Recent Advances on the Innate Immune Response to Coxiella burnetii
- 1Central Laboratory of Advanced Diagnostic and Biological Research (CLADIBIOR), Department of Biomedicine, Neurosciences and Advanced Diagnostics (BIND), University Hospital “Paolo Giaccone”, Università degli studi di Palermo, Palermo, Italy
- 2Istituto Zooprofilattico Sperimentale della Sicilia, Palermo, Italy
- 3SaBio Health and Biotechnology, Instituto de Investigación en Recursos Cinegéticos, IREC -Spanish National Research Council CSIC - University of Castilla-La Mancha UCLM - Regional Government of Castilla-La Mancha JCCM, Ciudad Real, Spain
- 4Department of Veterinary Pathobiology, Center for Veterinary Health Sciences, Oklahoma State University, Stillwater, OK, United States
Coxiella burnetii is an obligate intracellular Gram-negative bacterium and the causative agent of a worldwide zoonosis known as Q fever. The pathogen invades monocytes and macrophages, replicating within acidic phagolysosomes and evading host defenses through different immune evasion strategies that are mainly associated with the structure of its lipopolysaccharide. The main transmission routes are aerosols and ingestion of fomites from infected animals. The innate immune system provides the first host defense against the microorganism, and it is crucial to direct the infection towards a self-limiting respiratory disease or the chronic form. This review reports the advances in understanding the mechanisms of innate immunity acting during C. burnetii infection and the strategies that pathogen put in place to infect the host cells and to modify the expression of specific host cell genes in order to subvert cellular processes. The mechanisms through which different cell types with different genetic backgrounds are differently susceptible to C. burnetii intracellular growth are discussed. The subsets of cytokines induced following C. burnetii infection as well as the pathogen influence on an inflammasome-mediated response are also described. Finally, we discuss the use of animal experimental systems for studying the innate immune response against C. burnetii and discovering novel methods for prevention and treatment of disease in humans and livestock.
Introduction
Coxiella burnetii (order Legionellales) is an obligate intracellular Gram-negative bacterium (Dragan and Voth, 2020) that can be transmitted by aerosol with a very low infectious dose, as 1–10 viable organisms are sufficient to induce in humans an infection via the aerogenic route (Moos and Hackstadt, 1987; Sawyer et al., 1987; Gürtler et al., 2014). These characteristics, combined with the stability in the environment, make C. burnetii a potential biological weapon (Oyston and Davies, 2011). C. burnetii can infect many species in addition to humans, and cattle, goats, and sheep are the main reservoirs (Van den Brom et al., 2015). In animals, infection by C. burnetii can be asymptomatic or it can cause problems on the reproductive sphere. Other manifestations include pneumonia and eye infections (Van den Brom et al., 2015). Humans can acquire the infection by inhalation of infected aerosol or after exposure to urine, feces, placenta, sperm, and vaginal secretions of infected animals. In addition, the disease can be developed after consuming infected raw milk (Dabaja et al., 2020), even if the link between infection and clinical disease in humans through consumption of unpasteurised milk and milk products is unclear (EFSA, 2010). Indeed, although milk may contain large amounts of C. burnetii, it is probably a minor route of Q fever acquisition (Maurin and Raoult, 1999; Gale et al., 2015).
C. burnetii in humans causes Q fever, usually an acute illness (Waag, 2007; Marrie et al., 2015), but in some cases, it can become chronic (Tobin et al., 1982; Oyston and Davies, 2011). Coxiella burnetii life cycle comprehends the stages of Small Cell Variant (SCV) and Large Cell Variant (LCV). The first is the infectious stage, found in the environment and resistant to environmental stresses; it is characterized by the synthesis of molecular determinants of SCV differentiation. SCV development involves responses to protect against oxidative and nutritional stress (Sandoz et al., 2016). Some of these proteins are under the control of the alternative sigma factor RpoS, an essential regulator of stress responses and stationary-phase physiology in several bacterial species (Moormeier et al., 2019). These proteins are involved in regulating the remodeling of the peptidoglycan, in facilitating penetration of the cell envelope and promoting both intracellular and extracellular survival (Moormeier et al., 2019). Among them, cbu0419 gene encodes a peptidoglycan polysaccharide deacetylase (Caufrier et al., 2003), which renders the peptidoglycan resistant to lysozyme, reducing the release of cell wall fragments and thus the probability of detection by cytosolic immune cell sensors (Vollmer and Tomasz, 2000; Boneca et al., 2007; Davis and Weiser, 2011; Kaoukab-Raji et al., 2012). Another factor that is SCV-associated is enhC (cbu1136), whose function in C. burnetii is unknown, while in Legionella pneumophila, it is thought to aid intracellular replication by controlling lytic murein transglycosylase activity and reducing the subsequent release of peptidoglycan peptide fragments into the cytosol that might be recognized by the cytosolic innate immune system (Liu et al., 2012; Moreira and Zamboni, 2012). Another enzyme that is SCV-associated is the deacetylates N-acetylglucosamine of peptidoglycan, which in C. burnetii also encodes a lytic murein transglycosylase (CBU0925) that is also known to aid penetration of the cell envelope by large and complex structures (Scheurwater and Burrows, 2011).
LCV is the metabolically active form, replicating efficiently in eukaryotic cells, distinguishable by the production of proteins involved in cell division and in intracellular survival (Marrie et al., 2015; Ammerdorffer et al., 2017). SCVs are ingested by the host macrophages and the vacuole containing the bacterium merges with lysosomes to form the phagolysosome (Figure 1). Here, SCV becomes LCV, which, in turn, convert back to SCVs (Coleman et al., 2004). During the infection, C. burnetii creates a favorable environment inside the cell host to escape from the immune response and to replicate successfully. In particular, C. burnetii presents a type IV secretion system (T4SS) essential for renovation of a lysosome into a mature Coxiella-containing vacuole (CCV) permissive of intracellular replication. This secretion system has both sequence homology and functional similarity to the defect in organelle trafficking/intracellular multiplication (Dot/Icm) apparatus of L. pneumophila, which is designated as the type IVB (T4BSS) and it is involved in creating a vacuole that evades rapid endocytic maturation and matures into an endoplasmic reticulum-derived organelle that supports bacterial replication (Roy and Tilney, 2002).
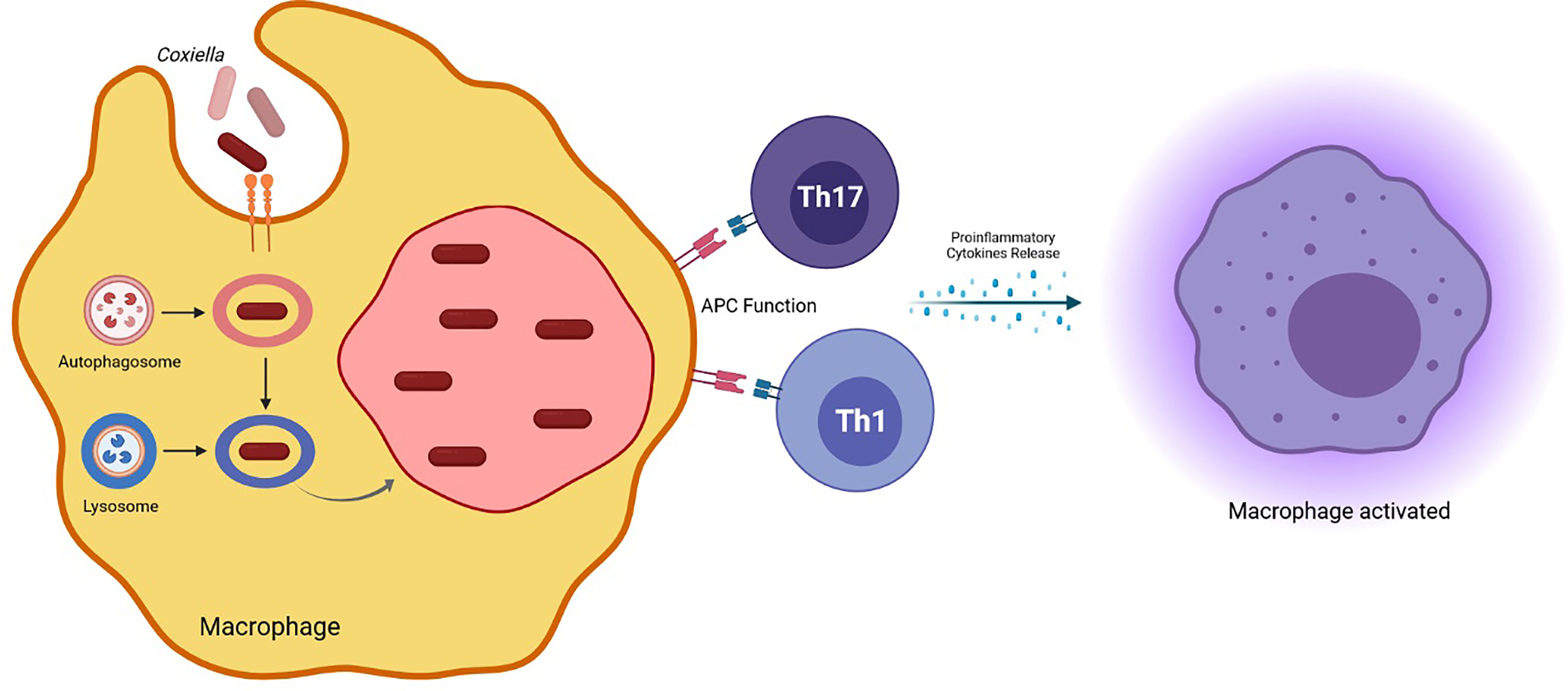
Figure 1 Coxiella burnetii phagolysosome formation in the host macrophage and induced effector mechanisms involved in protection against the pathogen.
Coxiella burnetii Dot/Icm T4SS consists primarily of bacterial membrane proteins that are assembled into the apparatus used to deliver effector proteins into the cytoplasm of the host cell. These effectors mediate the survival of the host cell and the development of CCV replicative compartment (Lührmann et al., 2017).
The comprehension of the immune response elicited in the host by the pathogen is crucial to understand pathogen transmission, establishment and pathogenesis and for identifying novel checkpoints for pathogen control (Torina et al., 2020a). Innate immunity, in particular, causes a rapid and intense protection for the acute phase of infectious diseases. However, even if effector mechanisms of innate immune responses, displayed by host in short time, are able to inhibit symptoms in the acute infection phases, they do not usually contain the infection (Torina et al., 2020b).
This review is focused on recent advances in the comprehension of innate immune mechanisms involved in protection against C. burnetii infection and the strategies elicited by the pathogen to invade the host cells and actively regulate the expression of specific host cell genes to subvert cellular processes. This information could be translated into new intervention for preventing, controlling, and improving Q fever symptoms.
Coxiella burnetii Lipopolysaccharide
One of the major virulence factors of C. burnetii is the lipopolysaccharide (LPS). Functional interactions of LPSs and proteins have been thoroughly investigated by glycomics and proteomics studies and a role of this complex emerged not only in growth and development of the microorganism, but also in pathogenesis and immunity of Q fever (Seshadri et al., 2003; Thompson et al., 2003; Skultety et al., 2005; Samoilis et al., 2007; Toman et al., 2009). C. burnetii LPS is involved in immune evasion strategies since its peculiar structure leads to repressive effects on the defensive mechanisms of cells (Abnave et al., 2017).
Wild-type C. burnetii strains possess a complex full-length LPS with typical O-chain saccharidic units that makes the bacterium virulent (it is the so-called phase I) (Hackstadt, 1986). Following repeated passage in chick embryo yolk sacs or in cell lines, i.e., in the absence of immune system intervention, C. burnetii wild-type strains convert to phase II and produce a phase II LPS determining the loss of virulence (Lukácová et al., 2008).
Phase II LPS is easily eliminated by immune response in immunocompetent hosts (Hackstadt, 1990). This passage is accompanied by modifications in both composition and structure of the LPS macromolecule (Toman et al., 2009). LPS II shows a rough (R) structure, unlike LPS I, characterized by a smooth (S) aspect. Moreover, LPS II is characterized by a peculiar composition, which also includes the sugars virenose and dihydrohydroxystreptose, not present in other LPS and which are therefore representative of the C. burnetii LPS (Narasaki and Toman, 2012). Phase variation is also used in the diagnosis of Q fever since during acute Q fever, C. burnetii induces antibodies against phase II (protein antigens), while chronic Q fever, often manifested as endocarditis, is associated with the production of high titers of antibodies directed against phase I (LPS antigen) (Peacock et al., 1983).
Truncated LPS is responsible for the avirulence of the Nine Mile phase II strain. Moreover, since phase I LPS of C. burnetii possesses low antigenic and immunogenic properties (Hitchcock et al., 1986), this LPS–protein complex is also used as a vaccine against Q fever.
Though LPS I and LPS II are weak endotoxins, their ability to induce TNF-α has been reported (Toman et al., 2009). In TNF-α and many other cytokines (IL1b, IL10, etc.), overproduction is involved (Dellacasagrande et al., 2000a) in C. burnetii survival inside the patient monocytes and may be related to specific inflammatory syndrome of Q fever endocarditis consisting of an increase in circulating TNF-α without variations in cytokine antagonist (Capo et al., 1999a). TNF production did not directly reflect the virulence of C. burnetii strains since avirulent organisms induced TNF production. The overproduction of TNF stimulated by avirulent C. burnetii is probably related to the increase in bacterial binding to monocytes and not to the potency of their LPS (Dellacasagrande et al., 2000b), even considering that the interaction of avirulent variants with monocytes was dramatically more efficient than that of virulent organisms (Capo et al., 1999b).
Coxiella burnetii Infection and Host Cell Toll-Like Receptors
Most pathogens are initially recognized by the innate immune system through one or more Toll-like receptors (TLRs) (Medzhitov, 2007), a family of pattern recognition receptors present on macrophages and other cells acting in innate immunity. TLRs are able to recognize different microbial structures/patterns, thus intervening in the early immune responses to several etiologic agents (Ramstead et al., 2016). Their activity is often mediated by MyD88, which acts as an adapter involved in signal transduction (Janssens and Beyaert, 2002).
Different studies investigated the role of TLR4 and TLR2 in C. burnetii infections (Honstettre et al., 2004; Zamboni et al., 2004; Meghari et al., 2005; Shannon et al., 2005). It was reported that phase I C. burnetii lacks the ability to stimulate TLR2 and TLR4 as a possible immune evasion strategy (Shannon et al., 2005).
Moreover, it was reported that C. burnetii LPS is able to avoid the activation of p38α-MAPK through the TLR4 recruitment. In particular, the re-organization of the macrophage cytoskeleton by C. burnetii LPS disrupts the interaction between TLR-2 and TLR-4, which is necessary for TLRs signaling in case of recognition of pathogenic C. burnetii LPS. Blocking the actin-mediated cytoskeleton re-organization was able to restore C. burnetii-induced TLR-2/TLR-4 association and the activation of p38α-MAPK (Conti et al., 2015).
A study investigating the involvement of TLR2, TLR4, and MyD88 in pulmonary C. burnetii infection found a different role for these factors according to the site of infection. Indeed, TLR2 and TLR4 were not necessary to reduce the growth of the pathogen following peripheral infection and to elicit the inflammatory response in the lungs following pulmonary infection. However, they were able to limit bacterium replication in the lung and spleen when pulmonary infection occurred. Moreover, MyD88 was involved in the infection-related morbidity (Ramstead et al., 2016).
