Diagnostic, Prognostic, and Therapeutic Roles of Gut Microbiota in COVID-19: A Comprehensive Systematic Review
- 1Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Biomedical Sciences, University of Sassari, Sassari, Italy
- 3Struttura Complessa (SC), Microbiologia e Virologia, Azienda Ospedaliera Universitaria, Sassari, Italy
- 4Clinician Scientist of Dental Materials and Restorative Dentistry, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 5Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 6Division of Pulmonary and Critical Care, College of Medicine-Jacksonville, University of Florida, Jacksonville, FL, United States
Introduction: The Coronavirus Disease 2019 (COVID-19) pandemic caused by Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) emerged in late December 2019. Considering the important role of gut microbiota in maturation, regulation, and induction of the immune system and subsequent inflammatory processes, it seems that evaluating the composition of gut microbiota in COVID-19 patients compared with healthy individuals may have potential value as a diagnostic and/or prognostic biomarker for the disease. Also, therapeutic interventions affecting gut microbial flora may open new horizons in the treatment of COVID-19 patients and accelerating their recovery.
Methods: A systematic search was conducted for relevant studies published from December 2019 to December 2021 using Pubmed/Medline, Embase, and Scopus. Articles containing the following keywords in titles or abstracts were selected: “SARS-CoV-2” or “COVID-19” or “Coronavirus Disease 19” and “gastrointestinal microbes” or “dysbiosis” or “gut microbiota” or “gut bacteria” or “gut microbes” or “gastrointestinal microbiota”.
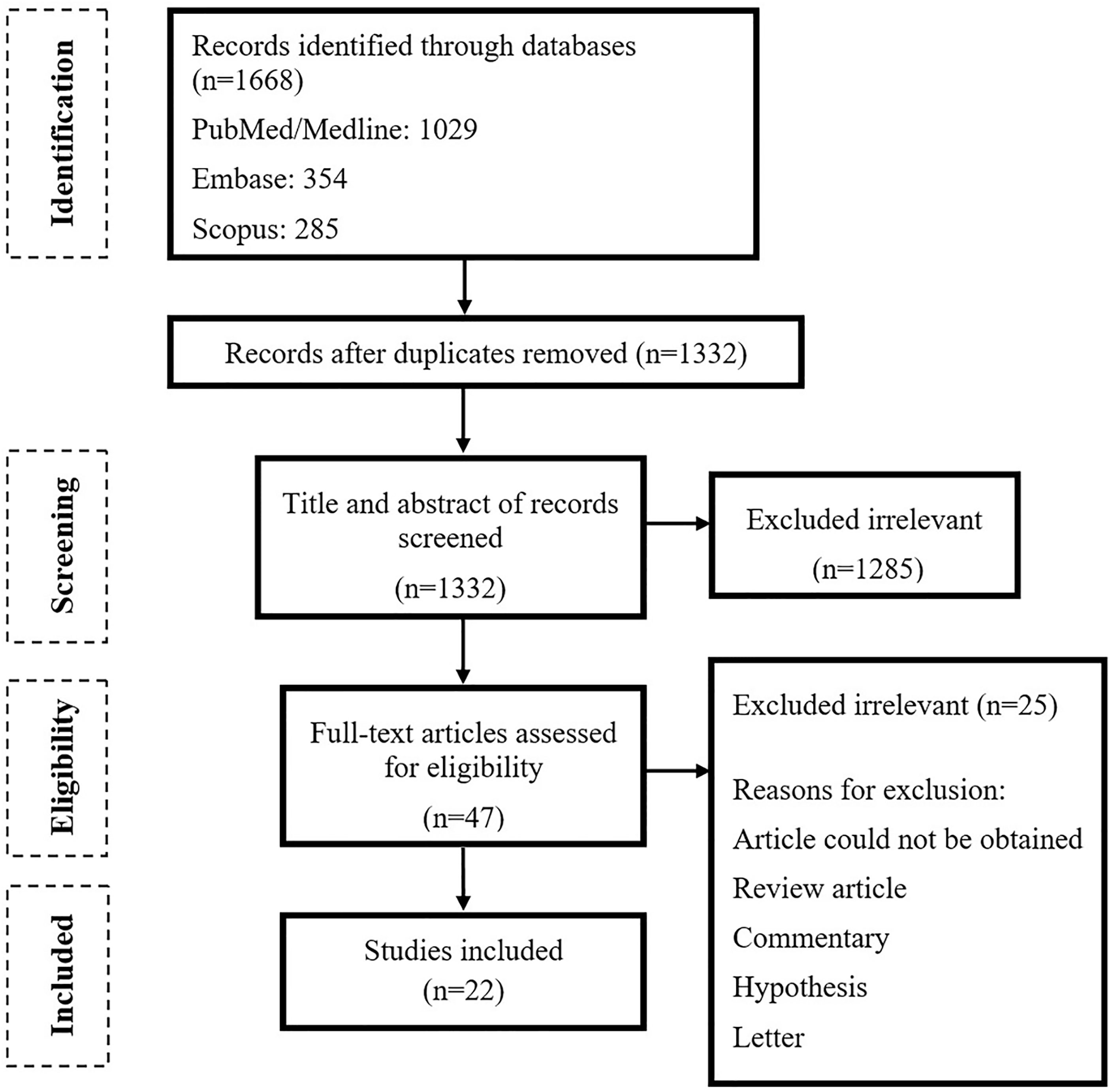
Results: Out of 1,668 studies, 22 articles fulfilled the inclusion criteria and a total of 1,255 confirmed COVID-19 patients were examined. All included studies showed a significant association between COVID-19 and gut microbiota dysbiosis. The most alteration in bacterial composition of COVID-19 patients was depletion in genera Ruminococcus, Alistipes, Eubacterium, Bifidobacterium, Faecalibacterium, Roseburia, Fusicathenibacter, and Blautia and enrichment of Eggerthella, Bacteroides, Actinomyces, Clostridium, Streptococcus, Rothia, and Collinsella. Also, some gut microbiome alterations were associated with COVID-19 severity and poor prognosis including the increment of Bacteroides, Parabacteroides, Clostridium, Bifidobacterium, Ruminococcus, Campylobacter, Rothia, Corynebacterium, Megasphaera, Enterococcus, and Aspergillus spp. and the decrement of Roseburia, Eubacterium, Lachnospira, Faecalibacterium, and the Firmicutes/Bacteroidetes ratio.
Conclusion: Our study showed a significant change of gut microbiome composition in COVID-19 patients compared with healthy individuals. This great extent of impact has proposed the gut microbiota as a potential diagnostic, prognostic, and therapeutic strategy for COVID-19. There is much evidence about this issue, and it is expected to be increased in near future.
Introduction
A pandemic caused by Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) emerged in late December 2019 (Zhu et al., 2020). The World Health Organization (WHO) named the consequent disease as Coronavirus Disease 2019 (COVID-19) and declared it as a global emergency due to the serious public health effects (Jamshidi et al., 2021). According to the report of the WHO, until February 1, 2022, there have been about 376 million confirmed cases and about 5.6 million deaths due to COVID-19 around the world.
The angiotensin-converting enzyme 2 (ACE2) receptor is a known SARS-CoV-2 receptor for entering host cells (Li et al., 2003; Zhou et al., 2020). This receptor is detected in various cells of the body such as the respiratory, digestive, renal, and skin epithelium, suggesting that each of these organs could be a potential target for the virus (Jamshidi et al., 2021; Xue et al., 2021). Moreover, virus RNA and viral particles have been identified in the fecal sample of COVID-19 patients, which may indicate the possibility of virus replication and activity in the human intestine (Gu et al., 2020a; Lamers et al., 2020; Xu et al., 2020).
Gut microbiota plays a well-known role in regulating immune system responses (Donaldson et al., 2016;; Schirmer et al., 2016). Recent studies indicate the role of gut dysbiosis in the pathogenesis of various diseases such as inflammatory bowel disease, type 1 and type 2 diabetes, and celiac disease, as well as chronic respiratory diseases such as asthma, COPD, and cystic fibrosis (Jamshidi et al., 2019; Enaud et al., 2020).
Bacteria in the human intestinal flora appear to affect the respiratory system and lungs (especially the lung microbiota) by producing metabolites, endotoxins, cytokines, and intestinal hormones reaching the bloodstream, which is called the gut–lung axis (Budden et al., 2017; Dang and Marsland, 2019; Zhang et al., 2020).
On the other hand, there is evidence of the role of gut dysbiosis in the severity and prognosis of bacterial (e.g., Streptococcus pneumonia, Klebsiella pneumonia, Pseudomonas aeruginosa, Mycobacterium tuberculosis) and viral (e.g., H1N1 influenza) respiratory infectious diseases in animal models (Ichinohe et al., 2011; Fagundes et al., 2012; Fox et al., 2012; Brown et al., 2017). The use of broad-spectrum antibiotics that target the gut microbiota has led to a poor prognosis in mouse models with infectious lung diseases (Enaud et al., 2020).
Considering the important role of gut microbiota in maturation, regulation, and induction of the immune system and subsequent inflammatory processes, it seems that evaluating the composition of gut microbiota in COVID-19 patients compared with healthy individuals may have potential value as a diagnostic and/or prognostic biomarker of the disease. Also, therapeutic interventions affecting gut microbial flora may open new horizons in the treatment of COVID-19 patients and accelerating their recovery.
Methods
This review conforms to the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement (Moher et al., 2009).
Search Strategy and Selection Criteria
To investigate the diagnostic, prognostic, and therapeutic role of the gut microbiota composition in COVID-19, a systematic search was conducted for relevant studies published from December 2019 to December 2021 using Pubmed/Medline, Embase, and Scopus.
Articles containing the following keywords in titles or abstracts were selected: “SARS-CoV-2” or “COVID-19” or “Coronavirus Disease 19” and “gastrointestinal microbes” or “dysbiosis” or “gut microbiota” or “gut bacteria” or “gut microbes” or “gastrointestinal microbiota”. Only studies included if they contained data about the gut microbiota composition in COVID-19 patients. There were no language restrictions. Review articles, duplicate publications, letters, commentary, animal studies, and articles with no relevant data were excluded from the analysis. Two authors (MA and FV) independently screened the articles by title and abstract. Full-text screening was conducted by two other authors independently (AT and YF). In each step, contrarieties were discussed with a third reviewer (PJ).
Data Extraction
A data extraction form designed by two authors (PJ and MJN) and, finally, selected data were extracted from the full text of eligible publications by PJ, YF, AT, MA, and FV. The following data were extracted for further analysis: first author’s name, year of publication, country where the study was executed, type of study, study population, mean age, gender, COVID-19 severity of the cases, comorbidity(ies), microbiota analysis technique, intestinal microbiota alterations, biochemical and immunological alterations, and studied value of gut microbiota alterations in COVID-19. The data were jointly reconciled, and disagreements were discussed and resolved by review authors (PJ, MJN).
Quality Assessment
The critical appraisal checklist for case reports provided by the Joanna Briggs Institute (JBI) was used to perform a quality assessment of the studies (Institute, 2021).
