Highly Potent 1H-1,2,3-Triazole-Tethered Isatin-Metronidazole Conjugates Against Anaerobic Foodborne, Waterborne, and Sexually-Transmitted Protozoal Parasites
- 1Department of Chemistry, Guru Nanak Dev University, Amritsar, India
- 2Center for Discovery and Innovation in Parasitic Diseases, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, La Jolla, CA, United States
- 3Department of Biological Sciences, University of the Pacific, Stockton, CA, United States
- 4Foodborne Toxin Detection and Prevention Research Unit, Agricultural Research Service, United States Department of Agriculture, Albany, CA, United States
Parasitic infections like amebiasis, trichomoniasis, and giardiasis are major health threats in tropical and subtropical regions of the world. Metronidazole (MTZ) is the current drug of choice for amebiasis, giardiasis, and trichomoniasis but it has several adverse effects and potential resistance is a concern. In order to develop alternative antimicrobials, a library of 1H-1,2,3-triazole-tethered metronidazole-isatin conjugates was synthesized using Huisgen's azide-alkyne cycloaddition reaction and evaluated for their amebicidal, anti-trichomonal, and anti-giardial potential. Most of the synthesized conjugates exhibited activities against Trichomonas vaginalis, Tritrichomonas foetus, Entamoeba histolytica, and Giardia lamblia. While activities against T. vaginalis and T. foetus were comparable to that of the standard drug MTZ, better activities were observed against E. histolytica and G. lamblia. Conjugates 9d and 10a were found to be 2–3-folds more potent than MTZ against E. histolytica and 8–16-folds more potent than MTZ against G. lamblia. Further analysis of these compounds on fungi and bacteria did not show inhibitory activity, demonstrating their specific anti-protozoal properties.
Introduction
Anaerobic protozoan parasites Trichomonas vaginalis and Tritrichomonas foetus are the major causes of reproductive tract infections viz. trichomoniasis and bovine trichomoniasis (Kumar et al., 2015; Ravaee et al., 2015). Though men remain asymptomatic, trichomoniasis leads to urogenital infection in women via sexual transmission, resulting infertility, urethritis, vaginitis, preterm delivery and low birth weight, Bovine trichomoniasis, acquired by direct sexual intercourse, is asymptomatic in nature but earlier death of developing foetus was observed in some cases. Multilocus genotyping confirmed that the isolates obtained from cattle and pig represented the “bovine genotype” of T. foetus and the cat isolates represented a closely related “feline genotype” of T. foetus (Fang et al., 2015). The prevalence of T. vaginalis infection in the United States is estimated to be 2.3 million among women of 14–49 years and this increases with age (Sutton et al., 2007; Conrad et al., 2013). Moreover, women without any past history of sexual intercourse can still be affected with trichomoniasis (Kumar et al., 2012). Major health threats associated with trichomoniasis include transmission of HIV-1 (Fichorova, 2009), benign prostatic hyperplasia (Mitteregger et al., 2012), prostate cancer and pelvic inflammatory disease (Stark et al., 2009).
Two other anaerobic intestinal protozoans, Giardia lamblia and Entamoeba histolytica cause the gastrointestinal diarrheal diseases (Halliez and Buret, 2013), giardiasis and amebiasis. Acute gastroenteritis is considered as one of the leading causes of illnesses and deaths in children under the age of 5 years (Heresi et al., 2000; Youssef et al., 2000). Infection occurs through the ingestion of cysts in contaminated water or food and direct person-to-person contact. Upon ingestion of G. lamblia or E. histolytica cysts, trophozoitese merge from the cysts and multiply in the lumen of the small intestine, where G. lamblia attach to the intestinal mucosa (Navaneethan and Giannella, 2008). E. histolytica trophozoites invade the colon and causes amoebic colitis. While 50% of G. lamblia infection is asymptomatic, major symptoms of amebiasis and giardiasis include weight loss, loss of appetite, watery or bloody diarrhea, dehydration, bloating and abdominal cramps, cognitive impairment in children, and chronic fatigue in adults (Berkman et al., 2002; Hanevik et al., 2007).
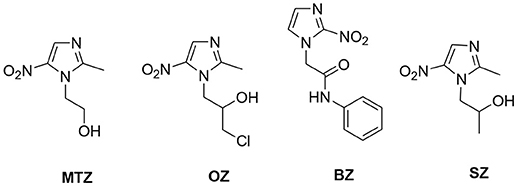
Nitro-imidazoles such as ornidazole (OZ), benznidazole (BZ), and secnidazole (SZ) (Figure 1) are the widely used medicament to treat anaerobic infections but have lower efficacies than MTZ (Nash, 2002). MTZ, an effective synthetic drug introduced in 1960, showed strong inhibitory efficacies against Gram-negative anaerobic bacteria like Helicobacter pylori and protozoans such as G. lamblia and E. histolytica. It is the only therapeutic drug available till date against trichomoniasis (Cudmore et al., 2004; Sutherland et al., 2010). These outstanding achievements have encouraged researchers to focus on the development of nitro-imidazoles with imminent medicinal application. However, MTZ resistance has been observed in E. histolytica, G. lamblia, and T. vaginalis and thus the development of novel, non-cytotoxic and efficient scaffolds against amebiasis, giardiasis, trichomoniasis, and bacterial infections is desirable (Meri et al., 2000; Upcroft et al., 2005; Debnath et al., 2013).
