Carbapenem-Resistant Enterobacterales in Long-Term Care Facilities: A Global and Narrative Review
- 1Division of Infectious Disease, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
- 2Department of Emergency, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 3Department of Emergency Medicine, Department of Emergency Medicine and Critical Care Medicine, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
- 4Department of Internal Medicine, Changhua Christian Hospital, Changhua, Taiwan
- 5Division of Infectious Diseases, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan
- 6Department of Microbiology and Immunology, School of Medicine, China Medical University, Taichung, Taiwan
- 7Department of Internal Medicine and Center for Infection Control, College of Medicine, National Cheng Kung University Hospital, Tainan, Taiwan
- 8Department of Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 9Rong Hsing Research Center for Translational Medicine, National Chung Hsing University, Taichung, Taiwan
- 10Ph.D. Program in Translational Medicine, National Chung Hsing University, Taichung, Taiwan
- 11Division of Infectious Disease, Department of Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
- 12Department of Laboratory Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
The emergence of carbapenem-resistant Enterobacterales (CRE) has become a major public health concern. Moreover, its colonization among residents of long-term care facilities (LTCFs) is associated with subsequent infections and mortality. To further explore the various aspects concerning CRE in LTCFs, we conducted a literature review on CRE colonization and/or infections in long-term care facilities. The prevalence and incidence of CRE acquisition among residents of LTCFs, especially in California, central Italy, Spain, Japan, and Taiwan, were determined. There was a significant predominance of CRE in LTCFs, especially in high-acuity LTCFs with mechanical ventilation, and thus may serve as outbreak centers. The prevalence rate of CRE in LTCFs was significantly higher than that in acute care settings and the community, which indicated that LTCFs are a vital reservoir for CRE. The detailed species and genomic analyses of CRE among LTCFs reported that Klebsiella pneumoniae is the primary species in the LTCFs in the United States, Spain, and Taiwan. KPC-2-containing K. pneumoniae strains with sequence type 258 is the most common sequence type of KPC-producing K. pneumoniae in the LTCFs in the United States. IMP-11- and IMP-6-producing CRE were commonly reported among LTCFs in Japan. OXA-48 was the predominant carbapenemase among LTCFs in Spain. Multiple risk factors associated with the increased risk for CRE acquisition in LTCFs were found, such as comorbidities, immunosuppressive status, dependent functional status, usage of gastrointestinal devices or indwelling catheters, mechanical ventilation, prior antibiotic exposures, and previous culture reports. A high CRE acquisition rate and prolonged CRE carriage duration after colonization were found among residents in LTCFs. Moreover, the patients from LTCFs who were colonized or infected with CRE had poor clinical outcomes, with a mortality rate of up to 75% in infected patients. Infection prevention and control measures to reduce CRE in LTCFs is important, and could possibly be controlled via active surveillance, contact precautions, cohort staffing, daily chlorhexidine bathing, healthcare-worker education, and hand-hygiene adherence.
Introduction
The emergence of antimicrobial resistance has become a major public health concern. Since the identification of carbapenem-resistant Enterobacterales (CRE) in the 1990s, CRE has spread worldwide during the past two decades (Centers for Disease Control and Prevention, 2013). The threat is not only confined to tertiary referral hospitals or academic health science centers. In a network of community hospitals in the southeastern United States, a CRE incidence of 0.26 per 100,000 patient-days in 2008 and 1.4 per 100,000 patient-days in 2012 was reported (Thaden et al., 2014). In a population-based study in seven states in the United States, CRE incidence of up to 2.93 per 100,000 persons (95% Confident Interval 2.65–3.23) was also reported (Guh et al., 2015). In addition, previous studies had reported high mortality rates among CRE-infected patients, ranging from 32.1%–48% (Patel et al., 2008; Gasink et al., 2009), which could even increase to 71% in one year among liver transplant patients (Kalpoe et al., 2012). Regarding the median cost of CRE infections with an incidence of 2.93 per 100,000 persons in the United States, it would cost hospitals $275 million, third party payers $147 million, and the society $553 million (Bartsch et al., 2017), indicating a high economic burden caused by CRE infections. Collectively, considering the rapid worldwide spreading of CRE, the association of CRE infections with poor clinical outcomes, high economic burden, and relatively limited antimicrobial treatment for CRE in the current era, the Centers of Disease Control and Prevention (CDC) announced CRE as the most urgent public health threat in 2013.
During the past decade, the demand for chronic rehabilitation and skilled nursing care after an acute illness has increased as the population ages. At the same time, a high prevalence of colonization by multi-drug resistant organisms (MDRO) among residents in long-term care facilities (LTCFs) was reported. The SHIELD Orange County Project demonstrated an MDRO prevalence of 65% among nursing homes (NHs) residents and 80% among long-term acute care hospital (LTACs) residents (Mckinnell et al., 2019). Furthermore, Guh et al. reported that most CRE isolates came from individuals with a history of health care exposure or hospitalization within one year, and most CRE hospitalized cases resulted in a discharge directly to a long-term care facility or LTAC hospitals (Guh et al., 2015). Another case-control study using the Illinois hospital discharge database reported that the CRE carriage rate at the time of admission was highly associated with prior health-care facility exposure (particularly LTACs) (Lin et al., 2019). That is, compared with the relatively low prevalence rate in the community (Prabaker et al., 2012) and in the acute care hospitals (ACHs), the high prevalence of CRE in LTCFs is an important public health issue and a critical component of large-scale antibiotic stewardship programs.
Tracing back, a systematic review and meta-analysis was conducted to highlight the importance of CRE in hospital settings (Van Loon et al., 2018). In this article, we conducted a literature review to summarize the current understanding of CRE in LTCF settings, and to demonstrate that the CRE colonization and/or infection rates are truly higher among LTCFs in different geographic regions.
We searched the English-language medical literature using PubMed/MEDLINE from 1990 to 2020, using the following keywords: carbapenem-resistant Enterobacteriaceae, carbapenem-resistant Enterobacterales, long-term care facilities, nursing home, long-term acute care hospitals. The references of articles found using this search were also reviewed to identify other potential studies that were not located using the search terms. Studies reporting CRE carriage and/or infection among elderly residents or patients who lived in or being admitted from long term care facilities were reviewed. Studies that provided data on previous long term care facilities exposure in the setting of acute care hospital or community or outbreak were also included. Exclusion criteria included studies that only documenting CRE carriage and/or infection in acute care hospital, tertiary medical centers, ICU or community; studies that tested for multiple-drug organism carriage and/or infection but did not evaluate for CRE; or studies that evaluated CRE carriage and/or infection among pediatric patients or health care workers. In total, 33 studies were reviewed in full. Other 12 articles involved in this study were searched while reviewing the similar articles in above mentioned papers.
Prevalence and Incidence of CRE Among Residents in LTCFs
An increasing number of studies have evaluated CRE in LTCFs between 2012 and 2020. In most studies, LTCF were facilities that provide long-term rehabilitation and skilled nursing care, such as long-term acute care hospitals or facilities (LTACs), skilled nursing facilities (SNFs) and nursing homes (NHs) in the United States, and residential care homes for the elderly (RCHE) in Hong-Kong. The study designs for CRE acquisition among LTCF residents could be broadly classified into four types: point prevalence survey (identifying the number of people with CRE at a specific point in time), incidence surveillance (determining the rate of CRE in LTCF for a specific period), outbreak investigations (reports of investigation during CRE outbreak), and network model formulation. CRE colonization and/or infection in LTCFs shared similar but not identical characteristics in different geographic regions; therefore, we separated the studies geographically, that is, the United States, Europe (including Turkey, Israel, and North Lebanon) and Asia.
The prevalence rate of CRE colonization among LTCFs in the United States varied widely, as demonstrated in Table 1, ranging from 1%–30.4% (Prabaker et al., 2012; Lin et al., 2013; Johnson et al., 2014; Van Duin et al., 2014; Cunha et al., 2016; Mckinnell et al., 2016; Prasad et al., 2016; Han et al., 2017; Reuben et al., 2017; Mckinnell et al., 2019). The prevalence rate of Carbapenem-resistant K. pneumoniae (CRKP) among residents of LTCFs in the United States demonstrated a high geographical variation, with the highest prevalence in the West (42.2%), followed by the South (12.2%), Midwest (7.3%), and Northeast (9.9%) (Han et al., 2017). The highest CRKP prevalence was found in California (45.5%, 701/1540) (Han et al., 2017). Among the CRKP endemic West region, the CRKP prevalence rate changed significantly across the study period: 48.5% in quarter 1, 2014; 40.4% in quarter 2, 2014; 49.5% in quarter 3, 2014; 32.5% in quarter 4, 2014; and 39.0% in quarter 1, 2015 (p<0.01, test for trend) (Han et al., 2017). The CRE incidence among residents of LTCFs in the United States varied from 1.07–6.83 cases per 10000 patient-days (Brennan et al., 2014; Chopra et al., 2018). The pooled mean incidence rate of CRE in the United States was 0.46 per 1000 patient-days (Marquez et al., 2013).