Results
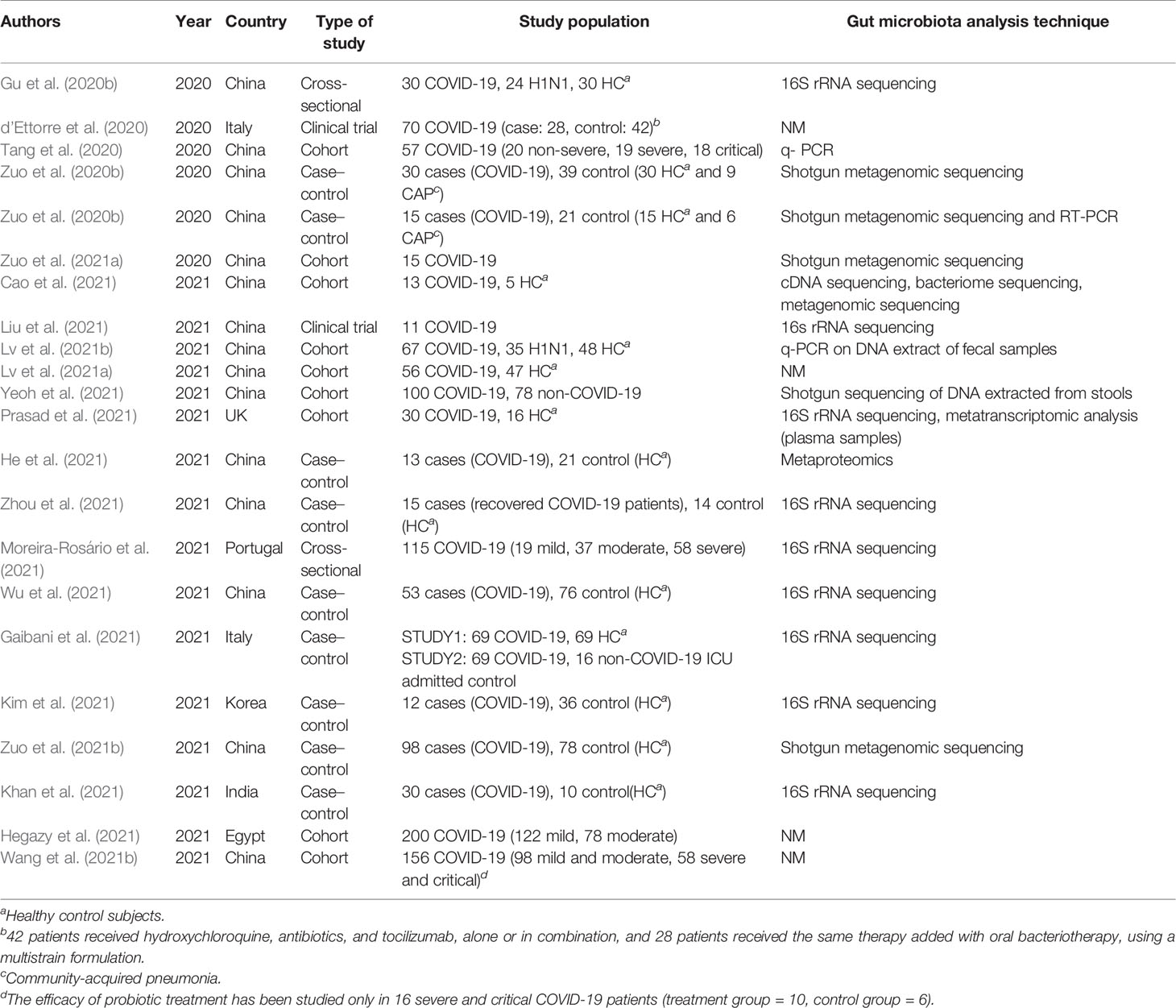
As shown in Figure 1, the primary search resulted in 1,668 relevant articles, of which 47 articles were selected after title and abstract screening. Following the full-text screening, 22 articles fulfilled the inclusion criteria. Most of the studies were case–control (n = 9) followed by cohort (n = 9), clinical trial (n = 2), and cross-sectional (n = 2) studies. Fifteen of the studies were executed in China, 2 in Italy and 1 in UK, Portugal, India, Egypt, and Korea (Table 1).
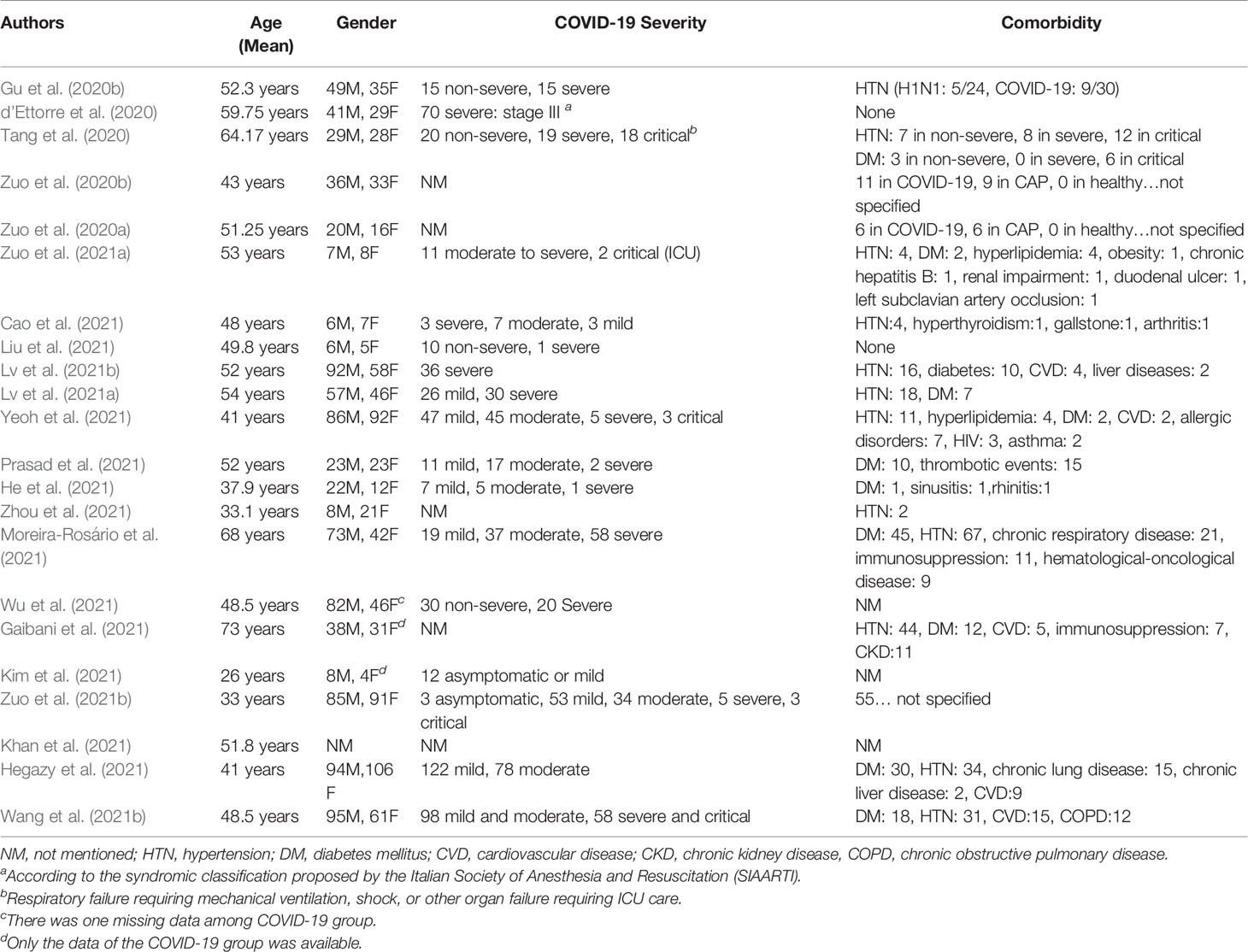
A total of 1,255 confirmed COVID-19 patients were examined in 22 included articles (Table 1). Among the COVID-19 patients whose comorbidity was mentioned by the authors, the most common ones were hypertension (32.8%) and diabetes mellitus (17.8%). Other reported comorbidities were chronic respiratory disease (6.9%), cardiovascular disease (3%), immunosuppression (2.9%), dyslipidemia (2.3%), thrombotic events (2%), and renal impairment (1.6%). See Table 2.
Ten studies assessed gut microbiota composition alteration by fecal samples; one study used plasma samples, and it was not mentioned by the rest. The most commonly used techniques in these studies for detection and assessment of gut microbiota were 16s rRNA sequencing and shotgun metagenomic sequencing analysis (Table 1).
Gut Microbiome Dysbiosis of COVID-19 Patients
All included studies showed a significant association between COVID-19 and gut microbiota dysbiosis (Table 3). The most alteration in the bacterial composition of COVID-19 patients was depletion in genera Ruminococcus, Alistipes, Eubacterium, Bifidobacterium, Faecalibacterium, Roseburia, Fusicathenibacter, and Blautia and enrichment of Eggerthella, Bacteroides, Actinomyces, Clostridium, Streptococcus, Rotia, and Collinsella. Details are shown in Table 4.
Three articles surveyed the gut mycobiota alterations, and different results have been reported for different species of the same genus. About the Candida spp., an increase in Candida albicans and a decrease in Candida glabrata and Candida parapsilosis were mentioned. In regard to Aspergillus spp., enrichment of Aspergillus flavus and Aspergillus niger and a depletion of Aspergillus rugulosus, Aspergillus tritici, and Aspergillus penicillioides were reported. Also, one study indicated a reduction in seven unclassified species belonging to order Helotiales, Pleosporales, and Sordariales, family Exidiaceae, and genera Microscypha and Emericellopsis in COVID-19 patients (Table 4).
According to all gut microbiota changes that were mentioned in the reviewed articles, a decrease in phyla Firmicutes and Bacteroidetes and an increase in phylum Actinobacteria among COVID-19 patients were inferred.
Association Between Gut Microbiota Composition and COVID-19 Severity
A few studies indicated the role of gut microbiome in COVID-19 severity (Table 4). In severe COVID-19 cases, Bacteroides spp., Parabacteroides spp., Clostridium spp., Bifidobacterium spp., Ruminococcus spp., Campylobacter spp., Rothia spp., Corynebacterium spp., Megasphaera spp., Enterococcus spp., and Aspergillus spp. were increased and Roseburia spp., Eubacterium spp., Lachnospira spp., Faecalibacterium spp., and Firmicutes/Bacteroidetes ratio were decreased significantly. In subjects with mild disease the observed significant change was in the enrichment of Eubacterium spp.
The alteration of the gut virome composition in severe COVID-19 cases was mentioned in two studies. In severe cases, fourteen Microviridae phages, one Inoviridae phage, one Podoviridae phage, and one unclassified virus were increased and plant-derived RNA virus, pepper chlorotic spot virus (PCSV), Myxococcus phage, Rheinheimera phage, Microcystis virus, Bacteroides phage, Murmansk poxvirus, Saudi moumouvirus, Sphaerotilus phage, Tomelloso virus, and Ruegeria phage were decreased significantly. See Table 4.
One study evaluated the associations between gut microbiota disturbance and SARS-CoV-2 viral loads and revealed that Prevotella copri and Eubacterium dolichum were positively correlated and Streptococcus anginosus, Dialister spp., Alistipes spp., Ruminococcus spp., Clostridium citroniae, Bifidobacterium spp., Haemophilus spp., and Haemophilus parainfluenzae were negatively correlated with the viral load of SARS-CoV-2.