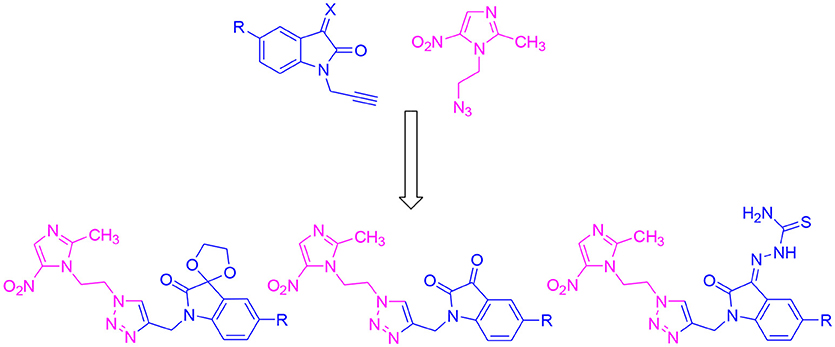
Isatin is one of the important pharmacophores with wide application in drug discovery and it is the core constituent of many alkaloids, dyes, pesticides, and analytical reagents. Various studies from the literature show the anti-bacterial (Sarangapani and Reddy, 1994), anti-fungal (Pandeya et al., 1999), anti-inflammatory (Bhattacharya and Chakrabarti, 1998) and anticonvulsant (Popp et al., 1980) properties of isatin-schiff bases. Thiosemicarbazones are the class of Schiff bases that exhibit anti-parastic and anti-bacterial properties (Chellan et al., 2012). Moreover, the spiro compounds of isatin are also known to exhibit versatile biological properties. Earlier results based on 1H-1,2,3-triazole and β-amino-alcohol tethered isatin-β-lactam conjugates showed efficient inhibitory activities against T. vaginalis (Nisha et al., 2013). Results obtained with N-propargylated-isatin-Mannich adducts and N-propargylated isatin-quinoline Mannich adducts against T. foetus have also prompted the use of isatin as a pharmacophore (Nisha et al., 2014). Based on the anticancer (Vine et al., 2007; Kumar et al., 2018; Singh et al., 2018), anticonvulsant (Verma et al., 2004), antidepressant (Singh et al., 1997), anti-HIV (Bal et al., 2005), and anti-bacterial (Pandeya et al., 2000) properties of isatin, the present study explicates the synthesis and biological evaluation of 1H-1,2,3-triazole-tethered isatin-metronidazole conjugates as shown in Figure 2. Substitutions at C-5 position of isatin-ring as well as the introduction of thiosemicarbazide and ketocarbonyl at C-3 carbonyl of isatin have been carried out to ascertain the structure-activity relationship of the synthesized conjugates.
Experimental Section
General Information
Melting points were determined with open capillaries using a Veego Precision Digital Melting Point apparatus (MP-D) and are uncorrected. 1H and 13C NMR were recorded on JEOL 400, Bruker 500 MHz spectrometer, respectively using CDCl3 as solvent. Chemical shifts were reported in parts per million (ppm) using tetramethylsilane (TMS) as an internal standard and coupling constants J indicated in Hertz. Splitting patterns were indicated as s: singlet, d: doublet, t: triplet, m: multiplet, dd: double doublet, ddd: doublet of a doublet of a doublet, and br: broad peak. Mass spectrometric analysis was carried out on BrukermicrOTOF QII equipment using ESI as the source. Column chromatography was performed on a silica gel (60–120 mesh) using an ethyl acetate: hexane mixture as eluent (Kumar et al., 2017).
General Procedure for the Synthesis of C-5 Substituted Isatin-Spiro-Ketals (2)
A solution of isatin 1 (1 mmol) in dry DMF was added drop-wise to a stirred suspension of NaH (1.2 mmol) in dry DMF at 0°C. The solution was then stirred for 15 min. followed by the drop-wise addition of 2-bromoethanol (1.2 mmol). The reaction mixture was allowed to stir at room temperature for 20 min. with subsequent heating at 80°C for 4 h. The progress was monitored by TLC. Upon completion, the reaction mixture was extracted with ethyl acetate (2 × 25 mL), washed with brine water (2 × 20 mL) and the combined organic layers were dried over anhydrous Na2SO4. The organic layer was concentrated under reduced pressure to afford the desired precursors.
General Procedure for the Synthesis of N-Propargylated C-5 Substituted Isatin (3)/Isatin Spiroketal (4)
To a stirred suspension of NaH (1 mmol) in dry DMF was added drop-wise a solution of C-5 substituted isatin (1 mmol) 3 /isatin-spiroketal 4 in dry DMF at 0°C. The mixture was stirred for 15 min followed by the drop-wise addition of propargyl bromide (1.2 mmol). The reaction mixture was allowed to stir at room temperature for 10 min. with subsequent heating at 60°C for 4 h with progress being monitored by TLC. Upon completion, the reaction mixture was extracted with ethyl acetate (2 × 25 mL) and washed with brine water (2 × 20 mL). The combined organic layers were dried over anhydrous Na2SO4, concentrated under vacuum to afford the desired precursors.
General Procedure for the Synthesis of Conjugates, 8a–e and 9a–e
CuSO4.5H2O (0.055 mmol) and sodium ascorbate (0.143 mmol) were added to a well stirred solution of 5-substituted 1-(prop-2-yn-1-yl)indoline-2,3-dione 3 (1 mmol) or 5-substituted 1-(prop-2-yn-1-yl)spiro[indoline-3,2/-[1,3]dioxolan]-2-one 4 and 1-(2-azidoethyl)-2-methyl-5-nitro-1H-imidazole 7 (1 mmol) in an ethanol:water (85: 15) mixture. The reaction mixture was allowed to stir at room temperature for 6–7 h and the progress was monitored by TLC. Upon completion, the reaction mixture was extracted with ethyl acetate and water and the combined organic layers were dried over anhydrous Na2SO4 and concentrated under vacuum to afford the desired conjugates which were purified via column chromatography using an ethyl acetate: hexane (70:30) mixture.
General Procedure for the Synthesis of Conjugate, 10a–e
Thiosemicarbazide (1 mmol) and glacial acetic acid in catalytic amount were added to a stirred solution of 5-substituted1-((1-(2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl)-1H-1,2,3-triazol-4-yl)methyl)indoline-2,3-dione (8) in ethanol. The reaction mixture was heated to reflux for 2 h and the progress was monitored using TLC. On completion, yellow colored solid resulted and this was re-crystallized using an absolute ethanol to afford the desired conjugates.
