Approaches to treatment of emerging Shiga toxin-producing Escherichia coli infections highlighting the O104:H4 serotype
- Department of Experimental Pathology, Immunology and Microbiology, Faculty of Medicine, American University of Beirut, Beirut, Lebanon
Shiga toxin-producing Escherichia coli (STEC) are a group of diarrheagenic bacteria associated with foodborne outbreaks. Infection with these agents may result in grave sequelae that include fatality. A large number of STEC serotypes has been identified to date. E. coli serotype O104:H4 is an emerging pathogen responsible for a 2011 outbreak in Europe that resulted in over 4000 infections and 50 deaths. STEC pathogenicity is highly reliant on the production of one or more Shiga toxins that can inhibit protein synthesis in host cells resulting in a cytotoxicity that may affect various organ systems. Antimicrobials are usually avoided in the treatment of STEC infections since they are believed to induce bacterial cell lysis and the release of stored toxins. Some antimicrobials have also been reported to enhance toxin synthesis and production from these organisms. Various groups have attempted alternative treatment approaches including the administration of toxin-directed antibodies, toxin-adsorbing polymers, probiotic agents and natural remedies. The utility of antibiotics in treating STEC infections has also been reconsidered in recent years with certain modalities showing promise.
Shiga toxin-producing Escherichia coli (STEC) are a group of bacterial organisms that are capable of producing one or more types of Shiga toxin (Stx). STEC are associated with a disease spectrum ranging from diarrhea and hemorrhagic colitis (HC) to the potentially fatal hemolytic uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP). STEC infections are typically food-borne (Dupont, 2007) and the production of Shiga toxins (Stx1, Stx2 or a variant) is believed to be central to the pathogenesis of these organisms. STEC strains are the result of an insertion of one of a group of lysogenic lambdoid bacteriophages that harbor an Stx1/2-encoding gene into the E. coli genome. The clinical syndromes, pathogenic characteristics, the pathobiology of these organisms and the toxins they produce are reviewed in Melton-Celsa et al. (2012); Farrokh et al. (2013); Kruger and Lucchesi (2015).
In recent years, novel serotypes have emerged culminating in a major outbreak in 2011 caused by a novel pathotype, E. coli O104:H4. The review at hand focuses on potential treatment strategies for STEC infections in light of a consensus contraindication of employing antimicrobials for these bacterial pathogens. The rise of E. coli O104:H4 and approaches employed in its treatment are highlighted.
Emerging STEC Serotypes
A large number of STEC serotypes has been documented; these have been isolated from various types of animals including cattle, sheep, and goats (Farrokh et al., 2013). More than 380 STEC serotypes have been associated with human disease; some of the most frequently reported serotypes include O111:H-, O26:H11/H-, O103:H2, O113:H21, O91:H21/H-, O117:H7, O118:H16, O121:H19, O145:H28, O128:H2/H-, and O146:H21. The O157:H7 serotype has been the most commonly isolated one in association with HC and HUS in both outbreaks and sporadic cases. It accounts for more than 30% of estimated STEC illness and mortality cases in the United States (Karmali et al., 2010; Scallan et al., 2011). However, there are some indications that non-O157 STEC are gaining traction in the United States and that they may be even more common than O157 strains in severe illnesses caused by STEC in parts of Europe, Latin America, Australia, and Africa (Blanco et al., 2005; Wang et al., 2013).
The epidemiology and pathogenic characteristics of non-O157 serotypes are not well studied; however, the limited reported data indicates some differences between the two types of infections. Non-O157 strains appear to induce a longer period of diarrhea which is less frequently of the hemorrhagic type (Johnson et al., 2006). Nevertheless, studies demonstrate that these non-O157 serotypes can be as virulent as O157 serotypes depending on the strain involved (Ethelberg et al., 2004).
Perhaps highlighting the relevance of monitoring these non-O157 serotypes was the emergence of the rather notorious E. coli O104:H4. This novel pathogen was the cause of a 2011 outbreak that affected 16 European countries with the majority of cases reported in Germany. Few cases were reported in Canada and the United States as well; nevertheless, these were travelers who had been to Europe prior to becoming ill. Reports of this novel pathogen started in May of 2011 and had peaked and then dwindled by July of the same year due to control measures that were implemented. The WHO indicates that 4075 cases and 50 deaths were caused by this STEC outbreak. Therefore, a 1.23% mortality rate was observed. On the other hand, the mortality rate of HUS due to E. coli O104:H4 in this outbreak was 3.74% (WHO, 2011). E. coli O104:H4 appears to be an enteroaggregative E. coli (EAEC) that has acquired the ability to produce Stx2, typically produced by enterohemorrhagic E. coli (EHEC) rather than EAEC group members. This may have occurred via horizontal gene transfer resulting in a new E. coli virotype dubbed the Enteroaggregative Hemorrhagic E. coli or EAHEC (Bloch et al., 2012). The O104:H4 serotype harbors two copies of the Stx2-encoding prophage. Therefore, this emergent bacterium seems to have a rather novel epidemiologic and pathogenic profile (Brzuszkiewicz et al., 2011; Mellmann et al., 2011). While ruminants are the reservoir of most STEC serotypes, no animal reservoir has been identified for E. coli O104:H4 and humans are believed to be the major reservoir for this organism (Wieler et al., 2011; Auvray et al., 2012; Karch et al., 2012). Whereas, the clinical profile of E. coli O104:H4 was relatively similar to that caused by other STEC infections some pertinent differences existed. For instance, about a quarter of subjects affected developed HUS during the 2011 outbreak, which is 2–5 fold higher than the rate usually observed for an STEC infection (WHO, 2011).
Treatment of an STEC Infection
The lack of an effective treatment strategy for an STEC infection has made these agents a prominent public health threat and a burden to the medical community at large. The currently recommended management of an STEC infection mainly relies on supportive therapy and hydration (Thorpe, 2004). The use of antimicrobial agents in treating these infections has been associated with an increased risk of HUS and is therefore contraindicated (Qadri and Kayali, 1998; Guerrant et al., 2001; Safdar et al., 2002).
Novel and Alternative STEC Treatment Strategies
The debatable use of antimicrobial agents for the treatment of an STEC infection has led to the rise of various alternative treatment approaches (Table 1). These have ranged from the use of natural products to the development of novel regimens that re-examine employing antimicrobials.