Biochemical and Immunologic Modifications in Relation to Gut Microbiota Alternations in COVID-19 Patients
In most studies, compared with healthy controls, COVID-19 patients had significantly higher levels of interleukin (IL)-2, IL-4, IL-6, IL-10, tumor necrosis factor (TNF)-α, and C-reactive protein (CRP) and lower lymphocyte counts.
According to one study, a positive correlation between Bifidobacterium spp. and prothrombin time (PT) and lactate dehydrogenase (LDH) was shown. Also, a negative correlation was reported between Atopobium spp. and D-dimer, Bacteroides spp. and LDH and creatine kinase (CK) level, Clostridium butyricum, and CRP and neutrophil count, and Faecalibacterium prausnitzii and CRP in critical COVID-19 patients. One study showed a specific relation between some genus of gut microbiota and immunological and biochemical modifications in critical and severe COVID-19 patients. In severe patients, Faecalibacterium prausnitzii and Clostridium leptum had a positive correlation with neutrophil count as well as Eubacterium rectale with IL-6 and Enterobacteriaceae with AST.
Another study indicated the specific relation of some species of gut microbiome and immune cells as the following: Bacteroides ovatus, Lachnospiraceae bacterium, and Eubacterium ventriosum had a positive correlation with CD4 and CD8 lymphocytes and other T-cells, in contrast to Bifidobacterium animalis and Escherichia spp. On the other hand, Faecalibacterium prausnitzii had a positive correlation with NK cells and Coprobacillus spp., Clostridium ramosum, and Clostridium symbiosum had a negative correlation with them.
Studied Value of Gut Microbiome in COVID-19
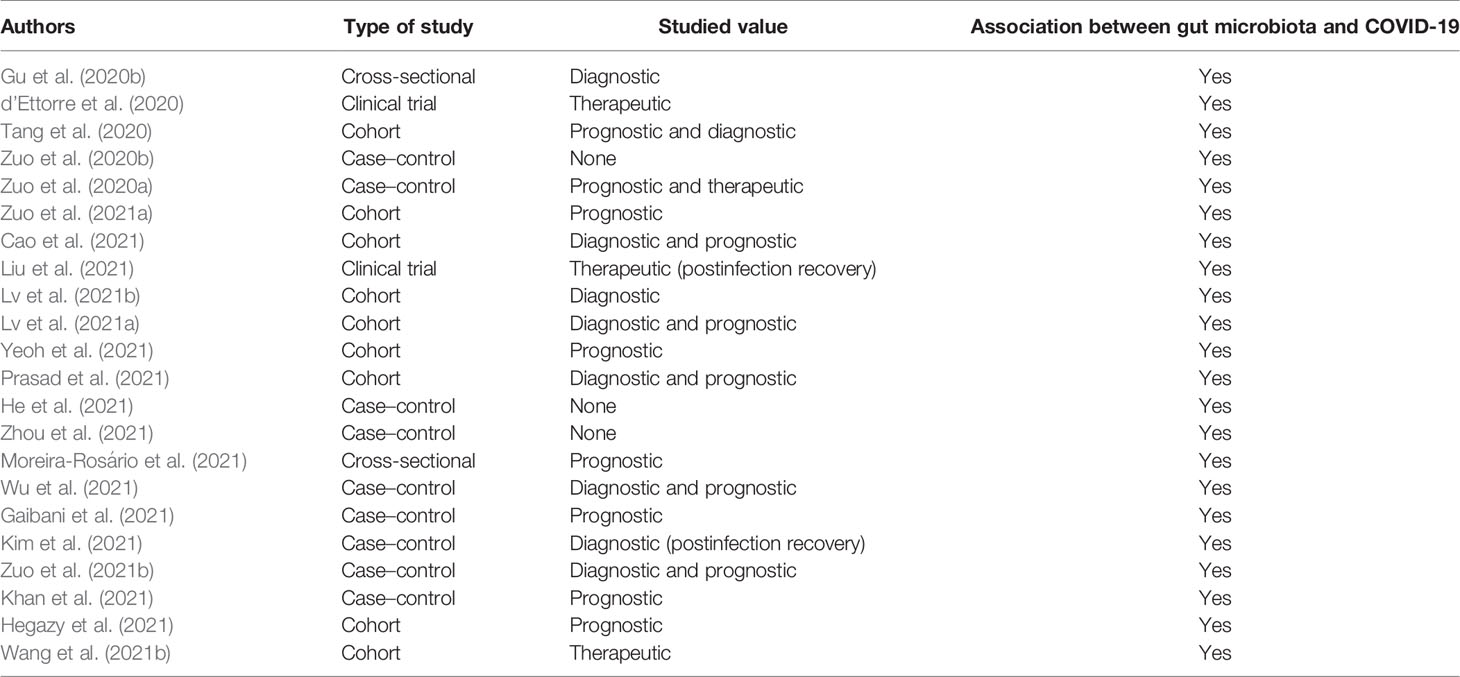
All of the included studies showed a correlation between intestinal microbiota and COVID-19, and they studied the correlation in different aspects as in the following.
Four studies suggested that microbiota could have therapeutic properties with reducing gastrointestinal (GI) symptoms. Streptococcus, Lactobacillus, and Bifidobacterium were the most common bacterial genera interventions used so far. Nine articles demonstrated intestinal microbiota modifications in infected cases with COVID-19 in which two of them confirmed the value of specified gut microbiota as a diagnostic tool and one of them studied gut microbiota changes during recovery time. Lachnospiraceae are a large family including Fusicathenibacter, Eubacterium hallii group, and Roseburia, and the Ruminococcaceae family including Faecalibacterium prausnitzii and Ruminococcus as well as Clostridium spp., Bacteroides spp., Lactobacillus spp., Rothia spp., Actinomyces spp., Lactobacillus spp., and Streptococcus spp. were the most common bacteria with diagnostic value. Thirteen studies demonstrated a relationship between gut microbiota changes and the intensity and prognosis of COVID-19. Eubacterium, Faecalibacterium, Ruminococcus, Bacteroides, Clostridium, Lactobacillus, Bifidobacterium, and Roseburia were the most notable bacterial genera with prognostic values. Details are shown in Table 3.
Discussion
The interaction between gut microbiota and viral respiratory diseases such as COVID-19 is a complex, bilateral, and dynamic association. The current study emphasizes the role of the gut–lung axis (GLA) in the pathogenesis of COVID-19. One of the important aspects of GLA is the impact of gut microbiota on the supply and maintenance of the lung immune system, and its correlation with respiratory diseases and infections (He et al., 2017; Dang and Marsland, 2019; Ahmadi Badi et al., 2021; Allali et al., 2021). The gut and lung microbiome are closely related in health or disease conditions (Dickson and Huffnagle, 2015; Enaud et al., 2020; Ahmadi Badi et al., 2021). SARS-CoV-2 may cause a dysbiosis in the lung microbiota to increase the population of inflammatory bacteria such as Klebsiella oxytoca and Rothia mucilaginosa which is associated with acute respiratory distress syndrome (Aktaş and Aslim, 2020; Han et al., 2020; van der Lelie and Taghavi, 2020; Battaglini et al., 2021; Chattopadhyay and Shankar, 2021). The high levels of inflammatory cytokine productions during SARS-CoV-2 invasion interfere with gut mucosal integrity and increasing risk of bacterial disposition to the bloodstream (Chattopadhyay and Shankar, 2021; Prasad et al., 2021).
Based on our findings, gut microbiota in patients with SARS-CoV-2 is significantly affected, possibly due to systemic inflammatory response. Although the underlying mechanism for the observed dysbiosis is unclear, it might happen via downregulation of ACE2 expression that alleviates the intestinal absorption of tryptophan leading to decreased secretion of antimicrobial peptides and changes the composition of gut microbiota (He et al., 2020). In a healthy individual, intact bacteria and their fragments or metabolites such as des-amino-tyrosine and short-chain fatty acids (SCFAs) pass across the intestinal barrier via the mesenteric lymphatic system, reach the lung, and activate the innate immune system by the production of cytokines like type 1 interferon (IFN1) (Antunes et al., 2019). In the current study, Faecalibacterium prausnitzii and Clostridium leptum have a positive correlation with neutrophil counts in COVID-19 patients and a negative correlation with Clostridium butyricum. In addition to the innate immunity, gut microbiota improves the function of CD8+ T-cell effectors (Trompette et al., 2018). There is also evidence that few bacterial species such as Bacteroides ovatus, Lachnospiraceae bacterium 5_1_63FAA, and Eubacterium ventriosum have an anti-inflammatory property in CD4+ and CD8+ T cells (Cao et al., 2021). Natural killer (NK) cells and B cells are also affected by gut microbiota; Coprobacillus spp., Clostridium ramosum, and Clostridium symbiosum are negatively associated with NK cell activity and Bacteroides uniformis, Faecalibacterium prausnitzii, and Subdoligranulum are positively correlated with B cells (Cao et al., 2021).
We found that the dysbiosis of gut microbiota may be a determinant factor in the clinical severity of COVID-19. Increase of the dominant Enterococcus and reduction of Ruminococcaceae and Lachnospiraceae are reported in severe cases of COVID-19 who were admitted to the medical intensive unit (MICU) (Gaibani et al., 2021).
Diversity of gut microbiota reduces in patients with COVID-19, which is associated with pro-inflammatory reaction and increased risk of opportunistic infections. GI symptoms of COVID-19 are reported to be strongly affected by gut microbial composition (Din et al., 2021; Katz-Agranov and Zandman-Goddard, 2021). The expression of the ACE-2 receptor on the surface of small intestine epithelial cells has been significantly associated with the extent of GI symptoms and fecal viral shedding during the course of disease (D’Amico et al., 2020; Patel et al., 2020; Wu et al., 2020; Katz-Agranov and Zandman-Goddard, 2021; Suárez-Fariñas et al., 2021). An increased expression of ACE-2 receptor in COVID-19 may occur due to a dominance of Coprobacillus in gut microbiota (Geva-Zatorsky et al., 2017; Enaud et al., 2020; Zuo et al., 2020a; Massip Copiz, 2021; Rajput et al., 2021; Walton et al., 2021). It is also noteworthy to imply that the expression of ACE-2 on luminal cells may be a determinant factor in microbial composition as the ACE-2 receptor plays some role in amino-acid absorption (Harmer et al., 2002; Dang and Marsland, 2019).
Eggerthella is another genus of bacteria which significantly increases in patients with COVID-19 (d’Ettorre et al., 2020; Cao et al., 2021). Eggerthella may induce colitis via abnormal activation of Th17 in patients with inflammatory diseases. It can interfere with gut integrity and make the patient more susceptible to pathogen invasion including SARS-CoV-2 (Alexander et al., 2019; Katz-Agranov and Zandman-Goddard, 2021).
There is a dominancy of genus Clostridium in patients with COVID-19 (Gu et al., 2020b; Zuo et al., 2020a; Cao et al., 2021; Yamamoto et al., 2021). The increase in Clostridium ramosum and Clostridium hathewayi is associated with the disease severity which may be a risk factor of acute portal vein thrombosis (Zuo et al., 2020; Rokkam et al., 2021). Clostridium difficile may complicate COVID-19 and worsen the GI symptoms (Ferreira et al., 2020; Oba et al., 2020; Sandhu et al., 2020; Khanna and Kraft, 2021). Two butyrate-producing members of this genus, Clostridium butyricum and Clostridium leptum, are decreased in patients with COVID-19 (Tang et al., 2020).