Materials and Methods
Biological Evaluation
In vitro Susceptibility Assay Against the Bovine Trichomonad T. foetus Strain D1 and the Feline Trichomonad T. foetus-Like Strain C1
To perform the initial susceptibility screens on T. foetus D1 (from Lynette Corbeil, University of California at San Diego, School of Medicine, San Diego, CA, USA) and T. foetus-like C1 (from Stanley Marks, University of California at Davis, School of Veterinary Medicine, Davis, CA, USA), compounds were dissolved in 100% DMSO to obtain concentrations of 100 mM. Stock solutions were kept at −20°C. 5 microliter aliquots of these solutions were diluted in 5 mL of TYM Diamond's media (Hardy Diagnostics, Santa Maria, CA, USA) to obtain a final concentration of 100 μM. Metronidazole-sensitive D1 and C1 strains were cultured under anaerobic conditions. After 24 h, cells were counted using a hemacytometer. The IC50 value for the series of compounds was determined by inoculating a constant number of parasite cells in TYM medium and running assays of increasing drug concentrations, 0.02–100 μM, and performing a regression analysis on percentage growth inhibition relative to DMSO control, using Prism software, from GraphPad. Predicted IC50 values of compounds were then confirmed by testing again using the same assay described above. The sample size consisted of four independent trials carried out on four different days (to account for possible variation in the parasite population).
In vitro Susceptibility Assay Against the Human Trichomonad T. vaginalis Strain G3
T. vaginalis G3 trophozoites (from Patricia Johnson, University of California at Los Angeles, CA, USA) were maintained in TYM media (pH 6.2) at 37°C for 24 h and every 24 h, 1000 μL of cells were passed into 10 ml of TYM media to maintain the culture. To perform the initial susceptibility screens on metronidazole-sensitive T. vaginalis G3, compounds were dissolved in DMSO to obtain concentrations of 100 mM; 5 μL aliquots of these solutions were diluted in 5 mL of TYM medium to obtain a final concentration of 100 μM. After 24 h, cells were counted using a hemacytometer. The assays were performed in 15 mL culture tubes with T. vaginalis G3 strain. 0.1% DMSO-only treated parasites served as control to normalize the effects of the solvent and in vitro conditions. TYM media only control was also included in the assays. After 24 h, cells were counted using a hemacytometer. The IC50 values were determined by inoculating a constant number of parasite cells in TYM medium and running assays of increasing compound concentrations, 0.02–100 μM, and performing a regression analysis on percentage growth inhibition relative to DMSO control, using Prism software from GraphPad. Predicted IC50 values of compounds were then confirmed by testing again using the same assay described above. The sample size consisted of four independent trials carried out on four different days (to account for possible variation in the parasite population).
G. lamblia and E. histolytica IC50 Assays
Axenic trophozoites of metronidazole-sensitive G. lamblia WB and E. histolytica HM1:IMSS were grown in TYI-S-33 medium supplemented with penicillin (100 U/mL) and streptomycin (100 μg/mL). (Diamod et al., 1978; Keister, 1983) For anti-E. histolytica and anti-G. lamblia IC50assays, 10 mM stocks of the test compounds were serially diluted in DMSO to achieve a concentration range of 10 mM−78 μM. 0.5 microliter of compound from this concentration range was transferred in triplicate to each well of 96-well plates and 5,000 trophozoites/well were added in a final volume of 100 μL/well. Cultures were grown for 2 days at 37°C under anaerobic conditions (GasPak EZ Anaerobe Gas Generating Pouch System (VWR). Cell growth and viability were determined by adding Cell Titer-Glo cell viability assay reagent (Promega) and measuring ATP-dependent luminescence in a microplate reader. Percent inhibition relative to maximum and minimum reference signal controls was calculated using the formula:
% Inhibition = [(mean of Maximum Signal Reference Control—Experimental Value)/(mean of Maximum Signal Reference Control—mean of Minimum Signal Reference Control)] × 100.
The 50% inhibitory concentration (IC50) and standard error (SE) was derived from the concentration-response curves using Prism software (GraphPad).
Anti-bacterial and Anti-fungal Susceptibility Analyses
To determine if the compound library had other antimicrobial activities, several fungal and bacterial species were analyzed for susceptibility to these compounds. Antifungal activity of compounds was tested in the filamentous fungal pathogen Aspergillus parasiticus 5862 (National Center for Agricultural Utilization and Research, USDA-ARS, Peoria, IL, USA) and the model yeast Saccharomyces cerevisiae BY4741 wild type (Mat a his3Δ1 leu2Δ0met15Δ0ura3Δ0) (Open Biosystems, Huntsville, AL, USA). A. parasiticus was cultured at 35°C on potato dextrose agar (PDA), and S. cerevisiae was grown on Synthetic Glucose (SG; Yeast nitrogen base without amino acids 0.67%, glucose 2% with appropriate supplements: uracil 0.02 mg/mL, amino acids 0.03 mg/mL) or Yeast Peptone Dextrose (YPD; Bacto yeast extract 1%, Bacto peptone 2%, glucose 2%) medium at 30°C. All chemicals for culturing fungi were procured from Sigma Co. (St. Louis, MO, USA).
To evaluate antifungal activity of compounds in A. parasiticus, bioassays were performed in microtiter plates (triplicate wells) (3 × 104 to 5 × 104 CFU/mL) with RPMI 1640 medium (Sigma Co., St. Louis, MO, USA). Compounds were tested at 500 μM, where fungal growth was monitored at 24–48 h after inoculation. A numerical score from 0 to 4 was provided to each well according to the protocol outlined by the Clinical and Laboratory Standards Institute (CLSI) M38-A2 (CLSI, 2008) as follows: 0 = optically clear/no visible growth, 1 = slight growth (25% of no treatment control), 2 = prominent growth reduction (50% of no treatment control), 3 = slight growth reduction (75% of no treatment control), 4 = no growth reduction. To test antifungal activity of compounds in S. cerevisiae, bioassays were performed in SG liquid medium (triplicate wells in microtiter plates). Compounds were examined at 500 μM, where antifungal activity was assessed 24–48 h after inoculation. A numerical score from 0 to 4 (See above) was provided to each well by the modified protocols outlined by European Committee on Antimicrobial Susceptibility Testing (EUCAST) (Keister, 1983).
