Epidemiological Study on Mycoplasma pneumoniae and Chlamydia pneumoniae Infection of Hospitalized Children in a Single Center During the COVID-19 Pandemic
- The Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, National Children’s Regional Medical Center, Hangzhou, China
Background: Since the outbreak of COVID-19, a series of preventive and control measures in China have been used to effectively curb the spread of COVID-19. This study aimed to analyze the epidemiological characteristics of Mycoplasma pneumoniae (MP) and Chlamydia pneumoniae (CP) in hospitalized children with acute respiratory tract infection during the COVID-19 pandemic.
Methods: MP IgM antibody and CP IgM antibody were detected in all hospitalized children due to acute respiratory tract infection in the Children’s Hospital Affiliated to Zhejiang University from January 2019 to December 2020. These data were compared between 2019 and 2020 based on age and month.
Results: The overall detection rate of MP and CP in 2020 was significantly lower than that in 2019 (MP: 21.5% vs 32.9%, P<0.001; CP: 0.3% vs 0.9%, P<0.001). This study found a 4-fold reduction in the number of children positive for MP and a 7.5-fold reduction in the number of children positive for CP from 2019 to 2020. The positive cases were concentrated in children aged >1 year old. In 2019, the positive rate of MP was detected more commonly in children 3 years of age or older than in younger children. In 2020, the higher positive rate of MP reached a peak in the 3- to 6-year age group (35.3%). CP was detected predominantly in children aged 6 years older in 2019 and 2020, with positive rates of 4.8% and 2.6%, respectively. Meanwhile, the positive rates of MP in 2019 were detected more commonly in July, August and September, with 47.2%, 46.7% and 46.3%, respectively. Nevertheless, the positive rates of MP from February to December 2020 apparently decreased compared to those in 2019. The positive rates of CP were evenly distributed throughout the year, with 0.5%-1.6% in 2019 and 0.0%-2.1% in 2020.
Conclusions: A series of preventive and control measures for SARS-CoV-2 during the COVID-19 pandemic can not only contain the spread of SARS-CoV-2 but also sharply improve the infection of other atypical pathogens, including MP and CP.
Introduction
COVID-19 pneumonia is characterized by acute respiratory infection, including fever, dry cough and fatigue. COVID-19 pneumonia is still in a pandemic state worldwide and is an urgent public health issue (Mallah et al., 2021). Since the outbreak of COVID-19, China has issued a series of preventive and control measures, including two overarching strategies, containment and suppression (Li et al., 2020; National Health Commission Of The People’s Republic Of, C., 2020). The core measures of containment are early finding infected people and actively treating in isolation, closely tracking and quarantining and reducing transmission, including staying at home, less aggregation and closing schools (Chen et al., 2021). Personal protection methods consist of wearing masks, washing hands frequently, keeping social distance, ventilation and gathering outside the house (Ju et al., 2021). These prevention and control measures refer to nonpharmaceutical interventions (Zhang et al., 2021a).
Zhejiang Province, located in East China, launched the first-level response to major public health emergencies on January 23, which was the first province in China to launch the “first-level emergency plan”. There were fewer children infected with COVID-19 in this area, including 36 children with COVID-19 in a previous multicenter study (Chen et al., 2020), and only one infant with COVID-19 was treated in our hospital. Recently, a few studies found that nonpharmaceutical interventions aimed at COVID-19 not only contain the spread of COVID-19 but also markedly reduce influenza virus infection (Fricke et al., 2021) and the transmission of other respiratory viruses, such as respiratory syncytial virus and adenovirus (Ye and Liu, 2021).
Acute respiratory tract infection is a common infectious disease in children. Mycoplasma pneumoniae (MP) and Chlamydia pneumoniae (CP) are atypical pathogens of acute respiratory tract infections in children. In particular, MP pneumonia accounts for 32.4%-39.5% of children’s community-acquired pneumonia (Ning et al., 2017; Gao LW et al., 2019). It is common in outpatients and hospitalized children and is mainly transmitted through the respiratory tract. This study aimed to analyze the epidemiological characteristics of MP and CP in hospitalized children with acute respiratory tract infection, which is transmitted by respiratory droplets similar to SARS-CoV-2, during the COVID-19 pandemic (Lansbury et al., 2020; Oliva et al., 2020; Zhu et al., 2020).
Methods
Study Subjects
The retrospective study included all children hospitalized due to acute respiratory tract infection in the Children’s Hospital Affiliated to Zhejiang University from January 2019 to December 2020. Demographic data, such as age, gender, and the patient’s clinical manifestations, were obtained from the electronic medical records. All enrolled children conformed to the following criteria: (1) one or more respiratory symptoms (cough, sore throat, combined with a body temperature > 37.5°C) (McCracken, 2001) and (2) children aged younger than 18 years. The exclusion criteria of this study were as follows: (1) children infected with COVID-19; (2) children with malignant tumors or congenital pulmonary airway obstruction; and (3) children with a recurrent chronic respiratory infection. All the children were divided into five age groups: under 28 days (0–28 d), 1-12 months (1-12 m), 1-3 years (1-3 y), 3-6 years (3-6 y) and six years older (> 6 y). The detection rate of pathogens was also compared by month.
Detection of Pathogens
After admission, blood was collected with a heparin anticoagulant tube and then centrifuged for 5 minutes at 2500 r/min. Centrifuged serum was used for detection. MP IgM and CP IgM antibodies were detected by a two-step indirect method of direct chemiluminescence technology (iFlash3000, YHLO, Shenzhen, China) using a commercial kit (YHLO, Biotechnology Co., Ltd., China). The steps of detection were as follows. The first step of incubation was that MP or CP IgM in the sample reacted with the corresponding antigen coated on superparamagnetic particles to form antigen-antibody complexes. Magnetic particles were adsorbed to the reaction tube wall under the action of a magnetic field, and unbound substances were washed away by the cleaning solution. The second incubation step was that mouse anti-human IgM labeled with acridine was added to the reaction tube to form an antigen-antibody-double antibody complex. Unbound substances were washed away again. Preexcitation solution and excitation solution were added to the reaction mixture, and then the relative luminous intensity (RLU) of the mixture was detected by the optical system of the tester. The amount of pathogen IgM in the sample was proportional to the RLU. The cutoff index (COI) value was automatically calculated according to the RLU value of each sample by the tester. The relative luminous intensity was compared with the cutoff value calculated by the corresponding IgM calibrator. When the COI < 0.9, the IgM antibody was negative. When the COI was between 0.9-1.1, the result needed to be rechecked or comprehensively judged. When the COI ≥ 1.1, the IgM antibody was positive.
