Enterotoxigenic Bacteroides fragilis: A Possible Etiological Candidate for Bacterially-Induced Colorectal Precancerous and Cancerous Lesions
- 1Laboratory Sciences Research Center, Golestan University of Medical Sciences, Gorgan, Iran
- 2Department of Microbiology, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
- 3Division of Gastroenterology and Hepatology, Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran
- 4Department of Microbiology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 5Department of Biomedical Sciences, University of Sassari, Sassari, Italy
Enterotoxigenic Bacteroides fragilis (ETBF) produces Bacteroides fragilis toxin (BFT), which is associated with acute diarrheal, inflammatory bowel disease, and colorectal cancer (CRC). In experimental models, ETBF has been shown to contribute to colon carcinogenesis. The present study was conducted to investigate mucosal colonization of ETBF in the colon to find a possible association between the presence of ETBF and precancerous and cancerous lesions. The mucosal biopsies of involved sites were obtained from 68 patients with precancerous and cancerous lesions and 52 healthy controls (HC). The samples were cultured on Bacteroides Bile Esculin agar. Then, specific primers were designed to detect B. fragilis and bft gene using quantitative real-time PCR, and the possible links of ETBF with clinicopathological characteristics was evaluated. Also real-time PCR was performed to detect the bft gene subtypes. Bacteroides fragilis was detected in 51% of the patients and 48% of HCs cultures. The 16SrRNA gene was found to be present in 63 and 81% of the patients and HCs' samples, respectively. Moreover, the bft gene was detected in 47 and 3.8% of the patients and HCs, respectively. Also, B. fragilis was significantly more abundant in the patients' samples compared to those of HCs. In the patient group, higher odds ratio (OR) of ETBF was significantly associated with serrated lesions and adenoma with low-grade dysplasia. The bft1 gene was the most prevalent subtype of bft gene, followed by the bft2 gene. This was the first study in Iran to demonstrate increased positivity of ETBF in patients with precancerous and cancerous lesions. In this study, the bft gene was found to be associated with CRC, especially in the patients with precancerous lesions and initial carcinogenic lesions. Moreover, the results suggest that mucosal BFT exposure is common and could be a risk factor and a screening marker for developing CRC.
Introduction
Bacteroides fragilis, which is found in the gastrointestinal flora of the humans and livestock, is an anaerobe bacterium. It is one of the prominent human commensal and one of most common isolated Bacteroides from the clinical samples which causes diarrhea, peritonitis, intra-abdominal abscesses, sepsis and endogenous purulent infections (Sears et al., 1995, 2014).
It has been shown that B. fragilis prevents intestinal inflammatory diseases in animal with colitis due to production of immunomodulatory molecule polysaccharide A (PSA) that induces an anti-inflammatory immune response in intestinal tissue (Mazmanian et al., 2008; Lee et al., 2018). The pathogenicity of B. fragilis is due to several factors, including the capsule, outer-membrane proteins (OMPs), and special enzymes that comprise a 20 kDa metalloprotease termed Bacteroides fragilis toxin (BFT) (Sears et al., 1995, 2014).
BFT-producing B. fragilis, enterotoxigenic B. fragilis (ETBF), has been known as a cause of diarrheal disorders in humans and animals (Myers et al., 1987; Purcell et al., 2017). ETBF is known as a risk factor for inflammatory bowel disease (IBD) and it is present in the stool and biopsy specimens of the patients (Prindiville et al., 2000; Basset et al., 2004; Zamani et al., 2017). BFT expression is revealed to cleave extracellular domain of E-cadherin, which is a major structural constituent of the zonula adherens and is responsible for cell adhesion. Moreover, BFT can activate B-catenin signaling and induce IL8 secretion in colonic epithelial cells (Wu et al., 1998). A study indicated that following BFT treatment of HT29/C1 cells, loss of membrane associated E-cadherin initiated the nuclear localization of ß-catenin, which induced c-myc translation and led to persistent cellular proliferation (Wu et al., 2003). The potency of BFT and its influence on gastrointestinal epithelial structure and physiology suggest that the presence of ETBF may contribute to chronic colonic diseases, including oncogenic transformation, intestinal inflammation, chronic colonic dysfunctions, and colorectal precancerous and cancerous lesions (Wu et al., 1998, 2003; Sears et al., 2014).