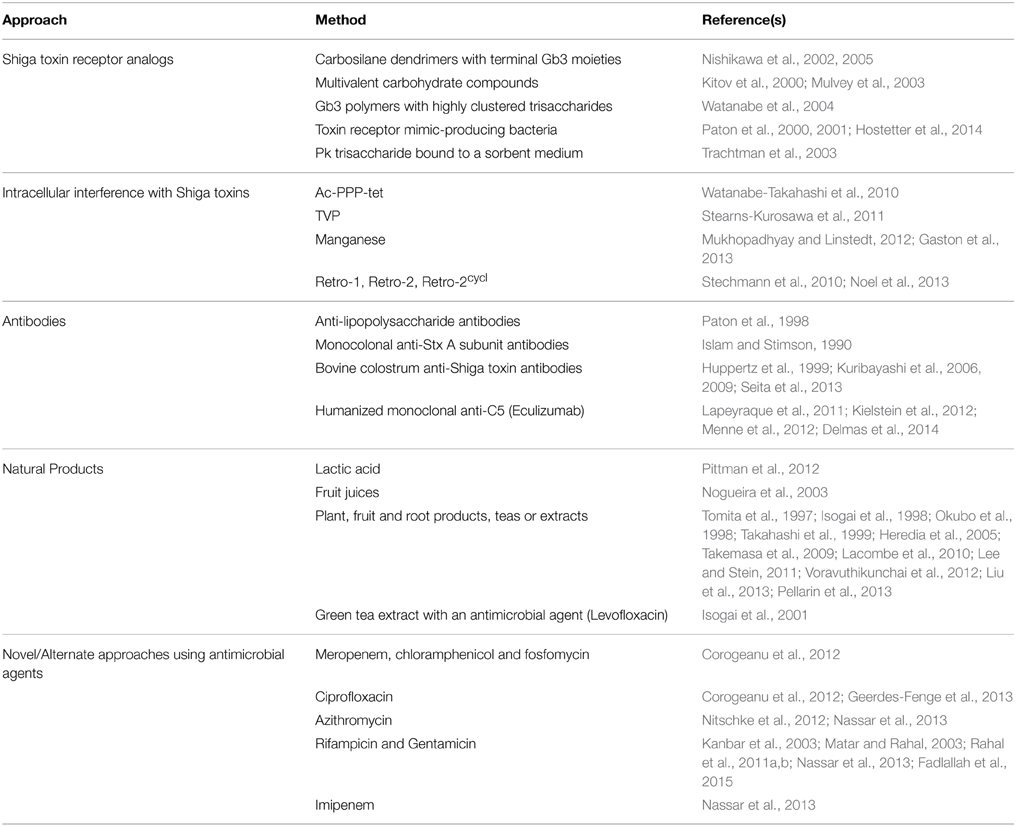
Table 1. Experimental approaches to the treatment of Shiga toxin-producing Escherichia coli infections.
Shiga Toxin Receptor Analog
Various agents that mimic Stx receptors and bind them thus reducing their availability to cellular receptors have been developed. Carbosilane dendrimers harboring Gb3 at their termini neutralize Shiga toxins in vitro and were demonstrated to protect challenged mice when administered intravenously (Nishikawa et al., 2002, 2005). Similarly, multivalent carbohydrate compounds, such as STARFISH and Daisy also neutralize Shiga toxins in vitro and in animals (Kitov et al., 2000; Mulvey et al., 2003). Gb3 polymers with highly clustered trisaccharides bind Shiga toxins with high affinity and protect challenged mice when administered orally (Watanabe et al., 2004). Recombinant bacterial strains that express toxin receptor mimics have also demonstrated a potential efficacy in vitro and upon testing in animals (Paton et al., 2000, 2001; Hostetter et al., 2014). SYNSORB Pk, a synthetic Stx receptor analog consisting of a Pk trisaccharide bound to Chromosorb® P, a multipurpose sorbent medium, was shown to have an abrogative effect on Shiga toxins in vitro. This agent, however, was not effective in clinical trials (Trachtman et al., 2003).
Intracellular Interference with Shiga Toxins
Cell permeable agents that can bind Stx2 and potentially interfere with its intracellular trafficking have been reported. These include Ac-PPP-tet (Watanabe-Takahashi et al., 2010) and TVP (Stearns-Kurosawa et al., 2011); both agents have been tested in animal models and have displayed Stx2 neutralization abilities. Manganese has also been reported to interfere with intracellular trafficking of the B subunits of Stx and to protect against Stx1 in mice (Mukhopadhyay and Linstedt, 2012). However, it did not protect against Stx1-S or Stx2a and hence it may be of limited use (Gaston et al., 2013). The small molecule inhibitors Retro-1 and Retro-2 have also been identified via high throughput screening as agents that interfere with Stx trafficking (Stechmann et al., 2010) and a derivative of Retro-2, referred to as Retro-2cycl, was shown to protect cells in culture against Stx (Noel et al., 2013).
Antibodies
Preparations of antibodies that can bind Shiga toxins and neutralize their effects have been reported. Anti-lipopolysaccharide antibodies have shown protective abilities upon laboratory assessment (Paton et al., 1998) and monocolonal anti-Stx A subunit antibodies have demonstrated potential utility in both laboratory and animal studies (Islam and Stimson, 1990). Bovine colostrum antibodies against Shiga toxins have also been demonstrated to protect challenged animals (Kuribayashi et al., 2006, 2009; Seita et al., 2013). A bovine colostrum preparation, rich in immunoglobulins and harboring a high titer of anti-Stx1 and anti-Stx2 antibodies, has also been assessed; a colostrum-treated group of 13 patients and 14 placebo-treated controls were compared. The median frequency of stool excretion was decreased in the colostrum-treated patients; however, the presence of the bacterial agent in subject stools was not notably affected. Study subjects were not monitored for the effect of this treatment on the development of HUS or other potential sequelae of infection (Huppertz et al., 1999). Eculizumab, a humanized monoclonal antibody against complement component 5 (C5), was shown in small clinical studies to have beneficial effects on recovery from STEC-associated HUS including cases during the 2011 E. coli O104:H4 outbreak (Lapeyraque et al., 2011; Delmas et al., 2014). However, some reports have indicated that inclusion of eculizumab in the treatment of E. coli O104:H4-induced HUS results in no additional benefits (Kielstein et al., 2012; Menne et al., 2012).
Natural Products
Various natural products have been considered as potential therapeutic agents for STEC infections. These have included lactic acid (Pittman et al., 2012), fruit juices (Nogueira et al., 2003) in addition to plant, fruit and root products, teas or extracts (Tomita et al., 1997; Isogai et al., 1998; Okubo et al., 1998; Takahashi et al., 1999; Heredia et al., 2005; Takemasa et al., 2009; Lacombe et al., 2010; Lee and Stein, 2011; Voravuthikunchai et al., 2012; Liu et al., 2013; Pellarin et al., 2013). These products have shown promise in vitro or in experimental animal models; however, they have not been evaluated in clinical studies. Worth noting is a study that showed a synergistic effect between green tea extract and an antibiotic, levofloxacin, in the treatment of an STEC-infected mouse model (Isogai et al., 2001) indicating that a potential risk imparted by an antibiotic treatment may be lessened by the inclusion of another agent.
Antimicrobial Agents
The use of antimicrobial agents in treating STEC infections has been controversial and the subject of an ongoing debate. While some studies indicated that the use of particular agents may increase the risk of HUS, others have reported a decrease of this risk upon implementation of antimicrobials. While these observations may be particular to certain agents at some doses, the potential risk of antimicrobial treatment inducing HUS has led to a general contraindication of such agents (Qadri and Kayali, 1998; Guerrant et al., 2001; Safdar et al., 2002). Antimicrobials are thought to augment the risk of HUS by enhancing the release of Shiga toxins from bacterial cells via a number of ways. DNA damage that can be caused by some antimicrobials may trigger the bacterial SOS response in STEC cells. The SOS response, whose function is to cope with genomic damage, results in the expression of a number of proteins that may activate the lytic cycle of the bacteriophage encoding a Stx thus enhancing its production. Other types of physiologic stresses caused by antimicrobial agents may also trigger the lytic cycle and result in increased toxin expression (Kimmitt et al., 2000; Los et al., 2009). On the other hand, Stx1 is known to be stored within the periplasmic space of STEC cells; therefore, cellular lysis induced by an antimicrobial agent may result in an enhanced release of this particular type of Stx (Strockbine et al., 1986; Yoh et al., 1997; Sato et al., 2003; Shimizu et al., 2009).
