Potential Biological Roles of Exosomal Long Non-Coding RNAs in Gastrointestinal Cancer
- 1Department of Pathology, Xiangya Changde Hospital, Changde, China
- 2Department of Medicine, Xizang Minzu University, Xianyang, China
- 3Department of Emergency, Xiangya Hospital, Central South University, Changsha, China
- 4National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
- 5Department of Emergency, Xiangya Changde Hospital, Changde, China
Exosomes, a type of extracellular vesicles (EVs), are secreted by almost all cells and contain many cellular constituents, such as nucleic acids, lipids, and metabolites. In addition, they play a crucial role in intercellular communication and have been proved to be involved in the development and treatment of gastrointestinal cancer. It has been confirmed that long non-coding RNAs (lncRNAs) exert a range of biological functions, such as cell metastasis, tumorigenesis, and therapeutic responses. This review mainly focused on the emerging roles and underlying molecular mechanisms of exosome-derived lncRNAs in gastrointestinal cancer in recent years. The biological roles of exosomal lncRNAs in the pathogenesis and therapeutic responses of gastrointestinal cancers were also investigated.
Introduction
Gastrointestinal cancer has a high incidence worldwide (Dekker et al., 2019; Global Burden of Disease Cancer et al., 2019) and is mainly treated with surgery and chemotherapy (Ajani et al., 2016; Benson et al., 2018; Benson et al., 2020). Due to the inconspicuous early clinical symptoms, gastrointestinal cancer is usually diagnosed at an advanced stage, resulting in high recurrence and mortality rates. Exosomes can transfer long non-coding RNAs (lncRNAs) to recipient cells, suggesting that they can affect biological functions such as regulating the occurrence and progression of gastrointestinal cancer (Li et al., 2019a; Kalluri and LeBleu, 2020). The studies of exosome-derived lncRNAs can help us to further elucidate the underlying molecular mechanisms of cancer progression and provide potential biomarkers for early diagnosis and targeted therapies for gastrointestinal cancer patients.
Gastrointestinal Cancer
Gastric cancer (GC) is the fifth most common cancer and the third most deadly cancer worldwide (Smyth et al., 2020). Colorectal cancer (CRC) is the fourth leading cause of cancer death in the world (Deng et al., 2021; Lu et al., 2021). Gastrointestinal cancer is usually treated with surgery, chemotherapy, targeted therapies, and so on. Patients receiving early diagnosis and treatment for gastrointestinal cancer have better prognosis than those diagnosed at an advanced stage (Luo and Li, 2019). Existing diagnostic methods almost exclusively rely on invasive procedures such as digestive endoscopy and pathological biopsy, which are difficult to be widely used for screening. Therefore, it is of great significance to investigate new biomarkers for early diagnosis and targeted therapies.
Exosomes
Exosomes are small, single-membrane, secreted organelles that contain selected proteins, nucleic acids, lipids, glycoconjugates metabolites, and so on (Kumar et al., 2019; Thakur et al., 2021; Thakur et al., 2020; Qiu et al., 2019), ranging from 50 to 150 nm in diameter (∼100 nM, medially) (Phan et al., 2018; Thakur et al., 2022). Exosomes play essential roles in intercellular communication. Additionally, exosome-associated nucleic acids, proteins, and metabolites can alter the functional consequence in recipient cells through autocrine and paracrine signaling (Figure 1), thus participating in the cancer progression and treatment (Zhang and Yu, 2019).