Colorectal cancer (CRC) is one of the most prevalent cancers worldwide and comprise 9% of all cancers and it is the fourth cause of cancer-related deaths worldwide (Sears et al., 1995; Ferlay et al., 2015). However, there is a significant difference between countries in age standardized incidence of this cancer; the incidence rate in the US and European countries is more than 25 times of that of African and Asian countries. The morbidity and mortality rate of CRC has been reducing in the last years because of enhanced screening tests that can detect colorectal modifications at earlier stages and improvements in medication and procedures (Boyle and Langman, 2000; Rafiemanesh et al., 2016).
CRC is one of the most common cancers in Iran and is the third most common cancer among Iranian men (8.1–8.3 per 100 000 populations) and the fourth most prevalent cancer among Iranian women (6.5–7.5 per 100,000 populations) (Moghimi-Dehkordi et al., 2008; Kolahdoozan et al., 2010). The risk factors of CRC are obesity, sedentary lifestyle, high fat diet, low vegetables and fruit diet, smoking, alcohol abuse, and non-steroidal anti-inflammatory drugs (NSAIDs). Chronic inflammation, IBD, polyps, adenoma, and dysplasia cause changes to colon cells and make them cancer prone (Boyle and Langman, 2000; Johnson et al., 2013).
The incidence and mortality of CRC is decreasing in developed Western nations, while its incidence is increasing among both sexes during the last decades in Iran due to lifestyle and dietary changes. Another reason for this decrease may be the increase in the number of facilities and improvement in equipment and technology, as more people refer to health care facilities for screening, while in the past, a person might have had a cancer and even have died, but the cancer went undiagnosed due to lack of equipment and facilities (Malekzadeh et al., 2009; Siegel et al., 2014).
In this study, the frequency and abundance of ETBF in biopsy samples of the patients with CRC and precancerous conditions were compared to those of the individuals with no personal or familial history of colorectal disease to investigate the association between the presence of BFT and tumor development.
Materials and Methods
Patients and Specimens
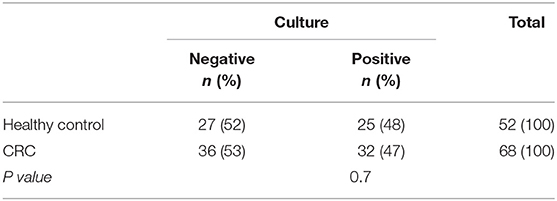
In this case control study, 120 mucosal biopsies were collected from Iranian patients with precancerous and CRC condition (n = 68) and control group (n = 52) using colonoscopy. Patients with precancerous (Serrated lesions, Adenoma include Low-grade Dysplasia: LGD and high-grade dysplasia: HGD) and cancerous conditions (Colorectal Cancer: CRC) who referred to Imam Khomeini hospital in Tehran between March 2015 and Jan 2017 were selected to participate in this study. The Ethics Committee of Tehran University of Medical Sciences approved the study protocol. Also, informed consent was obtained from all participants. All patients were diagnosed based on clinical symptoms as well as histologic and radiographic standards, which showed typical features with special distribution (Swiderska et al., 2014). All data on age, gender, and type of lesions were retrieved from patients' records. All of the patients that enter in the study diagnosed at the time of colonoscopy and chemotherapy didn't start for treating them.
During the same period, 52 healthy controls, with no personal or familial history of diagnostic colorectal disease, whose age and gender matched with those of the patients were included in the study as controls. A recent history of diarrhea and IBD was an exclusion criterion for controls. None of the individuals who took part in this study used any antibiotics or probiotics in the last 3 months. All the specimens were maintained in the sterile container comprising thioglycollate medium (Merck, Germany) and transported to the laboratory in an anaerobic condition for immediate handling. Also, 2 mucosal biopsies were collected from each patient for culture and DNA extraction.
Bacterial Culture
Two glass homogenizers were used for mechanical disruption and homogenization for all the biopsies; then, they were cultured on Bacteroides Bile Esculin agar (BBE) (Himedia Laboratories Pvt. Ltd, India) medium. In this study, Anoxomat system (MART Microbiology Drachten, Netherlands) was used to provide gaseous atmospheric conditions for anaerobes; then, the plates were incubated in an anaerobic chamber at 37°C for 72 h. Moreover, B. fragilis was confirmed using real-time PCR method.