Streptococcus is another important bacterial genus which increases in COVID-19 (d’Ettorre et al., 2020; Donati Zeppa et al., 2020; Gu et al., 2020b; Zuo et al., 2021a). The abundance of Streptococcus is also an indicator of the extent of opportunistic bacterial invasion (Weiser et al., 2018; Tao et al., 2020). Streptococcus abundance is associated with more expressions of IL-18, TNF-α, and IFN-γ and other inflammatory cytokines worsening clinical outcomes (Tao et al., 2020; van der Lelie and Taghavi, 2020; Chhibber-Goel et al., 2021). An altered gut integrity affected by dysbiosis and inflammatory cytokines seems to be the main cause of increase in Streptococcus abundance in COVID-19 (Donati Zeppa et al., 2020). Streptococcus also affects the lung microbiome with proinflammatory activity (Kyo et al., 2019; Yamamoto et al., 2021).
Genus Rothia dominancy increases in patients with COVID-19 (Gu et al., 2020b; Lv et al., 2021a). This genus seems to be associated with inflammatory lung injuries (Han et al., 2020; Chattopadhyay and Shankar, 2021).
The genus Collinsella is an opportunistic pathogenic genus which is widely found in the gut of patients with COVID-19 especially in severe cases and higher infectivity status (Chhibber-Goel et al., 2021; Liu et al., 2021; Massip Copiz, 2021; Rajput et al., 2021; Zuo et al., 2021a). Collinsella aerofaciens abundance altered with the gut mucosal integrity and production of inflammatory mediators such as IL-17, CXCL1, and CXCL5 from the luminal cells (Kalinkovich and Livshits, 2019).
The alteration of genus Parabacteroides in COVID-19 is an area of controversy (Tang et al., 2020; Tao et al., 2020; Chhibber-Goel et al., 2021; Zuo et al., 2021a; Yeoh et al., 2021). It has been suggested that higher levels of Parabacteroides in microbiota are associated with better gut mucosal integrity (Venegas et al., 2019; Tang et al., 2020; Chhibber-Goel et al., 2021).
Ruminococcus species such as Ruminococcus gnavus and Ruminococcus torques increase (Cao et al., 2021; Yeoh et al., 2021), and species including Ruminococcus bromii, Ruminococcus obeum, and Ruminococcus sp. 5139BFAA are reduced in patients with COVID-19 (Zuo et al., 2020a; Cao et al., 2021; Lv et al., 2021). Ruminococcus gnavus and Ruminococcus torques are known as proinflammatory bacteria which previously have been shown to be associated with proinflammatory status and higher production of inflammatory mediators (Matsuoka and Kanai, 2015; Hall et al., 2017; Henke et al., 2019; Yeoh et al., 2021). Reduction of Ruminococcus obeum seems to be secondary to the wide use/misuse of antibiotics in the management of COVID-19 patients (Cyprian et al., 2021). Among Alistipes genera, Alistipes_sp_AP11, Alistipes indistinctus, and Alistipes shahii are reduced and Alistipes onderdonkii increased in COVID-19 (Zuo et al., 2020a; Cao et al., 2021). Alistipes onderdonkii is one of the most important sources of short-chain fatty acid (SCFA) production in the gut that helps to the gut homeostasis (Venegas et al., 2019; Tang et al., 2020). Alistipes is also important in preservation of the gut immunity via being involved in tryptophan synthesis pathways (Gao et al., 2018).
Bacteroides alteration is reported in COVID-19, especially in critically ill patients (Tang et al., 2020; Cao et al., 2021; Chattopadhyay and Shankar, 2021; Yeoh et al., 2021; Zuo et al., 2021a). Bacteroides are the most critical commensal bacterial genera in gut whose alterations are associated with several conditions affecting human health and disease (Ley et al., 2006; Turnbaugh et al., 2006; Larsen et al., 2010; Claesson et al., 2012; Yu et al., 2015; Boursier et al., 2016; Salazar et al., 2017; Belizário et al., 2018; O’Toole and Jeffery, 2018; Yildiz et al., 2018; Antosca et al., 2019; Rahayu et al., 2019; Crovesy et al., 2020; Juárez-Fernández et al., 2021). Bacteroides also have immunomodulatory effects, which is mainly mediated by alterations in production of polysaccharide A, IL-6, IL-7, IL-10, dendritic cells, and CD4+ and CD8+ T cells (Abt et al., 2012; Zhang et al., 2018; Jia et al., 2018; Ramakrishna et al., 2019; Alvarez et al., 2020; Gautier et al., 2021). Bacteroides are also associated with reduction of the expression of the ACE-2 receptor (Chattopadhyay and Shankar, 2021). Use of antibiotics seems to result in increase of Bacteroides caccae in a COVID-19 patient. In antibiotic-naive COVID-19 patients, Bacteroides nordii are more common (Chattopadhyay and Shankar, 2021). On the other hand, species such as Bacteroides massiliensis, Bacteroides dorei, Bacteroides thetaiotaomicron, and Bacteroides ovatus decrease in SARS-CoV-2 infection (Zuo et al., 2020a; Cao et al., 2021). Bacteroides dorei itself is a controversial bacterium with both evidence of increase and decrease in COVID-19 patients. This species is associated with IL-6 and IL-8 and downregulation of the ACE-2 receptor (Yoshida et al., 2018; Yeoh et al., 2021).
Bifidobacterium, a major bacterial genus in the gut, reduces by SARS-CoV-2 (Gu et al., 2020b). In patients who received fecal microbial transplant (FMT), a re-expansion of Bifidobacterium in their gut was demonstrated (Liu et al., 2021). Bifidobacterium spp. are well known for their immunomodulatory effects especially on Th17 and in the amelioration of inflammatory process (de Vrese et al., 2005; Wang et al., 2011; Groeger et al., 2013; Jungersen et al., 2014; King et al., 2014; Han et al., 2016; Schiavi et al., 2016; Bozkurt et al., 2019; Bozkurt and Kara, 2020; Tian et al., 2020; Arenas-Padilla et al., 2021; Chen and Vitetta, 2021; Hong et al., 2021; Milner et al., 2021). Bifidobacterium may be considered as a supplemental therapeutic agent for controlling cytokine storm and inflammation in patients with COVID-19 (Bozkurt and Quigley, 2020; Bozkurt and Quigley, 2020a; Schett et al., 2020; Bhushan et al., 2021 Gautier et al., 2021; Mohseni et al., 2021).
Faecalibacterium decreases in COVID-19 and has been related to the severity of disease (Zuo et al., 2020; Gu et al., 2020; Tang et al., 2020; Yamamoto et al., 2021; Lv et al., 2021). Faecalibacterium is a butyrate-producing genus which positively impacts on intestinal mucosal integrity and is also known to have anti-inflammatory effects (Alameddine et al., 2019; Zuo et al., 2020a; Chhibber-Goel et al., 2021). Fecal transplantation significantly increases the abundance of Faecalibacterium in discharged patients with COVID-19 and has been shown to improve the inflammation states (Sokol et al., 2008; van den Munckhof et al., 2018; Venegas et al., 2019; Liu et al., 2021).
Lachnospiraceae, which is known as SCFA-producing bacteria, decreases in patients with COVID-19 (d’Ettorre et al., 2020; Gu et al., 2020b; Zuo et al., 2020a; Cao et al., 2021; Gaibani et al., 2021; Gautier et al., 2021; Zuo et al., 2021a). It may be attributed to common use of azithromycin and other antibiotics in the management of COVID-19 (Segal et al., 2020).
Genus Roseburia is another commensal gut microbiota which decreases in patients with COVID-19 and other viral diseases such as influenza (Wang et al., 2017; Gu et al., 2020b; Cao et al., 2021; Lv et al., 2021a). SCFAs produced by Roseburia maintain mucosal integrity in healthy adults via modulation of inflammatory mediators especially IL-10 (Koh et al., 2016; Zheng et al., 2017; Haak et al., 2018; Gautier et al., 2021). It has been shown previously that butyrate may preserve lung integrity from cytokine-induced injuries in influenza (Chakraborty et al., 2017; Dang and Marsland, 2019). If we assume that it is true in COVID-19, lower levels of Roseburia result in lower levels of butyrate and consequently more extensive lung injuries due to inflammatory processes.
Eubacterium is a genus with immunomodulatory effects which significantly decrease in gut microbiota of patients with COVID-19 (d’Ettorre et al., 2020; Zuo et al., 2020a; Gu et al., 2020b; Cao et al., 2021; Chattopadhyay and Shankar, 2021; Gautier et al., 2021; Lv et al., 2021; Yeoh et al., 2021). Wide use of antibiotics is considered to be associated with reduction of this genus (Zuo et al., 2020a). This genus similar to Roseburia spp. produces butyrate and modulates inflammation in inflammation-mediated injuries (Koh et al., 2016; Zheng et al., 2017; Haak et al., 2018; Gautier et al., 2021).
Fusicatenibacter is another bacterial genus that reduced during the course of COVID-19 (Gu et al., 2020; Chattopadhyay and Shankar, 2021; Lv et al., 2021a). Fusicatenibacter alteration is a very sensitive biomarker during COVID-19. It is proposed to be a diagnostic tool for COVID-19 (Gu et al., 2020b; Segal et al., 2020; Cyprian et al., 2021; Howell et al., 2021). This genus is also negatively correlated with CRP and procalcitonin levels in patients with COVID-19 (Gu et al., 2020b).
Other members of the gut microbiota are viruses and fungi. Although pathogenic gut viruses are known for more than a century, the term “gut virome” is recently introduced (Reyes et al., 2012). Most of the gut virome consists of bacteriophages that can explain the fact that the virome structure is related to gut bacterial composition in both healthy and COVID-19 people (Minot et al., 2011; Cao et al., 2021). There is a bidirectional relationship between gut virome and infectious diseases; bacteriophages have a significant role in protecting against bacterial infections (Wilks and Golovkina, 2012). Gut virome composition might be affected during COVID-19 (Cao et al., 2021). There are limited data on the alterations of commensal viral and fungal populations in the gut during COVID-19 infection.
Lv et al. showed a strong correlation between altered fungal gut microbiome and inflammatory blood biomarkers (Lv et al., 2021b). Further studies focusing on viral and fungal alterations during the COVID-19 are desired.
The gut microbiota alteration in COVID-19 patients should be considered as a dynamic process (d’Ettorre et al., 2020; Liu et al., 2021; Hussain et al., 2021; Zuo et al., 2020a). To the date of revising this manuscript (January 2022), several registered clinical trials are in progress and the results are not provided yet; however, growing evidence supports the effectiveness of microbiota modulatory actions on fastening the recovery of patients with COVID-19 (Chen and Vitetta, 2021; Hussain et al., 2021; Wang et al., 2021a).
Limitations and Suggestions
A few studies have documented the comorbidities of subjects. However, almost all the studies have missed the impact of comorbidities on gut microbiota alterations in COVID-19 patients compared with the healthy control. It has been shown that gut microbiota may change in patients with hypertension, cardiovascular diseases, diabetes mellitus, hyperlipidemia, and thrombotic events (Huynh, 2020; Avery et al., 2021; Kyriakidou et al., 2021; Mineshita et al., 2021). We strongly propose to investigate the effects of underlying comorbidities in gut microbial composition in patients with COVID-19.
