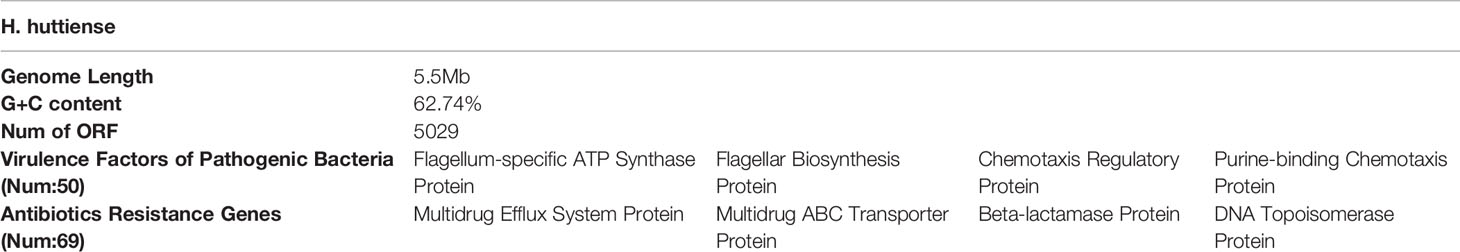
The bacterial colonies appeared white, gelatinous, and slightly raised after 24 h in culture and had diameters between 1 and 1.5 mm without apparent hemolytic rings (Figure 1). Stainings showed the bacterial colonies were Gram-negative bacilli (Figure 2). The MALDI-TOF MS results indicated 99.2% homology with H. huttiense (Figure 3). The 16S rRNA gene sequence was consistent with that of H. huttiense. Phylogenetic tree analysis showed that the isolate was present on the same branch as H. huttiense (Figure 4). The isolates were thus identified as H. huttiense for the 16S gene. Imipenem, meropenem, piperacillin-tazobactam and levofloxacin had good antibiotic activities and the MICs results were summarized in Table 1. The MIC was defined as the drug concentration that completely inhibited bacterial growth or caused a marked reduction (≥90%) compared with the drug-free control. The draft genome sequence of H. huttiense revealed chromosome size was 5.5Mb with a 62.74% G + C content. Automatic annotation revealed 5029 open reading frames (ORFs) covering 50 virulence associated genes and 69 antibiotic resistance associated genes. Virulence associated genes including flagellum-specific ATP synthase protein, flagellar biosynthesis protein, chemotaxis regulatory protein, and purine-binding chemotaxis protein were identified in the VFDB database. Antibiotic resistance associated genes including multidrug efflux system protein, multidrug ABC transporter protein, β-lactamase protein, and DNA topoisomerase protein were detected in the CARD database. The detailed genomic features are listed in Table 2 (Gan et al., 2020; Yang et al., 2021).