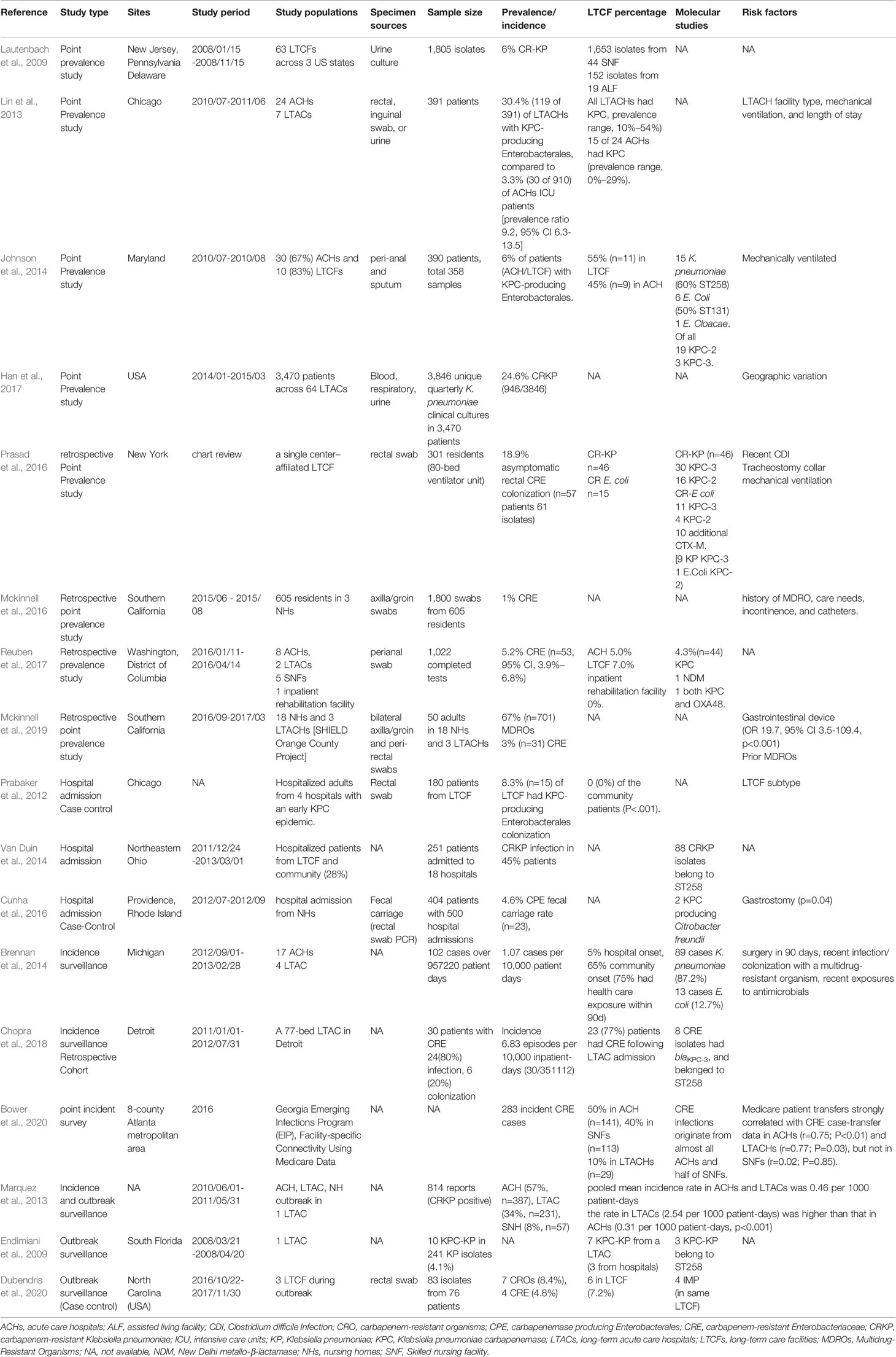
Table 1 Studies of carbapenem-resistant Enterobacterales colonization in long-term care facilities in the United States.
In Europe (including Turkey, Israel, and North Lebanon), CRE in LTCFs also demonstrated a high geographic variance (Table 2). Recent studies about CRE in Europe were few and most of them reported low CRE prevalence rates among residents of LTCFs. There was a 0.3% CRE prevalence rate in LTCFs in Switzerland (37/12423) (Kohler et al., 2018), and only one resident with CRE carriage was found in each LTCF in Belgium and the Netherlands (Latour et al., 2019; Van Dulm et al., 2019). Even though a low prevalence rate was noted, the high association of CRE colonization with LTCF was still noted from the hospital admission data in Spain, reporting that about 37% of cases were health-care associated, of which 42% were nursing home residents (Palacios-Baena et al., 2016). Different from mainland Europe, a higher prevalence of CRE carriage was found among residents in LTCFs in Israel (12%) (Ben-David et al., 2011) and North Lebanon (1.7%) (Dandachi et al., 2016). Though a relatively low prevalence rate of CRE colonization in LTCFs was found in Belgium, the Netherlands, and Switzerland, high CRE carriage rates (28.4%) were reported among a LTAC rehabilitation facility (LTACRF) in central Italy, which is a CRE endemic region since 2010 (Ambretti et al., 2019).
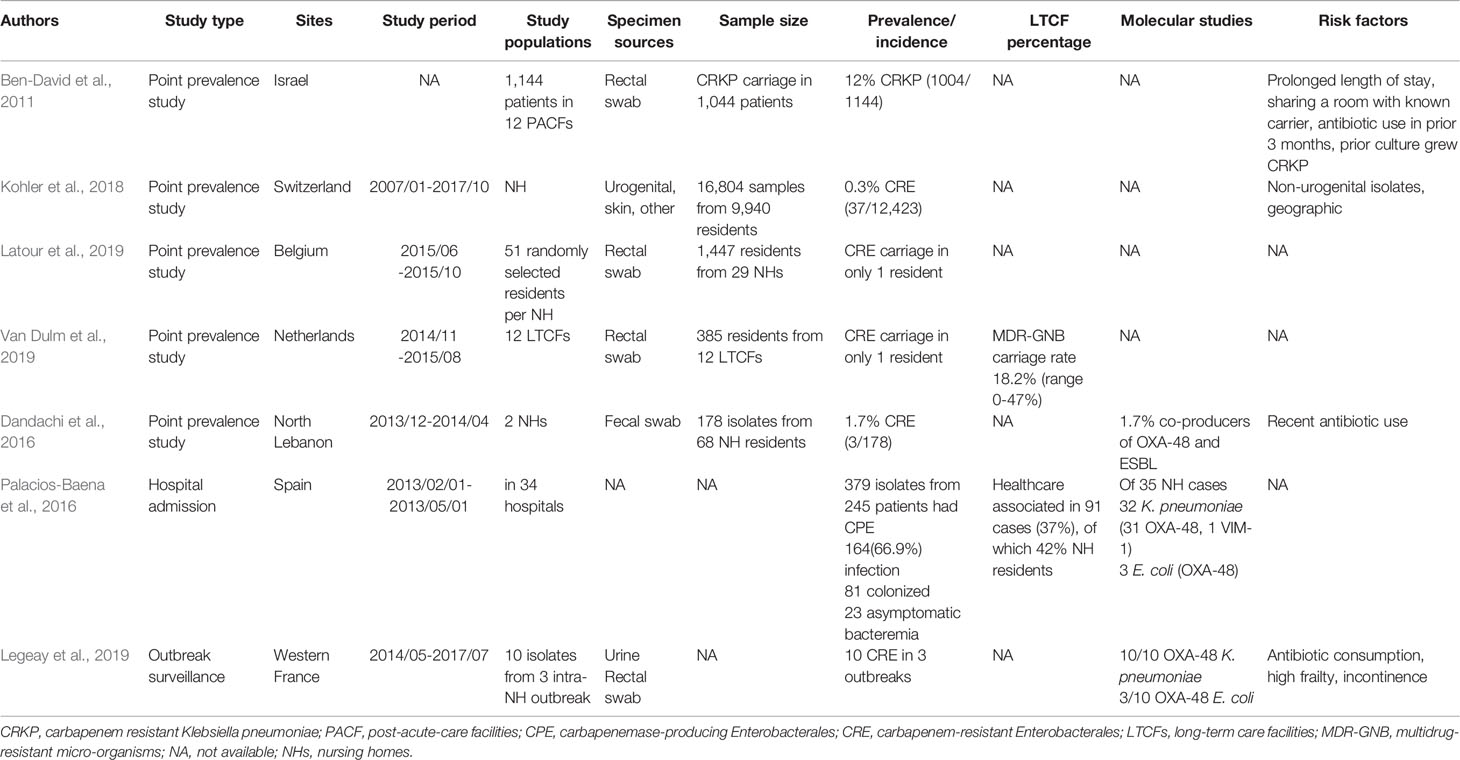
Table 2 Studies carbapenem-resistant Enterobacterales colonization in long-term care facilities in the Europe.
As shown in Table 3, the prevalence rate of CRE colonization among LTCFs in the Asia region ranged from 13%–22.7% (Dandachi et al., 2016; Lee et al., 2017; Chen et al., 2018; Hagiya et al., 2018; Jean et al., 2018; Mao et al., 2018; Le et al., 2020). The two-point prevalence studies in Japan showed a 13% prevalence rate of CRE colonization in LTCFs in Hiroshima and a 19.3% prevalence rate in Osaka hospitals (one of which had approximately 200 long-term care beds) (Hagiya et al., 2018; Le et al., 2020). The CRE prevalence among the LTCFs in Taiwan was 22.7% (Lee et al., 2017). In Korea, a 10-month active surveillance survey via rectal specimens among LTCFs reported a low carbapenemase-producing Enterobacterales (CPE) prevalence rate (1.4%, 4/282) (Hwang et al., 2018). In Hong-Kong, no CPE fecal carriage was found in residential care homes for the elderly (RCHEs) (Dandachi et al., 2016), compatible with a previous study of 28 RCHEs in Hong-Kong by (Cheng et al. (2016).
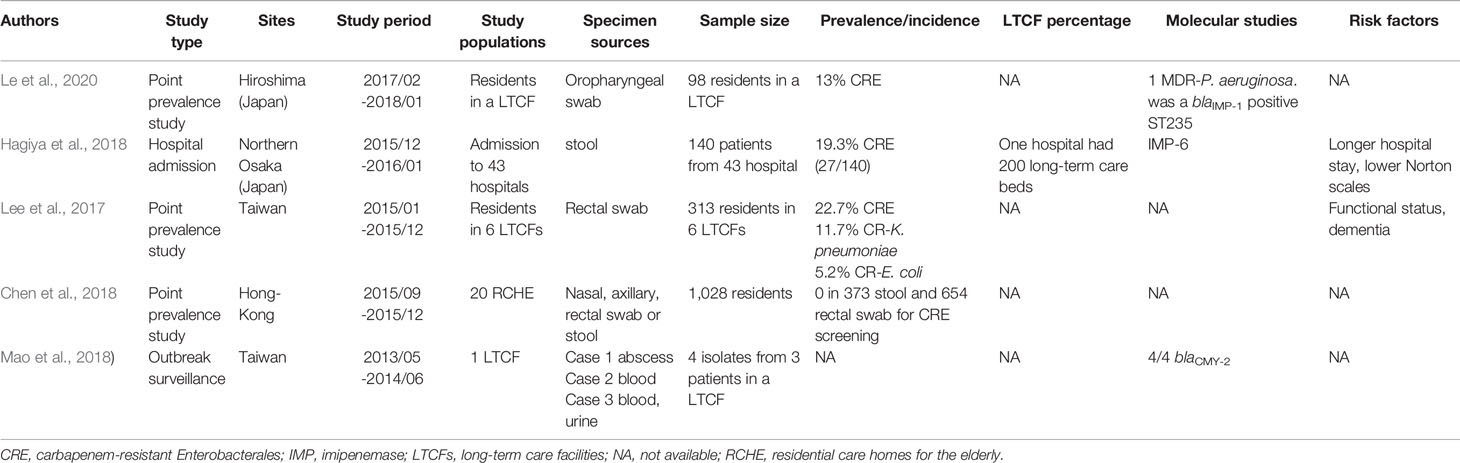
Table 3 Studies of carbapenem-resistant Enterobacterales colonization in long-term care facilities in the Asia.