The final diagnosis of M. pneumoniae and C. pneumoniae infection in our manuscript combined serological IgM antibody with the patient’s clinical symptoms, other laboratory indicators (leukocytes, hypersensitive C-reactive protein, cytokines, etc.) and imaging data (Meyer Sauteur et al., 2016).
Statistical Analysis
All the data were analyzed using SPSS version 26.0 software (IBM Corp., Armonk, N.Y., USA). Categorical variables were analyzed using the chi-squared test or Fisher’s exact test. A P value <0.05 was considered statistically significant.
Results
Patient Characteristics
From January 2019 to December 2020, a total of 18833 children were admitted to our hospital due to acute respiratory infection, including 13621 cases in 2019 and 5212 cases in 2020. The total number of patients in 2020 was significantly lower than that in 2019 (P<0.001). Among all the enrolled children, 10922 were male and 7911 were female, with a male to female ratio of 1.38:1. There was no significant difference in sex between patients in 2019 and those in 2020. In the five age groups, most patients were aged 1-3 years (7023, 37.3%), followed by children aged 1-12 months (5510, 29.3%). (Table 1)
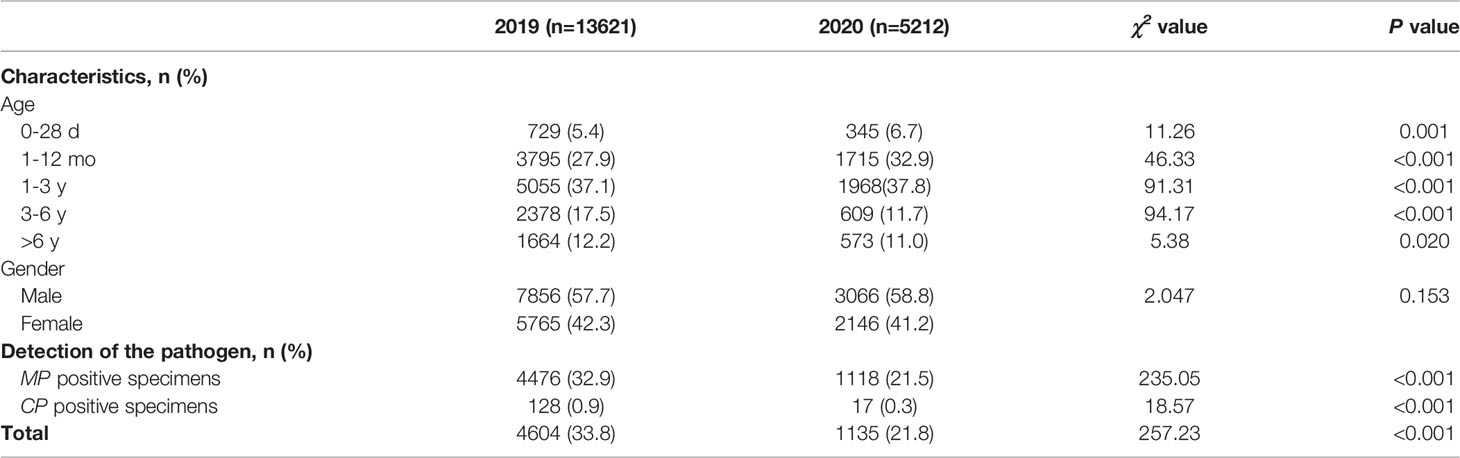
Table 1 Patient characteristics and detection of Mycoplasma pneumoniae and Chlamydia pneumoniae between 2020 (during the COVID-19 pandemic) and 2019 (before the COVID-19 pandemic).
Overall Detection of MP and CP
In 2019 (before the COVID-19 pandemic), MP was detected in 32.9% of patients (4476/13621), and CP was detected in 0.9% (128/13621). However, in 2020 (during the COVID-19 pandemic), MP was detected in 21.5% of patients (1118/5212), and CP was detected in 0.3% (17/5212). We found a 4-fold reduction in the number of children positive for MP and a 7.5-fold reduction in the number of children positive for CP from 2019 to 2020. The positive cases were concentrated in children aged >1 year old. The overall detection rate of MP and CP in 2020 was significantly lower than that in 2019 (χ2 = 235.05, P<0.001; χ2 = 18.57, P<0.001). (Table 1).