Due to the variations in data analysis techniques such as 16S rRNA sequencing, qPCR, and metagenome sequencing, there was a challenge to compare the bacterial taxa across studies. Since there was a fair diversity in geographical distribution of the current studies (most of them are from China), we cannot ignore the effect of diet and genetic predisposing factors like HLA in gut microbiome compositions. Future studies in different countries are required in this regard.
It is important to mention that different levels of p-value significance were reported in reviewed articles; however, in this study we used only statistically significant findings from the included studies.
Further studies with a larger study population, including the range of patients from mild to severe symptoms, involving the patients who are managed out patiently, focusing on the effectiveness of gut microbiota-targeted therapies for prevention and improvement of COVID-19 patients’ symptoms are desired to light up this topic.
Conclusion
Our study showed a significant alteration of gut microbiome composition in patients with COVID-19 compared to healthy individuals. This great extent of impact has proposed the gut microbiota as a potential diagnostic, prognostic, and potentially therapeutic strategy for COVID-19.
Author Contributions
MN and PJ designed the study. MN, YF, AT, PJ, MA, and FV performed the search and data extraction and wrote the first draft of the manuscript. LS, AHSB, and MM reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study was supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
Abt, M. C., Osborne, L. C., Monticelli, L. A., Doering, T. A., Alenghat, T., Sonnenberg, G. F., et al. (2012). Commensal Bacteria Calibrate the Activation Threshold of Innate Antiviral Immunity. Immunity 37 (1), 158–170. doi: 10.1016/j.immuni.2012.04.011
Ahmadi Badi, S., Tarashi, S., Fateh, A., Rohani, P., Masotti, A., Siadat, S. D. (2021). From the Role of Microbiota in Gut-Lung Axis to SARS-CoV-2 Pathogenesis. Mediators Inflamm. 2021, 6611222. doi: 10.1155/2021/6611222
Aktaş, B., Aslim, B. (2020). Gut-Lung Axis and Dysbiosis in COVID-19. Turkish J. Biol. 44 (SI-1), 265–272. doi: 10.3906/biy-2005-102
Alameddine, J., Godefroy, E., Papargyris, L., Sarrabayrouse, G., Tabiasco, J., Bridonneau, C., et al. (2019). Faecalibacterium Prausnitzii Skews Human DC to Prime IL10-Producing T Cells Through TLR2/6/JNK Signaling and IL-10, IL-27, CD39, and IDO-1 Induction. Front. Immunol. 10, 143. doi: 10.3389/fimmu.2019.00143
Alexander, M., Ang, Q. Y., Turnbaugh, P. J. (2019). A Diet-Dependent Enzyme From the Human Gut Microbiome Promotes Th17 Accumulation and Colitis. bioRxiv Preprint Server Biol., 766899. doi: 10.1101/766899
Allali, I., Bakri, Y., Amzazi, S., Ghazal, H. (2021). Gut-Lung Axis in COVID-19. Interdiscip. Perspect. Infect. Dis. 2021, 6655380. doi: 10.1155/2021/6655380
Alvarez, C. A., Jones, M. B., Hambor, J., Cobb, B. A. (2020). Characterization of Polysaccharide A Response Reveals Interferon Responsive Gene Signature and Immunomodulatory Marker Expression. Front. Immunol. 11 (2677). doi: 10.3389/fimmu.2020.556813
Antosca, K. M., Chernikova, D. A., Price, C. E., Ruoff, K. L., Li, K., Guill, M. F., et al. (2019). Altered Stool Microbiota of Infants With Cystic Fibrosis Shows a Reduction in Genera Associated With Immune Programming From Birth. J. Bacteriol. 201 (16), e00274–e00219. doi: 10.1128/JB.00274-19
Antunes, K. H., Fachi, J. L., de Paula, R., da Silva, E. F., Pral, L. P., Dos Santos, A., et al. (2019). Microbiota-Derived Acetate Protects Against Respiratory Syncytial Virus Infection Through a GPR43-Type 1 Interferon Response. Nat. Commun. 10 (1), 3273. doi: 10.1038/s41467-019-11152-6
Arenas-Padilla, M., González-Rascón, A., Hernández-Mendoza, A., Calderón de la Barca, A. M., Hernández, J., Mata-Haro, V. (2021). Immunomodulation by Bifidobacterium Animalis Subsp. Lactis Bb12: Integrative Analysis of miRNA Expression and TLR2 Pathway-Related Target Proteins in Swine Monocytes. Probiot. Antimicrob. Proteins 20, 1–13. doi: 10.1007/s12602-021-09816-1
Avery, E. G., Bartolomaeus, H., Maifeld, A., Marko, L., Wiig, H., Wilck, N., et al. (2021). The Gut Microbiome in Hypertension: Recent Advances and Future Perspectives. Circ. Res. 128 (7), 934–950. doi: 10.1161/CIRCRESAHA.121.318065
Battaglini, D., Robba, C., Fedele, A., Trancǎ, S., Sukkar, S. G., Di Pilato, V., et al. (2021). The Role of Dysbiosis in Critically Ill Patients With COVID-19 and Acute Respiratory Distress Syndrome. Front. Med. 8, 671714. doi: 10.3389/fmed.2021.671714
Belizário, J. E., Faintuch, J., Garay-Malpartida, M. (2018). Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediators Inflamm. 2018, 2037838. doi: 10.1155/2018/2037838
Bhushan, I., Sharma, M., Mehta, M., Badyal, S., Sharma, V., Sharma, I., et al. (2021). Bioactive Compounds and Probiotics–a Ray of Hope in COVID-19 Management. Food Sci. Hum. Wellness 10 (2), 131–140. doi: 10.1016/j.fshw.2021.02.001
Boursier, J., Mueller, O., Barret, M., Machado, M., Fizanne, L., Araujo-Perez, F., et al. (2016). The Severity of Nonalcoholic Fatty Liver Disease is Associated With Gut Dysbiosis and Shift in the Metabolic Function of the Gut Microbiota. Hepatology 63 (3), 764–775. doi: 10.1002/hep.28356
Bozkurt, H. S., Kara, B. (2020). A New Treatment for Ulcerative Colitis: Intracolonic Bifidobacterium and Xyloglucan Application. Eur. J. Inflamm. 18, 2058739220942626. doi: 10.1177/2058739220942626
Bozkurt, H. S., Quigley, E. M. (2020a). The Probiotic Bifidobacterium in the Management of Coronavirus: A Theoretical Basis. Int. J. Immunopathol. Pharmacol. 34, 2058738420961304. doi: 10.1177/2058738420961304
Bozkurt, H., Quigley, E. (2020b). Probiotic Bifidobacteria Against the COVİD-19: A New Way Out! Int. J. Immunopathol. Pharmacol. 34. doi: 10.1177/2058738420961304
Bozkurt, H. S., Quigley, E. M., Kara, B. (2019). Bifidobacterium Animalis Subspecies Lactis Engineered to Produce Mycosporin-Like Amino Acids in Colorectal Cancer Prevention. SAGE Open Med. 7, 2050312119825784. doi: 10.1177/2050312119825784
Brown, R. L., Sequeira, R. P., Clarke, T. B. (2017). The Microbiota Protects Against Respiratory Infection via GM-CSF Signaling. Nat. Commun. 8 (1), 1512. doi: 10.1038/s41467-017-01803-x
Budden, K. F., Gellatly, S. L., Wood, D. L., Cooper, M. A., Morrison, M., Hugenholtz, P., et al. (2017). Emerging Pathogenic Links Between Microbiota and the Gut-Lung Axis. Nat. Rev. Microbiol. 15 (1), 55–63. doi: 10.1038/nrmicro.2016.142
Cao, J., Wang, C., Zhang, Y., Lei, G., Xu, K., Zhao, N., et al. (2021). Integrated Gut Virome and Bacteriome Dynamics in COVID-19 Patients. Gut Microbes 13 (1), 1887722. doi: 10.1080/19490976.2021.1887722
Chakraborty, K., Raundhal, M., Chen, B. B., Morse, C., Tyurina, Y. Y., Khare, A., et al. (2017). The Mito-DAMP Cardiolipin Blocks IL-10 Production Causing Persistent Inflammation During Bacterial Pneumonia. Nat. Commun. 8, 13944. doi: 10.1038/ncomms13944
Chattopadhyay, I., Shankar, E. M. (2021). SARS-CoV-2-Indigenous Microbiota Nexus: Does Gut Microbiota Contribute to Inflammation and Disease Severity in COVID-19? Front. Cell. Infect. Microbiol. 11 (96). doi: 10.3389/fcimb.2021.590874
Chen, J., Vitetta, L. (2021). Modulation of Gut Microbiota for the Prevention and Treatment of COVID-19. J. Clin. Med. 10 (13), 2903. doi: 10.3390/jcm10132903
Chhibber-Goel, J., Gopinathan, S., Sharma, A. (2021). Interplay Between Severities of COVID-19 and the Gut Microbiome: Implications of Bacterial Co-Infections? Gut Pathogens 13 (1), 14. doi: 10.1186/s13099-021-00407-7
Claesson, M. J., Jeffery, I. B., Conde, S., Power, S. E., O’Connor, E. M., Cusack, S., et al. (2012). Gut Microbiota Composition Correlates With Diet and Health in the Elderly. Nature 488 (7410), 178–184. doi: 10.1038/nature11319
Crovesy, L., Masterson, D., Rosado, E. L. (2020). Profile of the Gut Microbiota of Adults With Obesity: A Systematic Review. Eur. J. Clin. Nutr. 74 (9), 1251–1262. doi: 10.1038/s41430-020-0607-6
Cyprian, F., Sohail, M. U., Abdelhafez, I., Salman, S., Attique, Z., Kamareddine, L., et al. (2021). SARS-CoV-2 and Immune-Microbiome Interactions: Lessons From Respiratory Viral Infections. Int. J. Infect. Dis. 105, 540–550. doi: 10.1016/j.ijid.2021.02.071