Other factors involved in the innate immune response against microorganisms are nucleotide-binding oligomerization domain receptor 1 (NOD1), NOD2, and the mitogen-activated protein kinases. Inhibition of TLR2 and mitogen-activated protein kinases as well as polymorphisms in TLR1 and NOD2 decreased cytokine responses in C. burnetii–stimulated human peripheral blood mononuclear cells (PBMCs), confirming the important role of both TLR1/TLR2 heterodimer and NOD2 for cytokine response induction against C. burnetii in humans (Ammerdorffer et al., 2015). In a subsequent study, the same authors found that the absence of TLR10 resulted in increased cytokine responses following C. burnetii infection. Similarly, the occurrence of SNPs in TLR10 in healthy volunteers increased cytokine production upon C. burnetii stimulation, suggesting an inhibitory effect of TLR10 (Ammerdorffer et al., 2016).
A different response of human TLR4 (hTLR4) and mouse TLR4 against differently hypo-acylated LPS has been reported. Since it is known that C. burnetii LPS is hypo-acylated and heavily glycosylated, a recent study investigated susceptibility to C. burnetii infection in mice expressing hTLR4. Although mice expressing hTLR4 and the human myeloid differentiation factor 2 (MD-2) adaptor protein showed a similar response with respect to wild-type mice, the authors observed differences in bacterial burdens in tissues and an increase in lung pathology in the first ones. An alteration of cytokine response was observed in hTLR4/MD2 mice, which showed the production of cytokines involved in myeloid cell recruitment accompanied by neutrophil recruitment to the lung. These responses were related to the subsequent observed increased bacterial load and worst pathology in the lung (Robison et al., 2019).
Macrophage Role in C. burnetii Infection
Coxiella burnetii is able to apply several survival strategies to persist within myeloid cells by subverting their microbicidal activity (Ghigo et al., 2009). An interesting study reported the innate immune reactions of neutrophils and macrophages within the lungs elicited following an aerosol-mediated C. burnetii infection. These authors reported that both virulent C. burnetii Nine Mile phase I (NMI) and avirulent Nine Mile phase II (NMII) were able to infect neutrophils, but the infection was limited since a low infection rate was observed. In addition, a possible C. burnetii evasive strategy to infect macrophage emerged since neutrophils did not kill C. burnetii and the bacterium inside neutrophils was still able to infect and replicate within macrophages. An early neutrophil-mediated immune response against aerosolized C. burnetii was detected as SCID mice exposed to aerosolized C. burnetii showed a delay in the influx of neutrophils into the lungs, both in case of NMI and in case of NMII infection (Elliott et al., 2013).
Human alveolar macrophages (hAMs) have been used as a model for defining novel innate immune responses to C. burnetii. These cells allowed the replication of C. burnetii in large, typical CCV, and in non-fused, atypical vacuole harboring replicating C. burnetii and recruiting lipid droplet. A functional type IV secretion system was necessary for the CCV formation and bacterial growth, suggesting production of effector proteins by C. burnetii able to control macrophage functions. Avirulent C. burnetii promoted the activation of pro-survival kinases, a short-term phosphorylation of stress-related factors and a robust, early pro-inflammatory response through the secretion of TNF-α and IL-6. On the contrary, virulent isolates elicited reduced secretion of these cytokines. Finally, IFN-γ treatment of infected hAMs was able to control the intracellular replication of C. burnetii (Graham et al., 2013).
Coxiella burnetii showed a limited ability of alveolar macrophage activation, as it was also supported by studies showing that the interactions between C. burnetii and the C-type lectin surfactant protein D (SP-D), a member of the innate immune response in different mucosal surfaces and in the lungs, result in bacterial aggregation provoking pathogen killing and clearance. It was observed that SP-D is able to bind C. burnetii in a calcium-dependent manner, but no bacterial aggregation or bactericidal effects have been observed. SP-D interactions with C. burnetii decreased the infectivity of C. burnetii towards mouse alveolar macrophage cells, probably due to a significant reduction in its adherence and phagocytosis. However, SP-D does not alter classical activation of alveolar macrophages (Soltysiak et al., 2015).
Coxiella burnetii phase II infection of primary murine alveolar macrophages (AMs) has been investigated in vitro (Fernandes et al., 2016), resulting in a pronounced M2 polarization of AMs and in the establishing of a highly permissive condition to C. burnetii multiplication in vitro. Murine AMs showed an increased susceptibility to infection in comparison to primary bone marrow-derived macrophages. Moreover, AMs from Nos2 or IFNγ knockout (KO) mice were significantly more permissive than wild-type cells when cultured in the absence of IFNγ and nitric oxide synthase 2 (NOS2). In contrast, AMs from Il4-/- mice (in C57BL/6 mouse genetic background) were more restrictive to C. burnetii replication. These results showed that M2 polarization is relevant to elicit the permissiveness of AMs to C. burnetii replication (Fernandes et al., 2016).
It was reported that C. burnetii NMII strain was able to replicate in both primary macrophages from C57BL/6J mice obtained differentiating bone marrow cells with recombinant murine macrophage-colony stimulating factor (M-CSF) as well as in C57BL/6J myeloid progenitors immortalized with an estrogen-regulated Hoxb8 oncogene. These studies indicated that both these ex vivo models can be useful to investigate the interplay between Coxiella and macrophages (Cockrell et al., 2017).
Coxiella burnetii infection was also analyzed in CD14+ macrophages collected from full-term placentas. The bacterium was detected in these macrophages after 4 h culture and was eliminated after 9 days. C. burnetii infection did not prevent placental macrophages from generating multinucleated giant cells, and it was able to induce an inflammatory profile, in particular stimulating IFN-γ production. Indeed, C. burnetii clearance was clearly correlated to IFN-γ production (Mezouar et al., 2019a).
An involvement of mast cells (MCs) has been supposed in Q fever pathophysiology since a decrease in MC progenitor count was observed in Q fever patients together with an increase of serum tryptase levels, which are markers of MCs activation (Mezouar et al., 2019b).
Cytokines in Q Fever
Cytokines are proteins involved in immunity and inflammation, which are mainly produced by monocytes and lymphocytes in response to an immune insult. Since C. burnetii is an obligate intracellular pathogen, it causes alterations in host immune response to survive inside this cell, and the cytokine response is significantly affected by modifications induced by the bacterium (Amara et al., 2012). For example, PBMCs from patients affected by Q fever Fatigue Syndrome (QFS), a debilitating fatigue syndrome lasting 5 to 10 years or longer after the acute illness, showed increased IL-6 and IFN-gamma and reduced IL-2 levels compared to controls (Penttila et al., 1998).
The role of cytokines in the pathogenesis of the acute and chronic Q fever and in the development of the QFS has been investigated. For instance, it has been hypothesized that cytokines may be responsible of persistent fever in patients affected by acute Q fever, which scarcely respond to antibiotic therapy (Lai et al., 2010). Moreover, serum levels of specific cytokines have been suggested as markers of acute or chronic Q fever.
It has been proposed that acute C. burnetii infection may cause an epigenetic remodeling of the promoter regions of pro-inflammatory cytokine genes, which determines an impaired inflammatory response. In particular, QFS patients affected by frequent and severe infections in the upper respiratory tract show a reduced cytokine response, probably due to a long-term change in monocytes induced by C. burnetii (Raijmakers et al., 2019).
Coxiella burnetii isolated from cattle induced higher levels of IL-1β, TNF-α, and IL-22 than bacteria isolated from goats, sheep, and chronic Q fever patients, which probably explains the low incidence of human outbreak associated with bovine disease. Cytokine responses depended on host origin more than on C. burnetii genotypes (Ammerdorffer et al., 2017).
Moreover, viable C. burnetii induces lower levels of TNF-α, IL-1β, IL-6, and IL-10 but similar levels of IFN-γ, IL-17, IL-22, and CXCL9 (IFN-γ-dependent chemokine) compared to the heat-killed bacteria. In contrast, the adaptive response is similar in both cases (Jansen et al., 2018).
In Q fever, IFN-γ promotes C. burnetii killing by the induction of TNF-mediated apoptosis in infected monocytes (Dellacasagrande et al., 1999; Dellacasagrande et al., 2002). In particular, IFN-γ is significantly increased in acute but not in vascular chronic Q fever patients compared to people with past infection, whereas IL-18 shows a similar rise in both acute and chronic patients. The IFN-γ-inducible protein 10 (IP-10) and the TGF-β instead are lower in patients affected by chronic vascular infection, suggesting that chronic patients had an impaired Type I immune response against C. burnetii (Pennings et al., 2015).
Patients affected by chronic Q fever showed intact IFN-γ response, so defects concerning this pathway are not involved in progression to chronic infection. Instead, since polymorphisms in the IL-12p40 gene are associated with chronic Q fever, the IL-12/IFN-γ pathway may play a role in this progression (Schoffelen et al., 2017). The QFS patients show similar levels of IFN-γ and decreased IFN-γ/IL-2 ratio compared to those affected by chronic Q fever. Symptom duration is positively correlated with IL-2 production and negatively correlated with the IFN-γ/IL-2 ratio, suggesting an impaired cell-mediated response in QFS (Keijmel et al., 2016).
Type I IFNs (IFN-α, β, κ, ω, τ, and ϵ) and other interferons are important in immune response against C. burnetii (Snyder et al., 2017). IFN-α administration at peritoneal level exacerbated disease, while at the lung level (site of infection), it reduced bacterial replication (Hedges et al., 2016). The negative impact of the peripheral delivery may be explained by the fact that type I IFNs reduce the inflammatory cytokine expression, whereas the beneficial effect of lung administration suggests that aerosolized type I IFNs may be an effective alternative to antibiotics to treat affected patients. There was also a report of an interaction between C. burnetii and plasmacytoid dendritic cells (pDCs), which play a key role in antiviral immunity via the production of I IFNs. C. burnetii was able to stimulate pDCs increasing the expression of activation and migratory markers and upregulating genes encoding for pro-inflammatory cytokines, chemokines, and type I IFN. Type I IFN upregulation was related to the increase in IFN-α release by C. burnetii-stimulated pDCs (Ka et al., 2016).
Coxiella burnetii also stimulates IL-10 production, a cytokine crucial for bacterial replication inside of monocytes. The mechanism involved probably consists in monocyte deactivation mediated by TNF suppression. It has been suggested that IL-10 overproduction promotes progression to chronic Q fever (Ghigo et al., 2001). Monocytes from patients affected by Q fever endocarditis instead show increased TNF production, which is associated with impaired C. burnetii elimination (Dellacasagrande et al., 2000b). Other studies, conversely, reported that the production of TNF is required to restrict C. burnetii Nine Mile II replication within macrophages (Bradley et al., 2016).
Q fever patients with hepatitis show serum cytokines levels higher than those affected by pneumonia or fever, and TNF and IL-10 are increased in patients with valvulopathy and extremely high in case of endocarditis. These observations indicate that acute Q fever is associated with cytokine overproduction and that TNF may be a marker of chronic evolution of Q fever (Honstettre et al., 2003). Besides, the activation marker of B cells sCD23 in Q fever endocarditis patients is significantly higher than in acute patients and controls, suggesting that it may be a marker of the Q fever endocarditis (Capo et al., 1999a).
Serum levels of cytokines such as IFN-γ and IL-6 may be helpful for distinguishing between past and chronic disease as well as for diagnosis of acute Q fever. For example, it has been observed that an IFN-γ/IL-2 ratio > 11 is strongly suggestive for chronic Q fever (Schoffelen et al., 2014b). Likewise, in sheep, goats and she-camels, cytokines, acute phase proteins, and oxidative stress markers may be useful tools to diagnose the Q fever, since animals that aborted due to coxiellosis show a significant increase of the proinflammatory cytokines (El-Deeb et al., 2019).
Finally, IFN-γ and IL-10 serum levels in dairy cattle may be helpful to value the health status of seropositive animals and to monitor response to vaccine (Małaczewska et al., 2018).
Apoptosis and Inflammasome in Coxiella burnetii Infections
Apoptosis is a programmed cell death process essential for the immune system homeostasis, acting also as a scavenger for damaged or infected cells (Byrne and Ojcius, 2004). Apoptosis can be triggered through two different pathways called the extrinsic and intrinsic pathway. In the first pathway, several ligands are involved, such as Fas Ligand (FasL) and TNF-related apoptosis-inducing ligand (TRAIL), their death receptors, and some adaptor proteins, which activate caspases (cysteinyl aspartate proteases). In the second pathway, the activation of the Bax group of proteins plays a crucial role. Their oligomerization leads to the permeabilization of the mitochondrial membrane, the resulting cytochrome c release, and the activation of effector caspases (caspases 3, 6, and 7). In detail, caspase 3 induces robust proteolysis and chromatin fragmentation (Enari et al., 1998; Cory and Adams, 2002).
Nowadays, it is well known that some pathogens are able to interfere positively or negatively with the apoptotic process through different pathways, such as Chlamydia pneumoniae, Rickettsia rickettsii, and Toxoplasma gondii. They act, for example, by degrading pro-apoptotic proteins, inducing the transcription of some genes encoding anti-apoptotic proteins or inhibiting the activation of the intrinsic pathway (Clifton et al., 1998; Byrne and Ojcius, 2004; Fischer et al., 2004; Carmen et al., 2006).
C. burnetii, as other intracellular pathogens, need to hinder the apoptosis process to survive and to establish a favorable environment inside the host cell. It has been seen that cells infected by C. burnetii show different susceptibility to apoptosis depending on their type, stage of maturation, and surface receptors expressed (Schoenlaub et al., 2016). For example, infection by NM II C. burnetii in murine bone marrow neutrophils inhibits apoptosis (Cherla et al., 2018), whereas in peritoneal B1a cells from the same species, it induces cell death (Schoenlaub et al., 2016). Similarly, Zhang et al. (2012) obseved that in human monocytic THP-1 cells, early C. burnetii infection induces apoptosis by a caspase-independent pathway involving translocation of Bcl-2-associated X protein (BAX) and Apoptosis-inducing factor (AIF), respectively, to the mitochondria and to the nucleus. The authors suggested that mantaining a certain level of apoptosis may be useful for C. burnetii to establish persistent infection.
It has been seen that C. burnetii infection affects both intrinsic and extrinsic apoptosis pathway (Bisle et al., 2016; Cordsmeier et al., 2019). Some experimental evidences showed that C. burnetii inhibits the activation of the host cell apoptotic process preventing the release of cytochrome c and the subsequent activation of caspase 3/7, probably through the upregulation of A1/bfl-1 and c-IAP2 (Lührmann and Roy, 2007; Voth et al., 2007). Effectors needed to inhibit apoptosis are injected inside the host cell through the type IVB secretion system (T4BSS) (Lührmann et al., 2010).
However, it is well known that microbial activation of TLRs in macrophages commonly leads to the induction of anti-apoptotic gene expression, suggesting that the C. burnetii could interfere with host cell survival through the activity of some specific bacterial protein produced upon the infection.
Among the C. burnetii proteins involved in the anti-apoptotic process, AnkG, CaeA, and CaeB are the most studied. AnkG is a 388-amino acid protein that belongs to the Ankyrin repeat family and is capable of blocking the p32-mediated apoptosis pathway in mammalian cells (Lührmann et al., 2010). It has been seen that the amino terminal 1–69 region codify a domain that is both necessary and sufficient to interact with p32 and to inhibit the apoptosis, whereas truncated variants are not able to block apoptosis. Moreover, to prevent pathogen-induced cell death, AnkG needs to be localized in the nucleus (Eckart et al., 2014; Schäfer et al., 2017; Schäfer et al., 2020).
CaeB is a T4BSS effector protein, which, in stressed mammalian cells, targets the endoplasmatic reticulum stress sensor IRE1, thereby inhibiting the ER stress-induced apoptosis. Due to its activity, CaeB is involved in pathogenity in vivo (Friedrich et al., 2021).