Age Distribution
The age group distributions of the positivity detection rates of MP and CP based on 2019 and 2020 are shown in Figure 1. The number of MP-positive patients reached a peak in the 1-3 year age group, with peaks of 2068 in 2019 and 548 in 2020. However, in 2019, the positive rate of MP was detected more commonly in children 3 years of age or older (47.8% in the age of 3-6 years, 50.7% in the age of >6 years) than in younger children (40.9% in the age of 1-3 years, 11.3% in the age of 1-12 months, 0.1% in the age of 0-28 days). In 2020, the higher positive rate of MP reached a peak in the 3- to 6-year age group (35.3%). MP-positive numbers in children aged 1-12 months decreased more than two times from 2019 to 2020 (427 vs. 190), and MP-positive numbers in children aged 3 years decreased more than 5 times from 2019 to 2020 (1136 vs. 215 in children aged 3-6 years; 844 vs. 164 in children aged 6 years). Nevertheless, the positive rate of MP in children older than 1 year was significantly decreased in 2020 (P<0.001). CP was detected predominantly in children aged 6 years older in 2019 and 2020, with positive numbers of 80 and 14, respectively. In children aged 6 years old, the positive rate of CP in 2020 was lower than that in 2019 (χ2 = 5.919, P=0.015). (Table 2)
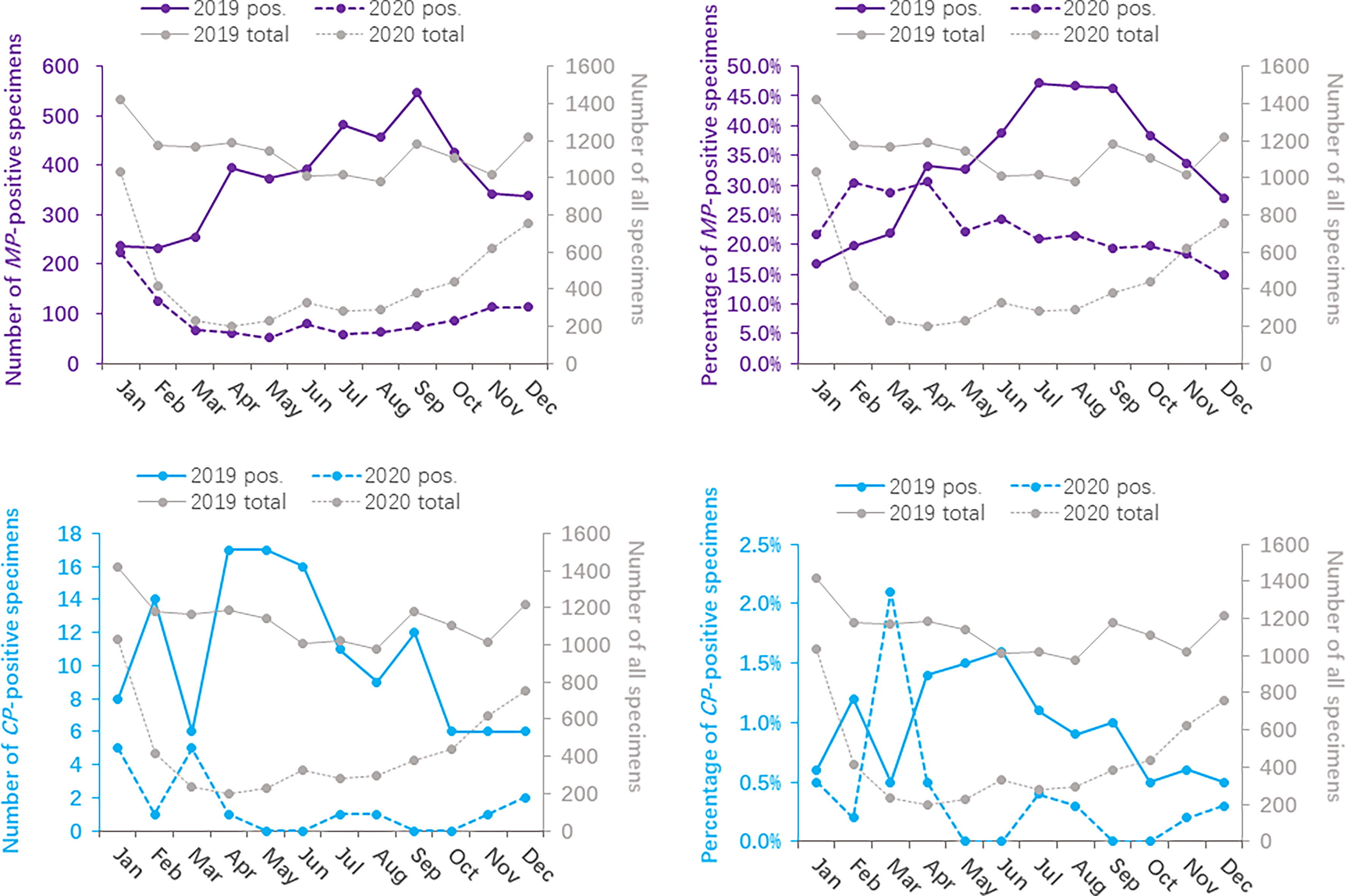
Figure 1 Distribution of Mycoplasma pneumoniae- and Chlamydia pneumoniae-positive specimens based on age between 2020 (during the COVID-19 pandemic) and 2019 (before the COVID-19 pandemic).
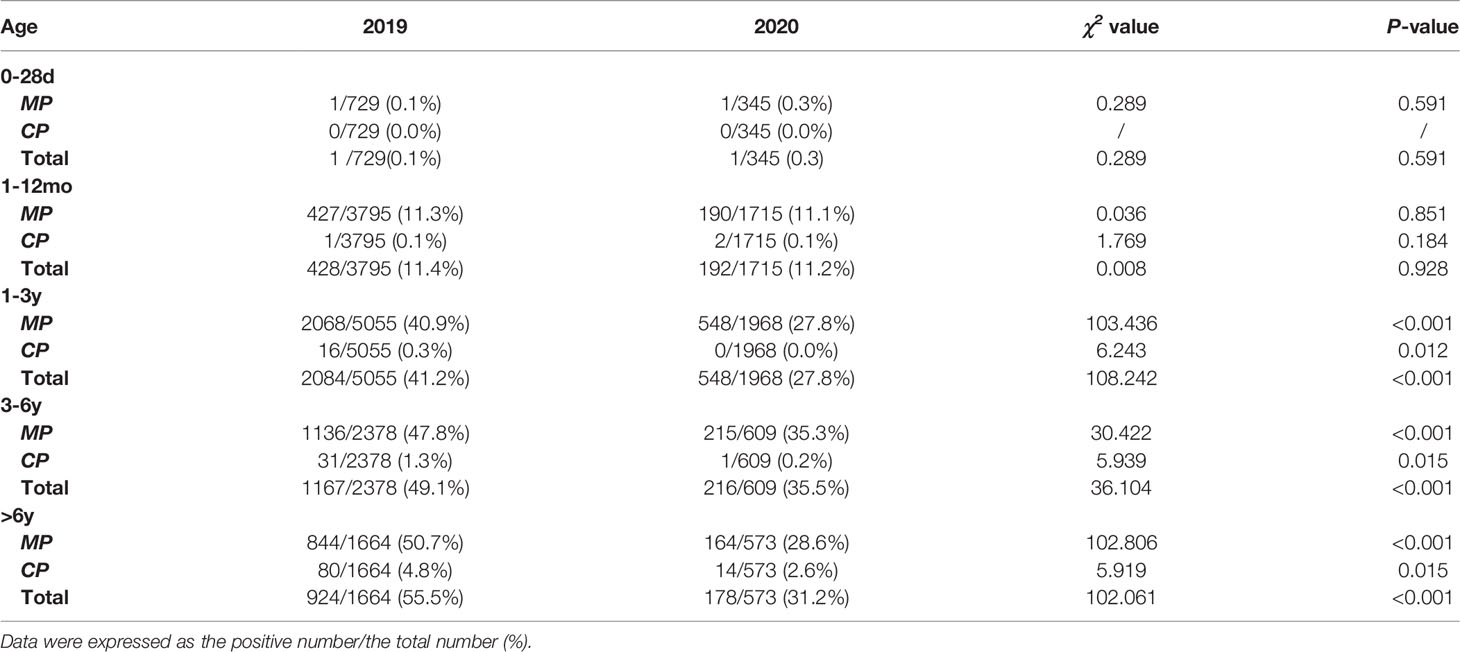
Table 2 The overall positive rate of Mycoplasma pneumoniae and Chlamydia pneumoniae based on age between 2020 (during the COVID-19 pandemic) and 2019 (before the COVID-19 pandemic).