Collectively, the high predominance of CRE in LTCFs was observed worldwide, especially in LTCFs in the West region of the United States (California), Spain, Italy, Japan, and Taiwan. Since very few studies have been conducted to evaluate the clinical epidemiology of CRE in LTCFs in some regions in Europe (Russia, Arabia) and Asia (such as the mainland China, and the southern east countries), we could not determine the clinical epidemiology of CRE colonization and/or infections in these countries or regions.
High-Acuity LTCFs Were an Important Reservoir of CRE
The high predominance of CRE in LTCFs reflects the local clinical epidemiology in the community or hospital settings, especially the ACHs or intensive care units (ICUs). In the following section, we compared recent studies evaluating CRE in LTCFs with previous studies about CRE in hospital settings and/or the community to demonstrate the issue.
In the United States, there were many studies supporting that the CRE acquisition and/or infections among residents of LTCFs was much more than that in acute care settings and/or the community. A population-based incidence study of CRKP among ACHs, LTACs, and SNHs in the Los Angeles County between 2010 and 2011 reported a higher CRE incidence rate in LTACs (2.54 per 1000 patient-days) than that in ACHs (0.31 per 1000 patient-days, p <0.001) (Marquez et al., 2013). Another outbreak investigation conducted among ACHs and LTACs in Indiana and Illinois in 2008 reported that one of the LTACs was the main locus of the outbreak, which accommodated 60% (24/60) of the cases, and only 10% (4/60) of the patients definitely had CRE colonization in ACHs (Gohil et al., 2017). A previous systematic review of CRE in the United States between 2000 and 2016 reported higher infection rates in LTACs than in ACHs and community settings (Livorsi et al., 2018), and community-onset cases mostly had health care exposure within the previous 90 days (Brennan et al., 2014). The multihospital case-control study in Chicago during an early KPC epidemic reported a higher prevalence of CRE carriage among LTCFs patients (8.3%, n=15) compared with patients admitted from the community (0%, n=0) (p<0.001) (Prabaker et al., 2012). Additionally, the HARP-DC studies (one of the first study to measure the prevalence of CRE colonization in a region aligning with CDC’s recommendation of collaborative approach), highlighted that the CRE prevalence in LTCFs was even higher than that in the ACHs (7.0% in LTCFs vs 5.0% in ACHs), with a relative prevalence ratio of 0.9% [0.5-1.5] in LTCFs and 1.5% [0.9-2.6] in ACHs) (Reuben et al., 2017). Furthermore, up to 30.4% prevalence rate of KPC-producing Enterobacterales among LTCFs was even reported (prevalence range 10–54%), compared with the relatively low prevalence rate in short-stay hospital ICU patients (3.3%, prevalence rate 0–29%) (Lin et al., 2013). That is, a 9-fold greater risk of KPC-producing Enterobacterales was found in the LTCF patients compared to the ACH patients (Lin et al., 2013). However, conflicting results of CRE prevalence between LTCFs and ACHs remain. A population based study conducted in Atlanta reported different results, of which CRE incidence was attributed mostly to the ACHs (n=141, 50%) and skilled nursing facilities (SNFs; n=113, 40%), rather than the LTACs (n=29, 10%) (Bower et al., 2020). A CRE incidence study by Guh et al. reported that most cases were collected from ACHs (33.9%) rather than from LTCFs (26.9%) or a LTAC (7.5%) (Guh et al., 2015).
In Europe, the European survey of carbapenemase-producing Enterobacterales (EuSCAPE study) evaluating CRE colonization and/or infections in hospitalized patients reported that CRE prevalence varied geographically, with the highest rate in the Mediterranean and Balkan countries (Grundmann et al., 2017). High incidence countries included Greece patients (5.78 per 10000 hospital admissions), Italy (5.96 per 10000 hospital admissions), Montenegro (5.65 per 10000 hospital admissions), Spain (4.01 per 10000 hospital admissions), and Serbia (3 per 10000 hospital admissions) (Grundmann et al., 2017). The high predominance of CRE in Spain and Italy were similar between ACHs and LTCFs, but the difference of CRE prevalence and/or incidence among LTCFs in other countries and/or regions in Europe had not been clarified in previous studies.
In Asia region, Kayoko Hayakawa et al. reported a 30% prevalence rate of carbapenemase-producing Enterobacterales (CPE) among tertiary hospitalized patients (Hayakawa et al., 2020), which was much higher than the CRE prevalence rate in LTCFs in another study in Japan (Le et al., 2020). However, Kayoko Hayakawa highlighted that patients with CPE were more likely to be residents in the nursing homes or the LTCFs prior to hospital admission (Hayakawa et al., 2020). Dokyun Kim et al. reported a low carbapenem resistance rate among K. pneumoniae in Korean secondary and tertiary hospitals (less than 0.1–2%), but an increasing trend of CRE (CRKP and E. coli) was reported in recent years (the carbapenem susceptibility rates of E. coli were 100% in 2011 and 99.3% in 2015; the carbapenem susceptibility rates of K. pneumoniae were 99.0% in 2011 and 97.0% in 2015) (Kim et al., 2017). In China, Qi Wang et al. conducted a longitudinal large scale CRE study between 2012 and 2016 among hospitals in China, and a high prevalence rate of carbapenem resistance among Enterobacterales was found (91% in K. pneumoniae, 80% in E. coli, and 72% in E. cloacae). However, there were few data about CRE prevalence in LTCFs in mainland China and other countries in Europe. Though little is known about the accurate difference of CRE prevalence between ACHs and LTCFs in the Asia region and Europe, studies in the United States, Italy, and Japan highlighted the threat of high prevalence of CRE among LTCFs, of which the prevalence and/or incidence of CRE acquisition and infections in LTCFs was much higher than that in ACHs, ICUs, and the community. Our review suggests that LTCFs are vital reservoirs for CRE and are important in regional outbreaks and/or dissemination of CRE (Chitnis et al., 2012; Prabaker et al., 2012; Lin et al., 2013; Guh et al., 2015; Gohil et al., 2017).
The CRE prevalence varied among different subtypes of LTCFs. One study reported a higher CRE prevalence in facilities that managed ventilated LTAC patients and ventilator-capable nursing homes (vNH) residents (8% vs < 1%, p<.001) and was rare in NHs that did not offer mechanical ventilation (NH <1%, vNH median 10% [0-12%], LTACs median 8%[8-10%]) (Mckinnell et al., 2019). Predominant CRE carriage among skilled nursing facilities with ventilator care (VSNFs) (27.3%) and LTACs (33.3%) (p <0.001) was found (Prabaker et al., 2012). Furthermore, patients from VSNFs and LTACs had a 7.0-fold greater odds ratio of KPC-producing Enterobacterales colonization (95% CI 1.3-42, p=0.022) than patients from SNFs (Prabaker et al., 2012). Collectively, high-acuity LTCFs that provided mechanical ventilation, such as ventilated LTAC, vNHs, and VSNFs, were particularly important for regional CRE spread.
Similar CRE Species Distribution Between LTCFs and ACHs
The species distribution of KPC-producing Enterobacterales was similar between the ACHs and LTCFs in the United States, of which K. pneumoniae was the prominent species (Brennan et al., 2014; Guh et al., 2015; Cunha et al., 2016; Satlin et al., 2017; Jean et al., 2018; Hayakawa et al., 2020). The KPC-producing K. pneumoniae was the predominant species (87%, n=129), followed by Enterobacter aerogenes (6%), E. coli (4%), E. cloacae (0.7%), and co-colonization with K. pneumoniae plus either E. coli or E. cloacae (2.7%) (Lin et al., 2013). Consistent with the CRE incidence study from the United States communities in 2012–2013, a high prevalence (58.6%, n=351) of K. pneumoniae among CRE isolates was found among the United States population, followed by E. coli (13.2%, n=79) and E. cloacae (12.5%, n=75) (Guh et al., 2015). A high percentage of K. pneumoniae (87.2%, n=89) was also noted in a state-wide surveillance study in Michigan (Brennan et al., 2014).
In Europe, the predominant CRE species were also similar between the ACHs and the LTCFs. A hospital admission survey demonstrated K. pneumoniae as the primarily species in Spain, of which 37% of cases were health-care related (42% were NH residents) (Palacios-Baena et al., 2016). The EuSCPAPE study reported predominant K. pneumoniae among CRE species, followed by E. coli. Wide geographic variations in CRE prevalence existed in Europe, with a high prevalence in Italy, Romania, Turkey, and Spain (Grundmann et al., 2017).
In the Asian region, among CPE in Japan, the most common species were E. cloacae (30%), followed by K. pneumoniae (22%), E. coli (14.8%), Citrobacter freundii (11.1%), Klebsiella oxytoca (7.4%), E. aerogenes (3.7%), and Serratia marcescens (3.7%) (Hayakawa et al., 2020). The prominent CRE strains identified among LTCFs in Taiwan were K. pneumoniae, compatible with previous studies (Jean et al., 2018).
Risk Factors for CRE Colonization and/or Infections Among Residents in LTCFs
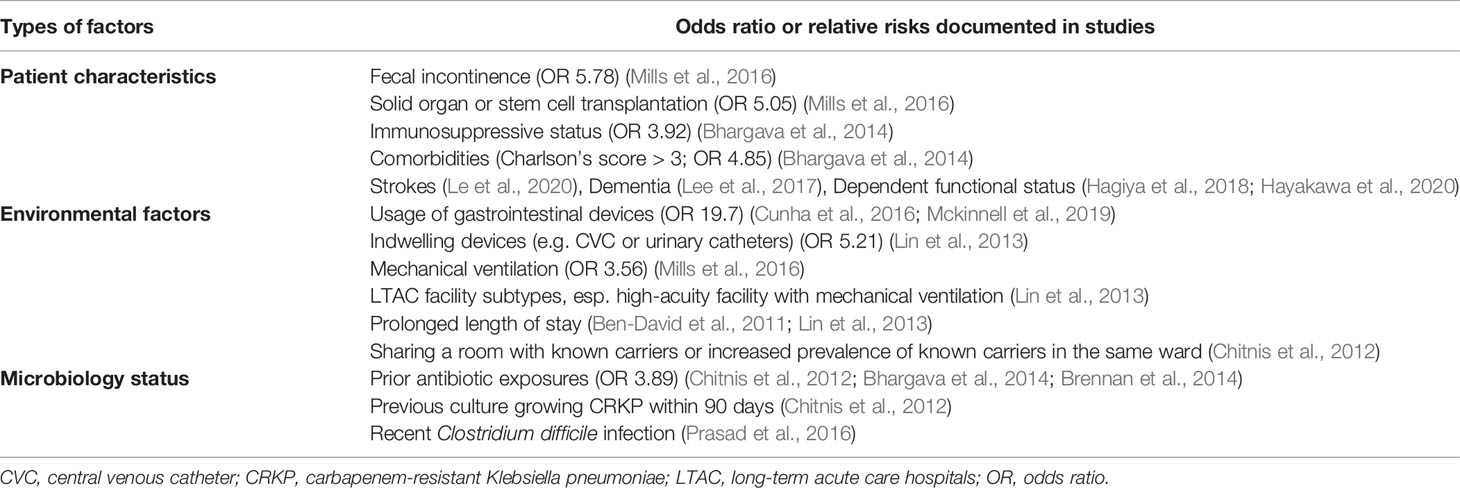
Multiple risk factors were reported to be associated with the increased vulnerability for colonization and/or infections with CRE or specifically KPC-producing Enterobacterales. The identified risk factors could be broadly classified into four groups: patient characteristics, environmental factors (including facility subtypes and the use of medical devices) and previous microbiology status or antibiotic exposures (including previous hospital stay and co-infection with other pathogens) (Table 4).