Several antimicrobial agents have been shown to enhance the release or the production of Shiga toxins from STEC cells in vitro; these include the quinolones, trimethoprim, and furazolidone (Kimmitt et al., 2000); however, observations indicate that these effect may be strain and antimicrobial agent-specific (Grif et al., 1998). For example some isolates of E. coli O104:H4 from the 2011 outbreak in Europe do not display an increase in toxin production upon treatment with meropenem, ciprofloxacin, chloramphenicol, or fosfomycin, unlike E. coli O157:H7 (Corogeanu et al., 2012). Our group assessed the effect of sub-MIC levels of various antimicrobial agents on triggering the SOS response and the production of Shiga toxins in E. coli O157:H7 and in E. coli O104:H4. A sub-MIC concentration may, after all, be the concentration available locally at the site of infection. We noted that the response is variable depending on the isolate used and the concentration of antimicrobial implemented (Nassar et al., 2013; Fadlallah et al., 2015).
Reconsideration of treating STEC infections with antimicrobial agents has nevertheless gained ground in recent years. Ciprofloxacin was recently reported to decrease the risk of HUS in subjects infected with E. coli O104:H4 during the 2011 outbreak (Geerdes-Fenge et al., 2013) and a reduced duration of carriage of the organism in subjects treated with azithromycin was detected during this outbreak as well (Nitschke et al., 2012). Worth noting, however, is that only a small number of treated subjects was included in both studies. Our group assessed the use of rifampicin at a concentration that decreases toxin production, but at which E. coli O157:H7 cells remain viable, followed by treatment with gentamicin at a bactericidal concentration. This strategy was effective in decreasing toxin release compared to solely treating the cells with a bactericidal gentamicin concentration (Kanbar et al., 2003; Matar and Rahal, 2003). Applying a similar strategy in an E. coli O157:H7 infection mouse model resulted in an improved animal survival rate (Rahal et al., 2011a,b). Utilizing the same strategy to treat E. coli O104:H4 infected mice similarly resulted in an improved survival rate compared to untreated control mice that were infected with the organism; however, the highest survival rate observed was with mice treated with gentamicin alone, unlike our observations with E. coli O157:H7 (Fadlallah et al., 2015). This again highlights observations indicating that different STEC serotypes and even isolates of the same serotype respond differently to antimicrobial treatments.
Probiotics, Phages and Vaccines
Although probiotics may not have a therapeutic benefit in the management of an STEC infection, they may have a relevant preventative utility. Probiotics are probably capable of disrupting host-infectious agent/toxin interactions by occupying cellular receptors themselves, by producing decoy receptors that take up the toxins or by modifying the local milieu, hence making these interactions unfavorable (Corr et al., 2009). Multiple studies have shown in vitro beneficial effects of probiotics and that inoculation of animal models with a probiotic prior to an experimental STEC infection has preventative capabilities (Asahara et al., 2004; Reissbrodt et al., 2009; Eaton et al., 2011; Mogna et al., 2012; Chen et al., 2013; Kakisu et al., 2013; Rund et al., 2013; Stanford et al., 2014). The extent of probiotic protective capabilities seen in experimental models is likely dependent on the probiotic strain used and its ability to modify the surrounding medium. For example, the production of acetate by the probiotic agent has been demonstrated to be an important factor (Fukuda et al., 2011, 2012) and the production of butyric acid and lactic acid may be of relevance as well (Ogawa et al., 2001; Takahashi et al., 2004). One in vitro study showed that cultivation of STEC organisms in the presence of various Bifidobacterium, Pediococcus, and Lactobacillus strains results in a decreased production of Stx2. This was attributed to a decrease in pH due to the acids produced by these agents (Carey et al., 2008). Worth noting is the recombinant probiotic agent that can produce toxin receptor mimics described in section 3.a. (Paton et al., 2000, 2001; Hostetter et al., 2014). Also of relevance are the various studies indicating that the administration of probiotic agents to cattle may reduce their carriage of STEC organisms (systematically reviewed in Sargeant et al., 2007), hence effectively reducing the risk of transmitting these toxigenic agents.
Another preventative measure proposed as a means of controlling STEC is the application of lytic phages. Lytic phages have been shown to reduce STEC numbers in vitro (Niu et al., 2009; Rivas et al., 2010), in phage-treated food products (Abuladze et al., 2008; Anany et al., 2011), on hard surfaces (Abuladze et al., 2008), in mice and in some ruminants (Raya et al., 2006; Sheng et al., 2006). Phage-containing products that can be sprayed on animal hides or on meat products for the control of STEC organisms are available on the market and are Food and Drug Administration (FDA) approved (Sillankorva et al., 2012). The efficacy of orally treating cattle with lytic phages, however, was reported to be limited and requires the development of an enhanced approach or delivery mode (Stanford et al., 2010). Bacteriophages used to eradicate STEC agents may also have a therapeutic utility should the safety and efficacy of such an application be demonstrated in humans.
Various vaccine approaches have also been attempted including the development of preparations that contain bacterial peptides and virulence factors (Wen et al., 2006; Tiels et al., 2008; Gu et al., 2009; McNeilly et al., 2010; Asper et al., 2011; Cai et al., 2011; Gupta et al., 2011; Wan et al., 2011; Zhang et al., 2012; Rossi et al., 2013; Sato et al., 2013; Cernicchiaro et al., 2014; Garcia-Angulo et al., 2014; Lu et al., 2014; Mejias et al., 2014; Paddock et al., 2014), attenuated bacterial cells (Rojas et al., 2010; Gu et al., 2011; Fujii et al., 2012), bacterial envelope/membrane derivatives (Cai et al., 2010; Choi et al., 2014) in addition to DNA vaccines (Bentancor et al., 2009; Ren et al., 2013). These vaccine preparations have been assessed in animal models with some showing promising results (reviewed in Garcia-Angulo et al., 2013).
In conclusion, despite the passage of more than three decades since STEC organisms were first associated with human clinical illness (CDC, 1982), a generally-accepted successful therapeutic method for these organisms remains undocumented. Various approaches have nevertheless been attempted including ones that reconsider the implementation of antimicrobial agents; beneficial effects have been reported for some agents with outcomes appearing dependent on the antimicrobials used, their dose and the STEC isolate itself. Further studies examining antimicrobial agents in the therapy of STEC infections should be conducted in animals to select the safest and most efficacious regimen that would then be assessed in clinical trials.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abuladze, T., Li, M., Menetrez, M. Y., Dean, T., Senecal, A., and Sulakvelidze, A. (2008). Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Appl. Environ. Microbiol. 74, 6230–6238. doi: 10.1128/AEM.01465-08
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Anany, H., Chen, W., Pelton, R., and Griffiths, M. W. (2011). Biocontrol of Listeria monocytogenes and Escherichia coli O157:H7 in meat by using phages immobilized on modified cellulose membranes. Appl. Environ. Microbiol. 77, 6379–6387. doi: 10.1128/AEM.05493-11
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Asahara, T., Shimizu, K., Nomoto, K., Hamabata, T., Ozawa, A., and Takeda, Y. (2004). Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 72, 2240–2247. doi: 10.1128/IAI.72.4.2240-2247.2004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Asper, D. J., Karmali, M. A., Townsend, H., Rogan, D., and Potter, A. A. (2011). Serological response of Shiga toxin-producing Escherichia coli type III secreted proteins in sera from vaccinated rabbits, naturally infected cattle, and humans. Clin. Vaccine Immunol. 18, 1052–1057. doi: 10.1128/CVI.00068-11
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Auvray, F., Dilasser, F., Bibbal, D., Kerouredan, M., Oswald, E., and Brugere, H. (2012). French cattle is not a reservoir of the highly virulent enteroaggregative Shiga toxin-producing Escherichia coli of serotype O104:H4. Vet. Microbiol. 158, 443–445. doi: 10.1016/j.vetmic.2012.02.029
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bentancor, L. V., Bilen, M., Brando, R. J., Ramos, M. V., Ferreira, L. C., Ghiringhelli, P. D., et al. (2009). A DNA vaccine encoding the enterohemorragic Escherichia coli Shiga-like toxin 2 A2 and B subunits confers protective immunity to Shiga toxin challenge in the murine model. Clin. Vaccine Immunol. 16, 712–718. doi: 10.1128/CVI.00328-08
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Blanco, M., Schumacher, S., Tasara, T., Zweifel, C., Blanco, J. E., Dahbi, G., et al. (2005). Serotypes, intimin variants and other virulence factors of eae positive Escherichia coli strains isolated from healthy cattle in Switzerland. Identification of a new intimin variant gene (eae-eta2). BMC Microbiol. 5:23. doi: 10.1186/1471-2180-5-23
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bloch, S. K., Felczykowska, A., and Nejman-Falenczyk, B. (2012). Escherichia coli O104:H4 outbreak—have we learnt a lesson from it? Acta Biochim. Pol. 59, 483–488.