During the COVID-19 epidemic, the number of MP infections in children except for infants aged 0-28 days decreased significantly compared to that in 2019. The MP-positive rate of children aged 3 years old reached a peak in 2019, while only children aged 3-6 years old showed a small peak in 2020. CP infections mainly occurred in children aged 6 years older.
Season Distribution
The monthly distribution of the positivity detection rate of MP and CP based on 2019 and 2020 is shown in Figure 2. The number of MP-positive patients in 2019 reached a peak in September (546/1178), followed by July (481/1020) and August (457/978), whereas the peak number of MP-positive patients in 2020 was in January (223/1032). Meanwhile, the positive rates of MP in 2019 were detected more commonly in July, August and September, with 47.2%, 46.7% and 46.3%, respectively. Nevertheless, the positive rates of MP from February to December 2020 apparently decreased compared to those in 2019. The numbers of MP-positive cases were not significantly different between January 2019 and January 2020 (237 vs 223, P>0.05). The positive rates of CP were evenly distributed throughout the year, with 0.5%-1.6% in 2019 and 0.0%-2.1% in 2020. (Table 3)
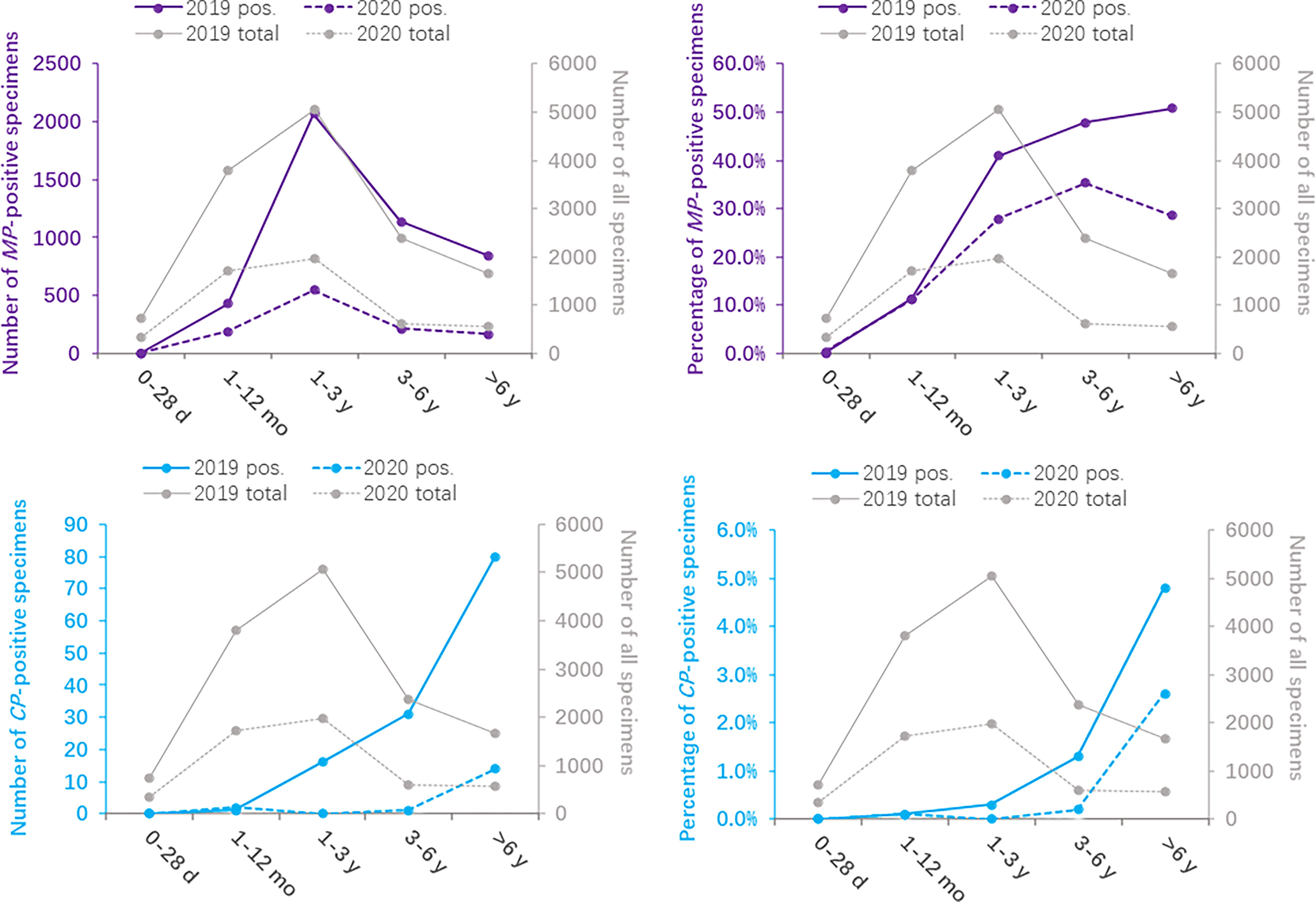
Figure 2 Distribution of Mycoplasma pneumoniae- and Chlamydia pneumoniae-positive specimens based on months between 2020 (during the COVID-19 pandemic) and 2019 (before the COVID-19 pandemic).