The identified patient characteristics associated with a significant risk factors for CRE colonization or infection among residents in LTCFs were fecal incontinence (OR 5.78, 95% CI 1.52 to 22.0, p=0.01) (Mills et al., 2016), solid organ or stem cell transplantation (OR 5.05, 95% CI 1.23 to 20.8, p=0.03) (Mills et al., 2016), comorbidity status with Charlson’s score greater than three (OR 4.85, 95% CI 1.64 to 14.41) (Bhargava et al., 2014), strokes (Le et al., 2020), dementia (Lee et al., 2017), residents in dependent functional status (Hagiya et al., 2018; Hayakawa et al., 2020), and immunosuppressive status (OR 3.92, 95% CI, 1.08 to 1.28) (Bhargava et al., 2014).
The environmental factors associated with a significant risk for CRE colonization or infection among residents of LTCFs were usage of gastrointestinal devices (OR 19.7, P <0.001) (Cunha et al., 2016; Mckinnell et al., 2019), mechanical ventilation (OR 3.56, 95% CI 1.24 to 5.28, p=0.01) (Mills et al., 2016), the presence of indwelling devices, such as central venous catheters or urinary catheters (OR 5.21, 95% CI 1.09 to 2.96), LTAC facility subtype (Lin et al., 2013), particularly high acuity facilities with mechanical ventilation, prolonged length of stay (Ben-David et al., 2011; Lin et al., 2013), and sharing a room with a known carrier (Chitnis et al., 2012).
Prior antimicrobial carriage status and associated antibiotics exposure were also associated with increased risk of CRE colonization or infection among residents in LTCFs (Ben-David et al., 2012). A high prevalence (41%) of residents in LTCFs had body cultures consistent with their prior CRE colonization status (Reuben et al., 2017). Prior antibiotic exposures (OR 3.89; 95% CI 0.71 to 21.47) (Chitnis et al., 2012; Bhargava et al., 2014; Brennan et al., 2014) and previous culture growing CRKP within 90 days were identified as independent risk factors for continued CRKP carriage (Chitnis et al., 2012). Furthermore, even short-term antimicrobial exposure in the prior one month was significantly associated with the increased risk of CRE colonization and infection among residents in LTCFs (meropenem OR 3.55, 95% CI 1.04–12.1, p=0.04; vancomycin OR 2.94, 95% CI 1.18–7.32, p=0.02; metronidazole OR 4.22, 95% CI 1.28–14.0, p=0.02) (Mills et al., 2016). In addition, recent Clostridium difficile infection was associated with increased risk of C. difficile and CRE colonization (Prasad et al., 2016), which indicated that prior or co-infection with other bacteria may increase CRE colonization risk in residents of LTCFs.
In the ACHs, the risk factors for CRE acquisition were exposure to antibiotics, high comorbidity indexes, deteriorated functional status and/or cognition at baseline, recent LTCF stay, and recent invasive procedures or permanent foreign devices (Lin et al., 2019). Our review of the risk factors associated with increased risk of CRE colonization and/or infection among residents in LTCFs was similar but not identical to the previous systematic review and meta-analysis (Van Loon et al., 2018), of which the systemic review identified risk factors associated with CRE acquisition among hospitalized patients between 2005 and 2017 in Europe, Asia, America, Australia, and Africa (Van Loon et al., 2018). Risk factors identified by Karlijn Van Loon et al. for CRE acquisition among hospitalized patients were patient’s underlying disease or condition (pooled OR 2.54; 95% CI 2.08 to 3.09, p <0.05), usage of medical devices (pooled OR 5.09; 95% CI, 3.38 to 7.67, p <0.05), mechanical ventilation (pooled OR 1.96; 95% CI 1.42 to 2.69, p<0.05), ICU admission (pooled OR 4.62; 95% CI, 2.46 to 8.69, p <0.05), antibiotic exposures (particularly carbapenem OR range 1.83 to 29.17 and cephalosporin OR 2.24 to 49.56), and CRE exposures (pooled OR 4.10; 96% CI, 1.46 to 11.52) (Van Loon et al., 2018).
Resistance Mechanisms of CRE From LTCFs Were Similar to ACHs
KPC is the major resistance determinant of CRE from LTCFs in the United States (Figure 1). Molecular study for CRE from LTCFs in the United States demonstrated a high prevalence of KPC-producing Enterobacterales (55%) (Johnson et al., 2014). Among KPC-producing Enterobacterales, the prominent species were KPC-producing K. pneumoniae (87%), followed by E. aerogenes (6%), E. coli (4%), and E. cloacae (0.7%) (Lin et al., 2013). Among LTCFs in the United States, most KPC-producing K. pneumoniae strains carried KPC-2 (19/21) and mostly belonged to the strain ST258 (60%) (Johnson et al., 2014). Regarding KPC-producing E. coli, half of the tested strains belong to ST131 (Johnson et al., 2014). Another report showed a higher carriage rate of KPC-3 in K. pneumoniae and E. coli, whereas other carbapenemase (NDM, IMP, VIM, OXA-48) were uncommon among CRE colonized in LTCF residents in the United States (Prasad et al., 2016; Reuben et al., 2017; Dubendris et al., 2020). Among these studies, the carriage of CTX-M (Reuben et al., 2017) or OXA-48 (Reuben et al., 2017) could be found in KPC-producing Enterobacterales. The predominance of KPC-producing CRE isolates was supported by previous CRE incidence study, of which 90% KPC was identified from CRE (79.3% were K. pneumoniae) (Guh et al., 2015); and previous outbreak investigation study (Gohil et al., 2017). Furthermore, clustering of the ST258 was also noted in the outbreak investigation study in Indiana and Illinois (Gohil et al., 2017). Similarly, in the ACHs, the predominance of KPC-producing CRE (KPC-3 48%, KPC-2 44%) and co-existence of NDM-1 and OXA-48 were identified in a multicenter prevalence study in the United States (Satlin et al., 2017). In addition, the prominent ST258 strain among CRKP was also identified in ACHs (Satlin et al., 2017).
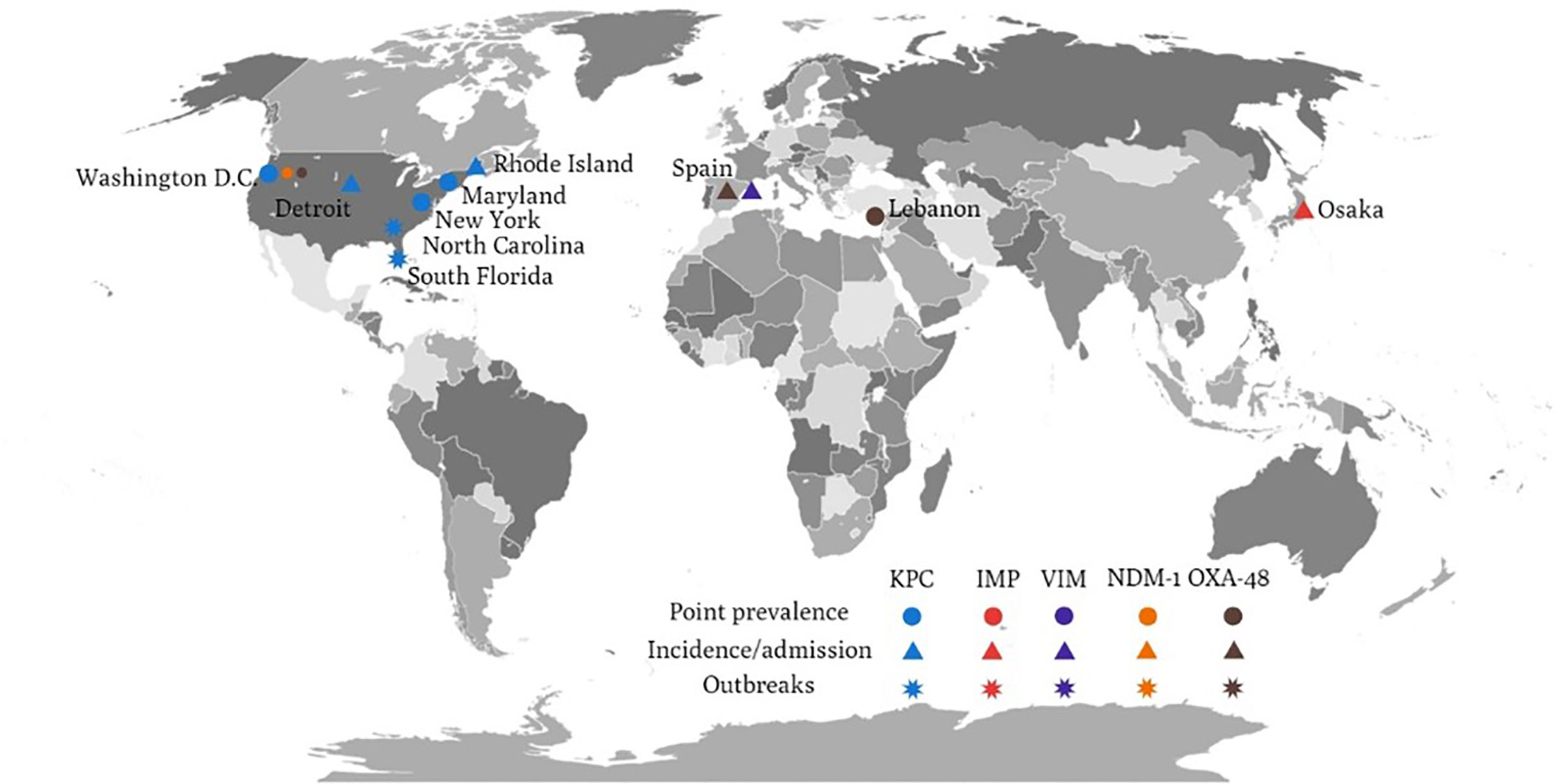
Figure 1 The global distribution of various carbapenemase-producing Enterobacteriaceae related to long-term care facilities.