For anti-bacterial susceptibility testing, disc diffusion methods were used. Vehicle control (DMSO) and 100 mM stock compounds were diluted to 100 μM in media and incubated with empty BDL-sensi-discs (6 mm) for 20 min at room temperature. These discs were placed upon plates streaked either with Lactobacillus reuteri (ATCC 23272), Lactobacillus acidophilus (ATCC 43560), Lactobacillus rhamnosus (ATCC 53103), Listeria monocytogenes 10403 (RM2194), Salmonella enterica pGFP, or Escherichia coli K-12 MG 1655. Additionally, various antibiotic discs [levofloxacin (5 μg), gentamicin (10 μg), and gentamicin (120 μg)] were placed upon these plates as controls for sensitivity. Plates were streaked for growth of Lactobacilli, grown in Lactobacilli MRS at 37°C under anaerobic conditions whereas the rest of the strains was grown at 37°C aerobically in Luria Broth or Brain Heart Infusion Broth. Zones of inhibition measured in millimeters were measured for each disc.
Results and Discussion
Synthetic Chemistry
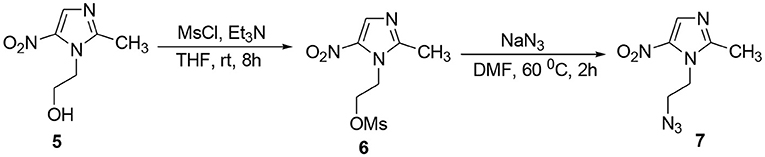
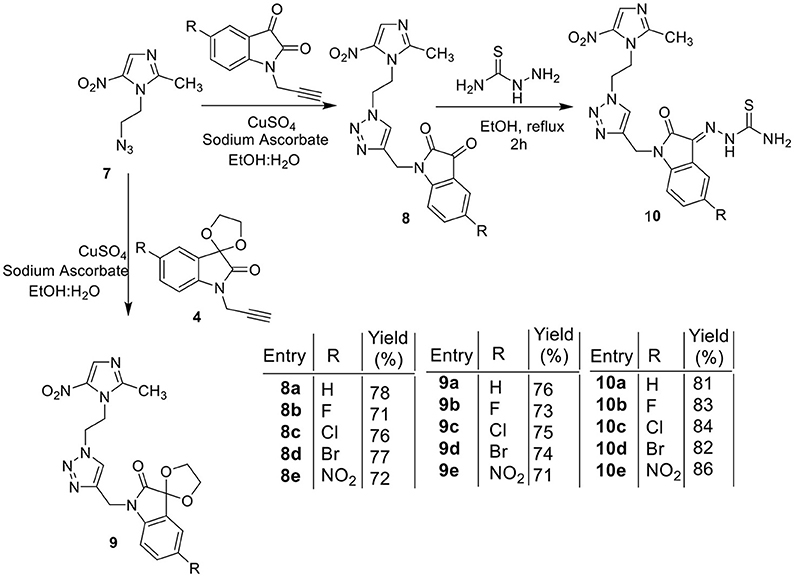
The methodology for the synthesis of isatin-spiroketal 2 involved sodium hydride promoted reaction of C-5 substituted isatin 1 with 1-bromoethanol (Sigma-Aldrich, Cat No. 48874, CAS No. 540-51-2) in dry DMF. N-propargylated C-5 substituted isatin and isatin-spiroketal were obtained via base mediated reaction of isatin/spiroisatin with propargyl bromide (Sigma-Aldrich, 80% weight in toluene, Cat No. P51001, CAS No. 106-96-7) in dry DMF at 60°C (Scheme 1). The precursor viz. 1-(2-azidoethyl)-2-methyl-5-nitro-1-H-imidazole 7, was synthesized via initial mesylation of metronidazole 5 (Sigma, St. Louis, MO, USA, Cat. No. M3761) in dry THF at 0°C to form the corresponding methane-sulfonic acid 2-(2-methyl-5-nitro-imidazol-1-yl)-ethyl ester 6 followed by its nucleophilic substitution reaction with sodium azide in dry DMF at 60°C to afford the corresponding precursor 1-(2-Azido-ethyl)-2-methyl-5-nitro-1H-imidazole 7 in good yields (Scheme 2).Cu promoted azide-alkyne cycloaddition reaction of 7 with N-propargylated-isatin 3 and N-propargylatedspiro-isatin 4 led to the isolation of desired conjugates 8 and 9 (Scheme 3). Conjugate 8 was further treated with thiosemicarbazide to obtain metronidazole-isatin-thiosemicarbazone conjugates 10. On the bases of spectral data and analytical evidence, structures were assigned to the synthesized 1H-1,2,3-triazole-tethered isatin-metronidazole conjugates (see Supplementary Material). The compound 8c, for example was characterized as 5-chloro-1-{1-[2-(2-methyl-5-nitro-imidazol-1-yl)-ethyl]-1H-[1,2,3]triazol-4-ylmethyl}-1H-indole-2,3-dione analyzed for C17H14ClN7O4 and showed molecular ion peak at m/z 416.4302 ([M+H]+) and 417.4318 ([M+2]+) in its mass spectrum. The salient feature of its (Ravaee et al., 2015) H NMR spectra include charterstick peak at δ 4.60 (t, J = 4.8 Hz, 2H); δ 4.75 (t, J = 5.8 Hz, 2H) and one singlet at 4.90 (2H) ppm corresponding to methylene group, respectively. Two singlets also appeared at δ 7.93 and 7.97 which correspond to triazole and imidazole proton (Navaneethan and Giannella, 2008). C NMR spectrum of compound 8c exhibited the appearance of characteristic peaks at δ 182.40 and 157.90 which correspond to isatin carbonyl (C = O) and amidic carbonyl of isatin (= N-C = O). Three methylenic carbons show characteristic peaks at δ 35.3, 46.5 and 49.3 along with one single peak at 13.3 corresponding to—CH3 group of imidazole ring.
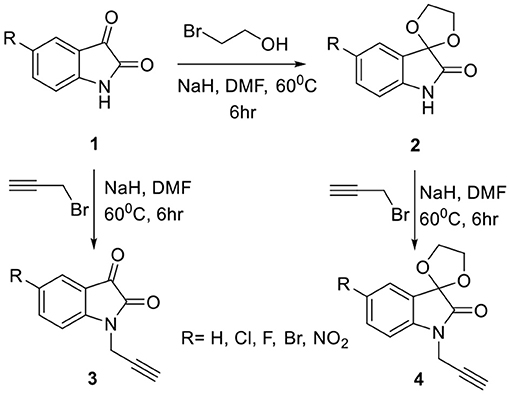
Scheme 1. Synthesis of N-propargylated C-5 substituted isatin 3 and spiroketal N-propargylated C-5 substituted isatin 4.