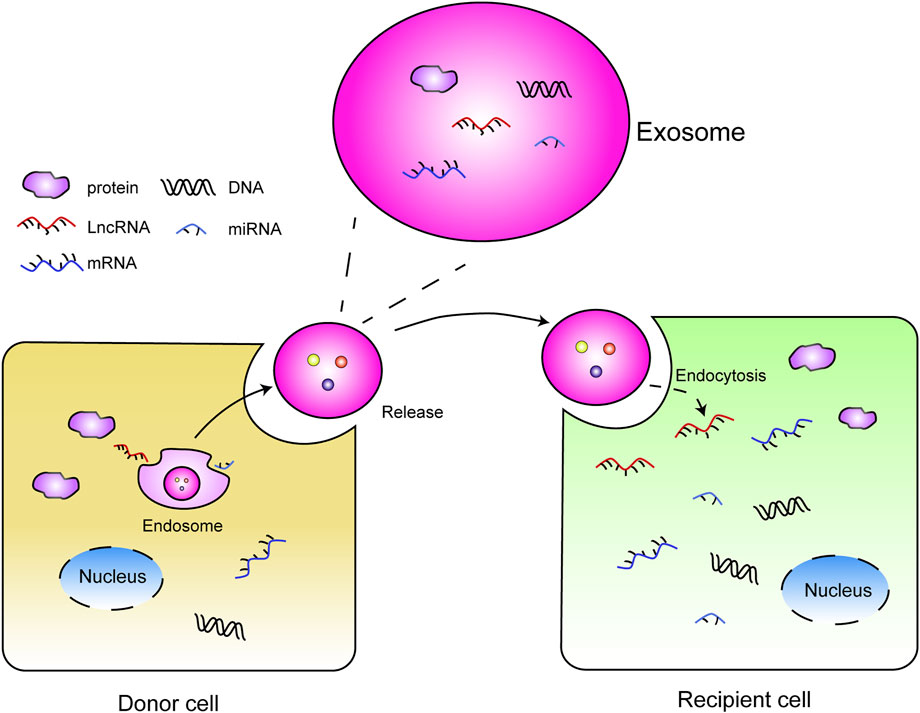
FIGURE 1. Intercellular communication: donor cells release the exosomes with contents (DNAs, RNAs, protein) that are received by recipient cells via endocytosis, and the cargoes contained in exosome exert function in recipient cells.
lncRNAs
The majority of expressed transcripts do not encode proteins, and the transcripts (>200 nt) in length are broadly classified as lncRNAs, once dismissed as “junk” RNA (Yan et al., 2017; Yan et al., 2019; Kong et al., 2021; Wei et al., 2021; Yue et al., 2021). Nowadays, the latest studies have demonstrated that lncRNAs can modulate gene expression by mediating translational inhibition or functioning as competitive endogenous RNAs (ceRNAs) (Iyer et al., 2015; Jacob et al., 2017; Richtig et al., 2017; Rinn and Chang, 2020). It has been confirmed that lncRNAs play critical roles in cancer progression and metastasis (Wang et al., 2021a; Ghafouri-Fard et al., 2021). Recently, exosomal lncRNAs have been reported to regulate multiple biological processes of cancers, such as apoptosis, proliferation, migration, and angiogenesis (Sun et al., 2018; Li et al., 2019b; Chen et al., 2019; Cheng et al., 2020; Kok and Yu, 2020; Behera et al., 2021). For instance, exosomal lncRNA LNMAT2 can promote lymphangiogenesis and lymph node metastasis in bladder cancers (Chen et al., 2019). In another study, exosomal FMR1-AS1 can activate TLR7-NFκB signaling pathway to induce and promote the progression of esophageal squamous cell carcinoma (Li et al., 2019b). These exosomal-derived lncRNAs have been proved to be potential biomarkers for the diagnosis and prognosis of various cancers, including gastrointestinal cancers (Wang et al., 2018; Guo et al., 2020).
Biological Roles of Exosomal lncRNAs in Gastrointestinal Cancer
LncRNAs have been proved to be significantly correlated with the occurrence and progression of gastrointestinal neoplasms. Exosomes are small-walled nanovesicles secreted by a variety of cells and involved in the progression of gastrointestinal cancer, such as proliferation, metastasis, and drug resistance by transferring lncRNAs (Table 1). Moreover, emerging evidence has showed the underlying molecular mechanisms of the exosomal lncRNAs in the biological processes of malignant cells.