In contrast to CaeB, CaeA affects both intrinsic and extrinsic apoptosis acting respectively on caspases and Fas pathways. An EK (glutamic acid/lysine) repetition motif is believed to be essential for CaeA anti-apoptotic activity (Bisle et al., 2016; Raghavan, 2016).
Finally, apart from those mentioned above, another C. burnetii effector worth mentioning is IcaA, secreted, similarly to AnkG, CaeA, and CaeB, by the Dot/Icm type IV secretion system. This protein is responsible for caspase-11 inhibition and plays an important role in preventing pyroptosis, which is an inflammatory cell death pathway massively involved in infection control (Cunha & Zamboni, 2013; Cunha et al., 2015; Cordsmeier et al., 2019).
In addition, C. burnetii, as well as other bacteria, implements several mechanisms to evade inflammasome activation and pyroptosis induction (Cunha and Zamboni, 2013).
Inflammasome is a protein scaffold involved in modulation of caspase-1 and in the maturation of IL-1β and IL-18 (Torina et al., 2020b). It is activated during infections by several pathogens, including Gram-negative and Gram-positive bacteria, virus, fungi, and protozoa (Hayward et al., 2018; Scorpio et al., 2018). It is generally activated by different Pathogen-Associated Molecular Patterns (PAMPs) or Damage-Associated Molecular Patterns (DAMPs) through various Nod-like receptors (NLRs), such as NLRP3. Inflammasome activation induces several processes, including the release of Caspase-1 active form. The protease allows pro-IL1β/pro-IL18 maturation and the release of active IL1β and IL18 with pyroptotic, or inflammatory, cell death induction.
Coxiella burnetii, as well as other bacteria, implements several mechanisms to evade inflammasome activation and pyroptosis induction (Cunha and Zamboni, 2013). For example, a study found that C. burnetii was able to prevent the non-canonical activation of the NLRP3 inflammasome mediated by caspase-11 in case of successive infection with other bacteria such as Escherichia coli or L. pneumophila. This activity was related to a novel C. burnetii gene (IcaA) codifying a protein interfering with caspase activation and with the non-canonical activation of the inflammasome mediated by caspase-11 (Cunha et al., 2015).
Coxiella burnetii is not involved in caspase-1 activation, IL-1b secretion, or cell death during the in vitro infection of murine macrophages. Moreover, C. burnetii-infected cells have no evident NLRP3 or ASC (apoptosis-associated speck-like protein containing a carboxy-terminal caspase activation and recruitment domain [CARD]) foci, indicating its ability to avoid cytosolic detection. C. burnetii is not able to inhibit and instead potentiates Caspase-1-mediated cell death, upregulating pro-IL-1b and priming NLRP3 inflammasomes via TLR2 and MyD88 signaling (Delaney et al., 2021).
Autophagy
Coxiella burnetii can subvert the autophagy process to obtain a better bacterial replication activity taking advantage of the host’s cellular response (Castrejón-Jiménez et al., 2015). It was argued that the autophagic compartments rich in nutrients (cell degradation products, small peptides, amino acids, etc.) such as those of the host cell autophagosomes, contribute to the conversion of SCV to LCV. Therefore, the morphogenesis and multiplication of the bacterium depend not only on the pH of the vacuoles, but also on the accessibility of nutrients (Gutierrez and Colombo, 2005; Colombo et al., 2006).
The role of the bacterial Dot/Icm type IV secretion system, through which C. burnetii translocates effector substrates from the bacterial cytosol directly into the cytosol of the host eukaryotic cell, has been investigated. Translocated effectors are involved in the pathogenesis mechanisms and therefore in the progression of infection. Through these effectors, C. burnetii modulates several cellular activities in order to maintain the infection. These activities include apoptosis suppression, lipid metabolism, and membrane trafficking alteration, gene transcription modification through the interaction of these effectors directly with targets in the nucleus, etc. (van Schaik et al., 2013).
One of these proteins is Cig2, also known as vacuolar protein B (CvpB) (Martinez et al., 2016). This C. bunetii effector interacts with phosphoinositides on host cell membranes and manipulates phosphatidylinositol 3-phosphate metabolism for optimal Coxiella-containing vacuole (CCV) development, promoting the fusion between autolysosomes and CCV and maintaining it in an autolysosomal stage. This allows distinguishing the CCV from the other vacuoles containing other pathogenic bacteria, which become targets of the host’s defensive pathway. This is a mechanism through which C. bunetii is able to subvert the host phagocytosis pathway in its favor, influencing the host’s immune response (Kohler and Roy, 2015).
Another study reported that GTPases of the Rho family have an important role in C. burnetii phagocytosis. More specifically, the active forms of the members of the Rho family RhoA, Cdc42, and Rac1 are involved in the entry of C. burnetii into the host cell, regulating the rearrangement of actin, necessary for the internalization process. The authors also showed that the effectors of RhoA mDia1 and ROCK are involved in the signal transduction mechanism, which favors the entry of C. burnetii (Salinas et al., 2015).
A recent study identified host proteins required for Dot/Icm effector translocation and for endocytic trafficking of the C. burnetii-containing vacuole to the lysosome. Moreover, many of the lysosomal proteins contribute to the virulence of C. burnetii, including the lysosomal serine protease TPP1. This tripeptidyl peptidase 1, also known as CLN2, is selectively transported to the lysosome by a lysosomal receptor, where it generates tripeptides from degraded proteins within the lysosome (Qian et al., 2008). The authors demonstrated that C. burnetii is capable of detecting specific amino acids present in lysosomes and of upregulating gene expression required for the activity of the Dot/Icm system itself (Newton et al., 2020).
A role also for galectin proteins, β-galactoside-binding lectins involved in many biological processes (Blanda et al., 2020), has been reported. Galectin-3, -8, and -9 monitor bacteria vacuolar rupture, and endosomal and lysosomal loss of membrane integrity through binding of host glycans exposed in the cytoplasm after membrane damage (Mansilla Pareja et al., 2017). Moreover, vacuoles containing C. burnetii are characterized by a reversible recruitment of members of the endosomal sorting complex required for transport (ESCRT) and the interference with this recruitment reduces intravacuolar bacterial replication (Radulovic et al., 2018).
Innate Immunity in Chronic Q Fever
Patients affected by Q fever usually recover entirely through antimicrobial therapy or even without any treatment, but in less than 5% of cases they develop chronic infection, a serious disease difficult to treat (a minimum of 18 months of antimicrobial therapy is required). Endocarditis and vascular infection are the most frequent clinical manifestations that can be lethal if untreated (Mazokopakis et al., 2010). Moreover, patients returning from C. burnetii acute infection can develop a chronic fatigue syndrome (Wildman et al., 2002; Morroy et al., 2016).
Mechanisms involved in the establishment of chronic infection are unknown, but it has been suggested that the chronicity may be due to a specific immune deficiency (e.g., lack of IFN-γ) and not to a general immunodeficiency. Pregnant women, people affected by heart valve disorders, and cancer patients are most at risk to develop chronic infection.
Several studies focused on the role of the immune response, and defects in both innate and adaptive immunity have been linked to the risk of developing the disease (Capo and Mege, 2012).
For example, it was reported that monocytes from patients with chronic Q fever in evolution, who do not control the infection, exhibited defective phagosome maturation and impaired C. burnetii killing. Both responses were restored in patients recovering from Q fever. A significant correlation was reported between phagosome maturation and C. burnetii killing. Defective phagosome maturation and impaired C. burnetii killing were induced by interleukin (IL)-10 addition to monocytes from convalescent patients and were restored by IL-10 neutralization in chronic Q fever in evolution (Ghigo et al., 2004).
The defective granuloma formation, an ineffective high level of TNF, a lack of antimicrobial activity of monocytes, and the failure of T-cell response are other examples of anomalies that appear involved in the chronic infection establishment. Genetically modified mice overexpressing IL-10 have been used as a model to analyze the role of IL-10 in chronic Q fever development. It has been demonstrated that IL-10 overproduction suppresses the microbicidal pathway (NO synthase and inflammatory cytokines), making the macrophages incapable of killing C. burnetii. This altered immune response causes sustained C. burnetii burden in tissues, high levels of antibody, and impaired granuloma formation, which are typical of the chronic Q fever (Meghari et al., 2008).
Some studies (Marmion et al., 2009; Sukocheva et al., 2010) performed by inoculating SCID mice with samples (bone marrow, PBMC, or heart valve) coming from Q fever patients showed the persistence of the so-called “Immunomodulatory complex” (IMC) even after a long time away from acute infection. This is composed by non-infective and non-biodegraded Coxiella cell components, including C. burnetii antigens, specific LPS, and traces of genomic DNA. The persistence of the IMC involve the capacity to pass repeatedly into the macrophages, and it induces an abnormal immune response, which may explain the development of chronic sequelae as the long fatigue syndrome after the acute infection in some patients.
Th-1 and IFN-γ response is another aspect that has become crucial to control acute C. burnetii infection. The development of the chronic Q fever reflects indeed its failure, whereas the antibody response seems less important (Eldin et al., 2017). The IFN-γ inducible chemokine CXCL9 is higher in sera from chronic Q fever patients than in people healed from C. burnetii infection, and so it has been proposed as a chronic Q fever biomarker (Jansen et al., 2017a). Even genetics host factors have a role in chronic Q fever progression. For example, SNPs in genes crucial for phagolysosome maturation, bacterial killing, and autophagy are involved in higher or decreased susceptibility to chronic Q fever (Jansen et al., 2019).
A further example can be found in the genes coding for matrix metalloproteinases (MMPs). Coxiella burnetii in fact induces their overexpression, contributing to the onset of chronic Q fever. Moreover, SNPs in MMP genes are more common in patients affected by chronic infection than in healthy people, so they can be considered as a risk factor (Jansen et al., 2017b).
Persistent infection by C. burnetii is a serious problem also for animal health. Affected Cows, sheep, and goats indeed show reproductive troubles, which result in economic damage for the farmers. In cattle, chronic Q fever often causes ipofertility, endometritis, and low birth weight, whereas goats and sheep show more frequent abortion and premature delivery. In another study (De Biase et al., 2018), the correlation between C. burnetii infection and reproductive problems in cows was proven through the detection of the bacterium inside the uterine macrophages of cattle affected by chronic endometritis and low fertility and PCR and aerobic culture negative for other pathogens.
In addition, another important problem relating to affected animals is that they expel C. burnetii through milk, feces, vaginal mucus, and birth products, which are vehicles for spreading the infection between the livestock and to the humans. Cattle, goats, and sheep are indeed considered as the most important reservoirs for the human infection (Georgiev et al., 2013; Pouquet et al., 2020).
New Immunotherapeutic Interventions in Experimental Models of C. burnetii Infections
Model organisms and cell culture are relevant for understanding complex biological mechanisms, as the immune response and the interaction between pathogen and host (Metters et al., 2019). Animals belonging to invertebrates, small mammals, and non-human primates have been used to analyze the interaction between C. burnetii and host cell, the course of disease, and the role of innate and adaptive immunity against the infection (Bewley, 2013) (Table 1).
The nematode Caenorhabditis elegans is susceptible to C. burnetii phase II and it develops acute infection. The fruit fly Drosophila melanogaster is susceptible to C. burnetii phase II; the infection can be induced by injection and it can cause mortality, which in females is dose-dependent (Bastos et al., 2017). Larvae of the greater wax moth, Galleria mellonella, have been used as a model of C. burnetii infection. G. mellonella can be maintained at 37°C and are simple to be manipulated and cheap. Moreover, they present several functional homologues of innate immune effectors in mammals (Bergin et al., 2005). This insect model has been used for different purposes such as to investigate virulence differences between C. burnetii NMI and NMII strains or to characterize of T4SS mutants (Norville et al., 2014), to identify virulence genes (Selim et al., 2018), or to map the transcriptome of C. burnetii during the infection (Kovacs-Simon et al., 2020).
In vertebrate models, such as mice, guinea pigs, and monkeys, the infection can be induced through aerosol inhalation, which is the natural route of transmission, or by intraperitoneal exposure. Susceptibility to the infection and signs experienced vary depending on species and strains (Bewley, 2013; Metters et al., 2019). Mice are often naturally resistant to the C. burnetii infection, but murine strains with specific immunologic alterations are available (Schoenlaub et al., 2016). For example, those with severe combined immunodeficiency (SCID) have been used to analyze the various components of immune response to the bacterium (Scott et al., 1978; Andoh et al., 2005). Ticks are also susceptible to C. burnetii infection with a possible role in the epidemiology of Q fever (Herrin et al., 2011; Chaligiannis et al., 2018; Varela-Castro et al., 2018).
Guinea pigs have been employed as models of human Q fever. These animals are more susceptible than mice and they develop high fever and splenic, hepatic, and bone marrow granulomas. In addition, they show blood abnormalities (as increased AST) and severe weight loss. In various studies, guinea pigs have been used to evaluate the histopathological alterations induced by C. burnetii infection after intraperitoneal or aerosol exposure (Heggers et al., 1975). Similar experiments have been conducted on murine models, and it was observed that, in mice, lesions were close to those seen in guinea pigs but more diffused (Scott et al., 1978).
As regards larger animals, nonhuman primates are particularly interesting as a model of the course of human Q fever (Gonder et al., 1979). Because of these characteristics, nonhuman primates can be useful to understand the disease caused by C. burnetii in humans and to develop a more reliable vaccine. However, monkeys are less employed as experimental models than mice and guinea pigs because of farming and use costs, space required, and ethical problems raised. Finally, in vitro and ex vivo models deserve to be cited as experimental models. They consist respectively in single cells (e.g., alveolar macrophages) and in perfused organ or tissue, and they have been employed to study the C. burnetii biology and its interaction with host at the cellular and tissue level. These models have been essential to comprehend C. burnetii intracellular replication and the host cell response to the infection (Voth and Heinzen, 2007; van Schaik et al., 2013). Other animal species (e.g., dogs, lions, and camels; Torina et al., 2007; Hornok et al., 2013; El Tigani-Asil et al., 2021) are susceptible to C. burnetii but have not been used as experimental models. Coxiella-related tick endosymbionts have also been identified as microbiota component of some tick species (Díaz-Sánchez et al., 2019).
As concerning molecular studies, a recent study aimed at characterization of the transcriptome of murine alveolar macrophages infected with a Coxiella strain mutant for type IVB secretion system (T4BSS) effector identified a set of inflammatory genes significantly upregulated in T4BSS mutant-infected cells compared to controls, suggesting a downregulating role of Coxiella T4BSS effector proteins for these genes. In particular, IL-17 signaling pathway was significantly affected with the T4BSS mutants that exhibited significantly more sensitivity to IL-17 than WT bacteria.
An increased expression of IL-17 downstream signaling genes, including the proinflammatory cytokine genes Il1a, Il1b, and Tnfa, the chemokine genes Cxcl2 and Ccl5, and the antimicrobial protein gene Lcn2, resulted in T4BSS mutant-infected cells compared to controls, confirming that Coxiella downregulates IL-17 signaling in a T4BSS-dependent manner in order to escape the macrophage immune response (Clemente et al., 2018).
Another study was aimed to discover new effector proteins involved in C. burnetii innate immune evasion process by the Dot/Icm T4SS. In particular, the authors found a new C. burnetii effector protein, NopA (nucleolar protein A), which interacts with Ras-related nuclear protein (Ran). Since it localizes at nucleoli of infected cells, NopA triggers the accumulation of Ran-GTP at nucleoli, thus perturbing the nuclear import of transcription factors of the innate immune signaling pathway. Cells exposed to C. burnetii strains mutant for NopA or for the Dot/Icm system presented a functional innate immune response, unlike cells exposed to wild-type C. burnetii or to a nopA complemented strain, confirming the role of NopA as C. burnetii regulator of the host innate immune response (Burette et al., 2020).