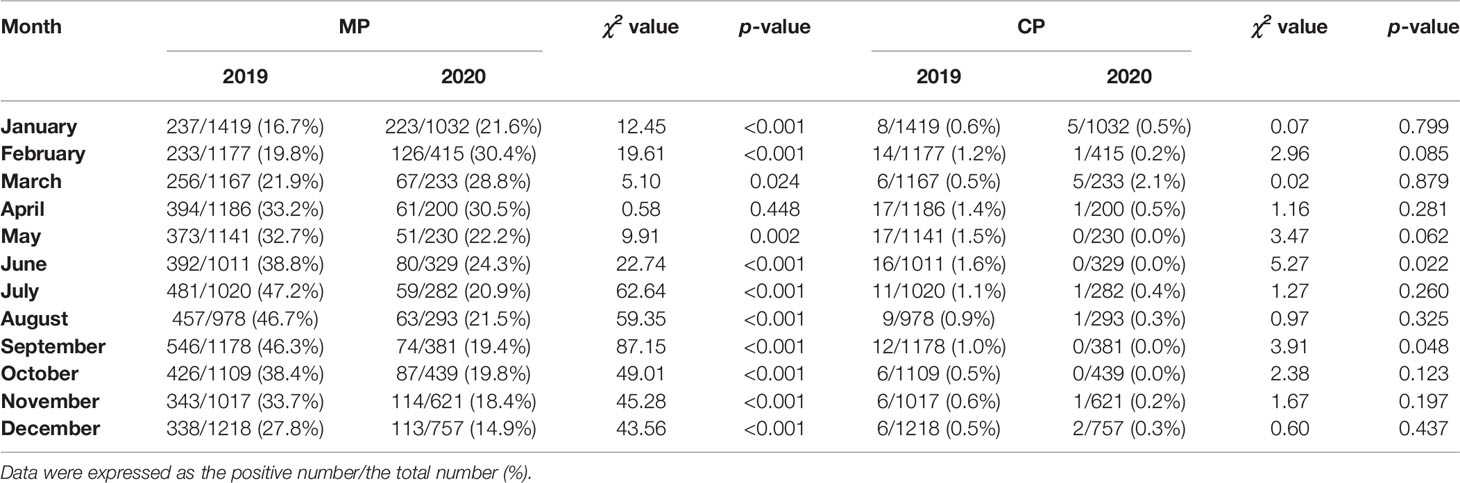
Table 3 Detection of Mycoplasma pneumoniae and Chlamydia pneumoniae based on months between 2020 (during the COVID-19 pandemic) and 2019 (before the COVID-19 pandemic).
Before the COVID-19 pandemic, MP-positive infections were detected more commonly in July, August and September, whereas no such regular seasonal variation was found in 2020. The study based on month showed a low prevalence of CP-positive infections in 2019 and 2020.
Discussion
Since COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first discovered in mid-December 2019 in Wuhan, China, a series of nonpharmaceutical interventions have been promptly taken to control the transmission of SARS-CoV-2 in China (Zhang et al., 2021a). Apart from controlling the spread of COVID-19, the epidemiology of other atypical pathogens, such as MP and CP, has undergone tremendous changes at the same time due to these nonpharmaceutical interventions. In our study, the number of children hospitalized due to acute respiratory tract infection in 2020 declined by 59.5% compared to that in 2019. Compared to 2019, the total positive rate of atypical pathogens decreased in 2020, with an emphasis on the positive rate of MP detection from 32.9% to 21.5%. This was consistent with previous results obtained in other regions of China (Li et al., 2021), in which the number of positive MP cases significantly decreased in 2020 for the public health response to COVID-19 (Zhang et al., 2021b). The positive rate of CP among children with acute respiratory infection decreased from 0.9% in 2019 to 0.3% in 2020, which was significantly lower than that of MP infection. Similar results were shown in a 5-year multicenter epidemiological study (Luo et al., 2021). In our present study, the positive rate of MP detection in children was significantly higher than that in adults (19%) (Chen et al., 2019), probably due to the mature immune system, paying more attention to hygiene and maintaining social distance at work. The decrease in the overall positive rate of atypical pathogens showed the significant additional effect of these nonpharmaceutical interventions in response to the COVID-19 epidemic.
In our study, children aged 1-3 years were most likely to develop acute respiratory tract infection symptoms in both 2019 and 2020. In addition, the positive rate of MP was detected more commonly in children 3 years of age or older than in younger children, which was corroborated by previous epidemiological studies (Jain et al., 2015; Schildgen et al., 2018; Liu et al., 2020). However, the positive rate of MP in our study is different from that in other previous studies (Gao LW et al., 2019; Jiang et al., 2020), which may be due to differences in MP detection methods and enrolled populations. Chemiluminescence was used to detect IgM antibodies of serum MP in our study, whereas atypical pathogen DNA was detected in nasopharyngeal samples by a polymerase chain reaction in some studies (Del Valle-Mendoza et al., 2017; Jiang et al., 2020; Kumar et al., 2020), or MP antibody was detected by the passive agglutination method (Gao CH et al., 2019; Gao LW et al., 2019). PCR-based techniques are now considered more reliable, however, this method also has limitations, such as low sensitivity and positive predictive values (Nilsson et al., 2008; Chang et al. 2014; Kurkela et al. 2019). So, each method, whether serological test or PCR method, has its advantages and disadvantages. Diagnosis needs to combined laboratory examination with clinical manifestation (Meyer Sauteur et al. 2016).