Brzuszkiewicz, E., Thurmer, A., Schuldes, J., Leimbach, A., Liesegang, H., Meyer, F. D., et al. (2011). Genome sequence analyses of two isolates from the recent Escherichia coli outbreak in Germany reveal the emergence of a new pathotype: Entero-Aggregative-Haemorrhagic Escherichia coli (EAHEC). Arch. Microbiol. 193, 883–891. doi: 10.1007/s00203-011-0725-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cai, K., Gao, X., Li, T., Hou, X., Wang, Q., Liu, H., et al. (2010). Intragastric immunization of mice with enterohemorrhagic Escherichia coli O157:H7 bacterial ghosts reduces mortality and shedding and induces a Th2-type dominated mixed immune response. Can. J. Microbiol. 56, 389–398. doi: 10.1139/W10-025
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cai, K., Gao, X., Li, T., Wang, Q., Hou, X., Tu, W., et al. (2011). Enhanced immunogenicity of a novel Stx2Am-Stx1B fusion protein in a mice model of enterohemorrhagic Escherichia coli O157:H7 infection. Vaccine 29, 946–952. doi: 10.1016/j.vaccine.2010.11.035
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Carey, C. M., Kostrzynska, M., Ojha, S., and Thompson, S. (2008). The effect of probiotics and organic acids on Shiga-toxin 2 gene expression in enterohemorrhagic Escherichia coli O157:H7. J. Microbiol. Methods 73, 125–132. doi: 10.1016/j.mimet.2008.01.014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
CDC. (1982). Isolation of E. coli O157:H7 from sporadic cases of hemorrhagic colitis—United States. MMWR Morb. Mortal. Wkly. Rep. 31, 580, 585.
Cernicchiaro, N., Renter, D. G., Cull, C. A., Paddock, Z. D., Shi, X., and Nagaraja, T. G. (2014). Fecal shedding of non-O157 serogroups of Shiga toxin-producing Escherichia coli in feedlot cattle vaccinated with an Escherichia coli O157:H7 SRP vaccine or fed a Lactobacillus-based direct-fed microbial. J. Food Prot. 77, 732–737. doi: 10.4315/0362-028X.JFP-13-358
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chen, Y. P., Lee, T. Y., Hong, W. S., Hsieh, H. H., and Chen, M. J. (2013). Effects of Lactobacillus kefiranofaciens M1 isolated from kefir grains on enterohemorrhagic Escherichia coli infection using mouse and intestinal cell models. J. Dairy Sci. 96, 7467–7477. doi: 10.3168/jds.2013-7015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Choi, K. S., Kim, S. H., Kim, E. D., Lee, S. H., Han, S. J., Yoon, S., et al. (2014). Protection from hemolytic uremic syndrome by eyedrop vaccination with modified enterohemorrhagic E. coli outer membrane vesicles. PLoS ONE 9:e100229. doi: 10.1371/journal.pone.0100229
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Corogeanu, D., Willmes, R., Wolke, M., Plum, G., Utermohlen, O., and Kronke, M. (2012). Therapeutic concentrations of antibiotics inhibit Shiga toxin release from enterohemorrhagic E. coli O104:H4 from the 2011 German outbreak. BMC Microbiol. 12:160. doi: 10.1186/1471-2180-12-160
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Corr, S. C., Hill, C., and Gahan, C. G. (2009). Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv. Food Nutr. Res. 56, 1–15. doi: 10.1016/S1043-4526(08)00601-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Delmas, Y., Vendrely, B., Clouzeau, B., Bachir, H., Bui, H. N., Lacraz, A., et al. (2014). Outbreak of Escherichia coli O104:H4 haemolytic uraemic syndrome in France: outcome with eculizumab. Nephrol. Dial. Transplant 29, 565–572. doi: 10.1093/ndt/gft470
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dupont, H. L. (2007). The growing threat of foodborne bacterial enteropathogens of animal origin. Clin. Infect. Dis. 45, 1353–1361. doi: 10.1086/522662
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Eaton, K. A., Honkala, A., Auchtung, T. A., and Britton, R. A. (2011). Probiotic Lactobacillus reuteri ameliorates disease due to enterohemorrhagic Escherichia coli in germfree mice. Infect. Immun. 79, 185–191. doi: 10.1128/IAI.00880-10
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ethelberg, S., Olsen, K. E., Scheutz, F., Jensen, C., Schiellerup, P., Enberg, J., et al. (2004). Virulence factors for hemolytic uremic syndrome, Denmark. Emerging Infect. Dis. 10, 842–847. doi: 10.3201/eid1005.030576
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fadlallah, S. M., Rahal, E. A., Sabra, A., Kissoyan, K. A., and Matar, G. M. (2015). Effect of rifampicin and gentamicin on Shiga toxin 2 expression level and the SOS response in Escherichia coli O104:H4. Foodborne Pathog. Dis. 12, 47–55. doi: 10.1089/fpd.2014.1824
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Farrokh, C., Jordan, K., Auvray, F., Glass, K., Oppegaard, H., Raynaud, S., et al. (2013). Review of Shiga-toxin-producing Escherichia coli (STEC) and their significance in dairy production. Int. J. Food Microbiol. 162, 190–212. doi: 10.1016/j.ijfoodmicro.2012.08.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fujii, J., Naito, M., Yutsudo, T., Matsumoto, S., Heatherly, D. P., Yamada, T., et al. (2012). Protection by a recombinant Mycobacterium bovis Bacillus Calmette-Guerin vaccine expressing Shiga toxin 2 B subunit against Shiga toxin-producing Escherichia coli in mice. Clin. Vaccine Immunol. 19, 1932–1937. doi: 10.1128/CVI.00473-12
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fukuda, S., Toh, H., Hase, K., Oshima, K., Nakanishi, Y., Yoshimura, K., et al. (2011). Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547. doi: 10.1038/nature09646
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fukuda, S., Toh, H., Taylor, T. D., Ohno, H., and Hattori, M. (2012). Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 3, 449–454. doi: 10.4161/gmic.21214
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Garcia-Angulo, V. A., Kalita, A., Kalita, M., Lozano, L., and Torres, A. G. (2014). Comparative genomics and immunoinformatics approach for the identification of vaccine candidates for enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 82, 2016–2026. doi: 10.1128/IAI.01437-13
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Garcia-Angulo, V. A., Kalita, A., and Torres, A. G. (2013). Advances in the development of enterohemorrhagic Escherichia coli vaccines using murine models of infection. Vaccine 31, 3229–3235. doi: 10.1016/j.vaccine.2013.05.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gaston, M. A., Pellino, C. A., and Weiss, A. A. (2013). Failure of manganese to protect from Shiga toxin. PLoS ONE 8:e69823. doi: 10.1371/journal.pone.0069823
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Geerdes-Fenge, H. F., Lobermann, M., Nurnberg, M., Fritzsche, C., Koball, S., Henschel, J., et al. (2013). Ciprofloxacin reduces the risk of hemolytic uremic syndrome in patients with Escherichia coli O104:H4-associated diarrhea. Infection 41, 669–673. doi: 10.1007/s15010-012-0387-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grif, K., Dierich, M. P., Karch, H., and Allerberger, F. (1998). Strain-specific differences in the amount of Shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agents. Eur. J. Clin. Microbiol. Infect. Dis. 17, 761–766. doi: 10.1007/s100960050181
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gu, J., Liu, Y., Yu, S., Wang, H., Wang, Q., Yi, Y., et al. (2009). Enterohemorrhagic Escherichia coli trivalent recombinant vaccine containing EspA, intimin and Stx2 induces strong humoral immune response and confers protection in mice. Microbes Infect. 11, 835–841. doi: 10.1016/j.micinf.2009.04.024
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gu, J., Ning, Y., Wang, H., Xiao, D., Tang, B., Luo, P., et al. (2011). Vaccination of attenuated EIS-producing Salmonella induces protective immunity against enterohemorrhagic Escherichia coli in mice. Vaccine 29, 7395–7403. doi: 10.1016/j.vaccine.2011.07.069
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Guerrant, R. L., Van Gilder, T., Steiner, T. S., Thielman, N. M., Slutsker, L., Tauxe, R. V., et al. (2001). Practice guidelines for the management of infectious diarrhea. Clin. Infect. Dis. 32, 331–351. doi: 10.1086/318514
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gupta, P., Singh, M. K., Singh, Y., Gautam, V., Kumar, S., Kumar, O., et al. (2011). Recombinant Shiga toxin B subunit elicits protection against Shiga toxin via mixed Th type immune response in mice. Vaccine 29, 8094–8100. doi: 10.1016/j.vaccine.2011.08.040
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Heredia, N., Escobar, M., Rodriguez-Padilla, C., and Garcia, S. (2005). Extracts of Haematoxylon brasiletto inhibit growth, verotoxin production, and adhesion of enterohemorrhagic Escherichia coli O157:H7 to HeLa cells. J. Food Prot. 68, 1346–1351.