A comparative microarray-based study found that C. burnetii protein synthesis modulated (≥2 fold change) 36 host cell genes, codifying proteins principally involved in innate immune response, cell death and proliferation, vesicle trafficking and development, lipid homeostasis, and cytoskeletal organization, as suggested by the ontological analysis. The authors suggested that the production of C. burnetii proteins modulates the host cell gene expression in order to avoid the immune response, promote the host cell survival, and direct the development and maintenance of a replicative parasitophorous vacuole by controlling vesicle formation and trafficking in the host cell (Mahapatra et al., 2010).
Current Diagnostic Approaches for Coxiella burnetii
Traditionally, laboratory diagnosis of C. burnetii can be carried out by detecting bacterial DNA (direct methods) or specific antibodies (indirect methods) in blood samples (Natale et al., 2012; Sahu et al., 2020).
Bacterium isolation is not a recommended diagnostic method since C. burnetii is classified as a level 3 containment microorganism and it requires biosafety level 3 conditions and prolonged incubation period (Sahu et al., 2020). Direct isolation can be obtained by inoculation of embryonated chicken eggs or cell culture, for example, in Vero cells or in L929 mouse fibroblasts. With heavily multi-contaminated samples, the inoculation of laboratory animals may be required and guinea pigs and mice are considered the most appropriate laboratory animals for this purpose (OIE, 2018). Also, a cell-free or axenic medium has been developed for C.burnetii culture, known as acidified citrate cysteine medium 2 (ACCM-2) (Omsland et al., 2009). The medium reduces oxidative stress (Sandoz et al., 2014) and mimics the intracellular environment of the host, allowing the propagation of the bacteria in both broth and solid agarose-based medium, at 37°C in a 2.5% O2 and 5% CO2 environment (Omsland et al., 2011). Another isolation medium, known as ACCM-D, has been formulated, including a supplementation of arginine, which further reduces the oxidative stress (Dresler et al., 2019). Axenic cultivation of C. burnetii will improve studies on its pathogenesis and genetics and will allow genetic manipulation of the pathogen (Omsland et al., 2009).
C. burnetii in tissue sections can be observed following staining with hematoxylin-eosin, periodic acid-schiff base, gomori methylamine silver stain, Giemsa, Warthin starry, and Gimenez technique (Woc-Colburn et al., 2008).
Microscopy-based observation can be carried out and electron microscopy can be used for pathogenicity studies, but it requires well equipped laboratory and skilled personnel and it is also a very expensive method (Justis et al., 2017). Other microscopic examinations include fluorescent microscopy, confocal microscopy, immunofluorescence, and/or immunoelectron microscopy, which can be used, respectively, to analyze intracellular growth and pathogenicity (Howe et al., 2003a; Howe et al., 2003b), to monitor phagolysosomal vacuole formation, localization, and multiplication of the microbes inside the CCV (Desnues et al., 2009; Miller et al., 2019; Moormeier et al., 2019), and to assess the location of a specific antigen or protein inside the bacterium (Morgan et al., 2010).
The polymerase chain reaction (PCR), an example of direct test, is particularly sensitive in the early stage of the infection, but its sensitivity decreases quickly following specific antibiotic treatment. Nowadays, C. burnetii infection is confirmed by real-time PCR detection, within 2 weeks after acute infections in sero-negative individuals together with clinical data and diagnostic imaging for the diagnosis of chronic Q fever (Schneeberger et al., 2010; Kampschreur et al., 2015; Eldin et al., 2017). The insertion sequence IS 1111 gene is considered as the target of choice in PCR detection of C. burnetii, as the repetitive element has a multi-copy gene that increases sensitivity of the method (Klee et al., 2006). Loop-mediated isothermal amplification (LAMP-PCR) assay presents with additional advantages such as rapidity, visual interpretation of results, and the possibility to carry out the reaction at a temperature between 60 and 65°C (Das et al., 2018).
Multi-spacer sequence typing (MST) and multi-locus variable number tandem repeat analysis (MLVA) are used for pathogen genotyping of C. burnetii (Galiero et al., 2016; Dabaja et al., 2020).
Immunological assays are useful once antibodies are detectable in the patient serum, about 10 days after the beginning of the disease, and the presence of antibodies against Coxiella in the serum still represent the gold standard for the diagnosis of the infection or past exposure to the Gram-negative coccobacillus. In the specific, complement fixation test was the previous reference test reported by OIE, but it was replaced due to its low sensitivity (OIE, 2018). The immunofluorescence assay (IFA) is more reliable even a year after acute infection or in chronic Q fever than other serological assays, such as ELISA or complement fixation test (Wegdam-Blans et al., 2012; Wegdam-Blans et al., 2014), although the absence of antibodies measured by IFA does not exclude a past exposure. Moreover, a different immune response is revealed in the presence of acute or chronic infection; as in acute Q fever, antibodies against C. burnetii phase II are prevalent, whereas in chronic Q fever, antibodies against C. burnetii phase I are the most represented (Mori et al., 2017).
Commercial ELISA kits able to detect both phase I and phase II stage antibodies or phase-specific antibodies are available (Kantsø et al., 2012; Meekelenkamp et al., 2012; Wegdam-Blans et al., 2014; El-Mahallawy et al., 2016) and ELISA represents a rapid, highly sensitive, and specific diagnostic assay (OIE, 2018).
In Australia, serology is also accompanied by a skin test, similar to tuberculin skin test, for evaluating cell-mediated response to C. burnetii (Marmion et al., 1990; Ruiz and Wolfe, 2014), but often the results coming from both assays are discordant. For this reason, other more sensitive and specific assays are under investigation.
Recently, it has been reported that C. burnetii-specific T-cell IFN-γ release may represent a sensitive and long-lasting marker of past C. burnetii exposure (Scholzen et al., 2021). The evaluation of cell-mediated responses to Coxiella through IFN-γ release assay (IGRA) could be very useful to detect past infections, since cellular response persists also in sero- and skin test-negative individuals (Schoffelen et al., 2014a; Schoffelen et al., 2013; Scholzen et al., 2021). It is still unknown which fraction of C. burnetii is responsible for IFN-γ release, but reported data show the existence of some immunodominant-specific peptides from its proteins that are recognized in IGRA-positive when compared with IGRA-negative subjects. Some endotoxins can also induce IFN-γ release from T and NK cells, probably mediated by monocytes, activated by Coxiella LPS, endothelial cells, and other cytokines (Le et al., 1986; Tennenberg and Weller, 1996; Lauw et al., 2000). Among T cells, preliminary data (Scholzen et al., 2021) indicate CD4+ cells as the major source of IFN-γ, followed by CD8+ T cells.
Protection of Coxiella burnetii Infected Mice Was Increased by TLR Triagonists Used as Adjuvants
Researchers observed in C. burnetii immunized mice a new successful component for vaccination strategies that takes advantage of a typical innate immunity receptor: TLR triagonists as good adjuvants to increase protection from infection symptoms (Gilkes et al., 2020). Using these new types of adjuvants that are able to stimulate immunity through binding simultaneously three different TLRs, the C. burnetii-specific Th1 and Ig responses were potentiated. Mice previously infected and treated with different TLR tritagonists do not suffer the typical symptoms of C. burnetii infections (e.g., fever, weight loss). These protective effects exerted by TLR tritagonists were accompanied by a beneficial Coxiella-specific adaptive immunity. In fact, mice receiving TLR tritagonists developed strong antigen-specific IgG and Th1 cells able to sustain a Coxiella-specific immune response (Figure 2).
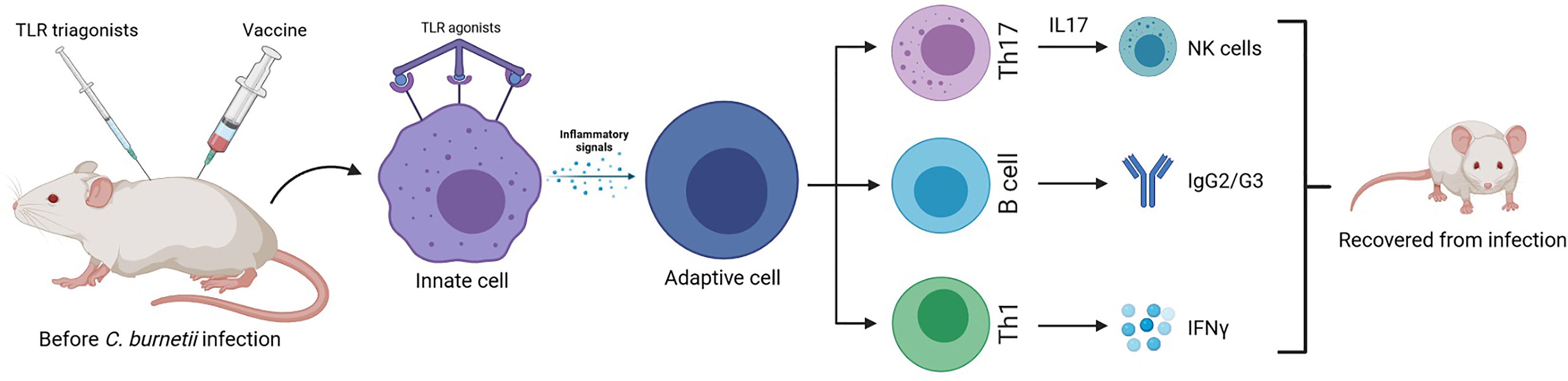
Figure 2 Murine model of enhanced protective response against C. burnetti infection: vaccines with TLR triagonists as adjuvants, activating multiple Toll-like receptors simultaneously, induce a strong antigen-specific humoral and cell-mediated (Th1) immune response against Coxiella infection, being a more successful vaccination strategy than the traditional one (Gilkes et al., 2020).
In addition, TLR stimulation of dendritic cells and other antigen-presenting cells represents a strong proinflammatory signal capable of overcoming even the control of regulatory T cells (Tregs). In fact, TLR expression has been reported also in adaptive immune lymphocytes, suggesting that the interaction with TLR ligands could stimulate or inhibit these cells (Hisaeda et al., 2004).
Further studies are needed to investigate the role of other cells potentially able to be involved in TLR triagonist effects such as Tregs, whose activation could be helpful for both host and pathogen, and innate lymphoid cells (ILCs) that could exert protection topically in the lungs and/or systemically. Another important effect necessary to better understand TLR tritagonist protections is represented by the analysis of T- and/or B-cell memory after the injection of TLR triagonists.
Conclusions
Non-human experimental models of protection are needed as a preclinical stage for the development of effective vaccination strategies. Taken together, a link between innate and adaptive immunity seems to be a good model to induce protection against C. burnetii infection, even if LPS detrimental effects are detected. Avoiding these side effects to design pre-clinical and clinical vaccination strategies could be a goal of future research in these fields. Definition of the protection exerted by other effector mechanisms such as ILCs or Th17-induced neutrophilia is necessary to complete the immunological scenario that supports protection against C. burnetii in TLR tritagonist animals.
An effort to define the effects of TLR triagonists on antigen-specific T- and B-cell memory could give a substantial completion of protection against C. burnetii infection (Fratzke et al., 2021). Furthermore, to translate this vaccination strategy using these adjuvants needs a complete definition of multiple immunological scenarios. Additionally, new immunodiagnostic assays, such as IGRA, could become more appreciable tools to diagnose past C. burnetii infections and they may result very helpful especially in the context of pre-vaccination screens. The application of latest omics, genetic, and in silico technologies for the identification of conserved T-cell epitopes in multiple host species, the development of genetically modified organisms, and the design of multi-epitope antigens are promising approaches for collaborative integration in the development of more effective and safe vaccines against Q fever (Gerlach et al., 2017; Reeves et al., 2017; Jaydari et al., 2020; Long et al., 2021; Nooroong et al., 2021; Piel et al., 2021).
Author Contributions
Conceptualization and design: GS and VB. Supervision: AT. Funding acquisition: AT and AG. Drafting the article: VB, LP, FG, and DL. Software: GB. Revision of article: GS and JF. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Italian Ministry of Health with grants RC IZSSI 08/19 and RC IZSSI 01/20.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abnave, P., Muracciole, X., Ghigo, E. (2017). Coxiella burnetii Lipopolysaccharide: What Do We Know? Int. J. Mol. Sci. 18, 2509. doi: 10.3390/ijms18122509
Amara, A. B., Bechah, Y., Mege, J. L. (2012). Immune Response and Coxiella burnetii Invasion. Adv. Exp. Med. Biol. 984, 287–298. doi: 10.1007/978-94-007-4315-1_15
Ammerdorffer, A., Kuley, R., Dinkla, A., Joosten, L. A. B., Toman, R., Roest, H. J., et al. (2017). Coxiella burnetii Isolates Originating From Infected Cattle Induce a More Pronounced Proinflammatory Cytokine Response Compared to Isolates From Infected Goats and Sheep. Pathog. Dis. 75, ftx040. doi: 10.1093/femspd/ftx040
Ammerdorffer, A., Schoffelen, T., Gresnigt, M. S., Oosting, M., den Brok, M. H., Abdollahi-Roodsaz, S., et al. (2015). Recognition of Coxiella burnetii by Toll-Like Receptors and Nucleotide-Binding Oligomerization Domain-Like Receptors. J. Infect. Dis. 211, 978–987. doi: 10.1093/infdis/jiu526
Ammerdorffer, A., Stappers, M. H., Oosting, M., Schoffelen, T., Hagenaars, J. C., Bleeker-Rovers, C. P., et al. (2016). Genetic Variation in TLR10 Is Not Associated With Chronic Q Fever, Despite the Inhibitory Effect of TLR10 on Coxiella burnetii-Induced Cytokines In Vitro. Cytokine 77, 196–202. doi: 10.1016/j.cyto.2015.09.005
Andoh, M., Russell-Lodrigue, K. E., Zhang, G., Samuel, J. E. (2005). Comparative Virulence of Phase I and II Coxiella burnetii in Immunodeficient Mice. Ann. N. Y. Acad. Sci. 1063, 167–170. doi: 10.1196/annals.1355.026
Bastos, R. G., Howard, Z. P., Hiroyasu, A., Goodman, A. G. (2017). Host and Bacterial Factors Control Susceptibility of Drosophila Melanogaster to Coxiella burnetii Infection. Infect. Immun. 85, e00218–e00217. doi: 10.1128/IAI.00218-17
Battisti, J. M., Watson, L. A., Naung, M. T., Drobish, A. M., Voronina, E., Minnick, M. F. (2017). Analysis of the Caenorhabditis Elegans Innate Immune Response to Coxiella burnetii. Innate Immun. 23, 111–127. doi: 10.1177/1753425916679255
Bergin, D., Reeves, E. P., Renwick, J., Wientjes, F. B., Kavanagh, K. (2005) Superoxide Production in Galleria Mellonella Hemocytes: Identification of Proteins Homologous to the NADPH Oxidase Complex of Human Neutrophils. Infect Immun 73(7):4161–70. doi: 10.1128/IAI.73.7.4161-4170.2005
Baumgärtner, W., Bachmann, S. (1992). Histological and Immunocytochemical Characterization of Coxiella burnetii-Associated Lesions in the Murine Uterus and Placenta. Infect. Immun. 60, 5232–5241. doi: 10.1128/iai.60.12.5232-5241.1992
Bisle, S., Klingenbeck, L., Borges, V., Sobotta, K., Schulze-Luehrmann, J., Menge, C., et al. (2016). The Inhibition of the Apoptosis Pathway by the Coxiella burnetii Effector Protein CaeA Requires the EK Repetition Motif, But Is Independent of Survivin. Virulence 7 (4), 400–412. doi: 10.1080/21505594.2016.1139280
Blanda, V., Bracale, U. M., Di Taranto, M. D., Fortunato, G. (2020). Galectin-3 in Cardiovascular Diseases. Int. J. Mol. Sci. 21, 9232. doi: 10.3390/ijms21239232
Boneca, I. G., Dussurget, O., Cabanes, D., Nahori, M. A., SoU. S. A, S., Lecuit, M., et al. (2007). A Critical Role for Peptidoglycan N-Deacetylation in Listeria Evasion From the Host Innate Immune System. Proc. Natl. Acad. Sci. U.S.A. 104, 997–1002. doi: 10.1073/pnas.0609672104
Bradley, W. P., Boyer, M. A., Nguyen, H. T., Birdwell, L. D., Yu, J., Ribeiro, J. M., et al. (2016). Primary Role for Toll-Like Receptor-Driven Tumor Necrosis Factor Rather Than Cytosolic Immune Detection in Restricting Coxiella burnetii Phase II Replication Within Mouse Macrophages. Infect. Immun. 84 (4), 998–1015. doi: 10.1128/IAI.01536-15
Burette, M., Allombert, J., Lambou, K., Maarifi, G., Nisole, S., Di Russo Case, E., et al. (2020). Modulation of Innate Immune Signaling by a Coxiella burnetii Eukaryotic-Like Effector Protein. Proc. Natl. Acad. Sci. U.S.A. 117 (24), 13708–13718. doi: 10.1073/pnas.1914892117
Byrne, G. I., Ojcius, D. M. (2004). Chlamydia and Apoptosis: Life and Death Decisions of an Intracellular Pathogen. Nat. Rev. Microbiol. 2, 802–808. doi: 10.1038/nrmicro1007
Capo, C., Amirayan, N., Ghigo, E., Raoult, D., Mege, J. (1999a). Circulating Cytokine Balance and Activation Markers of Leucocytes in Q Fever. Clin. Exp. Immunol. 115, 120–123. doi: 10.1046/j.1365-2249.1999.00786.x
Capo, C., Lindberg, F. P., Meconi, S., Zaffran, Y., Tardei, G., Brown, E. J., et al. (1999b). Subversion of Monocyte Functions by Coxiella burnetii: Impairment of the Cross-Talk Between Alphavbeta3 Integrin and CR3. J. Immunol. 163, 6078–6085.