There are only few studies concerning the resistance mechanisms of CRE from LTCFs in Asia. Molecular analyses showed that nearly all CRE harbored IMP-type carbapenemase in LTCFs in Japan, of which IMP-11 was the most prominent type (40.7% IMP-11, 22.2% IMP-42, 14.8% IMP-6, 11.1% IMP-10, 11.1% IMP-1) (Hayakawa et al., 2020), though another study reported IMP-6 as the most common carbapenemase in Japan (Hagiya et al., 2018). Besides, efflux pump genes (oqxA and oqxB) were mostly observed in the CP-CRE group compared with the non-CP-CRE group (Hayakawa et al., 2020). A study in Taiwan revealed blaCMY-2 in imipenem-resistant Providencia stuartii isolates associated with the outbreak in a LTCF, yet the true determinant remained unidentified (Mao et al., 2018).
OXA-48 has been reported in CPE isolates from LTCFs in Europe. In a hospital admission survey of 379 CPE isolates from 245 patients in Spain, OXA-48 was the predominant carbapenemase, followed by VIM-1, IMP, and KPC (74% OXA-48, 22% VIM-1, 2% IMP, 2% KPC) (Palacios-Baena et al., 2016). In the 35 isolates from NHs, 32 of them were K. pneumoniae, of which 31 isolates were OXA-48 and one isolate was VIM-1. On the contrary, among hospitalized patients in Europe, KPCs remained the predominant carbapenemase (42%, 393/927), followed by OXA-48-like enzymes (38%, 353/927), NDM-1 (12%, 113/927), and VIM (7%, 68/927) (Grundmann et al., 2017). At country level, KPC were predominantly detected in Italy (96%, 187/195), Israel (80%, 31/39), Greece (65%, 56/86), and Portugal (59%, 36/61) (Grundmann et al., 2017). OXA-48-like carbapenemases were common in Turkey (79%, 98/124), Romania (74%, 50/68), Spain (70%, 81/116), Belgium (38%, 18/48), France (37%, 10/27), and Germany (33%, 12/36) (Grundmann et al., 2017).
Outcome of CRE Colonization in LTCFs and ACHs
Acquisition of CRE is associated with a high economic burden and poor clinical outcomes. Among patients colonized or infected with CRE in LTCFs, the 30-day mortality rate was 10%–25% (Tischendorf et al., 2016; Chopra et al., 2018; Igbinosa et al., 2020). It is worth noting that the mortality rates in specific subgroups of patients with clinical CRE infections were as high as 30%–75% (Borer et al., 2012; Papadimitriou-Olivgeris et al., 2013; Lubbert et al., 2014). The condition is complicated by the prolonged CRE colonization which has been documented in various studies. Manon R. Haverkate et al. demonstrated that the duration of KPC-producing Enterobacterales colonization could be more than 9 months in KPC-positive patients (Haverkate et al., 2016).
The risk of infection after colonization with CRE varied in different studies. The cumulative risk of infection after CRE colonization was 16.5% in a systemic review (Tischendorf et al., 2016). The risk of infection varied, ranging from 7.6%–44.4% (Tischendorf et al., 2016), of which the most common site of infection was the lung (50% of the patients), followed by the urinary tract (20%), primary bloodstream, and skin and soft tissue infections (including surgical sites) (Tischendorf et al., 2016).
Regarding the hospitalized patients, a systemic review and meta-analysis revealed that the pooled risk ratio of CRE infection and mortality rate was 2.85 [95% CI, 1.88 to 4.30] (Soontaros and Leelakanok, 2019). Dickstein et al. conducted a matched cohort study in the ICU, and reported that colonization with CRE was independently associated with Enterobacterales infection (cause-specific hazard ratio was 2.06, 95% CI 1.31 to 8.43) (Dickstein et al., 2016). Zilberberg et al. conducted a retrospective cohort study among the hospitals in the United States, and reported that the presence of CRE was significantly associated with increased inappropriate empirical treatment than the absence of CRE (46.5% vs. 11.8%, p <0.001) (adjusted relative risk ratio 3.95, 95% CI 3.5 to 4.5, p <0.001) (Zilberberg et al., 2017). In addition, increased mortality rate (adjusted mortality 12%, 95% CI 3% to 23%) and prolonged length of hospital stay (an excess of 5.2 days, 95% CI 4.8 to 5.6, p <0.001) were found (Zilberberg et al., 2017).
Colonization with CRE poses an increasing threat to other residents in the same facility. CRE are mostly transmitted via patient-to-patient contact, and interestingly, the CRE transmission in the environment follows the 20/80 rule. That is, 20% super-spreaders are responsible for 80% bacterial transmission, which indicated that the super-spreaders play an important role in CRE transmission (Lerner et al., 2015). CRE super-spreaders were associated with high rectal CRE concentrations (modelled as blaKPC copies/16s rDNA copies ratio, OR 14.5, 95% CI, 1.09 to 192.0, p=0.042) and respiratory illnesses on admission (OR 20.5, 95% CI, 1.41 to 297.6, p=0.027) (Lerner et al., 2015).
The Consideration of Active Surveillance for CRE in LTCFs
In 2016, with the worsening threat of CRE, the European Centre for Disease Prevention and Control (ECDC) recommended infection prevention and control measures in hospitals and other healthcare settings, active screening in the epidemic region, and active surveillance in the endemic region. Even though active surveillance is one of the infection control measures for the prevention of CPE transmission and spread (Ambretti et al., 2019), and the 2013 European Society of Clinical Microbiology and Infectious Disease (ESCMID) strongly recommended contact precautions, using alert codes to identify known colonized patients at admission, pre-emptive contact precautions, isolation in a single room for infected or colonized patients, cohort staffing, antimicrobial stewardship and education, monitoring cleaning performance and active surveillance (Ambretti et al., 2019); there are limited national or international guidelines for optimal measures for active surveillance and management of CRE colonized patients in LTCFs (Ambretti et al., 2019). Some experts suggested that screening all hospital admission from the LTCFs for CRE may not be cost-effective (Hwang et al., 2018).
For CRE, targeting patients at high risk of CRE carriage is very important, and these high risk patients should be screened for digestive tract carriage, with concomitant pre-emptive contact precaution and followed isolation if colonization was confirmed (Magiorakos et al., 2017). Lau A. F. et al. recommended that the universal nucleic acid amplification technology (NAAT)-based method of CRE screening may not be universally affordable, but molecular rapid methods may be applicable for high-risk patients (e.g. from endemic region, LTCFs, extensive exposure to carbapenems), followed by susceptible culture-based method if the screening is negative, and considering to perform the carbapenemase confirmatory test if suspicious species are detected (Lau et al., 2015). Other than molecular and/or genomic analyses, Vincent J. LaBombardi et al. suggested that Remel Spectra CRE agar plates could provide faster (18 hours for Remel Spectra CRE agar versus 36 hours for the CDC method) and reliable results for KPC-type CRE detection (Labombardi et al., 2015). The sensitivity for common commercial chromogenic media were also reported, including chromID Carba media 96.5%, Remel Spectra CRE 97.8%, CHROMagar KPC 76.6%, and Direct ertapenem disk method 83% (Labombardi et al., 2015).
The CDC recommended perirectal swab for targeted CRE screening at hospital admission, especially for those admitted directly from LTACs, other LTCFs with known endemicity, or patients who are transferred directly from another ACHs (Tischendorf et al., 2016). Although KPC-producing Enterobacterales were mostly identified in perianal swab specimens (Johnson et al., 2014) or rectal swab culture (Cunha et al., 2016)), axilla/groin screening samples also detected 1% CRE prevalence in nursing home facilities (Mckinnell et al., 2016). CRE could be found in a variety of samples. Various studies reported urine as the most common source of CRE (Brennan et al., 2014; Guh et al., 2015), and CP-CRE were prominently isolated from sputum (40.7%) (Hayakawa et al., 2020). In a study conducted in Hiroshima, CRE were detected in oropharyngeal swab specimen (Le et al., 2020), and in a study in Osaka, CRE were detected from blood, sputum, urine, and intra-abdominal samples (Hayakawa et al., 2020).
Interventions to Reduce CRE Colonization and Infection in LTCFs
The importance of interventions to reduce the CRE carriage rate in LTCFs has already been documented. An outbreak investigation of CRE colonization among patients in LTAC between 2009 and 2011 revealed that surveillance testing and targeted interventions resulted in significant reductions in CRE prevalence (49% vs 8%), CRE incidence (44% vs 0%) and CRE bacteremia (2.5 vs 0.0 per 1000 patient-days) (Chitnis et al., 2012). In addition, the prevention measures for CRE in LTCFs from a nation-wide coordinated protocol in Israel between 2007 and 2008 successfully reduced nosocomial CRE cases from 55.5 cases per 100000 patient-years to 11.7 cases per 100000 patient-years (Schwaber et al., 2011). The infection prevention measures in Israel were contact isolation, self-contained nursing units (single room or cohorts), and re-isolation of known carriers when they are encountered for subsequent hospital admission (Schwaber et al., 2011). Furthermore, the subsequent nation-wide intervention (alcohol-based hand rub, appropriate use of gloves, and a policy of CRE surveillance at hospital admission) in 13 post-acute hospitals in Israel between 2008 and 2011 resulted in the reduction of the overall CRE carriage rate (16.8% vs 12.5%, p=0.013) (Ben-David et al., 2014). Collectively, the Israeli guidelines for CRE prevention were isolation or cohorting of CRE carriers, cohorting of nursing staff (mandatory only for ACHs), barrier precautions on room entrance, admission CRE screening for high-risk patients, screening of patients’ contacts, standard protocol for discontinuation of contact isolation, daily reporting of new cases to the Israeli National Center for Infection Control in the Ministry of Health, and periodic auditing of health care facilities’ compliance with national guidelines by the National Center for Infection Control in the Ministry of Health (Ben-David et al., 2014).