DNA Extraction
DNA was extracted directly from the biopsy tissue using RTP® Mycobacteria Kit (Invitek, Berlin, Germany). The optical density (OD) of the extracted DNA was determined at 260 nanometres. Then, DNA was preserved at −20°C for subsequent analysis and real-time PCR.
Real-Time PCR
The sequences of the bft and 16S rRNA gene were regained from the Gene bank. The primers and probes were designed using primer 3 plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi). All the primers and probes used for the detection of all bft gene types had been designed in our previous study (Zamani et al., 2017). In order to detect the bft gene subtypes, the real-time PCR was performed as previously described (Merino et al., 2011).
The genomic DNA of ETBF strain D-134 and RIGLD clone 1 were used as positive controls (Rashidan et al., 2018). The negative control was PCR TaqMan master mix with distilled water instead of DNA.
Standard DNA was prepared for amplification and the number of molecules of the template per gram was calculated by the defined formula (Zamani et al., 2017). The standard curve for bft and 16S rRNA gene was assessed using each primer and probe with a 10-fold serial dilution of B. fragilis DNA samples, corresponding to 101-106 mean value per gram of biopsies.
According to the standard curve and y-intercept, samples that did not show the fluorescent signal earlier than the Ct of 38 were determined as negative. Also, samples that produced fluorescence of a Ct value ≤ 10 were diluted. The efficacy of the real-time PCR was determined as E = 10(−1/slop) – 1 (Zamani et al., 2017).
After optimization of standard curves, the dilution series was put in each amplification run. Real-time PCR tests were evaluated using LinGene K Real Time PCR tool (Bioer, Hangzhou, PR, China). All the tests were done in a volume of 25 μL (Zamani et al., 2017). To ensure quality, all experiments were repeated for a second time independently and the means were reported.
To check the specificity of the PCR and the expected size of the product, the primers were applied in a conventional PCR and the amplicons were run on the agarose gel. Moreover, specificity of the positive amplified fragments was proved by sequencing. The PCR product was sent to the South Korea's Macrogen Corporation for sequencing. Sequencing results were analyzed with Chromas 2.6 software. Then, all the sequences were blasted in NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). In all of the sequenced isolates the percentage of similarity to the related genes (16s rRNA gene and bft gene) present in the NCBI database were more than 95%.