Hostetter, S. J., Helgerson, A. F., Paton, J. C., Paton, A. W., and Cornick, N. A. (2014). Therapeutic use of a receptor mimic probiotic reduces intestinal Shiga toxin levels in a piglet model of hemolytic uremic syndrome. BMC Res. Notes 7:331. doi: 10.1186/1756-0500-7-331
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Huppertz, H. I., Rutkowski, S., Busch, D. H., Eisebit, R., Lissner, R., and Karch, H. (1999). Bovine colostrum ameliorates diarrhea in infection with diarrheagenic Escherichia coli, shiga toxin-producing E. Coli, and E. coli expressing intimin and hemolysin. J. Pediatr. Gastroenterol. Nutr. 29, 452–456. doi: 10.1097/00005176-199910000-00015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Islam, M. S., and Stimson, W. H. (1990). Production and characterization of monoclonal antibodies with therapeutic potential against Shiga toxin. J. Clin. Lab. Immunol. 33, 11–16.
Isogai, E., Isogai, H., Hirose, K., Hayashi, S., and Oguma, K. (2001). In vivo synergy between green tea extract and levofloxacin against enterohemorrhagic Escherichia coli O157 infection. Curr. Microbiol. 42, 248–251. doi: 10.1007/s0028403357
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Isogai, E., Isogai, H., Takeshi, K., and Nishikawa, T. (1998). Protective effect of Japanese green tea extract on gnotobiotic mice infected with an Escherichia coli O157:H7 strain. Microbiol. Immunol. 42, 125–128. doi: 10.1111/j.1348-0421.1998.tb02260.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Johnson, K. E., Thorpe, C. M., and Sears, C. L. (2006). The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin. Infect. Dis. 43, 1587–1595. doi: 10.1086/509573
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kakisu, E., Abraham, A. G., Farinati, C. T., Ibarra, C., and De Antoni, G. L. (2013). Lactobacillus plantarum isolated from kefir protects vero cells from cytotoxicity by type-II shiga toxin from Escherichia coli O157:H7. J. Dairy Res. 80, 64–71. doi: 10.1017/S0022029912000659
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kanbar, A., Rahal, E., and Matar, G. M. (2003). In Vitro inhibition of the expression of Escherichia coli O157:H7 genes encoding the shiga-like toxins by antimicrobial agents: potential use in the treatment of human infection. J. Appl. Res. 3, 137–143. doi: 10.1179/000349803235002146
Karch, H., Denamur, E., Dobrindt, U., Finlay, B. B., Hengge, R., Johannes, L., et al. (2012). The enemy within us: lessons from the 2011 European Escherichia coli O104:H4 outbreak. EMBO Mol. Med. 4, 841–848. doi: 10.1002/emmm.201201662
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Karmali, M. A., Gannon, V., and Sargeant, J. M. (2010). Verocytotoxin-producing Escherichia coli (VTEC). Vet. Microbiol. 140, 360–370. doi: 10.1016/j.vetmic.2009.04.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kielstein, J. T., Beutel, G., Fleig, S., Steinhoff, J., Meyer, T. N., Hafer, C., et al. (2012). Best supportive care and therapeutic plasma exchange with or without eculizumab in Shiga-toxin-producing E. coli O104:H4 induced haemolytic-uraemic syndrome: an analysis of the German STEC-HUS registry. Nephrol. Dial. Transplant. 27, 3807–3815. doi: 10.1093/ndt/gfs394
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kimmitt, P. T., Harwood, C. R., and Barer, M. R. (2000). Toxin gene expression by shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 6, 458–465. doi: 10.3201/eid0605.000503
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kitov, P. I., Sadowska, J. M., Mulvey, G., Armstrong, G. D., Ling, H., Pannu, N. S., et al. (2000). Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature 403, 669–672. doi: 10.1038/35001095
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kruger, A., and Lucchesi, P. M. (2015). Shiga toxins and stx-phages: highly diverse entities. Microbiology 161, 451–462. doi: 10.1099/mic.0.000003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kuribayashi, T., Seita, T., Fukuyama, M., Furuhata, K., Honda, M., Matsumoto, M., et al. (2006). Neutralizing activity of bovine colostral antibody against verotoxin derived from enterohemorrhagic Escherichia coli O157:H7 in mice. J. Infect. Chemother. 12, 251–256. doi: 10.1007/s10156-006-0470-Y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kuribayashi, T., Seita, T., Matsumoto, M., Furuhata, K., Tagata, K., and Yamamoto, S. (2009). Bovine colostral antibody against verotoxin 2 derived from Escherichia coli O157:H7: resistance to proteases and effects in beagle dogs. Comp. Med. 59, 163–167.