In the United States, Mary K. Hayden et al. (Hayden et al., 2015) examined a bundled intervention in four LTACs in a high endemic KPC-producing Enterobacterales region (Chicago, Illinois) (Hayden et al., 2015). They adopted a rectal swab culture for active surveillance and preemptive contact isolation while awaiting the culture report. KPC intervention bundle was as follows: biweekly rectal culture surveillance, contact isolation, geographic separation of KPC-positive patients with ward cohort or single room, universal contact isolation of all patients in a high-acuity ward, daily 2% chlorhexidine gluconate (CHG) bathing for patients, healthcare-worker education, and adherence monitoring (particularly hand hygiene) (Hayden et al., 2015). During the pre-intervention period, the prevalence rate of KPC-producing Enterobacterales remained unchanged (average 45.8%, 95% CI 42.1% to 49.5%) (Hayden et al., 2015). During the intervention period, the prevalence rate of KPC-producing Enterobacterales declined significantly, then reached a plateau (34.3%, 95% CI 32.4 to 36.2%, p <0.001) (Hayden et al., 2015). The incidence of KPC-producing Enterobacterales also declined significantly during the intervention (from 4 to 2 acquisition per 100 patient-weeks, p=0.004) (Hayden et al., 2015). Other important clinical outcome indicators, including KPC identified in any clinical culture (32% reduction) and KPC bacteremia (56% reduction) also decreased (Hayden et al., 2015).
Similar to Hayden et al.’ s study (Hayden et al., 2015), Toth et al. designed an agent-based simulation model for CRE transmission in a single LTAC within a regional network of ten health-care facilities (including one LTAC, three ACHs, and six NHs). The CRE prevalence rate was 45.8% and clinical detection incidence was 3.7 per 1000 patient-days (Toth et al., 2017). Two models with different transmission rates were created, and significant reduction in CRE transmission (range 79% to 93%) and prevalence (decrease from 21% to 6% in model A, decrease from 9% to 0.5% in model B) in a five-year period were reported (Toth et al., 2017). Even when the intervention was delayed until the 20th CRE detection, CRE transmission was still reduced by 60%–79% over five years (Toth et al., 2017). Furthermore, among the infection control measures, Toth et al. (Toth et al., 2019) designed a model-based assessment in Chicago, and reported that contact precautions by itself could potentially explain the decline in CPE colonization among surveillance-detected carriers (Toth et al., 2019). Other interventions, including CHG bathing, hand hygiene, and adherence monitoring may play a role in the slowing down of colonization (Toth et al., 2019). Therefore, focusing infection control and prevention measures in LTCFs is an effective strategy to reduce CRE acquisition and transmission (Toth et al., 2017).
Moreover, the importance of controlling CRE in LTCFs could not be over emphasized, since it may become a tragedy without interventions. Bruce Y Lee et al. conducted a prediction model and reported that if infection control and prevention measures were not implemented properly, CRE would become endemic in almost all health-care facilities (in Orange County) within 10 years (Lee et al., 2016). Although benefits of CRE decolonization include reduced CRE-related infection incidence and all-cause mortality (Tacconelli et al., 2019), the potential of increased antimicrobial resistance to decolonizing agents was reported in nearly all studies (Tacconelli et al., 2019). Consequently, the ESCMID-EUCIC clinical guidelines do not recommend routine decolonization of CRE (Tacconelli et al., 2019).
Antimicrobial use in LTCFs remains a critical issue in long-term care (Ricchizzi et al., 2018). Antimicrobial stewardship programs are needed to control the spreading of multidrug-resistant organisms (Oliva et al., 2018). Current data suggested effective antimicrobial stewardship strategies in LTCF reduced antimicrobial use (Jump et al., 2012). The implementation strategies vary considerably in different setting and warrant more studies to define appropriate and quality appraisal tools (Wu et al., 2019).
Summary
The emergence of CRE has become a major public health concern. Moreover, its colonization among residents of LTCFs is associated with subsequent infections and mortality. LTCFs are also an important reservoir of CRE. There are increasing studies concerning the high predominance of CRE in LTCFs. Further studies are needed to develop effective control measures.
Author Contributions
H-YC, P-YL, S-SJ, Y-LL, M-CL, W-CK and P-RH collected and analyzed the data. S-SJ, Y-LL, M-CL, and W-CK conducted validation and supervision. H-YC, S-SJ, Y-LL, M-CL, W-CK and P-YL participated in the writing of the manuscript. H-YC, P-YL, S-SJ, Y-LL, M-CL, W-CK and P-RH read and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ambretti S., Bassetti M., Clerici P., Petrosillo N., Tumietto F., Viale P., et al. (2019). Screening for Carriage of Carbapenem-Resistant Enterobacteriaceae in Settings of High Endemicity: A Position Paper From an Italian Working Group on CRE Infections. Antimicrob. Resist. Infect. Control. 8, 136. doi: 10.1186/s13756-019-0591-6
Bartsch S. M., Mckinnell J. A., Mueller L. E., Miller L. G., Gohil S. K., Huang S. S., et al. (2017). Potential Economic Burden of Carbapenem-Resistant Enterobacteriaceae (CRE) in the United States. Clin. Microbiol. Infect. 23, 48.e49–48.e16. doi: 10.1016/j.cmi.2016.09.003
Ben-David D., Masarwa S., Adler A., Mishali H., Carmeli Y., Schwaber M. J. (2014). A National Intervention to Prevent the Spread of Carbapenem-Resistant Enterobacteriaceae in Israeli Post-Acute Care Hospitals. Infect. Control. Hosp. Epidemiol. 35, 802–809. doi: 10.1086/676876
Ben-David D., Masarwa S., Navon-Venezia S., Mishali H., Fridental I., Rubinovitch B., et al. (2011). Carbapenem-Resistant Klebsiella pneumoniae in Post-Acute-Care Facilities in Israel. Infect. Control. Hosp. Epidemiol. 32, 845–853. doi: 10.1086/661279
Ben-David D., Masarwa S., Navon-Venezia S., Mishali H., Fridental I., Rubinovitch B., et al. (2012). Risk Factors for Developing Clinical Infection With Carbapenem-Resistant Klebsiella pneumoniae in Hospital Patients Initially Only Colonized With Carbapenem-Resistant K. pneumoniae. Am. J. Infect. Control. 40, 421–425. doi: 10.1016/j.ajic.2011.05.022
Bhargava A., Hayakawa K., Silverman E., Haider S., Alluri K. C., Datla S., et al. (2014). Risk Factors for Colonization Due to Carbapenem-Resistant Enterobacteriaceae Among Patients Exposed to Long-Term Acute Care and Acute Care Facilities. Infect. Control. Hosp. Epidemiol. 35, 398–405. doi: 10.1086/675614
Borer A., Saidel-Odes L., Eskira S., Nativ R., Riesenberg K., Livshiz-Riven I., et al. (2012). Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K pneumoniae. Am. J. Infect. Control 40, 421–425.
Bower C. W., Fridkin D. W., Wolford H. M., Slayton R. B., Kubes J. N., Jacob J. T., et al. (2020). Evaluating Movement of Patients With Carbapenem-Resistant Enterobacteriaceae Infections in the Greater Atlanta Metropolitan Area Using Social Network Analysis. Clin. Infect. Dis. 70, 75–81. doi: 10.1093/cid/ciz154
Brennan B. M., Coyle J. R., Marchaim D., Pogue J. M., Boehme M., Finks J., et al. (2014). Statewide Surveillance of Carbapenem-Resistant Enterobacteriaceae in Michigan. Infect. Control. Hosp. Epidemiol. 35, 342–349. doi: 10.1086/675611
Centers for Disease Control and Prevention (2013). Vital Signs: Carbapenem-Resistant Enterobacteriaceae. MMWR Morb. Mortal. Wkly. Rep. 62, 165–170.
Chen H., Au K. M., Hsu K. E., Lai C. K., Myint J., Mak Y. F., et al. (2018). Multidrug-Resistant Organism Carriage Among Residents From Residential Care Homes for the Elderly in Hong Kong: A Prevalence Survey With Stratified Cluster Sampling. Hong Kong Med. J. 24, 350–360. doi: 10.12809/hkmj176949
Cheng V. C., Chen J. H., Ng W. C., Wong J. Y., Chow D. M., Law T. C., et al. (2016). Emergence of Carbapenem-Resistant Acinetobacter baumannii in Nursing Homes With High Background Rates of MRSA Colonization. Infect. Control Hosp. Epidemiol. 37, 983–986.