Statistical Analysis
The results were compared using Fisher's exact, Chi square, and Mann–Whitney tests. P < 0.05 was considered as statistically significant. Also, mean values ± Std. error of mean (SEM) were calculated for B. fragilis and ETBF. Data were analyzed using SPSS 13.0 statistical software.
In this study, conditional logistic regression was used to assess odds ratios (OR) and 95% confidence intervals. In this design, the odds ratio is a consistent estimator of the rate ratio of CRC when exposed with ETBF vs. unexposed subjects.
Results
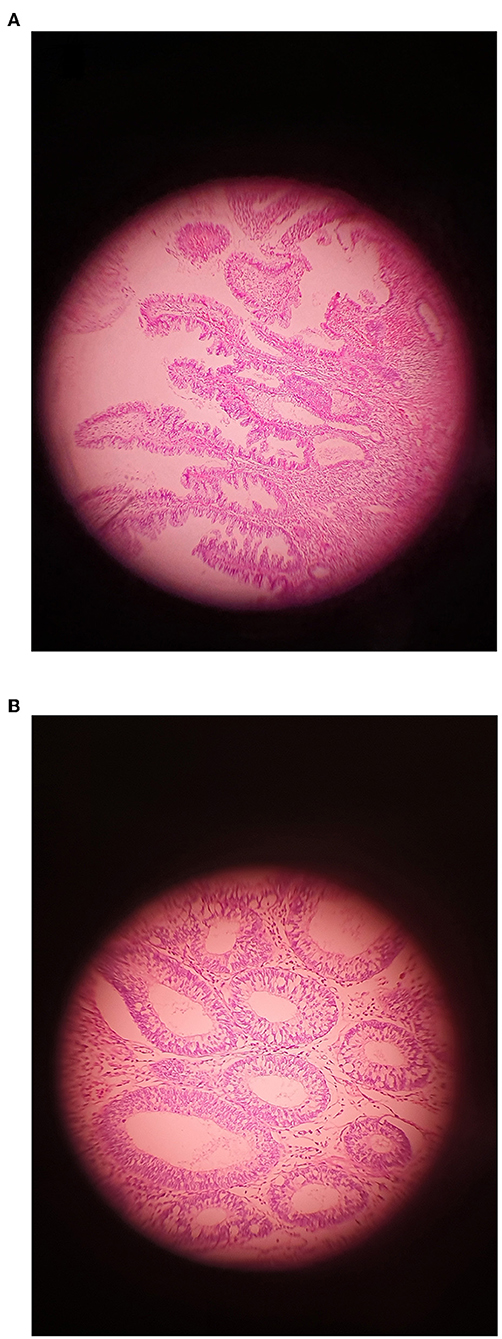
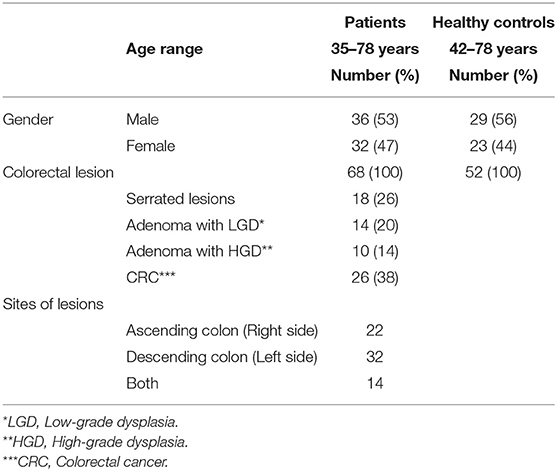
In this study, 68 samples were collected from CRC patients (36 males and 32 females; mean age: 55 yrs.; range: 35–78) and 52 samples were taken from healthy controls (HC) (30 males and 22 females; mean age: 56 yrs.; range: 42–78). All of the patients diagnosed based on colonoscopies procedures at the time of sampling. All of the patients diagnosed at the time of colonoscopy giving a total of 26 of patients with invasive CRC, 18 of patients with serrated lesions, 24 patients with adenoma include 14 patients with LGD and 10 of patients with HGD as per current classification (Figure 1). These biopsies were collected from right-side (ascending), left-side (descending), and both sides in 22, 32, and 14 patient, respectively (Table 1).
The results for B. fragilis culture was positive for 31(51%) and 25 (48%) samples of the patients and healthy controls, respectively (Table 2) (P = 0.7).
According to the standard curve, dilutions of ETBF (control positive) DNA at 101, 102, 103, 104, 105, and 106 provided Ct values of 15.08 ± 0.1, 18.04 ± 0.2, 21.42 ± 0.2, 25.02 ± 0.2, 28.02 ± 0.4, and 31.96 ± 0.4, respectively. The efficiency of the real-time PCR was between 98 and 100%.
Positive samples for 16S rRNA gene and bft gene were 63 and 47% in patients. However, positive results were shown in 81 and 3.8% of HC samples, respectively (Table 3). The difference between the positivity of bft gene in patients and HC was statistically significant (P = 0.00).
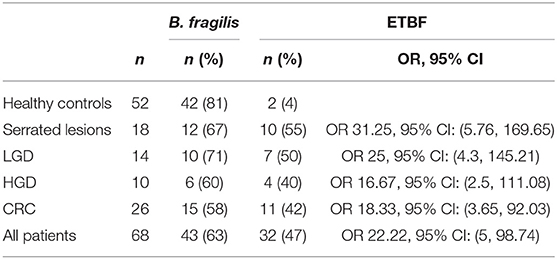
Table 3. The number of positive samples for 16S rRNA gene and bft Genes in clinicopathological groups and HC.
Also, the number of bft genes positive samples in CRC and HC within clinicopathological groups is shown in Table 3. The highest OR was found in serrated lesions group followed by adenomatous lesions with LGD group. Also the OR for all of the patients (OR 22.22, 95% CI: 5, 98.74) described the association of ETBF and the existence of lesions.