Roles of Exosomal lncRNAs in Cell Proliferation
The abundantly expressed lncRNA H19 has been found in many human cancers. In CRC, the expression level of H19 of carcinoma-associated fibroblasts (CAFs) was significantly higher than that of normal fibroblasts (NFs). Ren and his colleague found that the CAF-derived exosomes can transfer lncRNA H19 to neighboring cells and activate the Wnt/β-catenin signaling pathway in CRC cells, thus facilitating the tumorigenesis and cell proliferation (Ren et al., 2018). In both tissue and plasma exosomes of CRC patients, the expression level of lncRNA-UCA1 increases. Mechanistically, UCA1 can regulate the expression of MYO6 via miRNA-143 sponge as a ceRNA. Researchers observed that after treatment with exosomal UCA1 from CRC patients, the expression of miR-143 decreases but MYO6 expression increases, thus promoting CRC cell proliferation (Luan et al., 2020). lnc HEIH can be encapsulated by exosomes and then transferred into natural gastric cells to stimulate the expression of EZH2, causing high methylation of the GSDME promoter and promotion of tumorigenesis (Lu et al., 2020). LINC01559 can enhance the proliferation, migration, and stemness characteristics of GC cells. Wang et al. found that the expression of LINC01559 is upregulated in mesenchymal stem cells (MSCs) compared with that in GC cells, and then exosomes from MSCs can transfer LINC01559 into GC cells to promote the cell progression by activating the PI3K/AKT signaling pathway (Wang et al., 2020). lncRNA ZFAS1 was proved to be involved in cell cycle regulation. The exosomes can promote GC cell proliferation through the transfer of ZFAS1 (Pan et al., 2017). Zhang et al. found that GC cells treated with the exosomes containing FOXM1-related lncRNA (FRLnc1) can enhance GC cell proliferation and migration. The FRLnc1 knockdown in GC cells can induce cell cycle arrest and cell apoptosis (Zhang et al., 2021). In addition, some studies have shown that the exosomal lncRNAs derived from cancer cells can regulate the cancer immune microenvironment, such as immunosuppressants and immune escape, to promote cancer progression. Wang et al. observed that lncRNA RP11-323N12.5 secreted by GC cells is associated with Treg cell–induced immunosuppression. RP11-323N12.5 can be transferred into T cells by exosome delivery and then enhance YAP1 transcription in T cells, thus leading to promotion of GC cells (Wang et al., 2021b). Exosomal lncRNA SNHG10 derived from CRC cells can regulate NK cell function by upregulating INHBC expression. It can significantly downregulate the release of perforin-1 and granzyme B to inhibit NK cell growth and then promote CRC cell growth (Huang et al., 2021). Xian’s group found that lncRNA KCNQ1OT1 derived from CRC cells can promote CRC progression. Mechanistically, exosomes can transfer KCNQ1OT1 via autocrine to mediate the miR-30a-5p/USP22 pathway, then regulate the ubiquitination of PD-L1 and inhibit CD8+ T-cell responses (Xian et al., 2021). Taken together, these results suggested that dysregulated exosomal lncRNAs may be meaningful biomarkers for cancer cell proliferation.
Roles of Exosomal lncRNAs in Cell Metastasis
It has been reported that exosomal lncRNA CRNDE-h levels are significantly correlated with lymph node metastasis and distant metastasis in the CRC (Liu et al., 2016). Early studies have demonstrated that the level of T-helper 17 cells is closely related to regional lymph node metastasis in CRC (Lee et al., 2017). CRC-derived exosomes can promote Th17 cell differentiation by transmitting CRNDE-h, and then promote lymph node metastasis (Sun et al., 2021). lncRNA RPPH1 can bind to TUBB3 to prevent its ubiquitination and degradation and induce EMT and cell metastasis of CRC cells. In addition, RPPH1 can mediate macrophage M2 polarization by being transferred to exosomes-bearing macrophages to promote CRC cell metastasis (Liang et al., 2019). In another study, macrophage M2 polarization was observed in BRAFV600E mutation of CRC, resulting in more angiogenesis and lymphangiogenesis in the microenvironment. Zhi et al. believed that this phenomenon may be related to the abundance of some lncRNAs in exosomes (Zhi et al., 2021). As a vital mediator of APC, lncRNA-APC1 directly regulates the stability of Rab5b mRNA, hence reducing the exosome secretion of CRC cells. Exosomes derived from lncRNA-APC1–silenced CRC cells can activate the MAPK pathway and then enhance actin refactoring and angiogenesis, thereby accelerating cell metastasis (Wang et al., 2019a). Exosomal lncRNA MALAT1 can function as a ceRNA via miR-26a/26b sponge and then enhance phosphorylation in PI3K/Akt/mTOR pathway and FUT4-associated fucosylation, involved in CRC cell metastasis (Xu et al., 2020a). Piao et al. found that the PCGEM1 expression was dramatically higher in hypoxia-cultured GC cells (HGC) than in normoxic-cultured cells (NGC). Moreover, PCGEM1 can be transferred from HGC cells to NGC cells by being packaged into exosomes, enhancing invasion and metastasis of NGC cells (Piao et al., 2021). Therefore, investigating the roles of exosomal lncRNA in the cellular metastasis can provide a promising strategy for targeted anti-metastatic therapies in gastrointestinal cancer.