Capo, C., Mege, J. L. (2012). Role of Innate and Adaptive Immunity in the Control of Q Fever. Adv. Exp. Med. Biol. 984, 273–286. doi: 10.1007/978-94-007-4315-1_14
Carmen, J. C., Hardi, L., Sinai, A. P. (2006). Toxoplasma Gondii Inhibits Ultraviolet Light-Induced Apoptosis Through Multiple Interactions With the Mitochondrion-Dependent Programmed Cell Death Pathway. Cell Microbiol. 8 (2), 301–315. doi: 10.1111/j.1462-5822.2005.00622.x
Castrejón-Jiménez, N. S., Leyva-Paredes, K., Hernández-González, J. C., Luna-Herrera, J., García-Pérez, B. E. (2015). The Role of Autophagy in Bacterial Infections. Biosci. Trends 9, 149–159. doi: 10.5582/bst.2015.01035
Caufrier, F., Martinou, A., Dupont, C., Bouriotis, V. (2003). Carbohydrate Esterase Family 4 Enzymes: Substrate Specificity. Carbohydr. Res. 338, 687–692. doi: 10.1016/S0008-6215(03)00002-8
Chaligiannis, I., Fernández de Mera, I. G., Papa, A., Sotiraki, S., de la Fuente, J. (2018). Molecular Identification of Tick-Borne Pathogens in Ticks Collected From Dogs and Small Ruminants From Greece. Exp. Appl. Acarol. 74, 443–453. doi: 10.1007/s10493-018-0237-z
Cherla, R., Zhang, Y., Ledbetter, L., Zhang, G. (2018). Coxiella burnetii Inhibits Neutrophil Apoptosis by Exploiting Survival Pathways and Antiapoptotic Protein Mcl-1. Infect. Immun. 86 (4), e00504–e00517. doi: 10.1128/IAI.00504-17
Clemente, T. M., Mulye, M., Justis, A. V., Nallandhighal, S., Tran, T. M., Gilk, S. D. (2018). Coxiella burnetii Blocks Intracellular Interleukin-17 Signaling in Macrophages. Infect. Immun. 86, e00532–e00518. doi: 10.1128/IAI.00532-18
Clifton, D. R., Goss, R. A., Sahni, S. K., van Antwerp, D., Baggs, R. B., Marder, V. J., et al. (1998). NF-Kappa B-Dependent Inhibition of Apoptosis Is Essential for Host Cellsurvival During Rickettsia Rickettsii Infection. Proc. Natl. Acad. Sci. U. S. A. 95 (8), 4646–4651. doi: 10.1073/pnas.95.8.4646
Cockrell, D. C., Long, C. M., Robertson, S. J., Shannon, J. G., Miller, H. E., Myers, L., et al. (2017). Robust Growth of Avirulent Phase II Coxiella burnetii in Bone Marrow-Derived Murine Macrophages. PloS One 12, e0173528. doi: 10.1371/journal.pone.0173528
Coleman, S. A., Fischer, E. R., Howe, D., Mead, D. J., Heinzen, R. A. (2004). Temporal Analysis of Coxiella burnetii Morphological Differentiation. J. Bacteriol. 186 (21), 7344–7352. doi: 10.1128/JB.186.21.7344-7352.2004
Colombo, M. I., Gutierrez, M. G., Romano, P. S. (2006). The Two Faces of Autophagy: Coxiella and Mycobacterium. Autophagy 2, 162–164. doi: 10.4161/auto.2827
Conti, F., Boucherit, N., Baldassarre, V., Trouplin, V., Toman, R., Mottola, G., et al. (2015). Coxiella burnetii Lipopolysaccharide Blocks P38α-MAPK Activation Through the Disruption of TLR-2 and TLR-4 Association. Front. Cell Infect. Microbiol. 4, 182. doi: 10.3389/fcimb.2014.00182
Cordsmeier, A., Wagner, N., Lührmann, A., Berens, C. (2019). Defying Death - How Coxiella burnetii Copes With Intentional Host Cell Suicide. Yale J. Biol. Med. 92 (4), 619–628.
Cory, S., Adams, J. (2002). The Bcl2 Family: Regulators of the Cellular Life-or-Death Switch. Nat. Rev. Cancer 2, 647–656. doi: 10.1038/nrc883
Cunha, L. D., Ribeiro, J. M., Fernandes, T. D., Massis, L. M., Khoo, C. A., Moffatt, J. H., et al. (2015). Inhibition of Inflammasome Activation by Coxiella burnetii Type IV Secretion System Effector IcaA. Nat. Commun. 6, 10205. doi: 10.1038/ncomms10205
Cunha, L. D., Zamboni, D. S. (2013). Subversion of Inflammasome Activation and Pyroptosis by Pathogenic Bacteria. Front. Cell Infect. Microbiol. 3, 76. doi: 10.3389/fcimb.2013.00076
Dabaja, M. F., Greco, G., Blanda, V., Tempesta, M., Bayan, A., Torina, A., et al. (2020). Multispacer Sequence Typing of Coxiella burnetii From Milk and Hard Tick Samples From Ruminant Farms in Lebanon. Vet. Ital. 56, 289–296. doi: 10.12834/VetIt.1799.13290.1
Das, D. P., Malik, S. V. S., Sahu, R., Yadav, J. P., Rawool, D. B., Barbuddhe, S. B. (2018). Loop-Mediated Isothermal Amplification Assay for Detection of Coxiella burnetii Targeting the Com1 Gene. J. Microbiol. Methods 155, 55–58. doi: 10.1016/j.mimet.2018.11.011
Davis, K. M., Weiser, J. N. (2011). Modifications to the Peptidoglycan Backbone Help Bacteria to Establish Infection. Infect. Immun. 79, 562–570. doi: 10.1128/IAI.00651-10
De Biase, D., Costagliola, A., Del Piero, F., Di Palo, R., Coronati, D., Galiero, G., et al. (2018). Coxiella burnetii in Infertile Dairy Cattle With Chronic Endometritis. Vet. Pathol. 55, 539–542. doi: 10.1177/0300985818760376
Delaney, M. A., Hartigh, A. D., Carpentier, S. J., Birkland, T. P., Knowles, D. P., Cookson, B. T., et al. (2021). Avoidance of the NLRP3 Inflammasome by the Stealth Pathogen, Coxiella burnetii. Vet. Pathol. 58, 624–642. doi: 10.1177/0300985820981369
Dellacasagrande, J., Capo, C., Raoult, D., Mege, J. L. (1999). IFN-Gamma-Mediated Control of Coxiella burnetii Survival in Monocytes: The Role of Cell Apoptosis and TNF. J. Immunol. 162, 2259–2265.
Dellacasagrande, J., Ghigo, E., Capo, C., Raoult, D., Mege, J. L. (2000a). Coxiella burnetii Survives in Monocytes From Patients With Q Fever Endocarditis: Involvement of Tumor Necrosis Factor. Infect. Immun. 68, 160–164. doi: 10.1128/IAI.68.1.160-164.2000
Dellacasagrande, J., Ghigo, E., Hammami, S. M., Toman, R., Raoult, D., Capo, C., et al. (2000b). Alpha(V)Beta(3) Integrin and Bacterial Lipopolysaccharide Are Involved in Coxiella burnetii-Stimulated Production of Tumor Necrosis Factor by Human Monocytes. Infect. Immun. 68, 5673–5678. doi: 10.1128/IAI.68.10.5673-5678.2000
Dellacasagrande, J., Ghigo, E., Raoult, D., Capo, C., Mege, J. L. (2002). IFN-Gamma-Induced Apoptosis and Microbicidal Activity in Monocytes Harboring the Intracellular Bacterium Coxiella burnetii Require Membrane TNF and Homotypic Cell Adherence. J. Immunol. 169, 6309–6315. doi: 10.4049/jimmunol.169.11.6309
Desnues, B., Imbert, G., Raoult, D., Mege, J. L., Ghigo, E. (2009). Role of Specific Antibodies in Coxiella burnetii Infection of Macrophages. Clin. Microbiol. Infect. 15 Suppl 2, 161–162. doi: 10.1111/j.1469-0691.2008.02208.x
Díaz-Sánchez, S., Hernández-Jarguín, A., Torina, A., de Mera, I. G. F., Blanda, V., Caracappa, S., et al. (2019). Characterization of the Bacterial Microbiota in Wild-Caught Ixodes Ventalloi. Ticks Tick Borne Dis. 10, 336–343. doi: 10.1016/j.ttbdis.2018.11.014
Dragan, A. L., Voth, D. E. (2020). Coxiella burnetii: International Pathogen of Mystery. Microbes Infect. 22, 100–110. doi: 10.1016/j.micinf.2019.09.001
Dresler, J., Klimentova, J., Pajer, P., Salovska, B., Fucikova, A. M., Chmel, M., et al. (2019). Quantitative Proteome Profiling of Coxiella burnetii Reveals Major Metabolic and Stress Differences Under Axenic and Cell Culture Cultivation. Front. Microbiol. 10, 2022. doi: 10.3389/fmicb.2019.02022
Eckart, R. A., Bisle, S., Schulze-Luehrmann, J., Wittmann, I., Jantsch, J., Schmid, B., et al. (2014). Antiapoptotic Activity of Coxiella burnetii Effector Protein AnkG Is Controlled by P32-Dependent Trafficking. Infect. Immun. 82 (7), 2763–2771. doi: 10.1128/IAI.01204-13
EFSA (2010). Scientific Opinion on Q Fever. EFSA J 8, 1595.Eldin, C., Angelakis, E., Renvoise, A. And Raoult, D., (2013) Coxiella burnetii DNA, But Not Viable Bacteria, in Dairyproducts in France. Am. J. Trop. Med. Hyg. 88, 765–769. doi: 10.4269/ajtmh.12-0212
El-Deeb, W., Ghoneim, I., Fayez, M., Elsohaby, I., Alhaider, A., ElGioushy, M. (2019). Acute Phase Proteins, Proinflammatory Cytokines and Oxidative Stress Biomarkers in Sheep, Goats and She-Camels With Coxiella burnetii Infection-Induced Abortion. Comp. Immunol. Microbiol. Infect. Dis. 67, 101352. doi: 10.1016/j.cimid.2019.101352
Eldin, C., Mélenotte, C., Mediannikov, O., Ghigo, E., Million, M., Edouard, S., et al. (2017). From Q Fever to Coxiella burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 30, 115–190. doi: 10.1128/CMR.00045-16
Elliott, A., Peng, Y., Zhang, G. (2013). Coxiella burnetii Interaction With Neutrophils and Macrophages In Vitro and in SCID Mice Following Aerosol Infection. Infect. Immun. 81, 4604–4614. doi: 10.1128/IAI.00973-13
El-Mahallawy, H. S., Kelly, P., Zhang, J., Yang, Y., Wei, L., Tian, L., et al. (2016). Serological and Molecular Evidence of Coxiella burnetii in Samples From Humans and Animals in China. Ann. Agric. Environ. Med. 23 (1), 87–91. doi: 10.5604/12321966.1196859
El Tigani-Asil, E. T. A., Blanda, V., Abdelwahab, G. E., Hammadi, Z. M. A., Habeeba, S., Khalafalla, A. I., et al. (2021). Molecular Investigation on Tick-Borne Hemoparasites and Coxiella burnetii in Dromedary Camels (Camelus Dromedarius) in Al Dhafra Region of Abu Dhabi, UAE. Anim. (Basel) 11 (3):666. doi: 10.3390/ani11030666
Enari, M., Sakahira, H., Yokoyama, H., Okawa, K., Iwamatsu, A., Nagata, S. (1998). A Caspase-Activated DNase That Degrades DNA During Apoptosis, and Its Inhibitor ICAD. Nature 391 (6662), 43–50. doi: 10.1038/34112
Fernandes, T. D., Cunha, L. D., Ribeiro, J. M., Massis, L. M., Lima-Junior, D. S., Newton, H. J., et al. (2016). Murine Alveolar Macrophages Are Highly Susceptible to Replication of Coxiella burnetii Phase II In Vitro. Infect. Immun. 84, 2439–2448. doi: 10.1128/IAI.00411-16
Fischer, S. F., Vier, J., Kirschnek, S., Klos, A., Hess, S., Ying, S., et al. (2004). Chlamydia Inhibit Host Cell Apoptosis by Degradation of Proapoptotic BH3-Only Proteins. J. Exp. Med. 200 (7), 905–916. doi: 10.1084/jem.20040402
Fratzke, A. P., Jan, S., Felgner, J., Liang, L., Nakajima, R., Jasinskas, A., et al. (2021). Subunit Vaccines Using TLR Triagonist Combination Adjuvants Provide Protection Against Coxiella burnetii While Minimizing Reactogenic Responses. Front. Immunol. 12, 653092. doi: 10.3389/fimmu.2021.653092
Friedrich, A., Beare, P. A., Schulze-Luehrmann, J., Cordsmeier, A., Pazen, T., Sonnewald, S., et al. (2021). The Coxiella burnetii Effector Protein CaeB Modulates Endoplasmatic Reticulum (ER) Stress Signalling and Is Required for Efficient Replication in Galleria Mellonella. Cell. Microbiol. 23 (4), e13305. doi: 10.1111/cmi.13305
Gale, P., Kelly, L., Mearns, R., Duggan, J., Snary, E. L. (2015). Q Fever Through Consumption of Unpasteurised Milk and Milk Products - a Risk Profile and Exposure Assessment. J. Appl. Microbiol. 118 (5), 1083–1095. doi: 10.1111/jam.12778