Chitnis A. S., Caruthers P. S., Rao A. K., Lamb J., Lurvey R., Beau De Rochars V., et al. (2012). Outbreak of Carbapenem-Resistant Enterobacteriaceae At a Long-Term Acute Care Hospital: Sustained Reductions in Transmission Through Active Surveillance and Targeted Interventions. Infect. Control. Hosp. Epidemiol. 33, 984–992. doi: 10.1086/667738
Chopra T., Rivard C., Awali R. A., Krishna A., Bonomo R. A., Perez F., et al. (2018). Epidemiology of Carbapenem-Resistant Enterobacteriaceae At a Long-term Acute Care Hospital. Open Forum Infect. Dis. 5, ofy224. doi: 10.1093/ofid/ofy224
Cunha C. B., Kassakian S. Z., Chan R., Tenover F. C., Ziakas P., Chapin K. C., et al. (2016). Screening of Nursing Home Residents for Colonization With Carbapenem-Resistant Enterobacteriaceae Admitted to Acute Care Hospitals: Incidence and Risk Factors. Am. J. Infect. Control. 44, 126–130. doi: 10.1016/j.ajic.2015.09.019
Dandachi I., Salem Sokhn E., Najem E., Azar E., Daoud Z. (2016). Carriage of Beta-Lactamase-Producing Enterobacteriaceae Among Nursing Home Residents in North Lebanon. Int. J. Infect. Dis. 45, 24–31. doi: 10.1016/j.ijid.2016.02.007
Dickstein Y., Edelman R., Dror T., Hussein K., Bar-Lavie Y., Paul M. (2016). Carbapenem-Resistant Enterobacteriaceae Colonization and Infection in Critically Ill Patients: A Retrospective Matched Cohort Comparison With non-Carriers. J. Hosp. Infect. 94, 54–59. doi: 10.1016/j.jhin.2016.05.018
Dubendris H., Macfarquhar J., Kornegay R., Gable P., Boyd S., Walters M., et al. (2020). Imipenemase-Producing Carbapenem-Resistant Enterobacteriaceae Transmission in a Long-Term-Care Facility During a Community-Wide Multidrug Resistant Organism Outbreak-North Carolin. Am. J. Infect. Control. 48, 320–323. doi: 10.1016/j.ajic.2019.05.022
Endimiani A., Depasquale J. M., Forero S., Perez F., Hujer A. M., Roberts-Pollack D., et al. (2009). Emergence of blaKPC-containing Klebsiella pneumoniae in a long-term acute care hospital: a new challenge to our healthcare system. J. Antimicrob. Chemother. 64, 1102–1110. doi: 10.1016/j.ajic.2019.05.022
Gasink L. B., Edelstein P. H., Lautenbach E., Synnestvedt M., Fishman N. O. (2009). Risk Factors and Clinical Impact of Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae. Infect. Control. Hosp. Epidemiol. 30, 1180–1185. doi: 10.1086/648451
Gohil S. K., Singh R., Chang J., Gombosev A., Tjoa T., Zahn M., et al. (2017). Emergence of Carbapenem-Resistant Enterobacteriaceae in Orange County, California, and Support for Early Regional Strategies to Limit Spread. Am. J. Infect. Control. 45, 1177–1182. doi: 10.1016/j.ajic.2017.06.004
Grundmann H., Glasner C., Albiger B., Aanensen D. M., Tomlinson C. T., Andrasević A. T., et al. (2017). Occurrence of Carbapenemase-Producing Klebsiella pneumoniae and Escherichia Coli in the European Survey of Carbapenemase-Producing Enterobacteriaceae (Euscape): A Prospective, Multinational Study. Lancet Infect. Dis. 17, 153–163. doi: 10.1016/S1473-3099(16)30257-2
Guh A. Y., Bulens S. N., Mu Y., Jacob J. T., Reno J., Scott J., et al. (2015). Epidemiology of Carbapenem-Resistant Enterobacteriaceae in 7 US Communitie-2013. JAMA 314, 1479–1487. doi: 10.1001/jama.2015.12480
Hagiya H., Yamamoto N., Kawahara R., Akeda Y., Shanmugakani R. K., Ueda A., et al. (2018). Risk Factors for Fecal Carriage of IMP-6-producing Enterobacteriaceae At a Long-Term Care Hospital in Japan: A Follow-Up Report From the Northern Osaka Multicentre Study Group. J. Infect. Chemother. 24, 769–772. doi: 10.1016/j.jiac.2018.03.009
Han J. H., Goldstein E. J., Wise J., Bilker W. B., Tolomeo P., Lautenbach E. (2017). Epidemiology of Carbapenem-Resistant Klebsiella pneumoniae in a Network of Long-Term Acute Care Hospitals. Clin. Infect. Dis. 64, 839–844. doi: 10.1093/cid/ciw856
Haverkate M. R., Weiner S., Lolans K., Moore N. M., Weinstein R. A., Bonten M. J., et al. (2016). Duration of Colonization With Klebsiella pneumoniae Crbapenemase-Producing Bacteria At Long-Term Acute Care Hospitals in Chicago, Illinois. Open Forum Infect. Dis. 3, ofw178. doi: 10.1093/ofid/ofw178
Hayakawa K., Nakano R., Hase R., Shimatani M., Kato H., Hasumi J., et al. (2020). Comparison Between Imp Carbapenemase-Producing Enterobacteriaceae and non-Carbapenemase-Producing Enterobacteriaceae: A Multicentre Prospective Study of the Clinical and Molecular Epidemiology of Carbapenem-Resistant Enterobacteriaceae. J. Antimicrob. Chemother. 75, 697–708. doi: 10.1093/jac/dkz501
Hayden M. K., Lin M. Y., Lolans K., Weiner S., Blom D., Moore N. M., et al. (2015). Prevention of Colonization and Infection by Klebsiella pneumoniae Carbapenemase-Producing Enterobacteriaceae in Long-Term Acute-Care Hospitals. Clin. Infect. Dis. 60, 1153–1161. doi: 10.1093/cid/ciu1173
Hwang J. H., Park J. S., Lee E., Bae J. Y., Song K. H., Choe P. G., et al. (2018). Active Surveillance for Carbapenem-Resistant Enterobacteriaceae, Vancomycin-Resistant Enterococci and Toxigenic Clostridium Difficile Among Patients Transferred From Long-Term Care Facilities in Korea. J. Hosp. Infect. 99, 487–491. doi: 10.1016/j.jhin.2018.02.017
Igbinosa O., Dogho P., Osadiaye N. (2020). Carbapenem-Resistant Enterobacteriaceae: A Retrospective Review of Treatment and Outcomes in a Long-Term Acute Care Hospital. Am. J. Infect. Control. 48, 7–12. doi: 10.1016/j.ajic.2019.07.006
Jean S. S., Lee N. Y., Tang H. J., Lu M. C., Ko W. C., Hsueh P. R. (2018). Carbapenem-Resistant Enterobacteriaceae Infections: Taiwan Aspects. Front. Microbiol. 9, 2888. doi: 10.3389/fmicb.2018.02888
Johnson J. K., Wilson L. E., Zhao L., Richards K., Thom K. A., Harris A. D. (2014). Point Prevalence of Klebsiella pneumoniae Carbapenemase-Producing Enterobacteriaceae in Maryland. Infect. Control. Hosp. Epidemiol. 35, 443–445. doi: 10.1086/675610
Jump R. L., Olds D. M., Seifi N., Kypriotakis G., Jury L. A., Peron E. P., et al. (2012). Effective Antimicrobial Stewardship in a Long-Term Care Facility Through an Infectious Disease Consultation Service: Keeping a LID on Antibiotic Use. Infect. Control. Hosp. Epidemiol. 33, 1185–1192. doi: 10.1086/668429
Kalpoe J. S., Sonnenberg E., Factor S. H., Del Rio Martin J., Schiano T., Patel G., et al. (2012). Mortality Associated With Carbapenem-Resistant Klebsiella pneumoniae Infections in Liver Transplant Recipients. Liver Transpl. 18, 468–474. doi: 10.1002/lt.23374
Kim D., Ahn J. Y., Lee C. H., Jang S. J., Lee H., Yong D., et al. (2017). Increasing Resistance to Extended-Spectrum Cephalosporins, Fluoroquinolone, and Carbapenem in Gram-negative Bacilli and the Emergence of Carbapenem non-Susceptibility in Klebsiella pneumoniae: Analysis of Korean Antimicrobial Resistance Monitoring System (Karms) Data From 2013 to 2015. Ann. Lab. Med. 37, 231–239. doi: 10.3343/alm.2017.37.3.231
Kohler P., Fulchini R., Albrich W. C., Egli A., Balmelli C., Harbarth S., et al. (2018). Antibiotic Resistance in Swiss Nursing Homes: Analysis of National Surveillance Data Over an 11-Year Period Between 2007 and 2017. Antimicrob. Resist. Infect. Control. 7, 88. doi: 10.1186/s13756-018-0378-1
Labombardi V. J., Urban C. M., Kreiswirth B. N., Chen L., Osorio G., Kopacz J., et al. (2015). Evaluation of Remel Spectra CRE Agar for Cetection of Carbapenem-Resistant Bacteria From Rectal Swabs Obtained From Residents of a Long-Term-Care Facility. J. Clin. Microbiol. 53, 2823–2826. doi: 10.1128/JCM.00789-15
Latour K., Huang T. D., Jans B., Berhin C., Bogaerts P., Noel A., et al. (2019). Prevalence of Multidrug-Resistant Organisms in Nursing Homes in Belgium in 2015. PloS One 14, e0214327. doi: 10.1371/journal.pone.0214327
Lau A. F., Fahle G. A., Kemp M. A., Jassem A. N., Dekker J. P., Frank K. M. (2015). Clinical Performance of Check-Direct CPE, a Multiplex PCR for Direct Detection of Bla(KPC), Bla(NDM) and/or Bla(VIM), and Bla(OXA)-48 From Perirectal Swabs. J. Clin. Microbiol. 53, 3729–3737. doi: 10.1128/JCM.01921-15
Lautenbach E., Marsicano R., Tolomeo P., Heard M., Serrano S., Stieritz D. D. (2009). Epidemiology of antimicrobial resistance among gram-negative organisms recovered from patients in a multistate network of long-term care facilities. Infect. Control Hosp. Epidemiol. 30, 790–793. doi: 10.1128/JCM.01921-15
Lee B. Y., Bartsch S. M., Wong K. F., Mckinnell J. A., Slayton R. B., Miller L. G., et al. (2016). The Potential Trajectory of Carbapenem-Resistant Enterobacteriaceae, an Emerging Threat to Health-Care Facilities, and the Impact of the Centers for Disease Control and Prevention Toolkit. Am. J. Epidemiol. 183, 471–479. doi: 10.1093/aje/kwv299
Lee C. M., Lai C. C., Chiang H. T., Lu M. C., Wang L. F., Tsai T. L., et al. (2017). Presence of Multidrug-Resistant Organisms in the Residents and Environments of Long-Term Care Facilities in Taiwan. J. Microbiol. Immunol. Infect. 50, 133–144. doi: 10.1016/j.jmii.2016.12.001
Le M. N., Kayama S., Yoshikawa M., Hara T., Kashiyama S., Hisatsune J., et al. (2020). Oral Colonisation by Antimicrobial-Resistant Gram-Negative Bacteria Among Long-Term Care Facility Residents: Prevalence, Risk Factors, and Molecular Epidemiology. Antimicrob. Resist. Infect. Control. 9, 45. doi: 10.1186/s13756-020-0705-1
Legeay C., Hue R., Berton C., Cormier H., Chenouard R., Corvec S., et al. (2019). Control strategy for carbapenemase-producing Enterobacteriaceae in nursing homes: perspectives inspired from three outbreaks. J. Hosp. Infect. 101, 183–187.