Among the five age groups, the positive detection rate of MP in children over 3 years old in 2019 was higher than that in other age groups, while the infection rate of infants younger than 28 days was very low. This was due to cross-infection among kindergarten and school-age children, carefully taking care by parents and hardly contacting outsiders for infants within 28 days. Interestingly, the positive detection rate of MP in children over 3 years old improved significantly in 2020, which is inseparable from the strict COVID-19 prevention and control policy. The policies included delaying school opening in spring and wearing masks, hand hygiene and social distancing.
We found that the number of MP-positive patients in 2019 reached a peak in September, followed by July and August, whereas the peak number of MP-positive patients in 2020 was in January. Previous studies have reported that the climate environment can significantly affect the transmission of MP pathogens (Wright et al., 1969; Tian et al., 2017). Our previous study suggested that MP infection significantly correlated with temperature (Tian et al., 2017). The optimum growth temperature of MP is 37°C. In China, July, August and September are the hottest months every year, and MP grows best at these higher temperatures. Since Zhejiang initiated the 1-level emergency response on January 23, 2020, the number of children hospitalized due to acute respiratory tract infection decreased sharply in February 2020, but the positive rate was still 30.4%. This may be because the number of MP-positive patients did not decrease as fast as the total number of hospitalizations. The numbers of MP-positive patients and the total number of hospitalizations from March to December 2020 were obviously lower than those in 2019, which was consistent with previous results found in Chengdu, China (Zhang et al., 2021b). Although the positive rate of CP was low in both 2019 and 2020, the number of CP-positive patients monthly in 2020 was still significantly decreased compared with that in 2019. A low prevalence of CP infections was also found in other countries, such as Japan (Oishi et al., 2020; Ishimaru et al., 2021), or other regions in China (Li et al., 2021; Luo et al., 2021). Due to the strict prevention and control policy for COVID-19 in China, the positive rate of CP reached a lower degree (0.0%-0.5%) from April to December 2020.
Many studies have reported that atypical pathogens can cause respiratory diseases and may induce secondary diseases such as skin lesions, especially urticaria (De Luigi et al., 2021), Fuchs’ syndrome, varicella-like eruptions, Henoch-Schonlein syndrome (Terraneo et al., 2015), acute kidney injury and myositis (Simoni et al., 2020). Therefore, an enormous reduction in the number of children positive for MP and CP had great significance in reducing the burden of respiratory diseases and may reduce the occurrence of these secondary diseases.
Overall, these strict prevention and control measures to effectively curb the spread of atypical pathogens may be due to multiple factors. First, wearing masks and social distancing effectively blocked the transmission of atypical pathogens from infected people. During the COVID-19 epidemic, a study proved that masks could prevent infectious aerosols produced by infected people (Ju et al., 2021). Similarly, social distancing can effectively reduce aerosol transmission between close contacts (Kucharski et al., 2020). Second, staying at home, less aggregation, and closing schools can control the spread of atypical pathogens between children at the source. Third, frequent hand hygiene may eliminate atypical pathogens to a certain extent, which was also sensitive to 75% alcohol.
Nevertheless, our study was subject to some limitations. First, this is a single-center study, even though this hospital is the largest children’s hospital in Zhejiang Province, China. The prevalence of atypical pathogens may differ from other provinces in China with different climates, economic levels and lifestyles. Second, the pathogens detected in our study included only MP and CP and did not analyze other respiratory viruses and bacterial pathogens. Because this was a retrospective study, detailed data on other pathogens were lacking.
Conclusion
In conclusion, this is the first study to analyze the impact of preventive and control measures for SARS-CoV-2 on the transmission of MP and CP infection during the COVID-19 pandemic in Zhejiang Province, China. A series of preventive and control measures for SARS-CoV-2 during the COVID-19 pandemic not only contain the spread of SARS-CoV-2 but also sharply improve the infection of other atypical pathogens, including MP and CP.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The study was approved by the medical ethics committee of the Children’s Hospital of Zhejiang University School of Medicine (Hangzhou, China). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
QY conceived and designed the study and took responsibility for the integrity of the data and the accuracy of the data analysis. FC contributed to the writing of the report. All authors contributed to data acquisition, analysis, or interpretation and reviewed and approved the final version.