The results of the quantitative analysis of real-time PCR for 16S rRNA gene counted for B. fragilis and bft gene counted for ETBF per ng DNA were shown in Table 4. The difference between the copy numbers of 16S rRNA gene in patients and HC was not statistically significant (P ≥ 0.05). The copy numbers of bft gene were more in the samples of patients than in those of healthy controls (P = 0.00). Moreover, the copy numbers of bft gene were more in ETBF positive samples in the precursor lesions group compared to those with CRC; however, this difference was not statistically significant. The sequencing results confirmed the presence of bft and 16S rRNA genes (Data Sheet 1).
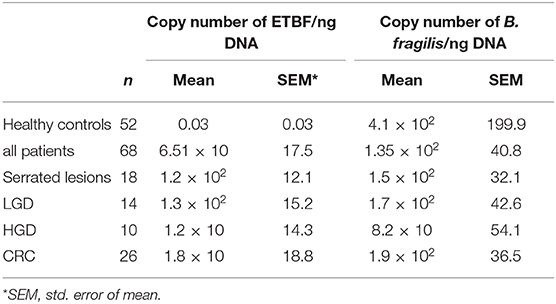
Table 4. Quantitative analysis of the 16S rRNA gene and bft genes in clinicopathological groups and HC.
Moreover, the results of these samples indicated that the most prevalent subtype of bft gene was bft1 followed by bft2. From 32 bft gene harboring isolates, 18 (56.2%) isolates subtyped as bft-1 and 14 (43.7%) isolates subtyped as bft-2. In addition, the bft gene was detected in 2 of HC samples, and all of them were subtyped as bft-1. No DNA sample harbored the subtype bft-3.
Discussion
There is growing evidence for the effect of microbial dysbiosis in the gut, initiation, and development of colorectal cancer (Sears and Garrett, 2014; Gagniere et al., 2016). Although the incidence of CRC was reported lower in Iran compared to other countries, its rate was anticipated to increase in the future (Hosseini et al., 2004; Malekzadeh et al., 2009). In previous studies, it was also reported that ETBF may have a role in diarrhea and IBD (Myers et al., 1987; Prindiville et al., 2000; Basset et al., 2004; Merino et al., 2011; Purcell et al., 2017; Zamani et al., 2017).
Some investigations have suggested that certain bacterial species (e.g., ETBF) may operate as pathogenic bacteria that clarify the development of dysbiosis in microbial community of the gut and trigger CRC (Hajishengallis et al., 2012; Hajishengallis and Lamont, 2016). The colorectal tumorigenesis was induced by immune responses and activation of proinflammatory cytokines due to BFT (Wu et al., 2004, 2009).
The findings of this study indicated that ETBF was significantly associated with the serrated lesions followed by LGD. The higher odds ratio in these lesions also showed that ETBF exposure is a risk factor for the cancerous and especially precancerous states. This supports the hypothesis that BFT producing strains may have an important role in triggering the inflammation and immunological response in genetically susceptible persons and may lead to CRC. The first study demonstrating an increased prevalence of ETBF in the stool specimens of colorectal cancer patients (38%) compared with the control group (12%) conducted by Ulger Toprak et al. (2006). Moreover, some previous reports have shown an association between bft gene and CRC, particularly in the late stage of CRC (Dejea et al., 2014; Boleij et al., 2015; Viljoen et al., 2015). A study conducted by Purcell et al. demonstrated significant associations of ETBF with tubular adenomas, serrated lesions, and low-grade dysplasia, which was similar to the results of the present study (Purcell et al., 2017).
In this study, precursor lesions, including those with low-grade dysplasia, showed an increasing trend in bft gene amount compared to those with CRC; however, this difference was not statistically significant. Moreover, the authors have previously reported that ETBF markers were observed in the colon and terminal ileum of the ulcerative colitis patients who are predisposed to CRC (Zamani et al., 2017; Rashidan et al., 2018). Similarly, in a previous study, only bft1 subtype of this gene was detected (Zamani et al., 2017). Although bft1 gene was the most prevalent subtype, the bft2 gene was also found in this study.