Lerner A., Adler A., Abu-Hanna J., Cohen Percia S., Kazma Matalon M., Carmeli Y. (2015). Spread of KPC-producing Carbapenem-Resistant Enterobacteriaceae: The Importance of Super-Spreaders and Rectal Kpc Concentration. Clin. Microbiol. Infect. 21, 470.e471–477. doi: 10.1016/j.cmi.2014.12.015
Lin M. Y., Lyles-Banks R. D., Lolans K., Hines D. W., Spear J. B., Petrak R., et al. (2013). The Importance of Long-Term Acute Care Hospitals in the Regional Epidemiology of Klebsiella pneumoniae Carbapenemase-Producing Enterobacteriaceae. Clin. Infect. Dis. 57, 1246–1252. doi: 10.1093/cid/cit500
Lin M. Y., Ray M. J., Rezny S., Runningdeer E., Weinstein R. A., Trick W. E. (2019). Predicting Carbapenem-Resistant Enterobacteriaceae Carriage At the Time of Admission Using a Statewide Hospital Discharge Database. Open Forum Infect. Dis. 6, ofz483. doi: 10.1093/ofid/ofz483
Livorsi D. J., Chorazy M. L., Schweizer M. L., Balkenende E. C., Blevins A. E., Nair R., et al. (2018). A Systematic Review of the Epidemiology of Carbapenem-Resistant Enterobacteriaceae in the United States. Antimicrob. Resist. Infect. Control. 7, 55. doi: 10.1186/s13756-018-0346-9
Lubbert C., Becker-Rux D., Rodloff A. C., Laudi S., Busch T., Bartels M., et al. (2014). Colonization of Liver Transplant Recipients With KPC-producing Klebsiella pneumoniae is Associated With High Infection Rates and Excess Mortality: A Case-Control Analysis. Infection 42, 309–316. doi: 10.1007/s15010-013-0547-3
Magiorakos A. P., Burns K., Rodríguez Baño J., Borg M., Daikos G., Dumpis U., et al. (2017). Infection Prevention and Control Measures and Tools for the Prevention of Entry of Carbapenem-Resistant Enterobacteriaceae Into Healthcare Settings: Guidance From the European Centre for Disease Prevention and Control. Antimicrob. Resist. Infect. Control. 6, 113. doi: 10.1186/s13756-017-0259-z
Mao Y. C., Chang C. L., Huang Y. C., Su L. H., Lee C. T. (2018). Laboratory Investigation of a Suspected Outbreak Caused by Providencia Stuartii With Intermediate Resistance to Imipenem At a Long-Term Care Facility. J. Microbiol. Immunol. Infect. 51, 214–219. doi: 10.1016/j.jmii.2016.07.004
Marquez P., Terashita D., Dassey D., Mascola L. (2013). Population-Based Incidence of Carbapenem-Resistant Klebsiella pneumoniae Along the Continuum of Care, Los Angeles County. Infect. Control. Hosp. Epidemiol. 34, 144–150. doi: 10.1086/669087
Mckinnell J. A., Miller L. G., Singh R., Kleinman K., Peterson E. M., Evans K. D., et al. (2016). Prevalence of and Factors Associated With Multidrug Resistant Organism (Mdro) Colonization in 3 Nursing Homes. Infect. Control. Hosp. Epidemiol. 37, 1485–1488. doi: 10.1017/ice.2016.215
Mckinnell J. A., Singh R. D., Miller L. G., Kleinman K., Gussin G., He J., et al. (2019). The SHIELD Orange County Project: Multidrug-resistant Organism Prevalence in 21 Nursing Homes and Long-Term Acute Care Facilities in Southern California. Clin. Infect. Dis. 69, 1566–1573. doi: 10.1093/cid/ciz119
Mills J. P., Talati N. J., Alby K., Han J. H. (2016). The Epidemiology of Carbapenem-Resistant Klebsiella pneumoniae Colonization and Infection Among Long-Term Acute Care Hospital Residents. Infect. Control. Hosp. Epidemiol. 37, 55–60. doi: 10.1017/ice.2015.254
Oliva A., Giacobbe D. R., Di Luca M., Miller N. S. (2018). New Insights Into Infections Due to Multidrug Resistant Gram Negative Bacteria: The Interplay Between Lab and Clinic. BioMed. Res. Int. 2018, 8905874. doi: 10.1155/2018/8905874
Palacios-Baena Z. R., Oteo J., Conejo C., Larrosa M. N., Bou G., Fernandez-Martinez M., et al. (2016). Comprehensive Clinical and Epidemiological Assessment of Colonisation and Infection Due to Carbapenemase-Producing Enterobacteriaceae in Spain. J. Infect. 72, 152–160. doi: 10.1016/j.jinf.2015.10.008
Papadimitriou-Olivgeris M., Marangos M., Fligou F., Christofidou M., Sklavou C., Vamvakopoulou S., et al. (2013). Kpc-Producing Klebsiella pneumoniae Enteric Colonization Acquired During Intensive Care Unit Stay: The Significance of Risk Factors for its Development and its Impact on Mortality. Diagn. Microbiol. Infect. Dis. 77, 169–173. doi: 10.1016/j.diagmicrobio.2013.06.007
Patel G., Huprikar S., Factor S. H., Jenkins S. G., Calfee D. P. (2008). Outcomes of Carbapenem-Resistant Klebsiella pneumoniae Infection and the Impact of Antimicrobial and Adjunctive Therapies. Infect. Control. Hosp. Epidemiol. 29, 1099–1106. doi: 10.1086/592412
Prabaker K., Lin M. Y., Mcnally M., Cherabuddi K., Ahmed S., Norris A., et al. (2012). Transfer From High-Acuity Long-Term Care Facilities is Associated With Carriage of Klebsiella pneumoniae Carbapenemase-Producing Enterobacteriaceae: A Multihospital Study. Infect. Control. Hosp. Epidemiol. 33, 1193–1199. doi: 10.1086/668435
Prasad N., Labaze G., Kopacz J., Chwa S., Platis D., Pan C. X., et al. (2016). Asymptomatic Rectal Colonization With Carbapenem-Resistant Enterobacteriaceae and Clostridium Difficile Among Residents of a Long-Term Care Facility in New York City. Am. J. Infect. Control. 44, 525–532. doi: 10.1016/j.ajic.2015.11.021
Reuben J., Donegan N., Wortmann G., Debiasi R., Song X., Kumar P., et al. (2017). Healthcare Antibiotic Resistance Prevalence - DC (Harp-Dc): A Regional Prevalence Assessment of Carbapenem-Resistant Enterobacteriaceae (CRE) in Healthcare Facilities in Washington, District of Columbia. Infect. Control. Hosp. Epidemiol. 38, 921–929. doi: 10.1017/ice.2017.110
Ricchizzi E., Latour K., Karki T., Buttazzi R., Jans B., Moro M. L., et al. (2018). Antimicrobial Use in European Long-Term Care Facilities: Results From the Third Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Usto 2017. Euro. Surveill. 23, 1800394. doi: 10.2807/1560-7917.ES.2018.23.46.1800394
Satlin M. J., Chen L., Patel G., Gomez-Simmonds A., Weston G., Kim A. C., et al. (2017). Multicenter Clinical and Molecular Epidemiological Analysis of Bacteremia Due to Carbapenem-Resistant Enterobacteriaceae (CRE) in the CRE Epicenter of the United States. Antimicrob. Agents. Chemother. 61, e02349–e02316. doi: 10.1128/AAC.02349-16
Schwaber M. J., Lev B., Israeli A., Solter E., Smollan G., Rubinovitch B., et al. (2011). Containment of a Country-Wide Outbreak of Carbapenem-Resistant Klebsiella pneumoniae in Israeli Hospitals via a Nationally Implemented Intervention. Clin. Infect. Dis. 52, 848–855. doi: 10.1093/cid/cir025
Soontaros S., Leelakanok N. (2019). Association Between Carbapenem-Resistant Enterobacteriaceae and Death: A Systematic Review and Meta-Analysis. Am. J. Infect. Control. 47, 1200–1212. doi: 10.1016/j.ajic.2019.03.020
Tacconelli E., Mazzaferri F., De Smet A. M., Bragantini D., Eggimann P., Huttner B. D., et al. (2019). Escmid-EUCIC Clinical Guidelines on Decolonization of Multidrug-Resistant Gram-Negative Bacteria Carriers. Clin. Microbiol. Infect. 25, 807–817. doi: 10.1016/j.cmi.2019.01.005
Thaden J. T., Lewis S. S., Hazen K. C., Huslage K., Fowler V. G. Jr., Moehring R. W., et al. (2014). Rising Rates of Carbapenem-Resistant Enterobacteriaceae in Community Hospitals: A Mixed-Methods Review of Epidemiology and Microbiology Practices in a Network of Community Hospitals in the Southeastern United States. Infect. Control. Hosp. Epidemiol. 35, 978–983. doi: 10.1086/677157
Tischendorf J., De Avila R. A., Safdar N. (2016). Risk of Infection Following Colonization With Carbapenem-Resistant Enterobactericeae: A Systematic Review. Am. J. Infect. Control. 44, 539–543. doi: 10.1016/j.ajic.2015.12.005
Toth D. J. A., Khader K., Slayton R. B., Kallen A. J., Gundlapalli A. V., O’hagan J. J., et al. (2017). The Potential for Interventions in a Long-Term Acute Care Hospital to Reduce Transmission of Carbapenem-Resistant Enterobacteriaceae in Affiliated Healthcare Facilities. Clin. Infect. Dis. 65, 581–587. doi: 10.1093/cid/cix370
Toth D. J. A., Khader K., Slayton R. B., Kallen A. J., Gundlapalli A. V., O’hagan J. J., et al. (2019). Model-Based Assessment of the Effect of Contact Precautions Applied to Surveillance-Detected Carriers of Carbapenemase-Producing Enterobacteriaceae in Long-Term Acute Care Hospitals. Clin. Infect. Dis. 69, S206–S213. doi: 10.1093/cid/ciz557
Van Duin D., Perez F., Rudin S. D., Cober E., Hanrahan J., Ziegler J., et al. (2014). Surveillance of Carbapenem-Resistant Klebsiella pneumoniae: Tracking Molecular Epidemiology and Outcomes Through a Regional Network. Antimicrob. Agents Chemother. 58, 4035–4041. doi: 10.1128/AAC.02636-14
Van Dulm E., Tholen A. T. R., Pettersson A., Van Rooijen M. S., Willemsen I., Molenaar P., et al. (2019). High Prevalence of Multidrug Resistant Enterobacteriaceae Among Residents of Long Term Care Facilities in Amsterdam, the Netherlands. PloS One 14, e0222200. doi: 10.1371/journal.pone.0222200
Van Loon K., Voor in ‘T Holt A. F., Vos M. C. (2018). A Systematic Review and Meta-analyses of the Clinical Epidemiology of Carbapenem-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 62, e01730–e01717. doi: 10.1128/AAC.01730-17
Wu J. H., Langford B. J., Daneman N., Friedrich J. O., Garber G. (2019). Antimicrobial Stewardship Programs in Long-Term Care Settings: A Meta-Analysis and Systematic Review. J. Am. Geriatr. Soc. 67, 392–399. doi: 10.1111/jgs.15675
Keywords: Enterobacteriaceae, long-term care facilities, oxacillinase, carbapenemases, metallo-beta-lactamase
Citation: Chen H-Y, Jean S-S, Lee Y-L, Lu M-C, Ko W-C, Liu P-Y and Hsueh P-R (2021) Carbapenem-Resistant Enterobacterales in Long-Term Care Facilities: A Global and Narrative Review. Front. Cell. Infect. Microbiol. 11:601968. doi: 10.3389/fcimb.2021.601968
Received: 02 September 2020; Accepted: 06 April 2021;
Published: 23 April 2021.
Edited by:
Alessandra Oliva, Sapienza University of Rome, ItalyReviewed by:
Krisztina M. Papp-Wallace, Louis Stokes Cleveland VA Medical Center, United StatesAlessandra D’Abramo, Istituto Nazionale per le Malattie Infettive Lazzaro Spallanzani (IRCCS), Italy
Copyright © 2021 Chen, Jean, Lee, Lu, Ko, Liu and Hsueh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Po-Ren Hsueh, hsporen@ntu.edu.tw; Po-Yu Liu, liupoyu@gmail.com
 Hsin-Yu Chen1
Hsin-Yu Chen1  Po-Yu Liu
Po-Yu Liu Po-Ren Hsueh
Po-Ren Hsueh