Galiero, A., Fratini, F., Cammà, C., Di Domenico, M., Curini, V., Baronti, I., et al. (2016). Occurrence of Coxiella burnetii in Goat and Ewe Unpasteurized Cheeses: Screening and Genotyping. Int. J. Food Microbiol. 237, 47–54. doi: 10.1016/j.ijfoodmicro.2016.08.008
Georgiev, M., Afonso, A., Neubauer, H., Needham, H., Thiery, R., Rodolakis, A., et al. (2013). Q Fever in Humans and Farm Animals in Four European Countrieto 2010. Euro Surveill. 18 (8), 20407. doi: 10.2807/ese.18.08.20407-en
Gerlach, C., Škultéty, Ľ., Henning, K., Neubauer, H., Mertens, K. (2017). Coxiella burnetii Immunogenic Proteins as a Basis for New Q Fever Diagnostic and Vaccine Development. Acta Virol. 61, 377–390. doi: 10.4149/av_2017_320
Ghigo, E., Capo, C., Raoult, D., Mege, J. L. (2001). Interleukin-10 Stimulates Coxiella burnetii Replication in Human Monocytes Through Tumor Necrosis Factor Down-Modulation: Role in Microbicidal Defect of Q Fever. Infect. Immun. 69, 2345–2352. doi: 10.1128/IAI.69.4.2345-2352.2001
Ghigo, E., Honstettre, A., Capo, C., Gorvel, J. P., Raoult, D., Mege, J. L. (2004). Link Between Impaired Maturation of Phagosomes and Defective Coxiella burnetii Killing in Patients With Chronic Q Fever. J. Infect. Dis. 190, 1767–1772. doi: 10.1086/425041
Ghigo, E., Pretat, L., Desnues, B., Capo, C., Raoult, D., Mege, J. L. (2009). Intracellular Life of Coxiella burnetii in Macrophages. Ann. N. Y. Acad. Sci. 1166, 55–66. doi: 10.1111/j.1749-6632.2009.04515.x
Gilkes, A. P., Albin, T. J., Manna, S., Supnet, M., Ruiz, S., Tom, J., et al. (2020). Tuning Subunit Vaccines With Novel TLR Triagonist Adjuvants to Generate Protective Immune Responses Against Coxiella burnetii. J. Immunol. 204, 611–621. doi: 10.4049/jimmunol.1900991
Gonder, J. C., Kishimoto, R. A., Kastello, M. D., Pedersen, C. E., Jr, Larson, E. W. (1979). Cynomolgus Monkey Model for Experimental Q Fever Infection. J. Infect. Dis. 139, 191–196. doi: 10.1093/infdis/139.2.191
Graham, J. G., MacDonald, L. J., Hussain, S. K., Sharma, U. M., Kurten, R. C., Voth, D. E. (2013). Virulent Coxiella burnetii Pathotypes Productively Infect Primary Human Alveolar Macrophages. Cell Microbiol. 15, 1012–1025. doi: 10.1111/cmi.12096
Gürtler, L., Bauerfeind, U., Blümel, J., Burger, R., Drosten, C., Gröner, A., et al. (2014). Coxiella burnetii - Pathogenic Agent of Q (Query) Fever. Transfus. Med. Hemother. 41 (1), 60–72. doi: 10.1159/000357107
Gutierrez, M. G., Colombo, M. I. (2005). Autophagosomes: A Fast-Food Joint for Unexpected Guests. Autophagy 1, 179–181. doi: 10.4161/auto.1.3.2063
Hackstadt, T. (1986). Antigenic Variation in the Phase I Lipopolysaccharide of Coxiella burnetii Isolates. Infect. Immun. 52, 337–340. doi: 10.1128/iai.52.1.337-340.1986
Hackstadt, T. (1990). The Role of Lipopolysaccharides in the Virulence of Coxiella burnetii. Ann. N. Y. Acad. Sci. 590, 27–32. doi: 10.1111/j.1749-6632.1990.tb42203.x
Hayward, J. A., Mathur, A., Ngo, C., Man, S. M. (2018). Cytosolic Recognition of Microbes and Pathogens: Inflammasomes in Action. Microbiol. Mol. Biol. Rev. 82, e00015–e00018. doi: 10.1128/MMBR.00015-18
Hedges, J. F., Robison, A., Kimmel, E., Christensen, K., Lucas, E., Ramstead, A., et al. (2016). Type I Interferon Counters or Promotes Coxiella burnetii Replication Dependent on Tissue. Infect. Immun. 84, 1815–1825. doi: 10.1128/IAI.01540-15
Heggers, J. P., Billups, L. H., Hinrichs, D. J., Mallavia, L. P. (1975). Pathophysiologic Features of Q Fever-Infected Guinea Pigs. Am. J. Vet. Res. 36 (7), 1047–1052.
Herrin, B., Mahapatra, S., Blouin, E. F., Shaw, E. I. (2011). Growth of Coxiella burnetii in the Ixodes Scapularis-Derived IDE8 Tick Cell Line. Vector Borne Zoonotic Dis. 11, 917–922. doi: 10.1089/vbz.2010.0126
Hisaeda, H., Maekawa, Y., Iwakawa, D., Okada, H., Himeno, K., Kishihara, K., et al. (2004). Escape of Malaria Parasites From Host Immunity Requires CD4+CD25+ Regulatory T Cells. Nat. Med. 10, 29–30. doi: 10.1038/nm975
Hitchcock, P. J., Leive, L., Makela, P. E., Rietchel, E. T., Strittmatter, W., Morrison, D. C. (1986). Lipopolysaccharide Nomenclature-Past, Present, and Future. J. Bacteriol. 166, 699–705.52. doi: 10.1128/jb.166.3.699-705.1986
Honstettre, A., Ghigo, E., Moynault, A., Capo, C., Toman, R., Akira, S., et al. (2004). Lipopolysaccharide From Coxiella burnetii Is Involved in Bacterial Phagocytosis, Filamentous Actin Reorganization, and Inflammatory Responses Through Toll-Like Receptor 4. J. Immunol. 172, 3695–3703. doi: 10.4049/jimmunol.172.6.3695
Honstettre, A., Imbert, G., Ghigo, E., Gouriet, F., Capo, C., Raoult, D., et al. (2003). Dysregulation of Cytokines in Acute Q Fever: Role of Interleukin-10 and Tumor Necrosis Factor in Chronic Evolution of Q Fever. J. Infect. Dis. 187, 956–962. doi: 10.1086/368129
Hornok, S., Dénes, B., Meli, M. L., Tánczos, B., Fekete, L., Gyuranecz, M., et al. (2013). Non-Pet Dogs as Sentinels and Potential Synanthropic Reservoirs of Tick-Borne and Zoonotic Bacteria. Vet. Microbiol. 167, 700–703. doi: 10.1016/j.vetmic.2013.08.011
Howe, D., Melnicáková, J., Barák, I., Heinzen, R. A. (2003a). Maturation of the Coxiella burnetii Parasitophorous Vacuole Requires Bacterial Protein Synthesis But Not Replication. Cell Microbiol. 5 (7), 469–480. doi: 10.1046/j.1462-5822.2003.00293.x
Howe, D., Melnicákova, J., Barák, I., Heinzen, R. A. (2003b). Fusogenicity of the Coxiella burnetii Parasitophorous Vacuole. Ann. N. Y. Acad. Sci. 990, 556–562. doi: 10.1111/j.1749-6632.2003.tb07426.x
Jansen, A. F. M., Dinkla, A., Roest, H. J., Bleeker-Rovers, C. P., Schoffelen, T., Joosten, L. A. B., et al. (2018). Viable Coxiella burnetii Induces Differential Cytokine Responses in Chronic Q Fever Patients Compared to Heat-Killed Coxiella burnetii. Infect. Immun. 86, e00333-18. doi: 10.1128/IAI.00333-18
Jansen, A. F. M., Schoffelen, T., Bleeker-Rovers, C. P., Wever, P. C., Jaeger, M., Oosting, M., et al. (2019). Genetic Variations in Innate Immunity Genes Affect Response to Coxiella burnetii and Are Associated With Susceptibility to Chronic Q Fever. Clin. Microbiol. Infect. 25, 631. doi: 10.1016/j.cmi.2018.08.011
Jansen, A. F. M., Schoffelen, T., Textoris, J., Mege, J. L., Bleeker-Rovers, C. P., Roest, H. I. J., et al. (2017b). Involvement of Matrix Metalloproteinases in Chronic Q Fever. Clin. Microbiol. Infect. 23, 487.e7–487.e13. doi: 10.1016/j.cmi.2017.01.022
Jansen, A. F. M., Schoffelen, T., Textoris, J., Mege, J. L., Nabuurs-Franssen, M., Raijmakers, R. P. H., et al. (2017a). CXCL9, a Promising Biomarker in the Diagnosis of Chronic Q Fever. BMC Infect. Dis. 17, 556. doi: 10.1186/s12879-017-2656-6
Janssens, S., Beyaert, R. (2002). A Universal Role for MyD88 in TLR/IL-1R-Mediated Signaling. Trends Biochem. Sci. 27, 474–482. doi: 10.1016/S0968-0004(02)02145-X
Jaydari, A., Nazifi, N., Forouharmehr, A. (2020). Computational Design of a Novel Multi-Epitope Vaccine Against Coxiella burnetii. Hum. Immunol. 81, 596–605. doi: 10.1016/j.humimm.2020.05.010
Justis, A. V., Hansen, B., Beare, P. A., King, K. B., Heinzen, R. A., Gilk, S. D. (2017). Interactions Between the Coxiella burnetii Parasitophorous Vacuole and the Endoplasmic Reticulum Involve the Host Protein ORP1L. Cell. Microbiol. 19 (1):10.1111/cmi.12637. doi: 10.1111/cmi.12637
Ka, M. B., Mezouar, S., Ben Amara, A., Raoult, D., Ghigo, E., Olive, D., et al. (2016). Coxiella burnetii Induces Inflammatory Interferon-Like Signature in Plasmacytoid Dendritic Cells: A New Feature of Immune Response in Q Fever. Front. Cell Infect. Microbiol. 6, 70. doi: 10.3389/fcimb.2016.00070
Kampschreur, L. M., Wegdam-Blans, M. C., Wever, P. C., Renders, N. H., Delsing, C. E., Sprong, T., et al. (2015). Chronic Q Fever Diagnosis- Consensus Guideline Versus Expert Opinion. Emerg. Infect. Dis. 21 (7), 1183–1188. doi: 10.3201/eid2107.130955
Kantsø, B., Svendsen, C. B., Jørgensen, C. S., Krogfelt, K. A. (2012). Comparison of Two Commercially Available ELISA Antibody Test Kits for Detection of Human Antibodies Against Coxiella burnetii. Scand. J. Infect. Dis. 44 (7), 489–494. doi: 10.1016/j.jinf.2016.01.004
Kaoukab-Raji, A., Biskri, L., Bernardini, M. L., Allaoui, A. (2012). Characterization of SfPgdA, a Shigella Flexneri Peptidoglycan Deacetylase Required for Bacterial Persistence Within Polymorphonuclear Neutrophils. Microbes Infect. 14, 619–627. doi: 10.1016/j.micinf.2012.01.009
Keijmel, S. P., Raijmakers, R.P., Bleeker-Rovers, C. P., van der Meer, J. W., Netea, M. G., Schoffelen, T., et al. (2016). Altered Interferon-γ Response in Patients with Q-Fever Fatigue Syndrome. J. Infect. 72:478–485. doi: 10.1016/j.jinf.2016.01.004
Klee, S. R., Tyczka, J., Ellerbrok, H., Franz, T., Linke, S., Baljer, G., et al. (2006). Highly Sensitive Real-Time PCR for Specific Detection and Quantification of Coxiella burnetii. BMC Microbiol. 6, 2. doi: 10.1186/1471-2180-6-2
Kohler, L. J., Roy, C. R. (2015). Biogenesis of the Lysosome-Derived Vacuole Containing Coxiella burnetii. Microbes Infect. 17 (11-12), 766–771. doi: 10.1016/j.micinf.2015.08.006
Kohl, L., Hayek, I., Daniel, C., Schulze-Lührmann, J., Bodendorfer, B., Lührmann, A., et al. (2019). MyD88 Is Required for Efficient Control of Coxiella burnetii Infection and Dissemination. Front. Immunol. 10, 165. doi: 10.3389/fimmu.2019.00165
Kovacs-Simon, A., Metters, G., Norville, I., Hemsley, C., Titball, R. W. (2020). Coxiella burnetii Replicates in Galleria Mellonella Hemocytes and Transcriptome Mapping Reveals In Vivo Regulated Genes. Virulence 11 (1), 1268–1278. doi: 10.1080/21505594.2020.1819111
Lai, C. H., Lin, J. N., Chang, L. L., Chen, Y. H., Lin, H. H. (2010). Circulating Cytokines and Procalcitonin in Acute Q Fever Granulomatous Hepatitis With Poor Response to Antibiotic and Short-Course Steroid Therapy: A Case Report. BMC Infect. Dis. 10, 193. doi: 10.1186/1471-2334-10-193
Lauw, F. N., Pajkrt, D., Hack, C. E., Kurimoto, M., van Deventer, S. J., van der Poll, T. (2000). Proinflammatory Effects of IL-10 During Human Endotoxemia. J. Immunol. 165 (5), 2783–2789. doi: 10.4049/jimmunol.165.5.2783
Le, J., Lin, J. X., Henriksen-DeStefano, D., Vilcek, J. (1986). Bacterial Lipopolysaccharide-Induced Interferon-Gamma Production: Roles of Interleukin 1 and Interleukin 2. J. Immunol. 136 (12), 4525–4530.