In vitro Evaluation Against T. vaginalis, T. foetus, T. foetus-Like, G. lamblia, E. histolytica, Fungal, and Bacterial Species
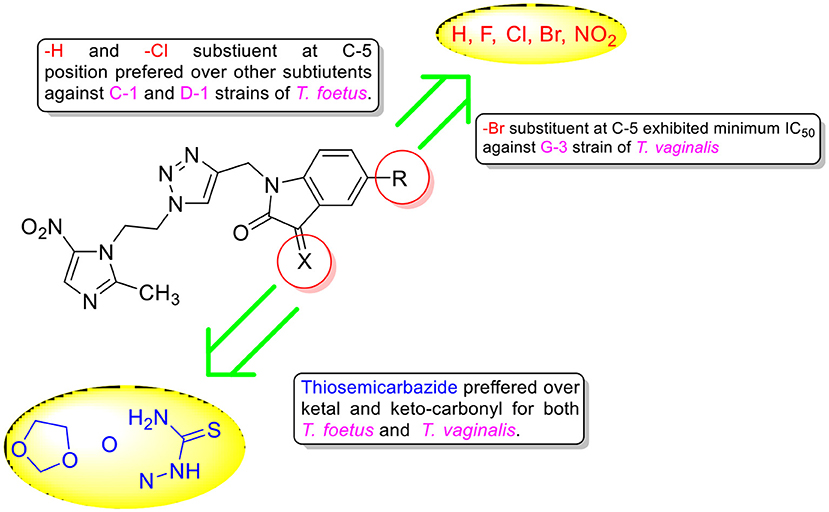
The chemical library of metronidazole-isatin conjugates was evaluated in a general inhibitory screen against protozoal pathogen T. vaginalis and the percentage inhibition results at different concentrations are enlisted in Table 1. As evident most of the conjugates exhibited 100% growth inhibition at 100 μM except 8a, 8d, and 10e (Table 1). The potent conjugates were further evaluated for their IC50 against different strains of T. vaginalis and T. foetus and compared with metronidazole, (Table 1). A closer inspection of Table 1 revealed an interesting structure activity relationship with activity being dependent upon the nature of substituent at C-3 and C-5 positions of isatin ring. In case of MTZ susceptible G-3 strain, introduction of a ketal and thiosemicarbazone substituent at C-3 position improved the activity profiles as evident from conjugates 9a–e and 10a–d. Further, the presence of halogen substituent (F, Cl, Br) improved the activity profiles with the most potent conjugate 10d (R = Br) exhibiting an IC50 value of 1.2 μM against G-3 strain, which is comparable to MTZ.
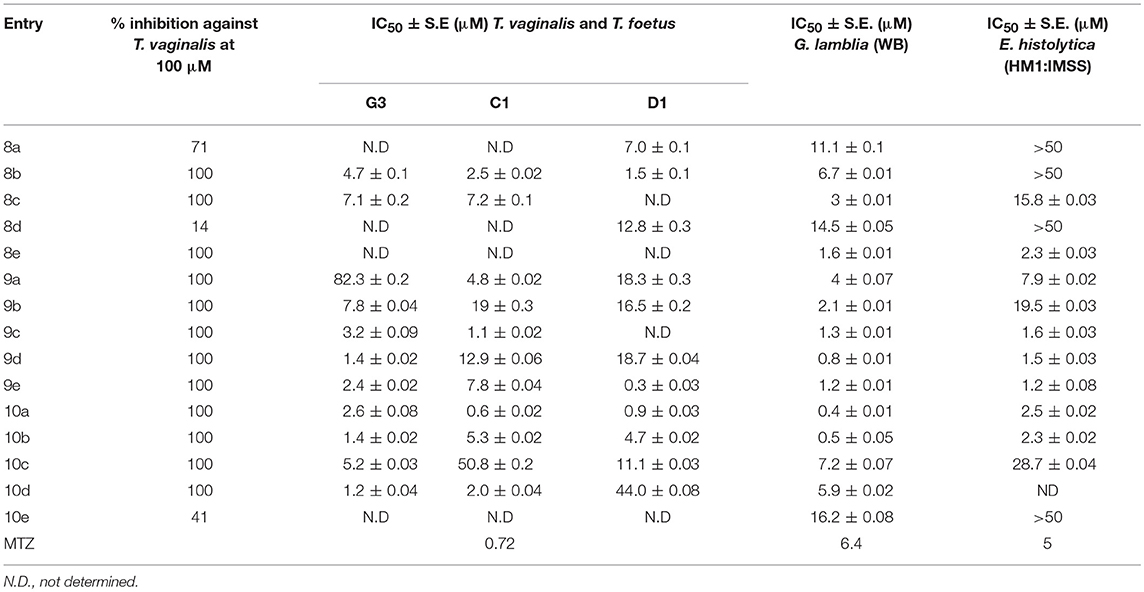
Table 1. In vitro activity of isatin-metronidazole conjugates against T. vaginalis, T. foetus, T. foetus-like pathogen, G. lamblia, and E. histolytica.
Similar SAR has been observed against C1 strain of T. foetus-like pathogen with activity mainly dependent upon the nature of substituent at C-3 position. The replacement of ketocarbonyl with ketal in general improved the activity profiles with conjugate 9c (R = Cl) exhibiting an IC50 value of 1.1 μM. However, the introduction of thiosemicarbazide functionality substantially improved the efficacy of the conjugates with compound 10a (R = H) displaying an IC50 value of 0.6 μM, better than the standard drug MTZ. The synthesized conjugates were also evaluated against D1 strain of T. foetus. The conjugate 10a again proved to be the most potent among the series, exhibiting an IC50 value of 0.9 μM. The generalized SAR of the synthesized conjugates against T. vaginalis and T. foetus has been provided in Figure 3.