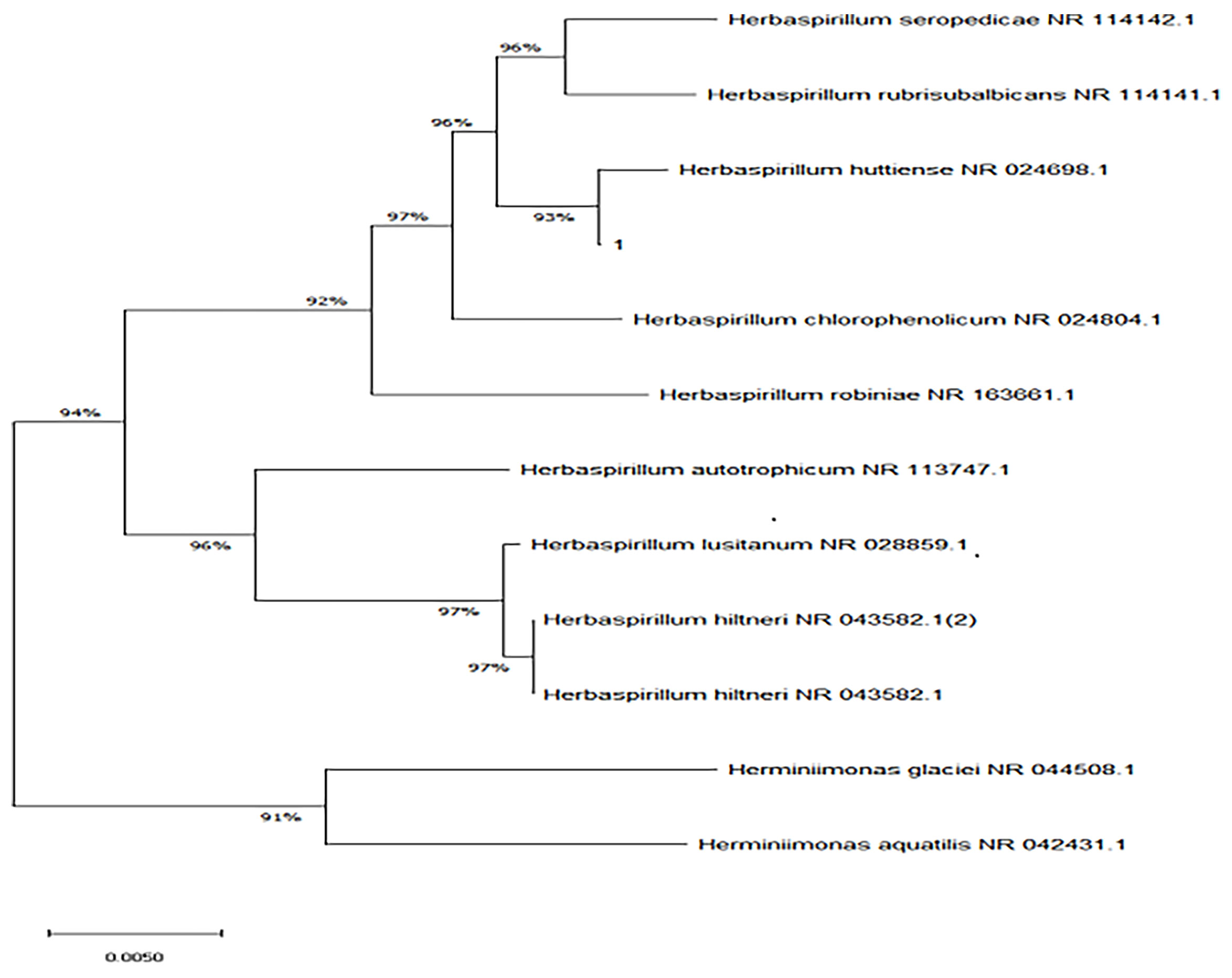
Figure 4 Neighbor-joining phylogenetic tree of H. huttiense indicated high homology (1:isolated strain).
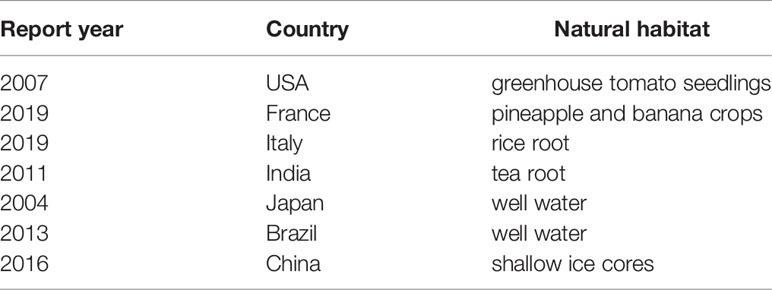
After a comprehensive literature search, it was evident that H. huttiense was an obviously rare cause of human infections and only 8 cases of H. huttiense detected in human samples had been reported. These cases were mainly observed in monomicrobial infections, such as infections of pneumonia (Regunath et al., 2015; Liu et al., 2019), acute myelocytic leukemia (Nurullah et al., 2021), breast cancer (Anonymity, 2021), thrombocytopenia (Berardino et al., 2019; Anonymity, 2020), intraventricular hemorrhage (Hernández et al., 2019), and infective endocarditis (Güngör et al., 2020). Another 7 reports described H. huttiense in its natural habitat. As H. huttiense was a nitrogen-fixing bacterium and was widely distributed in the environment, it had been investigated in the roots of rice and tea plants (Gulati et al., 2011; Andreozzi et al., 2019), greenhouse tomato seedlings, pineapple and banana crops (Obradovic et al., 2007; González et al., 2019), well water and shallow ice cores (Ding and Akira, 2004; Souza et al., 2013; Chen et al., 2016). The essential data from several of the H. huttiense studies were summarized in Tables 3, 4.