Funding
This study was supported by the Natural Science Foundation of Zhejiang Province (LY22H050001), the Key Project of Provincial Ministry Construction, Health Science and Technology Project Plan of Zhejiang Province (WKJ-ZJ-2128), Key Laboratory of Women’s Reproductive Health Research of Zhejiang Province (No. ZDFY2020-RH-0006), the National Natural Science Foundation of China (Grant/Award Number: U20A20351),and Key Research and Development Plan of Zhejiang Province (Grant/Award Number: 2021C03079).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Chang, H. Y., Chang, L. Y., Shao, P. L., Lee, P. I., Chen, J. M., Lee, C. Y., et al. (2020). Comparison of Real-Time Polymerase Chain Reaction and Serological Tests for the Confirmation of Mycoplasma Pneumoniae Infection in Children with Clinical Diagnosis of Atypical Pneumonia. J Microbiol. Immunol. Infect. 47, 137–144. doi: 10.1016/j.jmii.2013.03.015
Chen, J., Li, X., Wang, W., Jia, Y., Lin, F., Xu, J. (2019). The Prevalence of Respiratory Pathogens in Adults With Community-Acquired Pneumonia in an Outpatient Cohort. Infect. Drug Resist. 12, 2335–2341. doi: 10.2147/IDR.S213296
Chen, Z., Tong, L., Zhou, Y., Hua, C., Wang, W., Fu, J., et al. (2020). Childhood COVID-19: A Multicentre Retrospective Study. Clin. Microbiol. Infect. 26 (9), 1260.e1–1260.e4. doi: 10.1016/j.cmi.2020.06.015
Chen, Q., Rodewald, L., Lai, S., Gao, G. F. (2021). Rapid and Sustained Containment of Covid-19 Is Achievable and Worthwhile: Implications for Pandemic Response. BMJ 375, e066169. doi: 10.1136/BMJ-2021-066169
De Luigi, G., Zgraggen, L., Kottanattu, L., Simonetti, G. D., Terraneo, L., Vanoni, F., et al. (2021). Skin and Mucous Membrane Eruptions Associated With Chlamydophila Pneumoniae Respiratory Infections: Literature Review. Dermatology 237 (2), 230–235. doi: 10.1159/000506460
Del Valle-Mendoza, J., Orellana-Peralta, F., Marcelo-Rodríguez, A., Verne, E., Esquivel-Vizcarra, M., Silva-Caso, W., et al. (2017). High Prevalence of Mycoplasma Pneumoniae and Chlamydia Pneumoniae in Children With Acute Respiratory Infections From Lima, Peru. PloS One 12 (1), e0170787. doi: 10.1371/journal.pone.0170787
Fricke, L. M., Glöckner, S., Dreier, M., Lange, B. (2021). Impact of Non-Pharmaceutical Interventions Targeted at COVID-19 Pandemic on Influenza Burden - A Systematic Review. J. Infect. 82 (1), 1–35. doi: 10.1016/j.jinf.2020.11.039
Gao, L. W., Yin, J., Hu, Y., Liu, X. Y., Feng, X. L., He, J. X., et al. (2019). The Epidemiology of Paediatric Mycoplasma Pneumoniae Pneumonia in North China: 2006 to 2016. Epidemiol. Infect. 147, e192. doi: 10.1017/S0950268819000839
Gao, C. H., Ji, B. K., Han, C., Wang, M. S. (2019). Comparison of Enzyme-Linked Immunosorbent Assay With Indirect Immunofluorescence Assay for the Diagnosis of Mycoplasma Pneumoniae Infection. J. Clin. Lab. Anal. 33 (2), e22677. doi: 10.1002/jcla.22677
Ishimaru, N., Suzuki, S., Shimokawa, T., Akashi, Y., Takeuchi, Y., Ueda, A., et al. (2021). Predicting Mycoplasma Pneumoniae and Chlamydophila Pneumoniae in Community-Acquired Pneumonia (CAP) Pneumonia: Epidemiological Study of Respiratory Tract Infection Using Multiplex PCR Assays. Intern. Emerg. Med. 16 (8), 2129–2137. doi: 10.1007/s11739-021-02744-6
Jain, S., Williams, D. J., Arnold, S. R., Ampofo, K., Bramley, A. M., Reed, C., et al. (2015). Community-Acquired Pneumonia Requiring Hospitalization Among US Children. New Engl. J. Med. 372 (9), 835–845. doi: 10.1056/NEJMoa1405870
Jiang, Q., Yang, F., Peng, Y., Dong, X., Ge, Y. (2020). Epidemiology and Molecular Identification of Mycoplasma Pneumoniae Associated With Respiratory Infections in Zhejiang Province, China, 2008-2017. J. Clin. Lab. Anal. 34 (11), e23460. doi: 10.1002/jcla.23460
Ju, J. T. J., Boisvert, L. N., Zuo, Y. Y. (2021). Face Masks Against COVID-19: Standards, Efficacy, Testing and Decontamination Methods. Adv. Colloid Interface Sci. 292, 102435. doi: 10.1016/j.cis.2021.102435
Kucharski, A. J., Klepac, P., Conlan, A. J. K., Kissler, S. M., Tang, M. L., Fry, H., et al. (2020). Effectiveness of Isolation, Testing, Contact Tracing, and Physical Distancing on Reducing Transmission of SARS-Cov-2 in Different Settings: A Mathematical Modelling Study. Lancet Infect. Dis. 20 (10), 1151–1160. doi: 10.1016/S1473-3099(20)30457-6
Kumar, S., Kashyap, B., Kumar, S., Kapoor, S. (2020). Diagnostic Utility of Serology and Polymerase Chain Reaction for Detection of Mycoplasma Pneumoniae and Chlamydophila Pneumoniae in Paediatric Community-Acquired Lower Respiratory Tract Infections. Indian J. Med. Microbiol. 38 (2), 152–156. doi: 10.4103/ijmm.IJMM_20_145
Kurkela, S. M., Puolakkainen, M., Hokynar, K., Nieminen, T., Saxen, H., Mannonen, L. (2019). Mycoplasma Pneumoniae Outbreak, Southeastern Finland, 2017-2018: Molecular Epidemiology and Laboratory Diagnostic Lessons. Eur. J. Clin. Microbiol. Infect. Dis. 38, 1867–71. doi: 10.1007/s10096-019-03619-7
Lansbury, L., Lim, B., Baskaran, V., Lim, W. S. (2020). Co-Infections in People With COVID-19: A Systematic Review and Meta-Analysis. J. Infection 81 (2), 266–275. doi: 10.1016/j.jinf.2020.05.046
Li, Z., Chen, Q., Feng, L., Rodewald, L., Xia, Y., Yu, H., et al. (2020). Active Case Finding With Case Management: The Key to Tackling the COVID-19 Pandemic. Lancet 396 (10243), 63–70. doi: 10.1016/S0140-6736(20)31278-2
Li, Z. J., Zhang, H. Y., Ren, L. L., Lu, Q. B., Ren, X., Zhang, C. H., et al. (2021). Etiological and Epidemiological Features of Acute Respiratory Infections in China. Nat. Commun. 12 (1), 5026. doi: 10.1038/s41467-021-25120-6
Liu, J., Wang, M., Zhao, Z., Lin, X., Zhang, P., Yue, Q., et al. (2020). Viral and Bacterial Coinfection Among Hospitalized Children With Respiratory Tract Infections. Am. J. Infect. Control 48 (10), 1231–1236. doi: 10.1016/j.ajic.2020.01.013
Luo, M., Gong, C., Luo, Q., Li, A. H., Wang, X., Li, M. Z., et al. (2021). [Epidemiological Characteristics of Chlamydia Pneumoniae in Cases With Acute Respiratory Infection in Beijing, 2015-2019]. Zhonghua Liu Xing Bing Xue Za Zhi 42 (8), 1466–1474. doi: 10.3760/cma.j.cn112338-20210522-00421
Mallah, S. I., Ghorab, O. K., Al-Salmi, S., Abdellatif, O. S., Tharmaratnam, T., Iskandar, M. A., et al. (2021). COVID-19: Breaking Down a Global Health Crisis. Ann. Clin. Microbiol. Antimicrob. 20 (1), 35. doi: 10.1186/s12941-021-00438-7
McCracken, G. H., Jr. (2001). Clinical Practice Guidelines for the Diagnosis and Treatment of Respiratory Tract Infections. Am. J. Manag Care 7 (6 Suppl), S183–S191.