D’Amico, F., Baumgart, D. C., Danese, S., Peyrin-Biroulet, L. (2020). Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin. Gastroenterol. Hepatol. 18 (8), 1663–1672. doi: 10.1016/j.cgh.2020.04.001
Dang, A. T., Marsland, B. J. (2019). Microbes, Metabolites, and the Gut-Lung Axis. Mucosal Immunol. 12 (4), 843–850. doi: 10.1038/s41385-019-0160-6
d’Ettorre, G., Ceccarelli, G., Marazzato, M., Campagna, G., Pinacchio, C., Alessandri, F., et al. (2020). Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front. Med. 7, 389. doi: 10.3389/fmed.2020.00389
de Vrese, M., Winkler, P., Rautenberg, P., Harder, T., Noah, C., Laue, C., et al. (2005). Effect of Lactobacillus Gasseri PA 16/8, Bifidobacterium Longum SP 07/3, B. Bifidum MF 20/5 on Common Cold Episodes: A Double Blind, Randomized, Controlled Trial. Clin. Nutr. (Edinburgh Scotland) 24 (4), 481–491. doi: 10.1016/j.clnu.2005.02.006
Dickson, R. P., Huffnagle, G. B. (2015). The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease. PloS Pathogens 11 (7), e1004923. doi: 10.1371/journal.ppat.1004923
Din, A. U., Mazhar, M., Waseem, M., Ahmad, W., Bibi, A., Hassan, A., et al. (2021). SARS-CoV-2 Microbiome Dysbiosis Linked Disorders and Possible Probiotics Role. Biomed. Pharmacother. = Biomed. Pharmacother. 133, 110947. doi: 10.1016/j.biopha.2020.110947
Donaldson, G. P., Lee, S. M., Mazmanian, S. K. (2016). Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 14 (1), 20–32. doi: 10.1038/nrmicro3552
Donati Zeppa, S., Agostini, D., Piccoli, G., Stocchi, V., Sestili, P. (2020). Gut Microbiota Status in COVID-19: An Unrecognized Player? Front. Cell. Infect. Microbiol. 10 (742). doi: 10.3389/fcimb.2020.576551
Enaud, R., Prevel, R., Ciarlo, E., Beaufils, F., Wieërs, G., Guery, B., et al. (2020). The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 10 (9). doi: 10.3389/fcimb.2020.00009
Fagundes, C. T., Amaral, F. A., Vieira, A. T., Soares, A. C., Pinho, V., Nicoli, J. R., et al. (2012). Transient TLR Activation Restores Inflammatory Response and Ability to Control Pulmonary Bacterial Infection in Germfree Mice. J. Immunol. (Baltimore Md 1950) 188 (3), 1411–1420. doi: 10.4049/jimmunol.1101682
Ferreira, E., Penna, B., Yates, E. A. (2020). Should We Be Worried About Clostridioides Difficile During the SARS-CoV2 Pandemic? Front. Microbiol. 11 (2292). doi: 10.3389/fmicb.2020.581343
Fox, A. C., McConnell, K. W., Yoseph, B. P., Breed, E., Liang, Z., Clark, A. T., et al. (2012). The Endogenous Bacteria Alter Gut Epithelial Apoptosis and Decrease Mortality Following Pseudomonas Aeruginosa Pneumonia. Shock (Augusta Ga) 38 (5), 508–514. doi: 10.1097/SHK.0b013e31826e47e8
Gaibani, P., D’Amico, F., Bartoletti, M., Lombardo, D., Rampelli, S., Fornaro, G., et al. (2021). The Gut Microbiota of Critically Ill Patients With COVID-19. Front. Cell. Infect. Microbiol. 11 (554). doi: 10.3389/fcimb.2021.670424
Gao, J., Xu, K., Liu, H., Liu, G., Bai, M., Peng, C., et al. (2018). Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 8, 13. doi: 10.3389/fcimb.2018.00013
Gautier, T., David-Le Gall, S., Sweidan, A., Tamanai-Shacoori, Z., Jolivet-Gougeon, A., Loréal, O., et al. (2021). Next-Generation Probiotics and Their Metabolites in COVID-19. Microorganisms 9 (5), 941. doi: 10.3390/microorganisms9050941
Geva-Zatorsky, N., Sefik, E., Kua, L., Pasman, L., Tan, T. G., Ortiz-Lopez, A., et al. (2017). Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell 168 (5), 928–943.e11. doi: 10.1016/j.cell.2017.01.022
Groeger, D., O’Mahony, L., Murphy, E. F., Bourke, J. F., Dinan, T. G., Kiely, B., et al. (2013). Bifidobacterium Infantis 35624 Modulates Host Inflammatory Processes Beyond the Gut. Gut Microbes 4 (4), 325–339. doi: 10.4161/gmic.25487
Gu, J., Han, B., Wang, J. (2020a). COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology 158 (6), 1518–1519. doi: 10.1053/j.gastro.2020.02.054
Gu, S., Chen, Y., Wu, Z., Chen, Y., Gao, H., Lv, L., et al. (2020b). Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. 71 (10), 2669–2678. doi: 10.1093/cid/ciaa709
Haak, B. W., Littmann, E. R., Chaubard, J. L., Pickard, A. J., Fontana, E., Adhi, F., et al. (2018). Impact of Gut Colonization With Butyrate-Producing Microbiota on Respiratory Viral Infection Following Allo-HCT. Blood 131 (26), 2978–2986. doi: 10.1182/blood-2018-01-828996
Hall, A. B., Yassour, M., Sauk, J., Garner, A., Jiang, X., Arthur, T., et al. (2017). A Novel Ruminococcus Gnavus Clade Enriched in Inflammatory Bowel Disease Patients. Genome Med. 9 (1), 103. doi: 10.1186/s13073-017-0490-5
Han, C., Ding, Z., Shi, H., Qian, W., Hou, X., Lin, R. (2016). The Role of Probiotics in Lipopolysaccharide-Induced Autophagy in Intestinal Epithelial Cells. Cell. Physiol. Biochem. 38 (6), 2464–2478. doi: 10.1159/000445597
Han, Y., Jia, Z., Shi, J., Wang, W., He, K. (2020). The Active Lung Microbiota Landscape of COVID-19 Patients. medRxiv. doi: 10.1101/2020.08.20.20144014
Harmer, D., Gilbert, M., Borman, R., Clark, K. L. (2002). Quantitative mRNA Expression Profiling of ACE 2, a Novel Homologue of Angiotensin Converting Enzyme. FEBS Lett. 532 (1-2), 107–110. doi: 10.1016/S0014-5793(02)03640-2
Hegazy, M., Ahmed Ashoush, O., Tharwat Hegazy, M., Wahba, M., Lithy, R. M., Abdel-Hamid, H. M., et al. (2021). Beyond Probiotic Legend: ESSAP Gut Microbiota Health Score to Delineate SARS-COV-2 Infection Severity. Br. J. Nutr. 7, 1–10. doi: 10.1017/S0007114521001926
Henke, M. T., Kenny, D. J., Cassilly, C. D., Vlamakis, H., Xavier, R. J., Clardy, J. (2019). Ruminococcus Gnavus, a Member of the Human Gut Microbiome Associated With Crohn’s Disease, Produces an Inflammatory Polysaccharide. Proc. Natl. Acad. Sci. U. S. A. 116 (26), 12672–12677. doi: 10.1073/pnas.1904099116
He, Y., Wang, J., Li, F., Shi, Y. (2020). Main Clinical Features of COVID-19 and Potential Prognostic and Therapeutic Value of the Microbiota in SARS-CoV-2 Infections. Front. Microbiol. 11, 1302. doi: 10.3389/fmicb.2020.01302
He, Y., Wen, Q., Yao, F., Xu, D., Huang, Y., Wang, J. (2017). Gut-Lung Axis: The Microbial Contributions and Clinical Implications. Crit. Rev. Microbiol. 43 (1), 81–95. doi: 10.1080/1040841X.2016.1176988
He, F., Zhang, T., Xue, K., Fang, Z., Jiang, G., Huang, S., et al. (2021). Fecal Multi-Omics Analysis Reveals Diverse Molecular Alterations of Gut Ecosystem in COVID-19 Patients. Analyt. Chim. Acta 1180, 338881. doi: 10.1016/j.aca.2021.338881
Hong, N., Ku, S., Yuk, K., Johnston, T. V., Ji, G. E., Park, M. S. (2021). Production of Biologically Active Human Interleukin-10 by Bifidobacterium Bifidum BGN4. Microb. Cell Fact. 20 (1), 16. doi: 10.1186/s12934-020-01505-y
Howell, M. C., Green, R., McGill, A. R., Dutta, R., Mohapatra, S., Mohapatra, S. S. (2021). SARS-CoV-2-Induced Gut Microbiome Dysbiosis: Implications for Colorectal Cancer. Cancers 13 (11), 2676. doi: 10.3390/cancers13112676
Hussain, I., Cher, G. L. Y., Abid, M. A., Abid, M. B. (2021). Role of Gut Microbiome in COVID-19: An Insight Into Pathogenesis and Therapeutic Potential. Front. Immunol. 12. doi: 10.3389/fimmu.2021.765965
Huynh, K. (2020). Novel Gut Microbiota-Derived Metabolite Promotes Platelet Thrombosis via Adrenergic Receptor Signalling. Nat. Rev. Cardiol. 17 (5), 265. doi: 10.1038/s41569-020-0367-y
Ichinohe, T., Pang, I. K., Kumamoto, Y., Peaper, D. R., Ho, J. H., Murray, T. S., et al. (2011). Microbiota Regulates Immune Defense Against Respiratory Tract Influenza A Virus Infection. Proc. Natl. Acad. Sci. U. S. A. 108 (13), 5354–5359. doi: 10.1073/pnas.1019378108
Jamshidi, P., Hajikhani, B., Mirsaeidi, M., Vahidnezhad, H., Dadashi, M., Nasiri, M. J. (2021). Skin Manifestations in COVID-19 Patients: Are They Indicators for Disease Severity? A Systematic Review. Front. Med. 8, 634208. doi: 10.3389/fmed.2021.634208
Jamshidi, P., Hasanzadeh, S., Tahvildari, A., Farsi, Y., Arbabi, M., Mota, J. F., et al. (2019). Is There Any Association Between Gut Microbiota and Type 1 Diabetes? A Systematic Review. Gut Pathog. 11 (1), 49. doi: 10.1186/s13099-019-0332-7
Jia, W., Xie, G., Jia, W. (2018). Bile Acid–Microbiota Crosstalk in Gastrointestinal Inflammation and Carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 15 (2), 111–128. doi: 10.1038/nrgastro.2017.119
Joanna Briggs Institute. (2021). Critical Appraisal Tools (South Australia: Joanna Briggs Institute: University of Adelaide). Available at: https://jbi.global/critical-appraisal-tools.