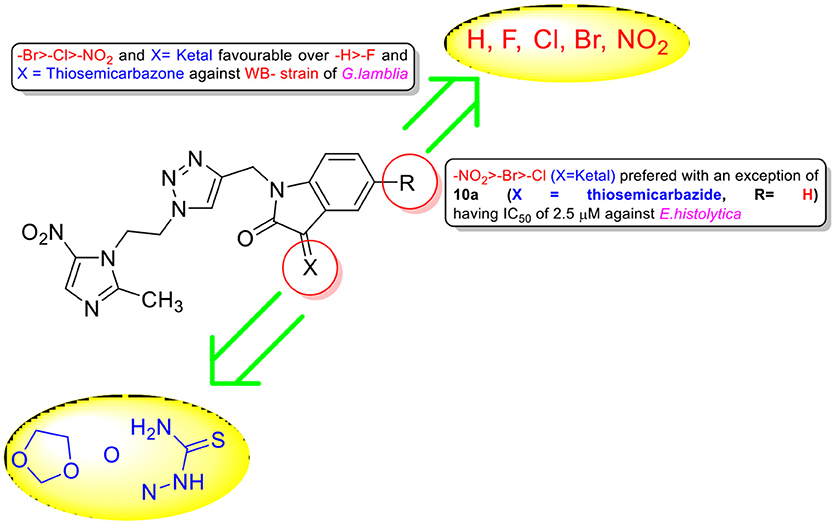
Encouraged with these biological results, it was considered worthwhile to examine the activities of synthesized conjugates against other anaerobic protozoans such as WB strain of G. lamblia and HM1 strain of E. histolytica. Since trophozoites are the relevant forms that cause trichomoniasis, amebiasis and giardiasis, we tested the activity of compounds against trophozoite forms. As evident from IC50 values enlisted in Table 1, all conjugates exhibited good activity profiles with IC50 values ranging from 0.4 to 16.2 μM against WB strain of G. lamblia. Activity profiles showed a preference for electron withdrawing substituent (R = F, Cl, Br, NO2) at C-5 position of isatin except 10a (R = H) which exhibited an IC50 value of 0.4 μM. The replacement of ketocarbonyl with ketal and thiosemicarbazide improved the activities except 8e which displayed an IC50 value of 1.6 μM. Conjugates 8c, 8e, 9a, 9b, 9c, 9d, 9e, 10a, 10b, and 10d proved to be more potent than the standard drug MTZ (IC50 = 6.4 μM) (Arendrup et al., 2012), with conjugates 9d, 10a, and 10b exhibiting IC50 values in sub-micromolar concentration. Similarly, conjugates 8e, 9c, 9d, 9e, 10a, and 10b showed better potency than the current standard of care MTZ (IC50 = 5 μM)(Bashyal et al., 2017) against E. histolytica (Table 1). The generalized SAR of the synthesized conjugates against E. histolytica and G. lamblia has been provided in Figure 4.
To determine if these compounds were specific for protozoal parasites, we also tested a number of different fungal and bacterial species. In all assays, the activities of these compounds were specific for protozoa and exerted no detectable antifungal and antibacterial properties.
In conclusion, the present study undertook the synthesis of 1H-1,2,3-triazole-tethered metronidazole-isatin conjugates and evaluated their activity against multiple protozoan parasites, namely T. vaginalis, T. foetus, T. foetus-like pathogen, G. lamblia and E. histolytica. SAR studies revealed the dependence of activity profiles on the nature of substituent at C-3 and C-5 positions of isatin ring with most of the synthesized conjugates exhibiting comparable efficacies to that of MTZ against different trichomonal strains while better inhibitory activities were observed against G. lamblia. Interestingly, introduction of thiosemicarbazide into the basic core of the synthesized conjugates improved the activities with most potent conjugate 10a being ~16 folds more active than MTZ against G. lamblia, ~2 folds more active than MTZ against E. histolytica and equipotent to MTZ against T. vaginalis and T. foetus. Further work on improving the activities of most promising conjugates of the synthesized library, is presently underway.
Author Contributions
SK synthesized and characterized metronidazole-isatin, metronidazole-spiroisatin, and metronidazole-isatin-thiosemicarbazones. TB performed E. histolytica and G. lamblia experiments. AD conceptualized the E. histolytica and G. lamblia studies, analyzed the data, reviewed, and edited the manuscript. KL conceptualized the T. vaginalis and T. foetus, and T. foetus-like pathogen studies, analyzed the data, reviewed, and edited the manuscript. AW performed T. vaginalis, T. foetus, and T. foetus-like pathogen experiments. LC conceptualized the anti-fungal and anti-bacterial studies, analyzed the data, reviewed, and edited the manuscript. JK and CT performed the anti-fungal and anti-bacterial experiments, respectively. VK designed and characterized the described conjugates, reviewed, and edited the manuscript.
Funding
VK acknowledges the Council of Scientific and Industrial Research (CSIR, New Delhi) for financial assistance [grant no. 02(0293)/17/EMR-I]. The project described was partially supported by the National Institutes of Health, 1KL2TR001444 (to AD). KL and AW were supported by the Department of Biological Sciences at the University of the Pacific. LC, JK, and CT were funded by the United States Department of Agriculture, Agricultural Research Service (National Program 108, Project #5325-42000-039-00D).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Department of Biological Sciences at the University of the Pacific for their continuous support of research in antimicrobials.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2018.00380/full#supplementary-material
Supplementary Data Sheet 1. 1H, 13C NMR data of all the synthesized compounds along with scanned 1H, 13C and 13C (DEPT) NMR of 8a, 8b, 8c, 9c, 10a, 10b.