Meyer Sauteur, P. M., Unger, W. W., Nadal, D., Berger, C., Vink, C., van Rossum, A. M., et al. (2016). Infection With and Carriage of Mycoplasma Pneumoniae in Children. Front. Microbiol. 7, 329. doi: 10.3389/fmicb.2016.00329
National Health Commission Of The People’s Republic Of, C. (2020). Protocol for Prevention and Control of COVID-19 (Edition 6). China CDC Wkly 2 (19), 321–326. doi: 10.46234/ccdcw2020.082
Nilsson, A. C., Bjorkman, P., Persson, K. (2008). Polymerase Chain Reaction Is Superior to Serology for the Diagnosis of Acute Mycoplasma Pneumoniae Infection and Reveals a High Rate of Persistent Infection. BMC Microbiol. 8, 93. doi: 10.1186/1471-2180-8-93
Ning, G., Wang, X., Wu, D., Yin, Z., Li, Y., Wang, H., et al. (2017). The Etiology of Community-Acquired Pneumonia Among Children Under 5 Years of Age in Mainland China, 2001-2015: A Systematic Review. Hum. Vaccin Immunother. 13 (11), 2742–2750. doi: 10.1080/21645515.2017.1371381
Oishi, T., Fukuda, T. Y., Wakabayashi, S., Kono, M., Ono, S., Kato, A., et al. (2020). Low Prevalence of Chlamydia Pneumoniae Infections During the Mycoplasma Pneumoniae Epidemic Season: Results of Nationwide Surveillance in Japan. J. Infect. Chemother. 26 (11), 1116–1121. doi: 10.1016/j.jiac.2020.04.015
Oliva, A., Siccardi, G., Migliarini, A., Cancelli, F., Carnevalini, M., D'Andria, M., et al. (2020). Co-Infection of SARS-Cov-2 With Chlamydia or Mycoplasma Pneumoniae: A Case Series and Review of the Literature. Infection 48 (6), 871–877. doi: 10.1007/s15010-020-01483-8
Schildgen, O., Søndergaard, M. J., Friis, M. B., Hansen, D. S., Jørgensen, I. M. (2018). Clinical Manifestations in Infants and Children With Mycoplasma Pneumoniae Infection. PloS One 13 (4), e0195288. doi: 10.1371/journal.pone.0195288
Simoni, C., Camozzi, P., Fare, P. B., Bianchetti, M. G., Kottanattu, L., Lava, S. A. G., et al. (2020). Myositis and Acute Kidney Injury in Bacterial Atypical Pneumonia: Systematic Literature Review. J. Infect. Public Health 13 (12), 2020–2024. doi: 10.1016/j.jiph.2020.10.007
Terraneo, L., Lava, S. A., Camozzi, P., Zgraggen, L., Simonetti, G. D., Bianchetti, M. G., et al. (2015). Unusual Eruptions Associated With Mycoplasma Pneumoniae Respiratory Infections: Review of the Literature. Dermatology 231 (2), 152–157. doi: 10.1159/000430809
Tian, D. D., Jiang, R., Chen, X. J., Ye, Q. (2017). Meteorological Factors on the Incidence of MP and RSV Pneumonia in Children. PloS One 12 (3), e0173409. doi: 10.1371/journal.pone.0173409
Wright, D. N., Bailey, G. D., Goldberg, L. J. (1969). Effect of Temperature on Survival of Airborne Mycoplasma Pneumoniae. J. Bacteriol 99 (2), 491–495. doi: 10.1128/jb.99.2.491-495.1969
Ye, Q., Liu, H. (2021). Impact of Non-Pharmaceutical Interventions During the COVID-19 Pandemic on Common Childhood Respiratory Viruses – A Epidemiological Study Based on Hospital Data. Microbes Infection 24, 104911. doi: 10.1016/j.micinf.2021.104911
Zhang, Y., Quigley, A., Wang, Q., MacIntyre, C. R. (2021a). Non-Pharmaceutical Interventions During the Roll Out of Covid-19 Vaccines. BMJ 375, n2314. doi: 10.1136/bmj.n2314
Zhang, Y., Huang, Y., Ai, T., Luo, J., Liu, H. (2021b). Effect of COVID-19 on Childhood Mycoplasma Pneumoniae Infection in Chengdu, China. BMC Pediatr. 21 (1), 202. doi: 10.1186/s12887-021-02679-z
Keywords: COVID-19, Mycoplasma pneumoniae, Chlamydia pneumoniae, acute respiratory tract infection, epidemiological study
Citation: Cai F, Shou X and Ye Q (2022) Epidemiological Study on Mycoplasma pneumoniae and Chlamydia pneumoniae Infection of Hospitalized Children in a Single Center During the COVID-19 Pandemic. Front. Cell. Infect. Microbiol. 12:843463. doi: 10.3389/fcimb.2022.843463
Received: 26 December 2021; Accepted: 02 March 2022;
Published: 21 March 2022.
Edited by:
Max Maurin, Université Grenoble Alpes, FranceCopyright © 2022 Cai, Shou and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Ye, qingye@zju.edu.cn
 Fengqing Cai
Fengqing Cai  Xinyi Shou
Xinyi Shou Qing Ye
Qing Ye