Juárez-Fernández, M., Porras, D., García-Mediavilla, M. V., Román-Sagüillo, S., González-Gallego, J., Nistal, E., et al. (2021). Aging, Gut Microbiota and Metabolic Diseases: Management Through Physical Exercise and Nutritional Interventions. Nutrients 13 (1), 16. doi: 10.3390/nu13010016
Jungersen, M., Wind, A., Johansen, E., Christensen, J. E., Stuer-Lauridsen, B., Eskesen, D. (2014). The Science Behind the Probiotic Strain Bifidobacterium Animalis Subsp. lactis BB-12(®). Microorganisms 2 (2), 92–110. doi: 10.3390/microorganisms2020092
Kalinkovich, A., Livshits, G. (2019). A Cross Talk Between Dysbiosis and Gut-Associated Immune System Governs the Development of Inflammatory Arthropathies. Semin. Arthritis Rheum. 49 (3), 474–484. doi: 10.1016/j.semarthrit.2019.05.007
Katz-Agranov, N., Zandman-Goddard, G. (2021). Autoimmunity and COVID-19 - The Microbiotal Connection. Autoimmun. Rev. 20 (8), 102865. doi: 10.1016/j.autrev.2021.102865
Khan, M., Mathew, B. J., Gupta, P., Garg, G., Khadanga, S., Vyas, A. K., et al. (2021). Gut Dysbiosis and IL-21 Response in Patients With Severe COVID-19. Microorganisms 9 (6), 1292. doi: 10.3390/microorganisms9061292
Khanna, S., Kraft, C. S. (2021). The Interplay of SARS-CoV-2 and Clostridioides Difficile Infection. Future Microbiol. 16 (6), 439–443. doi: 10.2217/fmb-2020-0275
Kim, H.-N., Joo, E.-J., Lee, C.-W., Ahn, K.-S., Kim, H.-L., Park, D.-I., et al. (2021). Reversion of Gut Microbiota During the Recovery Phase in Patients With Asymptomatic or Mild COVID-19: Longitudinal Study. Microorganisms 9 (6), 1237. doi: 10.3390/microorganisms9061237
King, S., Glanville, J., Sanders, M. E., Fitzgerald, A., Varley, D. (2014). Effectiveness of Probiotics on the Duration of Illness in Healthy Children and Adults Who Develop Common Acute Respiratory Infectious Conditions: A Systematic Review and Meta-Analysis. Br. J. Nutr. 112 (1), 41–54. doi: 10.1017/S0007114514000075
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., Bäckhed, F. (2016). From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 165 (6), 1332–1345. doi: 10.1016/j.cell.2016.05.041
Kyo, M., Nishioka, K., Nakaya, T., Kida, Y., Tanabe, Y., Ohshimo, S., et al. (2019). Unique Patterns of Lower Respiratory Tract Microbiota are Associated With Inflammation and Hospital Mortality in Acute Respiratory Distress Syndrome. Respir. Res. 20 (1), 246. doi: 10.1186/s12931-019-1203-y
Kyriakidou, A., Koufakis, T., Goulis, D. G., Vasilopoulos, Y., Zebekakis, P., Kotsa, K. (2021). Pharmacogenetics of the Glucagon-Like Peptide-1 Receptor Agonist Liraglutide: A Step Towards Personalized Type 2 Diabetes Management. Curr. Pharm. Des. 27 (8), 1025–1034. doi: 10.2174/1381612826666201203145654
Lamers, M. M., Beumer, J., van der Vaart, J., Knoops, K., Puschhof, J., Breugem, T. I., et al. (2020). SARS-CoV-2 Productively Infects Human Gut Enterocytes. Sci. (New York NY) 369 (6499), 50–54. doi: 10.1126/science.abc1669
Larsen, N., Vogensen, F. K., van den Berg, F. W., Nielsen, D. S., Andreasen, A. S., Pedersen, B. K., et al. (2010). Gut Microbiota in Human Adults With Type 2 Diabetes Differs From non-Diabetic Adults. PloS One 5 (2), e9085. doi: 10.1371/journal.pone.0009085
Ley, R. E., Turnbaugh, P. J., Klein, S., Gordon, J. I. (2006). Human Gut Microbes Associated With Obesity. Nature 444 (7122), 1022–1023. doi: 10.1038/4441022a
Li, W., Moore, M. J., Vasilieva, N., Sui, J., Wong, S. K., Berne, M. A., et al. (2003). Angiotensin-Converting Enzyme 2 is a Functional Receptor for the SARS Coronavirus. Nature 426 (6965), 450–454. doi: 10.1038/nature02145
Liu, F., Ye, S., Zhu, X., He, X., Wang, S., Li, Y., et al. (2021). Gastrointestinal Disturbance and Effect of Fecal Microbiota Transplantation in Discharged COVID-19 Patients. J. Med. Case Rep. 15 (1), 60. doi: 10.1186/s13256-020-02583-7
Lv, L., Gu, S., Jiang, H., Yan, R., Chen, Y., Chen, Y., et al. (2021b). Gut Mycobiota Alterations in Patients With COVID-19 and H1N1 Infections and Their Associations With Clinical Features. Commun. Biol. 4 (1), 480. doi: 10.1038/s42003-021-02036-x
Lv, L., Jiang, H., Chen, Y., Gu, S., Xia, J., Zhang, H., et al. (2021a). The Faecal Metabolome in COVID-19 Patients is Altered and Associated With Clinical Features and Gut Microbes. Analyt. Chim. Acta 1152, 338267. doi: 10.1016/j.aca.2021.338267
Massip Copiz, M. M. (2021). The Gut Microbiota in Patients With COVID-19 and Obesity. FASEB J. 35 (S1). doi: 10.1096/fasebj.2021.35.S1.05159
Matsuoka, K., Kanai, T. (Eds.) (2015). The Gut Microbiota and Inflammatory Bowel Disease. Seminars in Immunopathology (Springer).
Milner, E., Stevens, B., An, M., Lam, V., Ainsworth, M., Dihle, P., et al. (2021). Utilizing Probiotics for the Prevention and Treatment of Gastrointestinal Diseases. Front. Microbiol. 12, 689958. doi: 10.3389/fmicb.2021.689958
Mineshita, Y., Sasaki, H., H-k, K., Shibata, S. (2021). Relationship Between Fasting and Postprandial Glucose Levels and the Gut Microbiota, 24 September 2021, PREPRINT (Version 1). available at Research Square [https://doi.org/10.21203/rs.3.rs-917351/v1]
Minot, S., Sinha, R., Chen, J., Li, H., Keilbaugh, S. A., Wu, G. D., et al. (2011). The Human Gut Virome: Inter-Individual Variation and Dynamic Response to Diet. Genome Res. 21 (10), 1616–1625. doi: 10.1101/gr.122705.111
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Internal Med. 151 (4), 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
Mohseni, A. H., Casolaro, V., Bermúdez-Humarán, L. G., Keyvani, H., Taghinezhad, S. S. (2021). Modulation of the PI3K/Akt/mTOR Signaling Pathway by Probiotics as a Fruitful Target for Orchestrating the Immune Response. Gut Microbes 13 (1), 1–17. doi: 10.1080/19490976.2021.1886844
Moreira-Rosário, A., Marques, C., Pinheiro, H., Araújo, J. R., Ribeiro, P., Rocha, R., et al. (2021). Gut Microbiota Diversity and C-Reactive Protein Are Predictors of Disease Severity in COVID-19 Patients. Front. Microbiol. 12, 705020. doi: 10.3389/fmicb.2021.705020
Oba, J., Silva, C. A., Toma, R. K., Carvalho, W. B., Delgado, A. F. (2020). COVID-19 and Coinfection With Clostridioides (Clostridium) Difficile in an Infant With Gastrointestinal Manifestation. Einstein (Sao Paulo Brazil) 18, eRC6048. doi: 10.31744/einstein_journal/2020RC6048
O’Toole, P. W., Jeffery, I. B. (2018). Microbiome-Health Interactions in Older People. Cell. Mol. Life Sci. 75 (1), 119–128. doi: 10.1007/s00018-017-2673-z
Patel, K. P., Patel, P. A., Vunnam, R. R., Hewlett, A. T., Jain, R., Jing, R., et al. (2020). Gastrointestinal, Hepatobiliary, and Pancreatic Manifestations of COVID-19. J. Clin. Virol. 128, 104386. doi: 10.1016/j.jcv.2020.104386
Prasad, R., Patton, M. J., Floyd, J. L., Vieira, C. P., Fortmann, S., DuPont, M., et al. (2021). Plasma Microbiome in COVID-19 Subjects: An Indicator of Gut Barrier Defects and Dysbiosis. bioRxiv Preprint Server Biol. 33851159. doi: 10.1101/2021.04.06.438634
Rahayu, E. S., Utami, T., Mariyatun, M., Hasan, P. N., Kamil, R. Z., Setyawan, R. H., et al. (2019). Gut Microbiota Profile in Healthy Indonesians. World J. Gastroenterol. 25 (12), 1478–1491. doi: 10.3748/wjg.v25.i12.1478
Rajput, S., Paliwal, D., Naithani, M., Kothari, A., Meena, K., Rana, S. (2021). COVID-19 and Gut Microbiota: A Potential Connection. Indian J. Clin. Biochem. 36 (3), 1–12. doi: 10.1007/s12291-020-00948-9
Ramakrishna, C., Kujawski, M., Chu, H., Li, L., Mazmanian, S. K., Cantin, E. M. (2019). Bacteroides Fragilis Polysaccharide A Induces IL-10 Secreting B and T Cells That Prevent Viral Encephalitis. Nat. Commun. 10 (1), 2153. doi: 10.1038/s41467-019-09884-6
Reyes, A., Semenkovich, N. P., Whiteson, K., Rohwer, F., Gordon, J. I. (2012). Going Viral: Next-Generation Sequencing Applied to Phage Populations in the Human Gut. Nat. Rev. Microbiol. 10 (9), 607–617. doi: 10.1038/nrmicro2853
Rokkam, V. R. P., Kutti, G., Sridharan, Vegunta, R., Vegunta, R., Boregowda, U., Mohan, B. P. (2021). Clostridium Difficile and COVID-19: Novel Risk Factors for Acute Portal Vein Thrombosis. Case Rep. Vasc. Med. 2021, 8832638. doi: 10.1155/2021/8832638
Salazar, N., Valdés-Varela, L., González, S., Gueimonde, M., de Los Reyes-Gavilán, C. G. (2017). Nutrition and the Gut Microbiome in the Elderly. Gut Microbes 8 (2), 82–97. doi: 10.1080/19490976.2016.1256525
Sandhu, A., Tillotson, G., Polistico, J., Salimnia, H., Cranis, M., Moshos, J., et al. (2020). Clostridioides Difficile in COVID-19 Patients, Detroit, Michigan, USA, March-April 2020. Emerg. Infect. Dis. 26 (9), 2272–2274. doi: 10.3201/eid2609.202126
Schett, G., Sticherling, M., Neurath, M. F. (2020). COVID-19: Risk for Cytokine Targeting in Chronic Inflammatory Diseases? Nat. Rev. Immunol. 20 (5), 271–272. doi: 10.1038/s41577-020-0312-7
Schiavi, E., Gleinser, M., Molloy, E., Groeger, D., Frei, R., Ferstl, R., et al. (2016). The Surface-Associated Exopolysaccharide of Bifidobacterium Longum 35624 Plays an Essential Role in Dampening Host Proinflammatory Responses and Repressing Local TH17 Responses. Appl. Environ. Microbiol. 82 (24), 7185–7196. doi: 10.1128/AEM.02238-16
Schirmer, M., Smeekens, S. P., Vlamakis, H., Jaeger, M., Oosting, M., Franzosa, E. A., et al. (2016). Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 167 (4), 1125–1136.e8. doi: 10.1016/j.cell.2016.10.020
Segal, J. P., Mak, J. W. Y., Mullish, B. H., Alexander, J. L., Ng, S. C., Marchesi, J. R. (2020). The Gut Microbiome: An Under-Recognised Contributor to the COVID-19 Pandemic? Ther. Adv. Gastroenterol. 13, 1756284820974914. doi: 10.1177/1756284820974914
Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermúdez-Humarán, L. G., Gratadoux, J.-J., et al. (2008). Faecalibacterium Prausnitzii is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. 105 (43), 16731–16736. doi: 10.1073/pnas.0804812105
Suárez-Fariñas, M., Tokuyama, M., Wei, G., Huang, R., Livanos, A., Jha, D., et al. (2021). Intestinal Inflammation Modulates the Expression of ACE2 and TMPRSS2 and Potentially Overlaps With the Pathogenesis of SARS-CoV-2-Related Disease. Gastroenterology 160 (1), 287–301.e20. doi: 10.1053/j.gastro.2020.09.029
Tang, L., Gu, S., Gong, Y., Li, B., Lu, H., Li, Q., et al. (2020). Clinical Significance of the Correlation Between Changes in the Major Intestinal Bacteria Species and COVID-19 Severity. Eng. (Beijing China) 6 (10), 1178–1184. doi: 10.1016/j.eng.2020.05.013
Tao, W., Zhang, G., Wang, X., Guo, M., Zeng, W., Xu, Z., et al. (2020). Analysis of the Intestinal Microbiota in COVID-19 Patients and its Correlation With the Inflammatory Factor IL-18. Med. Microecol. 5, 100023. doi: 10.1016/j.medmic.2020.100023
Tian, T., Zhao, Y., Yang, Y., Wang, T., Jin, S., Guo, J., et al. (2020). The Protective Role of Short-Chain Fatty Acids Acting as Signal Molecules in Chemotherapy- or Radiation-Induced Intestinal Inflammation. Am. J. Cancer Res. 10 (11), 3508–3531.