Lacombe, A., Wu, V. C., Tyler, S., and Edwards, K. (2010). Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. Int. J. Food Microbiol. 139, 102–107. doi: 10.1016/j.ijfoodmicro.2010.01.035
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lapeyraque, A. L., Malina, M., Fremeaux-Bacchi, V., Boppel, T., Kirschfink, M., Oualha, M., et al. (2011). Eculizumab in severe Shiga-toxin-associated HUS. N. Engl. J. Med. 364, 2561–2563. doi: 10.1056/NEJMc1100859
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, J. H., and Stein, B. D. (2011). Antimicrobial activity of a combination of Mume fructus, Schizandrae fructus, and Coptidis rhizoma on enterohemorrhagic Escherichia coli O26, O111, and O157 and its effect on Shiga toxin releases. Foodborne Pathog. Dis. 8, 643–646. doi: 10.1089/fpd.2010.0710
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, Y., Song, M., Che, T. M., Almeida, J. A., Lee, J. J., Bravo, D., et al. (2013). Dietary plant extracts alleviate diarrhea and alter immune responses of weaned pigs experimentally infected with a pathogenic Escherichia coli. J. Anim. Sci. 91, 5294–5306. doi: 10.2527/jas.2012-6194
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Los, J. M., Los, M., Wegrzyn, G., and Wegrzyn, A. (2009). Differential efficiency of induction of various lambdoid prophages responsible for production of Shiga toxins in response to different induction agents. Microb. Pathog. 47, 289–298. doi: 10.1016/j.micpath.2009.09.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lu, X., Skurnik, D., Pozzi, C., Roux, D., Cywes-Bentley, C., Ritchie, J. M., et al. (2014). A Poly-N-acetylglucosamine-Shiga toxin broad-spectrum conjugate vaccine for Shiga toxin-producing Escherichia coli. MBio 5, e00974–e00914. doi: 10.1128/mBio.00974-14
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Matar, G. M., and Rahal, E. (2003). Inhibition of the transcription of the Escherichia coli O157:H7 genes coding for shiga-like toxins and intimin, and its potential use in the treatment of human infection with the bacterium. Ann. Trop. Med. Parasitol. 97, 281–287. doi: 10.1179/000349803235002146
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McNeilly, T. N., Mitchell, M. C., Rosser, T., McAteer, S., Low, J. C., Smith, D. G., et al. (2010). Immunization of cattle with a combination of purified intimin-531, EspA and Tir significantly reduces shedding of Escherichia coli O157:H7 following oral challenge. Vaccine 28, 1422–1428. doi: 10.1016/j.vaccine.2009.10.076
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mejias, M. P., Cabrera, G., Fernandez-Brando, R. J., Baschkier, A., Ghersi, G., Abrey-Recalde, M. J., et al. (2014). Protection of mice against Shiga toxin 2 (Stx2)-associated damage by maternal immunization with a Brucella lumazine synthase-Stx2 B subunit chimera. Infect. Immun. 82, 1491–1499. doi: 10.1128/IAI.00027-14
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mellmann, A., Harmsen, D., Cummings, C. A., Zentz, E. B., Leopold, S. R., Rico, A., et al. (2011). Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS ONE 6:e22751. doi: 10.1371/journal.pone.0022751
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Melton-Celsa, A., Mohawk, K., Teel, L., and O'Brien, A. (2012). Pathogenesis of Shiga-toxin producing Escherichia coli. Curr. Top. Microbiol. Immunol. 357, 67–103. doi: 10.1007/82_2011_176
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Menne, J., Nitschke, M., Stingele, R., Abu-Tair, M., Beneke, J., Bramstedt, J., et al. (2012). Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study. BMJ 345:e4565. doi: 10.1136/bmj.e4565
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mogna, L., Del Piano, M., Deidda, F., Nicola, S., Soattini, L., Debiaggi, R., et al. (2012). Assessment of the in vitro inhibitory activity of specific probiotic bacteria against different Escherichia coli strains. J. Clin. Gastroenterol. 46(Suppl.) S29–S32. doi: 10.1097/MCG.0b013e31826852b7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mukhopadhyay, S., and Linstedt, A. D. (2012). Manganese blocks intracellular trafficking of Shiga toxin and protects against Shiga toxicosis. Science 335, 332–335. doi: 10.1126/science.1215930
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mulvey, G. L., Marcato, P., Kitov, P. I., Sadowska, J., Bundle, D. R., and Armstrong, G. D. (2003). Assessment in mice of the therapeutic potential of tailored, multivalent Shiga toxin carbohydrate ligands. J. Infect. Dis. 187, 640–649. doi: 10.1086/373996
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nassar, F. J., Rahal, E. A., Sabra, A., and Matar, G. M. (2013). Effects of subinhibitory concentrations of antimicrobial agents on Escherichia coli O157:H7 Shiga toxin release and role of the SOS response. Foodborne Pathog. Dis. 10, 805–812. doi: 10.1089/fpd.2013.1510
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nishikawa, K., Matsuoka, K., Kita, E., Okabe, N., Mizuguchi, M., Hino, K., et al. (2002). A therapeutic agent with oriented carbohydrates for treatment of infections by Shiga toxin-producing Escherichia coli O157:H7. Proc. Natl. Acad. Sci. U.S.A. 99, 7669–7674. doi: 10.1073/pnas.112058999
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nishikawa, K., Matsuoka, K., Watanabe, M., Igai, K., Hino, K., Hatano, K., et al. (2005). Identification of the optimal structure required for a Shiga toxin neutralizer with oriented carbohydrates to function in the circulation. J. Infect. Dis. 191, 2097–2105. doi: 10.1086/430388
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nitschke, M., Sayk, F., Hartel, C., Roseland, R. T., Hauswaldt, S., Steinhoff, J., et al. (2012). Association between azithromycin therapy and duration of bacterial shedding among patients with Shiga toxin-producing enteroaggregative Escherichia coli O104:H4. JAMA 307, 1046–1052. doi: 10.1001/jama.2012.264
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Niu, Y. D., Johnson, R. P., Xu, Y., McAllister, T. A., Sharma, R., Louie, M., et al. (2009). Host range and lytic capability of four bacteriophages against bovine and clinical human isolates of Shiga toxin-producing Escherichia coli O157:H7. J. Appl. Microbiol. 107, 646–656. doi: 10.1111/j.1365-2672.2009.04231.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Noel, R., Gupta, N., Pons, V., Goudet, A., Garcia-Castillo, M. D., Michau, A., et al. (2013). N-methyldihydroquinazolinone derivatives of Retro-2 with enhanced efficacy against Shiga toxin. J. Med. Chem. 56, 3404–3413. doi: 10.1021/jm4002346
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nogueira, M. C., Oyarzabal, O. A., and Gombas, D. E. (2003). Inactivation of Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella in cranberry, lemon, and lime juice concentrates. J. Food Prot. 66, 1637–1641.
Ogawa, M., Shimizu, K., Nomoto, K., Tanaka, R., Hamabata, T., Yamasaki, S., et al. (2001). Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157:H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 68, 135–140. doi: 10.1016/S0168-1605(01)00465-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Okubo, S., Sasaki, T., Hara, Y., Mori, F., and Shimamura, T. (1998). [Bactericidal and anti-toxin activities of catechin on enterohemorrhagic Escherichia coli]. Kansenshogaku Zasshi 72, 211–217. doi: 10.11150/kansenshogakuzasshi1970.72.211
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Paddock, Z. D., Renter, D. G., Cull, C. A., Shi, X., Bai, J., and Nagaraja, T. G. (2014). Escherichia coli O26 in feedlot cattle: fecal prevalence, isolation, characterization, and effects of an E. coli O157 vaccine and a direct-fed microbial. Foodborne Pathog. Dis. 11, 186–193. doi: 10.1089/fpd.2013.1659
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Paton, A. W., Morona, R., and Paton, J. C. (2000). A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat. Med. 6, 265–270. doi: 10.1038/73111
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Paton, A. W., Morona, R., and Paton, J. C. (2001). Neutralization of Shiga toxins Stx1, Stx2c, and Stx2e by recombinant bacteria expressing mimics of globotriose and globotetraose. Infect. Immun. 69, 1967–1970. doi: 10.1128/IAI.69.3.1967-1970.2001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Paton, A. W., Voss, E., Manning, P. A., and Paton, J. C. (1998). Antibodies to lipopolysaccharide block adherence of Shiga toxin-producing Escherichia coli to human intestinal epithelial (Henle 407) cells. Microb. Pathog. 24, 57–63. doi: 10.1006/mpat.1997.0172
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pellarin, M. G., Albrecht, C., Rojas, M. J., Aguilar, J. J., Konigheim, B. S., Paraje, M. G., et al. (2013). Inhibition of cytotoxicity of Shiga toxin of Escherichia coli O157:H7 on vero cells by Prosopis alba Griseb (Fabaceae) and Ziziphus mistol Griseb (Rhamnaceae) extracts. J. Food Prot. 76, 1733–1739. doi: 10.4315/0362-028X.JFP-13-087
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pittman, C. I., Geornaras, I., Woerner, D. R., Nightingale, K. K., Sofos, J. N., Goodridge, L., et al. (2012). Evaluation of lactic acid as an initial and secondary subprimal intervention for Escherichia coli O157:H7, non-O157 Shiga toxin-producing E. coli, and a nonpathogenic E. coli surrogate for E. coli O157:H7. J. Food Prot. 75, 1701–1708. doi: 10.4315/0362-028X.JFP-11-520
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Qadri, S. M., and Kayali, S. (1998). Enterohemorrhagic Escherichia coli. A dangerous food-borne pathogen. Postgrad. Med. 103, 179–180, 185–177.