References
Arendrup, M. C., Cuenca-Estrella, M., Lass-Flörl, C., and Hope, W. (2012). EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 18, 246–247. doi: 10.1111/j.1469-0691.2012.03880.x
Bal, T. R., Anand, B., Yogeeswari, P., and Sriram, D. (2005). Synthesis and evaluation of anti-HIV activity of isatin β-thiosemicarbazone derivatives. Bioorg. Med. Chem. Lett. 15, 4451–4455. doi: 10.1016/j.bmcl.2005.07.046
Bashyal, B., Li, L., Bains, T., Debnath, A., and LaBarbera, D. V. (2017). Larreatrdentata: a novel source for anti-parasitic agents active against Entamoeba histolytica, Giardia lamblia and Naegleria fowleri. PloS. Negl. Trop. Dis. 11:0005832. doi: 10.1371/journal.pntd.0005832
Berkman, D. S., Lescano, A. G., Gilman, R. H., Lopez, S. L., and Black, M. M. (2002). Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet 359, 564–571. doi: 10.1016/S0140-6736(02)07744-9
Bhattacharya, S. K., and Chakrabarti, S. (1998). Dose-related proconvulsant and anticonvulsant activity ofisatin, a putative biological factor in rats. Indian J. Exp. Biol. 36, 118–121.
Chellan, P., Land, K. M., Shokar, A., Au, A., An, S. H., Clavel, C. M., et al. (2012). Exploring the versatility of cycloplatinated thiosemicarbazones as antitumor and antiparasitic agents. Organometallics 31, 5791–5799. doi: 10.1021/om300334z
Conrad, M. D., Bradic, M., Warring, S. D., Gorman, A. W., and Carlton, J. M. (2013). Getting trichy: tools and approaches to interrogating Trichomonas vaginalis in a post-genome world. Trends Parasitol. 29, 17–25. doi: 10.1016/j.pt.2012.10.004
Cudmore, S. L., Delgaty, K. L., Hayward-McClelland, S. F., Petrin, D. P., and Garber, G. E. (2004). Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis. Clin. Microbiol. Rev. 17, 783–793. doi: 10.1128/CMR.17.4.783-793.2004
Debnath, A., Ndao, M., and Reed, S. L. (2013). Reprofiled drug targets ancient protozoans. Gut Microbes 4, 66–71. doi: 10.4161/gmic.22596
Diamod, L. S., Harlow, D. R., and Cunnick, C. C. (1978). A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoba. Trans. R. Soc. Trop Med. Hyg. 72, 431–432. doi: 10.1016/0035-9203(78)90144-X
Fang, Y. K., Huang, K. Y., Huang, P. J., Lin, R., Chao, M., and Tang, P. (2015). Gene-expression analysis of cold-stress response in the sexually transmitted protest Trichomonas vaginalis. J. Micro. Immunol. Infect. 48, 662–675. doi: 10.1016/j.jmii.2014.07.013
Fichorova, R. N. (2009). Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J. Reprod. Immunol. 83, 185–189. doi: 10.1016/j.jri.2009.08.007
Halliez, M. C., and Buret, A. G. (2013). Extra-intestinal and long term consequences of Giardia duodenalis infections. World J. Gastroenterol. 19, 8974–8985. doi: 10.3748/wjg.v19.i47.8974
Hanevik, K., Hausken, T., Morken, M. H., Strand, E. A., Morch, K., Coll, P., et al. (2007). Persisting symptoms and duodenal inflammation related to Giardia duodenalis infection. J. Infect. 55, 524–530. doi: 10.1016/j.jinf.2007.09.004
Heresi, G. P., Murphy, J. R., and Cleary, T. G. (2000). Giardiasis. Semin. Pediatr. Infect. Dis. J. 11, 189–195. doi: 10.1053/pi.2000.6230
Keister, D. B. (1983). Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop Med. Hyg. 77, 487–488. doi: 10.1016/0035-9203(83)90120-7
Kumar, K., Liu, N., Yang, D., Na, D., Thompson, J., Wrischnik, L. A., et al. (2015). Synthesis and antiprotozoal activity of mono- and bis-uracil isatin conjugates against the human pathogen Trichomonas vaginalis. Bioorg. Med. Chem. 23, 5190–5197. doi: 10.1016/j.bmc.2015.04.075
Kumar, L., Jain, A., Lal, N., Sarswat, A., Jangir, S., Kumar, L., et al. (2012). Potentiating metronidazole scaffold against resistant trichomonas: design, synthesis, biology and 3D–QSAR analysis. ACS Med. Chem. Lett. 3, 83–87. doi: 10.1021/ml200161t
Kumar, S., Gu, L., Palma, G., Kaur, M., Pillay, A. S., Singh, P., et al. (2018). Design, synthesis, anti-proliferative evaluation and docking studies of 1H−1,2,3-triazole tethered ospemifene-isatin conjugates as selective estrogen receptor modulators. New. J. Chem. 42, 3703–3713. doi: 10.1039/C7NJ04964A
Kumar, S., Saini, A., Gut, J., Rosenthal, J. P., Raj, R., and Kumar, V. (2017). 4-Aminoquinoline-chalcone/-N-acetylpyrazoline conjugates: synthesis and antiplasmodial evaluation. Eur. J. Med. Chem. 138, 993–1001. doi: 10.1016/j.ejmech.2017.07.041
Meri, T., Jokiranta, T. S., Suhonen, L., and Meri, S. (2000). Resistance of Trichomonas vaginalis to metronidazole: report of the first three cases from Finland and optimization of in vitro susceptibility testing under various oxygen concentrations. J. Clin. Microbiol. 38, 763–767.