In this study, ETBF was detected in the lesions of the CRC patients, and similar results have also been previously reported by other investigators who studied ETBF in colonic mucosal samples (Boleij et al., 2015; Viljoen et al., 2015). Recent studies on the role of the gut microbiome have demonstrated that dysbiosis in the microbial community occurs in the non-tumor and tumor regions of the CRC patients (Dejea et al., 2014; Flemer et al., 2017; Purcell et al., 2017). In these studies showed that some of the bacteria including Fusobacterium nucleatum, ETBF, Escherichia coli, Streptococcus gallolyticus, and Enterococcus faecalis and butyrate-producing bacteria may play important roles in the development of CRC (Dejea et al., 2014; Flemer et al., 2017; Park et al., 2018).
The results of Real-time PCR for detection of B. fragilis showed that more percentage of the patients and HC contained B. fragilis compared to the culture method. The gold standard method for detection of bacteria is culture-based but it requires a high number of viable cells and specifically for anaerobic bacterial culture, some limitations could be occurs. May be these limitation affect the difference between the results.
The differences in B. fragilis cultures and Real-time PCR results between controls and CRC samples is not significant. These data suggest that probably the strains that harbor bft gene, not B. fragilis by itself, could contribute to CRC in this study. It has been shown that B. fragilis is a prominent human commensal, so this bacterium can be isolated from healthy controls like the patients too. But in the patients with cancerous and precancerous lesions some of the strains that contains bft genes increased and this dysbiosis likely can induce inflammation. Additionally some studies have shown that the human commensal B. fragilis prevented the development of colitis and may provide an effective therapeutic strategy for CRC while various studies suggest that enterotoxigenic strains of this bacterium is associated with intestinal tumors due to enterotoxin production (Sears et al., 1995, 2014; Mazmanian et al., 2008; Lee et al., 2018).
There were some limitations in this study. Different methods were used to diagnose the bft gene, which was extracted directly from tumor tissues in the colon of the patients. Thus, conducting a large population-based cohort study is highly recommended. Moreover, further investigations are needed to prove a possible correlation between the presence of bft gene and serrated lesions, LGD, and CRC.
Conclusions
Results of this study suggest that ETBF could be present in the mucosal biopsies of the patients with precancerous conditions, such as serrated lesions and LGD, in addition to CRC patients. A significant association was found between the presence of ETBF in the affected tissues and the number of these bacteria in the samples of the patients, especially in the precancerous carcinogenic lesions: adenomas with low-grade dysplasia and serrated lesions. In fact ETBF is more often detected in early lesions but further research with higher number of specimens seemed to be helpful to determine precisely.
Also additional research is necessary to determine whether age, sex, diet, and other environmental factors affect ETBF diagnosis in humans over time.
Finally, ETBF could be a marker of CRC prognosis, particularly in the precancerous lesions, and could be used to screen these disorders.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Tehran University of Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SZ is the first author who performed all laboratory experiments, collected and analyzed data, and drafted the manuscript. RT is the gastroenterologist who did colonoscopy and provided the specimens from all cases. LS participated in the study design and coordination and advised in all parts of the study. AS and SJ participated in collecting the samples and performing the tests. MF has supervised all parts of the study. All authors read and approved the manuscript.
Funding
This work was supported by Tehran University of Medical Sciences (Grant No: 32467).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the personnel of Imam Khomeini hospital for their kind support in gathering the samples.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2019.00449/full#supplementary-material
Data Sheet 1. The sequencing results of bft and 16S rRNA genes in one isolate.