Leone, M., Honstettre, A., Lepidi, H., Capo, C., Bayard, F., Raoult D and Mege, J. L. (2004). Effect of Sex on Coxiella burnetii Infection: Protective Role of 17beta-Estradiol. J. Infect. Dis. 189, 339–345. doi: 10.1086/380798
Liu, M., Haenssler, E., Uehara, T., Losick, V. P., Park, J. T., Isberg, R. R. (2012). The Legionella Pneumophila EnhC Protein Interferes With Immunostimulatory Muramyl Peptide Production to Evade Innate Immunity. Cell Host Microbe 12, 166 –1176. doi: 10.1016/j.chom.2012.06.004
Long, C. M., Beare, P. A., Cockrell, D. C., Fintzi, J., Tesfamariam, M., Shaia, C. I., et al. (2021). Contributions of Lipopolysaccharide and the Type IVB Secretion System to Coxiella burnetii Vaccine Efficacy and Reactogenicity. NPJ Vaccines 6 (1):38. doi: 10.1038/s41541-021-00296-6
Lührmann, A., Newton, H. J., Bonazzi, M. (2017). Beginning to Understand the Role of the Type IV Secretion System Effector Proteins in Coxiella burnetii Pathogenesis. Curr. Top. Microbiol. Immunol. 413, 243–268. doi: 10.1007/978-3-319-75241-9_10
Lührmann, A., Nogueira, C. V., Carey, K. L., Roy, C. R. (2010). Inhibition of Pathogen-Induced Apoptosis by a Coxiella burnetii Type IV Effector Protein. Proc. Natl. Acad. Sci. U. S. A. 107 (44), 18997–19001. doi: 10.1073/pnas.1004380107
Lührmann, A., Roy, C. R. (2007). Coxiella burnetii Inhibits Activation of Host Cell Apoptosis Through a Mechanism That Involves Preventing Cytochrome C Release From Mitochondria. Infect. Immun. 75 (11), 5282–5289. doi: 10.1128/IAI.00863-07
Lukácová, M., Barák, I., Kazár, J. (2008). Role of Structural Variations of Polysaccharide Antigens in the Pathogenicity of Gram-Negative Bacteria. Clin. Microbiol. Infect. 14, 200–206. doi: 10.1111/j.1469-0691.2007.01876.x
Mahapatra, S., Ayoubi, P., Shaw, E. I. (2010). Coxiella burnetii Nine Mile II Proteins Modulate Gene Expression of Monocytic Host Cells During Infection. BMC Microbiol. 10, 244. doi: 10.1186/1471-2180-10-244
Małaczewska, J., Kaczorek-Łukowska, E., Szymańska-Czerwińska, M., Rękawek, W., Wójcik, R., Niemczuk, K., et al. (2018). Early Cytokine Response After Vaccination With Coxiella burnetii Phase I in an Infected Herd of Dairy Cattle. J. Vet. Res. 62, 469–476. doi: 10.2478/jvetres-2018-0076
Mansilla Pareja, M. E., Bongiovanni, A., Lafont, F., Colombo, M. I. (2017). Alterations of the Coxiella burnetii Replicative Vacuole Membrane Integrity and Interplay With the Autophagy Pathway. Front. Cell Infect. Microbiol. 7, 112. doi: 10.3389/fcimb.2017.00112
Marmion, B. P., Ormsbee, R. A., Kyrkou, M., Wright, J., Worswick, D. A., Izzo, A. A., et al. (1990). Vaccine Prophylaxis of Abattoir-Associated Q Fever: Eight Years’ Experience in Australian Abattoirs. Epidemiol. Infect. 104 (2), 275–287. doi: 10.1017/S0950268800059458
Marmion, B. P., Sukocheva, O., Storm, P. A., Lockhart, M., Turra, M., Kok, T., et al (2009). Q Fever: Persistence of Antigenic Non-Viable Cell Residues of Coxiella burnetii in the Host–Implications for Post Q Fever Infection Fatigue Syndrome and Other Chronic Sequelae. QJM 102, 673–684. doi: 10.1093/qjmed/hcp077
Marrie, T. J., Minnick, M. F., Textoris, J., Capo, C., Mege, J. L. (2015). “Coxiella,” in In Molecular Medical Microbiology (Academic Press, Cambridge, Massachusetts, U. S. A.), 1941–1972.
Martinez, E., Allombert, J., Cantet, F., Lakhani, A., Yandrapalli, N., Neyret, A., et al. (2016). Coxiella burnetii Effector CvpB Modulates Phosphoinositide Metabolism for Optimal Vacuole Development. Proc. Natl. Acad. Sci. U. S. A. 113 (23), E3260–E3269. doi: 10.1073/pnas.1522811113
Maurin, M., Raoult, D. (1999). Q Fever. Clin. Microbiol. Rev. 12, 518–553. doi: 10.1128/CMR.12.4.518
Mazokopakis, E. E., Karefilakis, C. M., Starakis, I. K. (2010). Q Fever Endocarditis. Infect. Disord. Drug Targets 10, 27–31. doi: 10.2174/187152610790410918
Medzhitov, R. (2007). TLR-Mediated Innate Immune Recognition. Semin. Immunol. 19, 1–2. doi: 10.1016/j.smim.2007.02.001
Meekelenkamp, J. C., Schneeberger, P. M., Wever, P. C., Leenders, A. C. (2012). Comparison of ELISA and Indirect Immunofluorescent Antibody Assay Detecting Coxiella burnetii IgM Phase II for the Diagnosis of Acute Q Fever. Eur. J. Clin. Microbiol. Infect. Dis. 31 (6), 1267–1270. doi: 10.1007/s10096-011-1438-0
Meghari, S., Bechah, Y., Capo, C., Lepidi, H., Raoult, D., Murray, P. J., et al. (2008). Persistent Coxiella burnetii Infection in Mice Overexpressing IL-10: An Efficient Model for Chronic Q Fever Pathogenesis. PloS Pathog. 4, e23. doi: 10.1371/journal.ppat.0040023
Meghari, S., Honstettre, A., Lepidi, H., Ryffel, B., Raoult, D., Mege, J. L. (2005). TLR2 is Necessary to Inflammatory Response in Coxiella burnetii Infection. Ann. N. Y. Acad. Sci. 1063, 161–166. doi: 10.1196/annals.1355.025
Metters, G., Norville, I. H., Titball, R. W., Hemsley, C. M. (2019). From Cell Culture to Cynomolgus Macaque: Infection Models Show Lineage-Specific Virulence Potential of Coxiella burnetii. J. Med. Microbiol. 68, 1419–1430. doi: 10.1099/jmm.0.001064
Mezouar, S., Benammar, I., Boumaza, A., Diallo, A. B., Chartier, C., Buffat, C., et al. (2019a). Full-Term Human Placental Macrophages Eliminate Coxiella burnetii Through an IFN-γ Autocrine Loop. Front. Microbiol. 10, 2434. doi: 10.3389/fmicb.2019.02434
Mezouar, S., Morel, V., Leveille, L., Resseguier, N., Chartier, C., Raoult, D., et al. (2019b). Progenitor Mast Cells and Tryptase in Q Fever. Comp. Immunol. Microbiol. Infect. Dis. 64, 159–162. doi: 10.1016/j.cimid.2019.03.011
Miller, H. E., Hoyt, F. H., Heinzen, R. A. (2019). Replication of Coxiella burnetii in a Lysosome-Like Vacuole Does Not Require Lysosomal Hydrolases. Infect. Immun. 87 (11), e00493–e00419. doi: 10.1128/IAI.00493-19
Moormeier, D. E., Sandoz, K. M., Beare, P. A., Sturdevant, D. E., Nair, V., Cockrell, D. C., et al. (2019). Coxiella burnetii RpoS Regulates Genes Involved in Morphological Differentiation and Intracellular Growth. J. Bacteriol. 201 (8), e00009–e00019. doi: 10.1128/JB.00009-19
Moos, A., Hackstadt, T. (1987). Comparative Virulence of Intra- and Interstrain Lipopolysaccharide Variants of Coxiella burnetii in the Guinea Pig Model. Infect. Immun. 55, 1144–1150. doi: 10.1128/iai.55.5.1144-1150.1987
Moreira, L. O., Zamboni, D. S. (2012). NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 3, 328. doi: 10.3389/fimmu.2012.00328
Morgan, J. K., Luedtke, B. E., Shaw, E. I. (2010). Polar Localization of the Coxiella burnetii Type IVB Secretion System. FEMS Microbiol. Lett. 305 (2), 177–183. doi: 10.1111/j.1574-6968.2010.01926.x
Mori, M., Mertens, K., Cutler, S. J., Santos, A. S. (2017). Critical Aspects for Detection of Coxiella burnetii. Vector Borne Zoonotic Dis. 17, 33–41. doi: 10.1089/vbz.2016.1958
Morroy, G., Keijmel, S. P., Delsing, C. E., Bleijenberg, G., Langendam, M., Timen, A., et al. (2016). Fatigue Following Acute Q-Fever: A Systematic Literature Review. PloS One 11, e0155884. doi: 10.1371/journal.pone.0155884
Narasaki, C. T., Toman, R. (2012). Lipopolysaccharide of Coxiella burnetii. Adv. Exp. Med. Biol. 984, 65–90. doi: 10.1007/978-94-007-4315-1_4
Natale, A., Bucci, G., Capello, K., Barberio, A., Tavella, A., Nardelli, S., et al. (2012). Old and New Diagnostic Approaches for Q Fever Diagnosis: Correlation Among Serological (CFT, ELISA) and Molecular Analyses. Comp. Immunol. Microbiol. Infect. Dis. 35, 375–379. doi: 10.1016/j.cimid.2012.03.002
Newton, P., Thomas, D. R., Reed, S. C. O., Lau, N., Xu, B., Ong, S. Y., et al. (2020). Lysosomal Degradation Products Induce Coxiella burnetii Virulence. Proc. Natl. Acad. Sci. U.S.A. 117 (12), 6801–6810. doi: 10.1073/pnas.1921344117
Nooroong, P., Trinachartvanit, W., Baimai, V., Anuracpreeda, P., Ahantarig, A. (2021). Partial DnaK Protein Expression From Coxiella-Like Endosymbiont of Rhipicephalus Annulatus Tick. PloS One 16, e0249354. doi: 10.1371/journal.pone.0249354
Norville, I. H., Hartley, M. G., Martinez, E., Cantet, F., Bonazzi, M., Atkins, T. P. (2014). Galleria Mellonella as an Alternative Model of Coxiella burnetii Infection. Microbiology (Reading) 160 (Pt 6):1175–1181.
Ochoa-Repáraz, J., Sentissi, J., Trunkle, T., Riccardi, C., Pascual, D. W. (2007). Attenuated Coxiella burnetii Phase II Causes a Febrile Response in Gamma Interferon Knockout and Toll-Like Receptor 2 Knockout Mice and Protects Against Reinfection. Infect. Immun. 75, 5845–5858. doi: 10.1128/IAI.00901-07
OIE (2018). “Q Fever. Chapter 3.1.16 [WWW Document],” in OIE Terr. Man. Paris, France. Available at: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.16_Q_FEVER.pdf.
Omsland, A., Beare, P. A., Hill, J., Cockrell, D. C., Howe, D., Hansen, B., et al. (2011). Isolation From Animal Tissue and Genetic Transformation of Coxiella burnetii Are Facilitated by an Improved Axenic Growth Medium. Appl. Environ. Microbiol. 77 (11), 3720–3725. doi: 10.1128/AEM.02826-10
Omsland, A., Cockrell, D. C., Howe, D., Fischer, E. R., Virtaneva, K., Sturdevant, D. E., et al. (2009). Host Cell-Free Growth of the Q Fever Bacterium Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 106 (11), 4430–4434. doi: 10.1073/pnas.0812074106
Oyston, P. C. F., Davies, C. (2011). Q Fever: The Neglected Biothreat Agent. J. Med. Microbiol. 60, 9–21. doi: 10.1099/jmm.0.024778-0
Peacock, M. G., Philip, R. N., Williams, J. C., Faulkner, R. S. (1983). Serological Evaluation of Q Fever in Humans: Enhancedphase I Titers of Immunoglobulins G and A Arediagnostic for Q Fever Endocarditis. Infect. Immun. 41, 1089–1098. doi: 10.1128/iai.41.3.1089-1098.1983
Pennings, J. L., Kremers, M. N., Hodemaekers, H. M., Hagenaars, J. C., Koning, O. H., Renders, N. H., et al. (2015). Dysregulation of Serum Gamma Interferon Levels in Vascular Chronic Q Fever Patients Provides Insights Into Disease Pathogenesis. Clin. Vaccine Immunol. 22, 664–671. doi: 10.1128/CVI.00078-15
Penttila, I. A., Harris, R. J., Storm, P., Haynes, D., Worswick, D. A., Marmion, B. P. (1998). Cytokine Dysregulation in the Post-Q-Fever Fatigue Syndrome. QJM 91 (8), 549–560. doi: 10.1093/qjmed/91.8.549
Piel, L. M. W., Durfee, C. J., White, S. N. (2021). Proteome-Wide Analysis of Coxiella burnetii for Conserved T-Cell Epitopes With Presentation Across Multiple Host Species. BMC Bioinf. 22, 296. doi: 10.1186/s12859-021-04181-w
Pouquet, M., Bareille, N., Guatteo, R., Moret, L., Beaudeau, F. (2020). Coxiella burnetii Infection in Humans: To What Extent do Cattle in Infected Areas Free From Small Ruminants Play a Role? Epidemiol. Infect. 148, e232. doi: 10.1017/S0950268820001880
Qian, M., Sleat, D. E., Zheng, H., Moore, D., Lobel, P. (2008). Proteomics Analysis of Serum From Mutant Mice Reveals Lysosomal Proteins Selectively Transported by Each of the Two Mannose 6-Phosphate Receptors. Mol. Cell Proteomics 7 (1), 58–70. doi: 10.1074/mcp.M700217-MCP200
Radulovic, M., Schink, K. O., Wenzel, E. M., Nähse, V., Bongiovanni, A., Lafont, F., et al. (2018). ESCRT-Mediated Lysosome Repair Precedes Lysophagy and Promotes Cell Survival. EMBO J. 37, e99753. doi: 10.15252/embj.201899753
Raghavan, R. (2016). A Repeat Motif on a Coxiella Effector Protein Facilitates Apoptosis Inhibition. Virulence 7:369–71. doi: 10.1080/21505594.2016.1156834
Raijmakers, R. P. H., Koeken, V. A. C. M., Jansen, A. F. M., Keijmel, S. P., Roerink, M. E., Joosten, L. A. B., et al. (2019). Cytokine Profiles in Patients With Q Fever Fatigue Syndrome. J. Infect. 78, 349–357. doi: 10.1016/j.jinf.2019.01.006
Ramstead, A. G., Robison, A., Blackwell, A., Jerome, M., Freedman, B., Lubick, K. J., et al. (2016). Roles of Toll-Like Receptor 2 (TLR2), TLR4, and MyD88 During Pulmonary Coxiella burnetii Infection. Infect. Immun. 84, 940–949. doi: 10.1128/IAI.00898-15
Reeves, P. M., Paul, S. R., Sluder, A. E., Brauns, T. A., Poznansky, M. C. (2017). Q-Vaxcelerate: A Distributed Development Approach for a New Coxiella burnetii Vaccine. Hum. Vaccin. Immunother. 13, 2977–2981. doi: 10.1080/21645515.2017.1371377
Robison, A., Snyder, D. T., Christensen, K., Kimmel, E., Hajjar, A. M., Jutila, M. A., et al. (2019). Expression of Human TLR4/myeloid Differentiation Factor 2 Directs an Early Innate Immune Response Associated With Modest Increases in Bacterial Burden During Coxiella burnetii Infection. Innate Immun. 25, 401–411. doi: 10.1177/1753425919855420
Roy, C. R., Tilney, L. G. (2002). The Road Less Traveled: Transport of Legionella to the Endoplasmic Reticulum. J. Cell Biol. 158 (3), 415–419. doi: 10.1083/jcb.200205011
Ruiz, S., Wolfe, D. N. (2014). Vaccination Against Q Fever for Biodefense and Public Health Indications. Front. Microbiol. 5, 726. doi: 10.3389/fmicb.2014.00726