Discussion
A review of the literatures of H. huttiense infections in human showed that the ages of the patients ranged two months to 93 years old with a male-to-female ratio was 4:3. The gender ratio was balanced. The first case was described in 2015, and another 7 sporadic cases were reported since 2015. Of the eight documented cases, three (3/8) were from Spain, and two each (2/8 and 2/8) respectively came from Turkey and the United States of America. Only one (1/8) originated from Korea. Based on the world prevalence of H. huttiense, majority of the reported cases came from Europe, followed by America and Asia. Nonetheless, no cases were reported from Africa, and it was probable that regional differences or underdiagnosis due to lack of technical resources led to this trend. In the present study, we successfully identified the pathogen to the species level using both Biotyper and Vitek MS systems. In terms of the reported cases, four (4/8) was identified on the Bruker Biotyper system and one infection (1/8) was diagnosed using Vitek MS system. Only two cases (2/8) were investigated using 16S rRNA gene sequence. H. huttiense infections were usually associated with risk factors, such as pneumonia, hematological system disease and cardiovascular disease. Our study showed that H. huttiense infection was associated with cardiovascular disease. In the reported studies, most of H. huttiense were isolated from blood, as in the present study.
The cut-off points for the interpretation of H. huttiense MICs were essentially in line with the CLSI recommendations for Gram-negative non-fermenters or non-enterobacteriaceae. Güngör et al. (2020) observed that H. huttiense was sensitive to teicoplanin, ceftazidime and meropenem. Hernández et al. (2019) reported that H. huttiense was sensitive to levofloxacin, ceftazidime, trimethoprim-sulfamethoxazole, minocycline and meropenem and resistant to amikacin and colistin (Hernández et al., 2019; Güngör et al., 2020). Currently, neither CLSI nor EUCAS provided a definite breakpoint for H. huttiense and its antibiotic sensitivity test was difficult to perform. In our research, piperacillin-tazobactam, imipenem, meropenem and levofloxacin were found to be effective against H. huttiense which was consistent with the results of Güngör ‘s and Hernández ‘s. Antibiotic susceptibility could serve as a means for differentiating H. huttiense from Burkholderia cepacia complex as the latter was usually multidrug-resistant, whereas the former was not (Berardino et al., 2019).Therefore, there was no definitive consensus reached on the precise antibiotic therapy for this infection and empirical therapy played an important role in the clinical treatment of H. huttiense infections. The publications showed that meropenem and piperacillin/tazobactom were the most commonly used clinical drugs and had good clinical effects. Our patient demonstrated a good clinical response due to the treatment with meropenram and tigecycline, followed by moxifloxacin and piperacillin/tazobactam. Of the 50 virulence associated genes detected in all genomes, several categories of flagellum-associated proteins were detected. Flagellar movement could enhance the invasion of bacteria to the host, because the movement was often chemically oriented and thus could avoid harmful environments or move toward the direction of high concentration environments. It was possible that the virulence of H. huttiense was flagella-related. Of the 69 antibiotic resistance genes identified, multidrug-resistance associated genes predominated, suggesting that antibiotic resistance of H. huttiense might be due to the possession of these multidrug-resistance associated genes.
Many environmental microorganisms have evolved into human pathogens, and H. huttiense is one of these. In the past, the isolation H. huttiense had proved esspecially challenging as it was easily be misidentified due to its phylogenetic and phenotypic resemblance to other strains. VITEK 2 and other biochemical identification systems had been unable to identify H. huttiense.. H. huttiense was frequently confused with organisms such as B. cepacia complex, Cupriavidus pauculus, Ralstonia spp., or Ochrobactrum anthropic. These limitations had retarded the investigation and knowledge of H. huttiense. However, the recent wide establishment of MALDI-TOF MS in clinical microbiology had resulted in the identification of bacteria and fungi with an accuracy of 90% or higher. MALDI-TOF MS was a spectroscopic method which required a reliable and complete database. The prompt (less than 1 h) identification and high discriminatory power of MALDI-TOF MS made it a useful tool for the characterization of rare bacteria that were previously difficult to identify using routine methods. In addition, the detection probability of rare bacteria was improved by the application of bioMérieux MS scientific research database. In our research, MALDI-TOF MS was used to identify an isolated strain of H. huttiense with a confidence of 99.2% in the scientific research database. This is the first report of the identification of H. huttiense in China by MALDI-TOF MS technology. The 16S rRNA gene sequencing and NGS were used to verify the MALDI-TOF results and a phylogenetic tree was constructed to confirm the findings. The results of MALDI-TOF MS, 16S rRNA gene sequencing, and NGS were consistent, and achieved high accuracy.
Conclusions
In conclusion, H. huttiense was isolated from one clinical sample and identified by MALDI-TOF MS, 16S rRNA gene sequencing and NGS. MALDI-TOF MS and 16S rRNA gene sequencing represented prompt and accurate detection methods and were completed within 24 h. The isolates had good antibiotic activities to imipenem, meropenem, piperacillin-tazobactam and levofloxacin. This demonstration of the prompt identification of a rare pathogen and its antibiotic activities might increase awareness of these uncommon infections.