References
Basset, C., Holton, J., Bazeos, A., Vaira, D., and Bloom, S. (2004). Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in in?ammatory bowel disease? Dig. Dis. Sci. 49, 1425–1432. doi: 10.1023/B:DDAS.0000042241.13489.88
Boleij, A., Hechenbleikner, E. M., Goodwin, A. C., Badani, R., Stein, E. M., Lazarev, M. G., et al. (2015). The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 60, 208–215. doi: 10.1093/cid/ciu787
Boyle, P., and Langman, J. (2000). ABC of colorectal cancer: epidemiology. BMJ 321, 805–808. doi: 10.1136/bmj.321.7264.805
Dejea, C. M., Wick, E. C., Hechenbleikner, E. M., White, J. R., Mark Welch, J. L., Rossetti, B. J., et al. (2014). Microbiota organization is a distinct feature of proximal colorectal cancers. Proc. Natl. Acad. Sci. U.S.A. 111, 18321–18326. doi: 10.1073/pnas.1406199111
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., et al. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386. doi: 10.1002/ijc.29210
Flemer, B., Lynch, D. B., Brown, J. M., Jeffery, I. B., Ryan, F. J., Claesson, M. J., et al. (2017). Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 66, 633–643. doi: 10.1136/gutjnl-2015-309595
Gagniere, J., Raisch, J., Veziant, J., Barnich, N., Bonnet, R., Buc, E., et al. (2016). Gut microbiota imbalance and colorectalcancer. World J. Gastroenterol. 22, 501–518. doi: 10.3748/wjg.v22.i2.501
Hajishengallis, G., Darveau, R. P., and Curtis, M. A. (2012). The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 10, 717–725. doi: 10.1038/nrmicro2873
Hajishengallis, G., and Lamont, R. J. (2016). Dancing with the stars: how choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol. 24, 477–489. doi: 10.1016/j.tim.2016.02.010
Hosseini, S. V., Izadpanah, A., and Yarmohammadi, H. (2004). Epidemiological changes in colorectal cancer in Shiraz, Iran: 1980–2000. ANZ J. Surg. 74, 547–549. doi: 10.1111/j.1445-2197.2004.03064.x
Johnson, C. M., Wei, C., Ensor, J. E., Smolenski, D. J., Amos, C. I., Levin, B., et al. (2013). Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 24:120722. doi: 10.1007/s10552-013-0201-5
Kolahdoozan, S., Sadjadi, A., Radmard, A. R., and Khademi, H. (2010). Five common cancers in Iran. Arch. Iran Med. 13, 143–146.
Lee, Y. K., Mehrabian, P., Boyajian, S., Wu, W. L., Selicha, J., Vonderfecht, S., et al. (2018). The protective role of bacteroides fragilis in a murine model of colitis-associated colorectal cancer. mSphere. 3:e00587–e00518. doi: 10.1128/mSphere.00587-18
Malekzadeh, R., Bishehsari, F., Mahdavinia, M., and Ansari, R. (2009). Epidemiology and molecular genetics of colorectal cancer in iran: a review. Arch. Iran Med. 12:161–169.
Mazmanian, S. K., Round, J. L., and Kasper, D. L. (2008). A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 453, 620–625. doi: 10.1038/nature07008
Merino, V. R., Nakano, V., Liu, C., Song, Y., Finegold, S. M., and Avila-Campos, M. J. (2011). Quantitative detection of enterotoxigenic Bacteroides fragilis subtypes isolated from children with and without diarrhea. J. Clin. Microbiol. 49, 416–418. doi: 10.1128/JCM.01556-10
Moghimi-Dehkordi, B., Safaee, A., and Zali, M. (2008). Prognostic factors in 1,138 Iranian colorectal cancer patients. Int. J. Colorect. Dis. 23, 683–688. doi: 10.1007/s00384-008-0463-7
Myers, L. L., Shoop, D. S., Stackhouse, L. L., Newman, F. S., Flaherty, R. J., Letson, G. W., et al. (1987). Isolation of enterotoxigenic Bacteroides fragilis from humans with diarrhoea. J. Clin. Microbiol. 25, 2230–2233.