Sahu, R., Rawool, D. B., Vinod, V. K., Malik, S. V. S., Barbuddhe, S. B. (2020). Current Approaches for the Detection of Coxiella burnetii Infection in Humans and Animals. J. Microbiol. Methods 179, 106087. doi: 10.1016/j.mimet.2020.106087
Salinas, R. P., Ortiz Flores, R. M., Distel, J. S., Aguilera, M. O., Colombo, M. I., Berón, W. (2015). Coxiella burnetii Phagocytosis Is Regulated by GTPases of the Rho Family and the RhoA Effectors Mdia1 and ROCK. PloS One 10 (12), e0145211. doi: 10.1371/journal.pone.0145211
Samoilis, G., Psaroulaki, A., Vougas, K., Tselentis, Y., Tsiotis, G. (2007). Analysis of Whole Cell Lysate From the Intercellular Bacterium Coxiella burnetii Using Two Gel-Based Protein Separation Techniques. J. Proteome Res. 6 (8), 3032–3041. doi: 10.1021/pr070077n
Sandoz, K. M., Popham, D. L., Beare, P. A., Sturdevant, D. E., Hansen, B., Nair, V., et al. (2016). Transcriptional Profiling of Coxiella burnetii Reveals Extensive Cell Wall Remodeling in the Small Cell Variant Developmental Form. PloS One 11, e0149957. doi: 10.1371/journal.pone.0149957
Sandoz, K. M., Sturdevant, D. E., Hansen, B., Heinzen, R. A. (2014). Developmental Transitions of Coxiella burnetii Grown in Axenic Media. J. Microbiol. Methods 96, 104–110. doi: 10.1016/j.mimet.2013.11.010
Sawyer, L. A., Fishbein, D. B., McDade, J. E. (1987). Q Fever: Current Concepts. Rev. Infect. Dis. 9 (5), 935–946. doi: 10.1093/clinids/9.5.935
Schäfer, W., Eckart, R. A., Schmid, B., Cagköylü, H., Hof, K., Muller, Y. A., et al. (2017). Nuclear Trafficking of the Anti-Apoptotic Coxiella burnetii Effector Protein AnkG Requires Binding to P32 and Importin-α1. Cell. Microbiol. 19 (1):10.1111/cmi.12634. doi: 10.1111/cmi.12634
Schäfer, W., Schmidt, T., Cordsmeier, A., Borges, V., Beare, P. A., Pechstein, J., et al. (2020). The Anti-Apoptotic Coxiella burnetii Effector Protein AnkG Is a Strain Specific Virulence Factor. Sci. Rep. 10 (1), 15396. doi: 10.1038/s41598-020-72340-9
Scheurwater, E. M., Burrows, L. L. (2011). Maintaining Network Security: How Macromolecular Structures Cross the Peptidoglycan Layer. FEMS Microbiol. Lett. 318, 1–9. doi: 10.1111/j.1574-6968.2011.02228.x
Schneeberger, P. M., Hermans, M. H., van Hannen, E. J., Schellekens, J. J., Leenders, A. C., Wever, P. C. (2010). Real-Time PCR With Serum Samples Is Indispensable for Early Diagnosis of Acute Q Fever. Clin. Vaccine Immunol. 17 (2), 286–290. doi: 10.1128/CVI.00454-09
Schoenlaub, L., Cherla, R., Zhang, Y., Zhang, G. (2016). Coxiella burnetii Avirulent Nine Mile Phase II Induces Caspase-1-Dependent Pyroptosis in Murine Peritoneal B1a B Cells. Infect. Immun. 84, 3638–3654. doi: 10.1128/IAI.00694-16
Schoffelen, T., Herremans, T., Sprong, T., Nabuurs-Franssen, M., Wever, P. C., Joosten, L. A., et al. (2013). Limited Humoral and Cellular Responses to Q Fever Vaccination in Older Adults With Risk Factors for Chronic Q Fever. J. Infect. 67 (6), 565–573. doi: 10.1016/j.jinf.2013.08.008
Schoffelen, T., Sprong, T., Bleeker-Rovers, C. P., Wegdam-Blans, M. C., Ammerdorffer, A., Pronk, M. J., et al. (2014b). A Combination of Interferon-Gamma and Interleukin-2 Production by Coxiella burnetii-Stimulated Circulating Cells Discriminates Between Chronic Q Fever and Past Q Fever. Clin. Microbiol. Infect. 20, 642–650. doi: 10.1111/1469-0691.12423
Schoffelen, T., Textoris, J., Bleeker-Rovers, C. P., Ben Amara, A., van der Meer, J. W., Netea, M. G., et al. (2017). Intact Interferon-γ Response Against Coxiella burnetii by Peripheral Blood Mononuclear Cells in Chronic Q Fever. Clin. Microbiol. Infect. 23, 209.e9-209.e15. doi: 10.1016/j.cmi.2016.11.008
Schoffelen, T., Wong, A., Rumke, H. C., Netea, M. G., Timen, A., van Deuren, M., et al. (2014a). Adverse Events and Association With Age, Sex and Immunological Parameters of Q Fever Vaccination in Patients at Risk for Chronic Q Fever in the Netherlands 2011. Vaccine 32 (49), 6622–6630. doi: 10.1016/j.vaccine.2014.09.061
Scholzen, A., de Vries, M., Duerr, H. P., Roest, H. J., Sluder, A. E., Poznansky, M. C., et al. (2021). Whole Blood Interferon G Release Is a More Sensitive Marker of Prior Exposure to Coxiella burnetii Than Are Antibody Responses. Front. Immunol. 12, 701811. doi: 10.3389/fimmu.2021.701811
Scorpio, D. G., Choi, K. S., Dumler, J. S. (2018). Anaplasma Phagocytophilum-Related Defects in CD8, NKT, and NK Lymphocyte Cytotoxicity. Front. Immunol. 9, 710. doi: 10.3389/fimmu.2018.00710
Scott, G. H., Burger, G. T., Kishimoto, R. A. (1978). Experimental Coxiella burnetii Infection of Guinea Pigs and Mice. Lab. Anim. Sci. 28, 673–675.
Scott, G. H., Williams, J. C., Stephenson, E. H. (1987). Animal Models in Q Fever: Pathological Responses of Inbred Mice to Phase I Coxiella burnetii. J. Gen. Microbiol. 133, 691–700. doi: 10.1099/00221287-133-3-691
Selim, A., Yang, E., Rousset, E., Thiéry, R., Sidi-Boumedine, K. (2018). Characterization of Coxiella burnetii Strains From Ruminants in a Galleria Mellonella Host-Based Model. New Microbes New Infect. 24, 8–13. doi: 10.1016/j.nmni.2018.02.008
Seshadri, R., Paulsen, I. T., Eisen, J. A., Read, T. D., Nelson, K. E., Nelson, W. C., et al. (2003). Complete Genome Sequence of the Q-Fever Pathogen Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 100 (9), 5455–5460. doi: 10.1073/pnas.0931379100
Shannon, J. G., Howe, D., Heinzen, R. A. (2005). Virulent Coxiella burnetii Does Not Activate Human Dendritic Cells: Role of Lipopolysaccharide as a Shielding Molecule. Proc. Natl. Acad. Sci. U.S.A. 102, 8722–8727. doi: 10.1073/pnas.0501863102
Skultety, L., Hernychova, L., Toman, R., Hubalek, M., Slaba, K., Zechovska, J., et al. (2005). Coxiella burnetii Whole Cell Lysate Protein Identification by Mass Spectrometry and Tandem Mass Spectrometry. Ann. N. Y. Acad. Sci. 1063, 115–122. doi: 10.1196/annals.1355.019
Snyder, D. T., Hedges, J. F., Jutila, M. A. (2017). Getting "Inside" Type I IFNs: Type I IFNs in Intracellular Bacterial Infections. J. Immunol. Res. 2017, 9361802. doi: 10.1155/2017/9361802
Soltysiak, K. A., van Schaik, E. J., Samuel, J. E. (2015). Surfactant Protein D Binds to Coxiella burnetii and Results in a Decrease in Interactions With Murine Alveolar Macrophages. PloS One 10 (9), e0136699. doi: 10.1371/journal.pone.0136699
Stein, A., Lepidi, H., Mege, J. L., Marrie, T. J., Raoult, D. (2000). Repeated Pregnancies in BALB/c Mice Infected With Coxiella burnetii Cause Disseminated Infection, Resulting in Stillbirth and Endocarditis. J. Infect. Dis. 181, 188–194. doi: 10.1086/315166
Sukocheva, O. A., Marmion, B. P., Storm, P. A., Lockhart, M., Turra, M., Graves, S. (2010). Long-Term Persistence After Acute Q Fever of Non-Infective Coxiella burnetii Cell Components, Including Antigens. QJM 103, 847–863. doi: 10.1093/qjmed/hcq113
Tennenberg, S. D., Weller, J. J. (1996). Endotoxin Activates T Cell Interferon-Gamma Secretion in the Presence of Endothelium. J. Surg. Res. 63 (1), 73–76. doi: 10.1006/jsre.1996.0225
Thompson, H. A., Hoover, T. A., Vodkin, M. H., Shaw, E. I. (2003). Do Chromosomal Deletions in the Lipopolysaccharide Biosynthetic Regions Explain All Cases of Phase Variation in Coxiella burnetii Strains? Update. Ann. N. Y. Acad. Sci. 990, 664–670. doi: 10.1111/j.1749-6632.2003.tb07441.x
Tobin, M. J., Cahill, N., Gearty, G., Maurer, B., Blake, S., Daly, K., et al. (1982). Q Fever Endocarditis. Am. J. Med. 72, 396–400. doi: 10.1016/0002-9343(82)90495-8
Toman, R., Skultety, L., Ihnatko, R. (2009). Coxiella burnetii Glycomics and Proteomics–Tools for Linking Structure to Function. Ann. N. Y. Acad. Sci. 1166, 67–78. doi: 10.1111/j.1749-6632.2009.04512.x
Torina, A., Blanda, V., Villari, S., Piazza, A., La Russa, F., Grippi, F., et al. (2020a). Immune Response to Tick-Borne Hemoparasites: Host Adaptive Immune Response Mechanisms as Potential Targets for Therapies and Vaccines. Int. J. Mol. Sci. 21, 8813. doi: 10.3390/ijms21228813
Torina, A., Naranjo, V., Pennisi, M. G., Patania, T., Vitale, F., Laricchiuta, P., et al. (2007). Serologic and Molecular Characterization of Tickborne Pathogens in Lions (Panthera leo) From the Fasano Safari Park, Italy. J. Zoo Wildl. Med. 38 (4), 591–593. doi: 10.1638/2007-0043R1.1
Torina, A., Villari, S., Blanda, V., Vullo, S., La Manna, M. P., Shekarkar Azgomi, M., et al. (2020b). Innate Immune Response to Tick-Borne Pathogens: Cellular and Molecular Mechanisms Induced in the Hosts. Int. J. Mol. Sci. 21, 5437. doi: 10.3390/ijms21155437
Van den Brom, R., van Engelen, E., Roest, H. I., van der Hoek, W., Vellema, P. (2015). Coxiella burnetii Infections in Sheep or Goats: An Opinionated Review. Vet. Microbiol. 181, 119–129. doi: 10.1016/j.vetmic.2015.07.011
van Schaik, E. J., Chen, C., Mertens, K., Weber, M. M., Samuel, J. E. (2013). Molecular Pathogenesis of the Obligate Intracellular Bacterium Coxiella burnetii. Nat. Rev. Microbiol. 11, 561–573. doi: 10.1038/nrmicro3049
Varela-Castro, L., Zuddas, C., Ortega, N., Serrano, E., Salinas, J., Castellà, J., et al. (2018). On the Possible Role of Ticks in the Eco-Epidemiology of Coxiella burnetii in a Mediterranean Ecosystem. Ticks Tick Borne Dis. 9, 687–694. doi: 10.1016/j.ttbdis.2018.02.014
Vollmer, W., Tomasz, A. (2000). The pgdA Gene Encodes for a Peptidoglycan N-Acetylglucosamine Deacetylase in Streptococcus Pneumoniae. J. Biol. Chem. 275, 20496–20501. doi: 10.1074/jbc.M910189199
Voth, D. E., Heinzen, R. A. (2007). Lounging in a Lysosome: The Intracellular Lifestyle of Coxiella burnetii. Cell Microbiol. 9, 829–840. doi: 10.1111/j.1462-5822.2007.00901.x
Voth, D. E., Howe, D., Heinzen, R. A. (2007). Coxiella burnetii Inhibits Apoptosis in Human THP-1 Cells and Monkey Primary Alveolar Macrophages. Infect. Immun. 75 (9), 4263–4271. doi: 10.1128/IAI.00594-07
Waag, D. M. (2007). Coxiella burnetii: Host and Bacterial Responses to Infection. Vaccine 25, 7288–7295. doi: 10.1016/j.vaccine.2007.08.002
Waag, D. M., England, M. J., Tammariello, R. F., Byrne, W. R., Gibbs, P., Banfield, C. M., et al. (2002). Comparative Efficacy and Immunogenicity of Q Fever Chloroform:Methanol Residue (CMR) and Phase I Cellular (Q-Vax) Vaccines in Cynomolgus Monkeys Challenged by Aerosol. Vaccine 20, 2623–2634. doi: 10.1016/s0264-410x(02)00176-7
Wegdam-Blans, M. C., Tjhie, H. T., Korbeeck, J. M., Nabuurs-Franssen, M. N., Kampschreur, L. M., Sprong, T., et al. (2014). Serology in Chronic Q Fever Is Still Surrounded by Question Marks. Eur. J. Clin. Microbiol. Infect. Dis. 33 (7), 1089–1094. doi: 10.1007/s10096-014-2048-4
Wegdam-Blans, M. C., Wielders, C. C., Meekelenkamp, J., Korbeeck, J. M., Herremans, T., Tjhie, H. T., et al. (2012). Evaluation of Commonly Used Serological Tests for Detection of Coxiella burnetii Antibodies in Well-Defined Acute and Follow-Up Sera. Clin. Vaccine Immunol. 19 (7), 1110–1115. doi: 10.1128/CVI.05581-11
Wildman, M. J., Smith, E. G., Groves, J., Beattie, J. M., Caul, E. O., Ayres, J. G. (2002). Chronic Fatigue Following Infection by Coxiella burnetii (Q Fever): Ten-Year Follow-Up of the 1989 UK Outbreak Cohort. QJM 95, 527–538. doi: 10.1093/qjmed/95.8.527
Woc-Colburn, A. M., Garner, M. M., Bradway, D., West, G., D’Agostino, J., Trupkiewicz, J., et al. (2008). Fatal Coxiellosis in Swainson’s Blue Mountain Rainbow Lorikeets (Trichoglossus Haematodus Moluccanus). Vet. Pathol. 45, 247–254. doi: 10.1354/vp.45-2-247
Zamboni, D. S., Campos, M. A., Torrecilhas, A. C., Kiss, K., Samuel, J. E., Golenbock, D. T., et al. (2004). Stimulation of Toll-Like Receptor 2 by Coxiella burnetii Is Required for Macrophage Production of Pro-Inflammatory Cytokines and Resistance to Infection. J. Biol. Chem. 279, 54405–54415. doi: 10.1074/jbc.M410340200
Keywords: Coxiella burnetii, innate immunity, Toll-like receptors, cytokine—immunological terms, inflammasome, autophagia, immunotherapeutics, experimental model
Citation: Sireci G, Badami GD, Di Liberto D, Blanda V, Grippi F, Di Paola L, Guercio A, de la Fuente J and Torina A (2021) Recent Advances on the Innate Immune Response to Coxiella burnetii. Front. Cell. Infect. Microbiol. 11:754455. doi: 10.3389/fcimb.2021.754455
Received: 06 August 2021; Accepted: 12 October 2021;
Published: 02 November 2021.
Edited by:
Luciana Balboa, Academia Nacional de Medicina, ArgentinaReviewed by:
Deepak B. Rawool, Indian Veterinary Research Institute, IndiaAnja Lührmann, University Hospital Erlangen, Gernmany
Copyright © 2021 Sireci, Badami, Di Liberto, Blanda, Grippi, Di Paola, Guercio, de la Fuente and Torina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria Blanda, valeria.blanda@izssicilia.it
 Guido Sireci1
Guido Sireci1  Giusto Davide Badami
Giusto Davide Badami Diana Di Liberto
Diana Di Liberto Valeria Blanda
Valeria Blanda Francesca Grippi
Francesca Grippi Laura Di Paola
Laura Di Paola José de la Fuente
José de la Fuente Alessandra Torina
Alessandra Torina