Data Availability Statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Local Research Ethics committee of the First Affiliated Hospital of Anhui Medical University (Quick-PJ2022-02-14). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XL, XB, GQ, LW, CS, SC, YX, MZ, and ZW conceived and designed the study. YX, MZ, and ZW were responsible for data interpretation. XL and XB wrote the paper. YX, MZ, and ZW revised subsequent versions. GQ, LW, CS, and SC carried out the experimental works in the clinical microbiology laboratory. All authors have read and approved the final manuscript.
Funding
This work was supported by Youth Project of National Natural Science Foundation of China (82100613), the Opening Project of Anhui Province Key Laboratory of Reproductive Health and Genetics, Doctoral Research Foundation of the First Affiliated Hospital of Anhui Medical University (Bsky2019038), Scientific Research Fund of Anhui Medical University (2021xkj137) and Anhui Provincial Key Research and Development Plan Project (201904a07020049). The grant number of the Opening Project of Anhui Province Key Laboratory of Reproductive Health and Genetics should be added and the grant number is 9021701201.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andreozzi, A., Prieto, P., Mercado-Blanco, J., Monaco, S., Zampieri, E., Romano, S., et al. (2019). Efficient Colonization of the Endophytes Herbaspirillum Huttiense RCA24 and Enterobacter Cloacae RCA25 Influences the Physiological Parameters of Oryza Sativa L. Cv. Baldo Rice. Environ. Microbiol. 21 (9), 3489–3504. doi: 10.1111/1462-2920.14688
Anonymity (2020). Methylprednisolone: Herbaspirillum Huttiense Bacteraemia: Case Report. Reactions Wkly. 1791 (1), 171.
Baldani, J. I., Pot, B., Kirchhof, G., Falsen, E., Baldani, V. L., Olivares, F. L., et al. (1996). Emended Description of Herbaspirillum; Inclusion of [Pseudomonas] Rubrisubalbicans, A Milk Plant Pathogen, as Herbaspirillum Rubrisubalbicans Comb. Nov.; and Classification of a Group of Clinical Isolates (EF Group 1) as Herbaspirillum Species 3. Int. J. Syst. Bacteriol. 46, 802–810. doi: 10.1099/00207713-46-3-802
Berardino, M. A., Rodríguez-Czaplicki, E., Sánchez-Hellín, V. (2019). Herbaspirillum Huttiense Pneumonia in a Patient With Essential Thrombocythaemia. Rev. Esp. Quimioter 32 (1), 83–84.
Chen, Y., Li, X. K., Si, J., Wu, G. J., Tian, L. D., Xiang, S. R. (2016). Changes of the Bacterial Abundance and Communities in Shallow Ice Cores From Dunde and Muztagata Glaciers, Western China. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.01716
Ding, L. X., Akira, Y. (2004). Proposals of Curvibacter Gracilis Gen. Nov., Sp. Nov. And Herbaspirillum Putei Sp. Nov. For Bacterial Strains Isolated From Well Water and Reclassification of [Pseudomonas] Huttiensis, [Pseudomonas] Lanceolata, [Aquaspirillum] Delicatum and [Aquaspirillum] Autotrophicum as Herbaspirillum Huttiense Comb. Nov., Curvibacter Lanceolatus Comb. Nov., Curvibacter Delicatus Comb. Nov. Int. J. Syst. Evol. Microbiol. 54 (Pt 6), 2223–2230. doi: 10.1099/ijs.0.02975-0
Dobritsa, P. A., Reddy, M. C. S., Samadpour, M. (2010). Reclassification of Herbaspirillum Putei as a Later Heterotypic Synonym of Herbaspirillum Huttiense, With the Description of H. Huttiense Subsp. Huttiense Subsp. Nov. And H. Huttiense Subsp. Putei Subsp. Nov., Comb. Nov., and Description of Herbaspirillum Aquaticum Sp. Nov. Int. J. Syst. Evol. Microbiol. 60, 1418–1426. doi: 10.1099/ijs.0.009381-0
Gan, L. Z., Li, X. G., Tian, Y. Q., Peng, B. (2020). Genomic Insights Into the Salt Tolerance and Cold Adaptation of Planococcus Halotolerans SCU63T. Arch. Microbiol. 202 (10), 2841–2847. doi: 10.1007/s00203-020-01979-9