Park, C. H., Eun, C. S., and Han, D. S. (2018). Intestinal microbiota, chronic inflammation, and colorectal cancer. Intest. Res. 16:338. doi: 10.5217/ir.2018.16.3.338
Prindiville, T., Sheikh, R., Cohen, S., Tang, Y., Cantrell, M., and Silva, J. (2000). Bacteroides fragilis enterotoxin gene sequences in patients with in?ammatory bowel disease. Emerg. Infect. Dis. 6, 171–174. doi: 10.3201/eid0602.000210
Purcell, R. V., Pearson, J., Aitchison, A., Dixon, L., Frizelle, F. A., and Keenan, J. I. (2017). Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS ONE 12:e0171602. doi: 10.1371/journal.pone.0171602
Rafiemanesh, H., Pakzad, R., Abedi, M., Kor, Y., Moludi, J., Towhidi, F., et al. (2016). Colorectal cancer in Iran: epidemiology and morphology trends. EXCLI J. 15, 738–744. doi: 10.17179/2Fexcli2016-346
Rashidan, M., Azimirad, M., Alebouyeh, M., Ghobakhlou, M., Aghdaei, H. A., and Zali, M. R. (2018). Detection of B. fragilis group and diversity of bft enterotoxin and antibiotic resistance markers cepA, cfiA and nim among intestinal Bacteroides fragilis strains in patients with inflammatory bowel disease. Anaerobe 50, 93–100. doi: 10.1016/j.anaerobe.2018.02.005
Sears, C. L., and Garrett, W. S. (2014). Microbes, microbiota, and colon cancer. Cell Host Microbe 15, 317–328. doi: 10.1016/j.chom.2014.02.007
Sears, C. L., Geis, A. L., and Housseau, F. (2014). Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis. J. Clin. Invest. 124, 4166–4172. doi: 10.1172/JCI72334
Sears, C. L., Myers, L. L., Lozenby, A., and Van Tassel, R. L. (1995). Enterotoxigenic Bacteroides fragilis. Clin Infect Dis. 20(suppl):142–148. doi: 10.1093/clinids/20.Supplement_2.S142
Siegel, R., DeSantis, C., and Jemal, A. (2014). Colorectal cancer statistics, 2014. Cancer J. Clinic. 64, 104–117. doi: 10.3322/caac.21220
Swiderska, M., Choromanska, B., Dabrowska, E., Konarzewska-Duchnowska, E., Choromanska, K., Szczurko, G., et al. (2014). The diagnostics of colorectal cancer. Contempor Oncol. 18, 1–6. doi: 10.5114/wo.2013.39995
Ulger Toprak, N., Yagci, A., Gulluoglu, B. M., Akin, M. L., Demirkalem, P., Celenk, T., et al. (2006). A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 12, 782–786. doi: 10.1111/j.1469-0691.2006.01494.x
Viljoen, K. S., Dakshinamurthy, A., Goldberg, P., and Blackburn, J. M. (2015). Quantitative profiling of colorectal cancerassociated bacteria reveals associations between fusobacterium spp., enterotoxigenic Bacteroides fragilis (ETBF) and clinicopathological features of colorectal cancer. PLoS ONE 10:e0119462. doi: 10.1371/journal.pone.0119462
Wu, S., Lim, K. C., Huang, J., Saidi, R. F., and Sears, C. L. (1998). Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc. Natl. Acad. Sci. U.S.A. 95, 14979–14984. doi: 10.1073/pnas.95.25.14979
Wu, S., Morin, P. J., Mauyo, D. J. I. K., and Sears, C. (2003). Bacteroides fragilis enterotoxin induces c-myc expression and cellular proliferation. Gastroenterology 124, 392–400. doi: 10.1053/gast.2003.50047
Wu, S., Powell, J., Mathioudakis, N., Kane, S., Fernandez, E., and Sears, C. L. (2004). Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway. Infect. Immun. 72, 5832–5839. doi: 10.1128/IAI.72.10.5832-5839.2004
Wu, S., Rhee, K. J., Albesiano, E., Rabizadeh, S., Wu, X., Yen, H. R., et al. (2009). A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15, 1016–1022. doi: 10.1038/nm.2015
Keywords: enterotoxigenic Bacteroides fragilis (ETBF), colorectal cancer, bft gene, adenoma, precancerous lesions
Citation: Zamani S, Taslimi R, Sarabi A, Jasemi S, Sechi LA and Feizabadi MM (2020) Enterotoxigenic Bacteroides fragilis: A Possible Etiological Candidate for Bacterially-Induced Colorectal Precancerous and Cancerous Lesions. Front. Cell. Infect. Microbiol. 9:449. doi: 10.3389/fcimb.2019.00449
Received: 07 July 2019; Accepted: 12 December 2019;
Published: 17 January 2020.
Edited by:
Eric Charles Martens, University of Michigan, United StatesReviewed by:
César López-Camarillo, Universidad Autónoma de la Ciudad de México, MexicoAshu Sharma, University at Buffalo, United States
Copyright © 2020 Zamani, Taslimi, Sarabi, Jasemi, Sechi and Feizabadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Mehdi Feizabadi, mfeizabadi@tums.ac.ir; Leonardo A. Sechi, sechila@uniss.it
 Samin Zamani
Samin Zamani Reza Taslimi3
Reza Taslimi3  Leonardo A. Sechi
Leonardo A. Sechi Mohammad Mehdi Feizabadi
Mohammad Mehdi Feizabadi