Trompette, A., Gollwitzer, E. S., Pattaroni, C., Lopez-Mejia, I. C., Riva, E., Pernot, J., et al. (2018). Dietary Fiber Confers Protection Against Flu by Shaping Ly6c(-) Patrolling Monocyte Hematopoiesis and CD8(+) T Cell Metabolism. Immunity 48 (5), 992–1005.e8. doi: 10.1016/j.immuni.2018.04.022
Turnbaugh, P. J., Ley, R. E., Mahowald, M. A., Magrini, V., Mardis, E. R., Gordon, J. I. (2006). An Obesity-Associated Gut Microbiome With Increased Capacity for Energy Harvest. Nature 444 (7122), 1027–1031. doi: 10.1038/nature05414
van den Munckhof, I. C., Kurilshikov, A., ter Horst, R., Riksen, N. P., Joosten, L., Zhernakova, A., et al. (2018). Role of Gut Microbiota in Chronic Low-Grade Inflammation as Potential Driver for Atherosclerotic Cardiovascular Disease: A Systematic Review of Human Studies. Obes. Rev. 19 (12), 1719–1734. doi: 10.1111/obr.12750
van der Lelie, D., Taghavi, S. (2020). COVID-19 and the Gut Microbiome: More Than a Gut Feeling. Msystems 5 (4), e00453–e00420. doi: 10.1128/mSystems.00453-20
Venegas, D., Marjorie, K., Landskron, G., González, M., Quera, R., Dijkstra, G., et al. (2019). Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 10, 277. doi: 10.3389/fimmu.2019.00277
Walton, G. E., Gibson, G. R., Hunter, K. A. (2021). Mechanisms Linking the Human Gut Microbiome to Prophylactic and Treatment Strategies for COVID-19. Br. J. Nutr. 126 (2), 219–227. doi: 10.1017/S0007114520003980
Wang, Z., Wang, J., Cheng, Y., Liu, X., Huang, Y. (2011). Secreted Factors From Bifidobacterium Animalis Subsp. Lactis Inhibit NF-κb-Mediated Interleukin-8 Gene Expression in Caco-2 Cells. Appl. Environ. Microbiol. 77 (22), 8171–8174. doi: 10.1128/AEM.06145-11
Wang, H., Wang, H., Sun, Y., Ren, Z., Zhu, W., Li, A., et al. (2021a). Potential Associations Between Microbiome and COVID-19. Front. Med. 8. doi: 10.3389/fmed.2021.785496
Wang, H., Wang, Y., Lu, C., Qiu, L., Song, X., Jia, H., et al. (2021b). The Efficacy of Probiotics in Patients With Severe COVID-19. Ann. Palliat. Med. 10 (12), 12374–12380. doi: 10.21037/apm-21-3373
Wang, B., Yao, M., Lv, L., Ling, Z., Li, L. (2017). The Human Microbiota in Health and Disease. Engineering 3 (1), 71–82. doi: 10.1016/J.ENG.2017.01.008
Weiser, J. N., Ferreira, D. M., Paton, J. C. (2018). Streptococcus Pneumoniae: Transmission, Colonization and Invasion. Nat. Rev. Microbiol. 16 (6), 355–367. doi: 10.1038/s41579-018-0001-8
Wilks, J., Golovkina, T. (2012). Influence of Microbiota on Viral Infections. PloS Pathogens 8 (5), e1002681. doi: 10.1371/journal.ppat.1002681
Wu, Y., Cheng, X., Jiang, G., Tang, H., Ming, S., Tang, L., et al. (2021). Altered Oral and Gut Microbiota and its Association With SARS-CoV-2 Viral Load in COVID-19 Patients During Hospitalization. NPJ Biofilms Microbiomes 7 (1), 61. doi: 10.1038/s41522-021-00232-5
Wu, Y., Guo, C., Tang, L., Hong, Z., Zhou, J., Dong, X., et al. (2020). Prolonged Presence of SARS-CoV-2 Viral RNA in Faecal Samples. Lancet Gastroenterol. Hepatol. 5 (5), 434–435. doi: 10.1016/S2468-1253(20)30083-2
Xue, X., Mi, Z., Wang, Z., Pang, Z., Liu, H., Zhang, F. (2021). High Expression of ACE2 on Keratinocytes Reveals Skin as a Potential Target for SARS-CoV-2. J. Invest. Dermatol. 141 (1), 206–209.e1. doi: 10.1016/j.jid.2020.05.087
Xu, Y., Li, X., Zhu, B., Liang, H., Fang, C., Gong, Y., et al. (2020). Characteristics of Pediatric SARS-CoV-2 Infection and Potential Evidence for Persistent Fecal Viral Shedding. Nat. Med. 26 (4), 502–505. doi: 10.1038/s41591-020-0817-4
Yamamoto, S., Saito, M., Tamura, A., Prawisuda, D., Mizutani, T., Yotsuyanagi, H. (2021). The Human Microbiome and COVID-19: A Systematic Review. PloS One 16 (6), e0253293. doi: 10.1371/journal.pone.0253293
Yeoh, Y. K., Zuo, T., Lui, G. C., Zhang, F., Liu, Q., Li, A. Y., et al. (2021). Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients With COVID-19. Gut 70 (4), 698–706. doi: 10.1136/gutjnl-2020-323020
Yildiz, S., Mazel-Sanchez, B., Kandasamy, M., Manicassamy, B., Schmolke, M. (2018). Influenza A Virus Infection Impacts Systemic Microbiota Dynamics and Causes Quantitative Enteric Dysbiosis. Microbiome 6 (1), 9. doi: 10.1186/s40168-017-0386-z
Yoshida, N., Emoto, T., Yamashita, T., Watanabe, H., Hayashi, T., Tabata, T., et al. (2018). Bacteroides Vulgatus and Bacteroides Dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation 138 (22), 2486–2498. doi: 10.1161/CIRCULATIONAHA.118.033714
Yu, B., Dai, C. -Q., Chen, J., Deng, L., Wu, X. -I., Wu, S., et al. (2015). Dysbiosis of Gut Microbiota Induced the Disorder of Helper T Cells in Influenza Virus-Infected Mice. Hum. Vaccines Immunother. 11 (5), 1140–1146. doi: 10.1080/21645515.2015.1009805
Zhang, D., Li, S., Wang, N., Tan, H. Y., Zhang, Z., Feng, Y. (2020). The Cross-Talk Between Gut Microbiota and Lungs in Common Lung Diseases. Front. Microbiol. 11, 301. doi: 10.3389/fmicb.2020.00301
Zhang, W., Zhu, B., Xu, J., Liu, Y., Qiu, E., Li, Z., et al. (2018). Bacteroides Fragilis Protects Against Antibiotic-Associated Diarrhea in Rats by Modulating Intestinal Defenses. Front. Immunol. 9 (1040). doi: 10.3389/fimmu.2018.01040
Zheng, L., Kelly, C. J., Battista, K. D., Schaefer, R., Lanis, J. M., Alexeev, E. E., et al. (2017). Microbial-Derived Butyrate Promotes Epithelial Barrier Function Through IL-10 Receptor-Dependent Repression of Claudin-2. J. Immunol. (Baltimore Md 1950) 199 (8), 2976–2984. doi: 10.4049/jimmunol.1700105
Zhou, P., Yang, X. L., Wang, X. G., Hu, B., Zhang, L., Zhang, W., et al. (2020). A Pneumonia Outbreak Associated With a New Coronavirus of Probable Bat Origin. Nature 579 (7798), 270–273. doi: 10.1038/s41586-020-2012-7
Zhou, Y., Zhang, J., Zhang, D., Ma, W. L., Wang, X. (2021). Linking the Gut Microbiota to Persistent Symptoms in Survivors of COVID-19 After Discharge. J. Microbiol. (Seoul Korea) 59 (10), 941–948. doi: 10.1007/s12275-021-1206-5
Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., et al. (2020). A Novel Coronavirus From Patients With Pneumonia in China, 2019. New Engl. J. Med. 382 (8), 727–733. doi: 10.1056/NEJMoa2001017
Zuo, T., Liu, Q., Zhang, F., Lui, G. C.-Y., Tso, E. Y., Yeoh, Y. K., et al. (2021a). Depicting SARS-CoV-2 Faecal Viral Activity in Association With Gut Microbiota Composition in Patients With COVID-19. Gut 70 (2), 276–284. doi: 10.1136/gutjnl-2020-322294
Zuo, T., Liu, Q., Zhang, F., Yeoh, Y. K., Wan, Y., Zhan, H., et al. (2021). Temporal Landscape of Human Gut RNA and DNA Virome in SARS-CoV-2 Infection and Severity. Microbiome 9 (1), 91. doi: 10.1186/s40168-021-01008-x
Zuo, T., Zhang, F., Lui, G. C. Y., Yeoh, Y. K., Li, A. Y. L., Zhan, H., et al. (2020a). Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 159 (3), 944–955.e8. doi: 10.1053/j.gastro.2020.05.048
Keywords: COVID-19, SARS-CoV-2, gastrointestinal microbiome, dysbiosis, prognosis, diagnosis, gut microbiota, therapeutic
Citation: Farsi Y, Tahvildari A, Arbabi M, Vazife F, Sechi LA, Shahidi Bonjar AH, Jamshidi P, Nasiri MJ and Mirsaeidi M (2022) Diagnostic, Prognostic, and Therapeutic Roles of Gut Microbiota in COVID-19: A Comprehensive Systematic Review. Front. Cell. Infect. Microbiol. 12:804644. doi: 10.3389/fcimb.2022.804644
Received: 29 October 2021; Accepted: 07 February 2022;
Published: 04 March 2022.
Edited by:
Yongqun Oliver He, University of Michigan, United StatesReviewed by:
Almagul Kushugulova, Nazarbayev University, KazakhstanHaihe Wang, Harbin Medical University at Daqing, China
Copyright © 2022 Farsi, Tahvildari, Arbabi, Vazife, Sechi, Shahidi Bonjar, Jamshidi, Nasiri and Mirsaeidi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Mirsaeidi, m.mirsaeidi@ufl.edu; Parnian Jamshidi, P.jamshidi@sbmu.ac.ir; Mohammad Javad Nasiri, mj.nasiri@hotmail.com
†These authors share first authorship
 Yeganeh Farsi
Yeganeh Farsi Azin Tahvildari1†
Azin Tahvildari1†  Mahta Arbabi
Mahta Arbabi Leonardo A. Sechi
Leonardo A. Sechi Parnian Jamshidi
Parnian Jamshidi Mohammad Javad Nasiri
Mohammad Javad Nasiri