Rahal, E. A., Kazzi, N., Kanbar, A., Abdelnoor, A. M., and Matar, G. M. (2011a). Role of rifampicin in limiting Escherichia coli O157:H7 Shiga-like toxin expression and enhancement of survival of infected BALB/c mice. Int. J. Antimicrob. Agents 37, 135–139. doi: 10.1016/j.ijantimicag.2010.10.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rahal, E. A., Kazzi, N., Sabra, A., Abdelnoor, A. M., and Matar, G. M. (2011b). Decrease in Shiga toxin expression using a minimal inhibitory concentration of rifampicin followed by bactericidal gentamicin treatment enhances survival of Escherichia coli O157:H7-infected BALB/c mice. Ann. Clin. Microbiol. Antimicrob. 10:34. doi: 10.1186/1476-0711-10-34
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Raya, R. R., Varey, P., Oot, R. A., Dyen, M. R., Callaway, T. R., Edrington, T. S., et al. (2006). Isolation and characterization of a new T-even bacteriophage, CEV1, and determination of its potential to reduce Escherichia coli O157:H7 levels in sheep. Appl. Environ. Microbiol. 72, 6405–6410. doi: 10.1128/AEM.03011-05
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reissbrodt, R., Hammes, W. P., Dal Bello, F., Prager, R., Fruth, A., Hantke, K., et al. (2009). Inhibition of growth of Shiga toxin-producing Escherichia coli by nonpathogenic Escherichia coli. FEMS Microbiol. Lett. 290, 62–69. doi: 10.1111/j.1574-6968.2008.01405.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ren, W., Yu, R., Liu, G., Li, N., Peng, Y., Wu, M., et al. (2013). DNA vaccine encoding the major virulence factors of Shiga toxin type 2e (Stx2e)-expressing Escherichia coli induces protection in mice. Vaccine 31, 367–372. doi: 10.1016/j.vaccine.2012.10.107
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rivas, L., Coffey, B., McAuliffe, O., McDonnell, M. J., Burgess, C. M., Coffey, A., et al. (2010). In vivo and ex vivo evaluations of bacteriophages e11/2 and e4/1c for use in the control of Escherichia coli O157:H7. Appl. Environ. Microbiol. 76, 7210–7216. doi: 10.1128/AEM.01530-10
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rojas, R. L., Gomes, P. A., Bentancor, L. V., Sbrogio-Almeida, M. E., Costa, S. O., Massis, L. M., et al. (2010). Salmonella enterica serovar Typhimurium vaccine strains expressing a nontoxic Shiga-like toxin 2 derivative induce partial protective immunity to the toxin expressed by enterohemorrhagic Escherichia coli. Clin. Vaccine Immunol. 17, 529–536. doi: 10.1128/CVI.00495-09
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rossi, L., Di Giancamillo, A., Reggi, S., Domeneghini, C., Baldi, A., Sala, V., et al. (2013). Expression of verocytotoxic Escherichia coli antigens in tobacco seeds and evaluation of gut immunity after oral administration in mouse model. J. Vet. Sci. 14, 263–270. doi: 10.4142/jvs.2013.14.3.263
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rund, S. A., Rohde, H., Sonnenborn, U., and Oelschlaeger, T. A. (2013). Antagonistic effects of probiotic Escherichia coli Nissle 1917 on EHEC strains of serotype O104:H4 and O157:H7. Int. J. Med. Microbiol. 303, 1–8. doi: 10.1016/j.ijmm.2012.11.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Safdar, N., Said, A., Gangnon, R. E., and Maki, D. G. (2002). Risk of hemolytic uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 enteritis: a meta-analysis. JAMA 288, 996–1001. doi: 10.1001/jama.288.8.996
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sargeant, J. M., Amezcua, M. R., Rajic, A., and Waddell, L. (2007). Pre-harvest interventions to reduce the shedding of E. coli O157 in the faeces of weaned domestic ruminants: a systematic review. Zoonoses Public Health 54, 260–277. doi: 10.1111/j.1863-2378.2007.01059.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sato, T., Matsui, T., Takita, E., Kadoyama, Y., Makino, S., Kato, K., et al. (2013). Evaluation of recombinant forms of the shiga toxin variant Stx2eB subunit and non-toxic mutant Stx2e as vaccine candidates against porcine edema disease. J. Vet. Med. Sci. 75, 1309–1315. doi: 10.1292/jvms.13-0118
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sato, T., Shimizu, T., Watarai, M., Kobayashi, M., Kano, S., Hamabata, T., et al. (2003). Genome analysis of a novel Shiga toxin 1 (Stx1)-converting phage which is closely related to Stx2-converting phages but not to other Stx1-converting phages. J. Bacteriol. 185, 3966–3971. doi: 10.1128/JB.185.13.3966-3971.2003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M. A., Roy, S. L., et al. (2011). Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17, 7–15. doi: 10.3201/eid1701.P11101
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Seita, T., Kuribayashi, T., Honjo, T., and Yamamoto, S. (2013). Comparison of efficacies of bovine immune colostral antibody and each immunoglobulin class against verotoxin 2, flagellum and somatic cells of Escherichia coli O157:H7 in mice. J. Microbiol. Immunol. Infect. 46, 73–79. doi: 10.1016/j.jmii.2012.01.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sheng, H., Knecht, H. J., Kudva, I. T., and Hovde, C. J. (2006). Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants. Appl. Environ. Microbiol. 72, 5359–5366. doi: 10.1128/AEM.00099-06
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shimizu, T., Ohta, Y., and Noda, M. (2009). Shiga toxin 2 is specifically released from bacterial cells by two different mechanisms. Infect. Immun. 77, 2813–2823. doi: 10.1128/IAI.00060-09
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sillankorva, S. M., Oliveira, H., and Azeredo, J. (2012). Bacteriophages and their role in food safety. Int. J. Microbiol. 2012:863945. doi: 10.1155/2012/863945
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stanford, K., Bach, S., Baah, J., and McAllister, T. (2014). A mixture of Lactobacillus casei, Lactobacillus lactis, and Paenibacillus polymyxa reduces Escherichia coli O157:H7 in finishing feedlot cattle. J. Food Prot. 77, 738–744. doi: 10.4315/0362-028X.JFP-13-433
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stanford, K., McAllister, T. A., Niu, Y. D., Stephens, T. P., Mazzocco, A., Waddell, T. E., et al. (2010). Oral delivery systems for encapsulated bacteriophages targeted at Escherichia coli O157:H7 in feedlot cattle. J. Food Prot. 73, 1304–1312.