Mitteregger, D., Aberle, S. W., Makristathis, A., Walochnlk, J., Wolfganag, B., Malberger, M., et al. (2012). High detection rate ofTrichomonas vaginalis in benign hyperplastic prostatic tissue. Med. Microbiol. Immunol. 201, 113–116. doi: 10.1007/s00430-011-0205-2
Nash, T. E. (2002). Surface antigenic variation in Giardia lamblia. Mol. Microbiol. 45, 585–590. doi: 10.1046/j.1365-2958.2002.03029.x
Navaneethan, U., and Giannella, R. A. (2008). Nat. Clin. Pract. Gastroenterol. Hepatol. 5, 637–647. doi: 10.1038/ncpgasthep1264
Nisha Kumar, K., Bhargava, G., Land, K. M., Chang, K. H., Arora, R., et al. (2014). N-Propargylatedisatin-mannich mono- and bis-adducts: synthesis and preliminary analysis of in vitro activity against Tritrichomonas foetus. Eur. J. Med. Chem. 74, 657–663. doi: 10.1016/j.ejmech.2014.01.015
Nisha Mehra, V., Hopper, M., Patel, N., Hall, D., and Wrischnik, L. A. (2013). Design and synthesis of β-amino alcohol based β-lactam–isatin chimeras and preliminary analysis of in vitro activity against the protozoal pathogen Trichomonas vaginalis. Med. Chem. Comm. 4, 1018–1024. doi: 10.1039/c3md00057e
Pandeya, S. N., Sriram, D., Nath, G., and De Clercq, E. (1999). Synthesis, antibacterial, antifungal andantiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Pharm. Acta Helv. 74, 11–17. doi: 10.1016/S0031-6865(99)00010-2
Pandeya, S. N., Sriram, D., Nath, G., and DeClercq, E. (2000). Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin and its derivatives with triazole. Arzneim. Arzneimittelforschung 50, 55–59. doi: 10.1002/chin.200015118
Popp, F. D., Parson, R., and Donigan, B. E. (1980). Potential anticonvulsants the condensation of isatin with cyclic-ketones. J. Heterocycl. Chem. 17, 1329–1330. doi: 10.1002/jhet.5570170639
Ravaee, R., Ebadi, P., Hatam, G., Vafafar, A., and Ghahraman Seno, M. M. (2015). Synthetic siRNAs effectively target cystein protease 12 and a-actinin transcripts in Trichomonas vaginalis. Exp. Parasitol. 157, 30–34. doi: 10.1016/j.exppara.2015.06.012
Sarangapani, M., and Reddy, V. M. (1994). Pharmacological evaluation of 1-(N, Ndisubstituted aminomethyl)-3-imino-(2-phenyl-3,4-dihydro-4-oxo-quinazolin-3-yl) indolin-2-ones. Indian J. Pharm. Sci. 56, 174–177.
Singh, A., Saha, S. T., Perumal, S., Kaur, M., and Kumar, V. (2018). Azide–alkyne cycloaddition en route to 1H-1,2,3-triazole-tethered isatin–ferrocene, ferrocenylmethoxy–isatin, and isatin–ferrocenylchalcone conjugates: synthesis and antiproliferative evaluation. ACS Omega 3, 1263–1268. doi: 10.1021/acsomega.7b01755
Singh, G. S., Singh, T., and Lakhan, R. (1997). Synthesis 13C-NMR and anti-convulsant activity of new isatin based spiroazetidinoes. Indian J. Chem. 36:951.
Stark, J. R., Judson, G., Alderete, J. F., Mundodi, V., Kucknoor, A. S., Giovannucci, E. L., et al. (2009). Prospective study of Trichomonas vaginalisinfection and prostate cancer incidence and mortality: physicians' health study. J. Natl. Cancer Inst. 101, 1406–1411. doi: 10.1093/jnci/djp306
Sutherland, H. S., Blaser, A., Kmentova, I., Franzblau, S. G., Wan, B., Wang, Y., et al. (2010). Synthesis and structures-activity relationships of antitubercular2-nitroimidazooxazines bearing heterocyclic side chains. J. Med. Chem. 53, 855–866. doi: 10.1021/jm901378u
Sutton, M., Sternberg, M., Koumans, E. H., McQuillan, G., Berman, S., and Markowitz, L. (2007). The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001-2004. Clin. Infect. Dis. 45, 1319–1326. doi: 10.1086/522532
Upcroft, J. A., Dunn, L. A., Wright, J. M., Benakli, K., Upcroft, P., and Vanelle, P. (2005). 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob. Agents Chemother. 50, 344–347. doi: 10.1128/AAC.50.1.344-347.2006
Verma, M., Pandeya, S. N., Singh, K. N., and James, P. S. (2004). Anticonvulsant activities of Schiff bases of isatin derrivatives. Acta Pharm. 54, 49–56.
Vine, K. L., Locke, J. M., Ranson, M., Pyne, S. G., and Bremner, J. B. (2007). In vitro cytotoxicity evaluation of some substituted isatin derivatives. Bioorg. Med. Chem. 15, 931–938. doi: 10.1016/j.bmc.2006.10.035
Youssef, M., Shurman, A., Bougnoux, M., Rawashdeh, M., Bretagne, S., and Strockbine, N. (2000). Bacterial, viral and parasitic enteric pathogens associated with acute diarrhea in hospitalized children from northern Jordan FEMS. Immunol. Med. Microbiol. 28, 257–263. doi: 10.1111/j.1574-695X.2000.tb01485.x
Keywords: Entamoeba histolytica, Trichomonas vaginalis, Tritrichomonas foetus, Giardia lamblia, metronidazole, cytotoxicity, isatin-metronidazole conjugates
Citation: Kumar S, Bains T, Won Kim AS, Tam C, Kim J, Cheng LW, Land KM, Debnath A and Kumar V (2018) Highly Potent 1H-1,2,3-Triazole-Tethered Isatin-Metronidazole Conjugates Against Anaerobic Foodborne, Waterborne, and Sexually-Transmitted Protozoal Parasites. Front. Cell. Infect. Microbiol. 8:380. doi: 10.3389/fcimb.2018.00380
Received: 15 June 2018; Accepted: 09 October 2018;
Published: 30 October 2018.
Edited by:
Herbert Leonel de Matos Guedes, Universidade Federal do Rio de Janeiro, BrazilReviewed by:
Veeranoot Nissapatorn, Walailak University, ThailandAbeer Elhenawy, Mansoura University, Egypt
Floriano Paes Silva-Jr, Fundação Oswaldo Cruz (Fiocruz), Brazil
Copyright © 2018 Kumar, Bains, Won Kim, Tam, Kim, Cheng, Land, Debnath and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anjan Debnath, adebnath@ucsd.edu
Vipan Kumar, vipan_org@yahoo.com
 Sumit Kumar1
Sumit Kumar1  Trpta Bains
Trpta Bains Ashley Sae Won Kim
Ashley Sae Won Kim Jong Kim
Jong Kim Kirkwood M. Land
Kirkwood M. Land Anjan Debnath
Anjan Debnath Vipan Kumar
Vipan Kumar