González, R. M., Mendoza, J. R., Pérez, D. V., Soler, A. B. (2019). Antimicrobial Activity of Endogenous Bacteria Against Phytophthora Nicotianae Var. Parasitica. Acta Hortic. 1239, 195–202. doi: 10.17660/ActaHortic.2019.1239.24
Gulati, A., Sood, S., Rahi, P., Thakur, R., Chauhan, S., Chawla, I. (2011). Diversity Analysis of Diazotrophic Bacteria Associated With the Roots of Tea. J. Microbiol. Biotechnol. 21, 545–555. doi: 10.4014/jmb.1012.12022
Güngör, A. A., Demirdağ, T. B., Dinc, B., Azak, E., Erdem, A. Y., Kurtipek, B., et al. (2020). A Case of Infective Endocarditis Due to Herbaspirillum Huttiense in a Pediatric Oncology Patient. J. Infect. Dev. Ctries. 24, 232–233. doi: 10.3855/jidc.13001
Hernández, M. G. L., Sada, P. G. V., Romero, I. F., Gómez, M. P. R. (2019). Bacteremia Caused by Herbaspirillum Huttiense in a Newborn. Enferm. Infecc. Microbiol. Clin. 37, 491. doi: 10.1016/j.eimc.2018.12.011
Liu, C., Kwon, M. J., Kim, M., Byun, H. J., Yong, D., Lee, K., et al. (2019). Septicemia Caused by Herbaspirillum Huttiense Secondary to Pneumonia. Ann. Lab. Med. 39, 340–342. doi: 10.3343/alm.2019.39.3.340
Nurullah, U., Nezahat, A., Mehmet, K. (2021). A Case of Bacteremia Caused by Herbaspirillum Huttiense in an Immunosuppressive Patient and Literature Review. Flora 26 (1), 220–226. doi: 10.5578/flora.20219924
Obradovic, A., Jones, B. J., Minsavage, V. G., Dickstein, R. E., Momol, M. T. (2007). A Leaf Spot and Blight of Greenhouse Tomato Seedlings Incited by a Herbaspirillum Sp. Plant Dis. 91, 886–890. doi: 10.1094/PDIS-91-7-0886
Regunath, H., Kimball, J., Smith, L. P., Salzer, W. (2015). Severe Community-Acquired Pneumonia With Bacteremia Caused by Herbaspirillum Aquaticum or Herbaspirillum Huttiense in an Immune-Competent Adult. J. Clin. Microbiol. 53, 3086–3088. doi: 10.1128/JCM.01324-15
Souza, D. V., Piro, V. C., Faoro, H., Tadra-Sfeir, M. Z., Chicora, K. V., Guizelini, D., et al. (2013). Draft Genome Sequence of Herbaspirillum Huttiense Subsp. Putei IAM 15032, A Strain Isolated From Well Water. Genome. Announc. 1, e002521–e0025212. doi: 10.1128/genomeA.00252-12
Tayeb, A. L., Lefevre, M., Passet, V., Diancourt, L., Brisse, S., Grimont, A. D. P. (2008). Grimont Comparative Phylogenies of Burkholderia, Ralstonia, Comamonas, Brevundimonas and Related Organisms Derived From Rpob, gyrB and Rrs Gene Sequences. Res. Microbiol. 159, 169–177. doi: 10.1016/j.resmic.2007.12.005
Keywords: first study, Herbaspirillum huttiense (H. huttiense), bacteremia, prompt identification, antibiotic sensitivity
Citation: Li X, Bao X, Qiao G, Wang L, Shi C, Chen S, Xu Y, Zheng M and Wang Z (2022) First Study of Bacteremia Caused by Herbaspirillum huttiense in China: A Brief Research Report and Literature Review. Front. Cell. Infect. Microbiol. 12:882827. doi: 10.3389/fcimb.2022.882827
Received: 24 February 2022; Accepted: 19 May 2022;
Published: 17 June 2022.
Edited by:
Percy Schröttner, Technische Universität Dresden, GermanyReviewed by:
Fupin Hu, Fudan University, ChinaJeong Hwan Shin, Inje University Busan Paik Hospital, South Korea
Copyright © 2022 Li, Bao, Qiao, Wang, Shi, Chen, Xu, Zheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongxin Wang, liuyajing62@163.com; Meijuan Zheng, mjzheng@mail.ustc.edu.cn; Yuanhong Xu, xyhong1964@163.com
†These authors share first authorship
 Xiangyun Li
Xiangyun Li Xundi Bao
Xundi Bao Guanhua Qiao
Guanhua Qiao Lianzi Wang
Lianzi Wang Cuixiao Shi
Cuixiao Shi Shuyi Chen3
Shuyi Chen3  Yuanhong Xu
Yuanhong Xu Zhongxin Wang
Zhongxin Wang