Stearns-Kurosawa, D. J., Collins, V., Freeman, S., Debord, D., Nishikawa, K., Oh, S. Y., et al. (2011). Rescue from lethal Shiga toxin 2-induced renal failure with a cell-permeable peptide. Pediatr. Nephrol. 26, 2031–2039. doi: 10.1007/s00467-011-1913-y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stechmann, B., Bai, S. K., Gobbo, E., Lopez, R., Merer, G., Pinchard, S., et al. (2010). Inhibition of retrograde transport protects mice from lethal ricin challenge. Cell 141, 231–242. doi: 10.1016/j.cell.2010.01.043
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Strockbine, N. A., Marques, L. R., Newland, J. W., Smith, H. W., Holmes, R. K., and O'Brien, A. D. (1986). Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 53, 135–140.
Takahashi, M., Taguchi, H., Yamaguchi, H., Osaki, T., Komatsu, A., and Kamiya, S. (2004). The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunol. Med. Microbiol. 41, 219–226. doi: 10.1016/j.femsim.2004.03.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Takahashi, T., Taguchi, H., Yamaguchi, H., Osaki, T., Sato, S., Kamei, M., et al. (1999). [Antibacterial effects of cacao mass on enterohemorrhagic Escherichia coli O157:H7]. Kansenshogaku Zasshi 73, 694–701. doi: 10.11150/kansenshogakuzasshi1970.73.694
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Takemasa, N., Ohnishi, S., Tsuji, M., Shikata, T., and Yokoigawa, K. (2009). Screening and analysis of spices with ability to suppress verocytotoxin production by Escherichia coli O157. J. Food Sci. 74, M461–M466. doi: 10.1111/j.1750-3841.2009.01326.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thorpe, C. M. (2004). Shiga toxin-producing Escherichia coli infection. Clin. Infect. Dis. 38, 1298–1303. doi: 10.1086/383473
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tiels, P., Verdonck, F., Coddens, A., Goddeeris, B., and Cox, E. (2008). The excretion of F18+ E. coli is reduced after oral immunisation of pigs with a FedF and F4 fimbriae conjugate. Vaccine 26, 2154–2163. doi: 10.1016/j.vaccine.2008.01.054
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tomita, T., Sato, N., Arai, T., Shiraishi, H., Sato, M., Takeuchi, M., et al. (1997). Bactericidal activity of a fermented hot-water extract from Stevia rebaudiana Bertoni towards enterohemorrhagic Escherichia coli O157:H7 and other food-borne pathogenic bacteria. Microbiol. Immunol. 41, 1005–1009. doi: 10.1111/j.1348-0421.1997.tb01961.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Trachtman, H., Cnaan, A., Christen, E., Gibbs, K., Zhao, S., Acheson, D. W., et al. (2003). Effect of an oral Shiga toxin-binding agent on diarrhea-associated hemolytic uremic syndrome in children: a randomized controlled trial. JAMA 290, 1337–1344. doi: 10.1001/jama.290.10.1337
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Voravuthikunchai, S. P., Suwalak, S., and Mitranan, W. (2012). Ellagitannin from Quercus infectoria eradicates intestinal colonization and prevents renal injuries in mice infected with Escherichia coli O157: H7. J. Med. Microbiol. 61, 1366–1372. doi: 10.1099/jmm.0.044495-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wan, C. S., Zhou, Y., Yu, Y., Peng, L. J., Zhao, W., and Zheng, X. L. (2011). B-cell epitope KT-12 of enterohemorrhagic Escherichia coli O157:H7: a novel peptide vaccine candidate. Microbiol. Immunol. 55, 247–253. doi: 10.1111/j.1348-0421.2011.00316.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, F., Yang, Q., Kase, J. A., Meng, J., Clotilde, L. M., Lin, A., et al. (2013). Current trends in detecting non-O157 Shiga toxin-producing Escherichia coli in food. Foodborne Pathog. Dis. 10, 665–677. doi: 10.1089/fpd.2012.1448
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Watanabe, M., Matsuoka, K., Kita, E., Igai, K., Higashi, N., Miyagawa, A., et al. (2004). Oral therapeutic agents with highly clustered globotriose for treatment of Shiga toxigenic Escherichia coli infections. J. Infect. Dis. 189, 360–368. doi: 10.1086/381124
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Watanabe-Takahashi, M., Sato, T., Dohi, T., Noguchi, N., Kano, F., Murata, M., et al. (2010). An orally applicable Shiga toxin neutralizer functions in the intestine to inhibit the intracellular transport of the toxin. Infect. Immun. 78, 177–183. doi: 10.1128/IAI.01022-09
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wen, S. X., Teel, L. D., Judge, N. A., and O'Brien, A. D. (2006). A plant-based oral vaccine to protect against systemic intoxication by Shiga toxin type 2. Proc. Natl. Acad. Sci. U.S.A. 103, 7082–7087. doi: 10.1073/pnas.0510843103
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
WHO. (2011). Outbreaks of E. coli O104:H4 Infection: Update 30. [Online]. Available online at: http://www.euro.who.int/en/what-we-do/health-topics/emergencies/international-health-regulations/news/news/2011/07/outbreaks-of-e.-coli-o104h4-infection-update-30 (Accessed November 26, 2014).
Wieler, L. H., Semmler, T., Eichhorn, I., Antao, E. M., Kinnemann, B., Geue, L., et al. (2011). No evidence of the Shiga toxin-producing E. coli O104:H4 outbreak strain or enteroaggregative E. coli (EAEC) found in cattle faeces in northern Germany, the hotspot of the 2011 HUS outbreak area. Gut Pathog. 3:17. doi: 10.1186/1757-4749-3-17
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yoh, M., Frimpong, E. K., and Honda, T. (1997). Effect of antimicrobial agents, especially fosfomycin, on the production and release of Vero toxin by enterohaemorrhagic Escherichia coli O157:H7. FEMS Immunol. Med. Microbiol. 19, 57–64. doi: 10.1111/j.1574-695X.1997.tb01072.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, X. H., He, K. W., Zhao, P. D., Ye, Q., Luan, X. T., Yu, Z. Y., et al. (2012). Intranasal immunisation with Stx2B-Tir-Stx1B-Zot protein leads to decreased shedding in goats after challenge with Escherichia coli O157:H7. Vet. Rec. 170, 178. doi: 10.1136/vr.100325
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: Shiga toxin-producing Escherichia coli, hemorrhagic colitis, hemolytic uremic syndrome, antimicrobial agents, Shiga toxin 1, Shiga toxin 2
Citation: Rahal EA, Fadlallah SM, Nassar FJ, Kazzi N and Matar GM (2015) Approaches to treatment of emerging Shiga toxin-producing Escherichia coli infections highlighting the O104:H4 serotype. Front. Cell. Infect. Microbiol. 5:24. doi: 10.3389/fcimb.2015.00024
Received: 19 February 2015; Accepted: 04 March 2015;
Published: 18 March 2015.
Edited by:
Robert Heinzen, National Institutes of Health, USAReviewed by:
Dan Drecktrah, University of Montana, USAJeff Shannon, National Institutes of Health, USA
Copyright © 2015 Rahal, Fadlallah, Nassar, Kazzi and Matar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elias A. Rahal, Department of Experimental Pathology, Immunology and Microbiology, American University of Beirut, PO Box 11-0236/ Riad El-Solh/Beirut 1107 2020, Lebanon er00@aub.edu.lb
†These authors have contributed equally to this work.
 Elias A. Rahal
Elias A. Rahal Sukayna M. Fadlallah
Sukayna M. Fadlallah Farah J. Nassar
Farah J. Nassar  Ghassan M. Matar
Ghassan M. Matar