Roles of Exosomal lncRNAs in Cell Chemoresistance
In addition to regulating cell proliferation, exosomal lncRNA UCA1 also mediates chemoresistance in CRC. UCA1 expression in cetuximab-resistant cells is significantly higher than that in cetuximab-sensitive cells. Further studies showed that recipient cells can obtain greater cetuximab resistance via exosomal transmission of UCA1 from cetuximab-resistant CRC cells (Yang et al., 2018). Exosomal lncRNA HOTTIP promotes cisplatin resistance by activating HMGA1. Mechanistically, exosomal HOTTIP can sponge miR-218 to mediate HMGA1 expression (Wang et al., 2019b). lncRNA CCAL can reduce the sensitivity of oxaliplatin (Oxa) and 5-FU, and CAF-derived exosomes can transfer CCAL to CRC cells, thus promoting Oxa resistance of CRC cells (Deng et al., 2020). In in vitro experiments, the expression level of lncRNA CRNDE was overexpressed in M2-polarized macrophage-derived exosomes (M2-exo) and it was encapsulated into exosomes to be transferred from M2 macrophages to GC cells. Studies showed that after GC cells are treated with M2-exo of silenced CRNDE, their cisplatin sensitivity was significantly enhanced (Xin et al., 2021). Collectively, exosomal lncRNA may help clarify the underlying molecular mechanisms of therapeutic resistance in gastrointestinal cancer and provide promising therapeutic strategies.
Clinical Application of Exosomal lncRNAs in Gastrointestinal Cancer
Exosomal lncRNAs from serum, plasma, and other body fluids are stable due to the particularity of their molecular structures, serving as ideal biomarkers and therapeutic targets for gastrointestinal cancer patients (Table 2).
Exosomal lncRNAs as Diagnostic and Prognostic Biomarkers
Hu’s group found that a group of six exosomal lncRNAs (LNCV_108266, LNCV6_84003, LNCV6_116109, LNCV6_98390, LNCV6_38772, and LNCV6_98602) are significantly overexpressed in the plasma of CRC patients, and they may serve as a promising non-invasion biomarker for diagnosis of CRC (Hu et al., 2018). The low/mediate expression of exosomal-derived lncRNA HOTTIP has been found to be significantly associated with poor overall survival. Oehme et al. found that patients with low/mediate expression of HOTTIP in primary CRC tissue may have a poor prognosis (Oehme et al., 2019). It was also found that with the increase in expression levels of exosomal HOPPIT, the depth of tumor invasion and TNM stages also increased in GC patients, indicating that exosomal HOPPIT may serve as a potential biomarker for the diagnosis and prognosis of GC (Zhao et al., 2018). The expression of serum exosomal lncRNA ADAMTS9-AS1 in CRC patients is significantly downregulated compared with that in healthy controls, suggesting that the exosomal ADAMTS9-AS1 may be a novel biomarker for the diagnosis of CRC (Li et al., 2020a). In GC patients, the expression of serum exosomal lnc-GNAQ-6:1 is reduced, but more studies are needed to determine whether it can be used as a new diagnostic biomarker for GC (Li et al., 2020b). In stage I GC patients, plasma exosomal lncRNA lncUEGC1 exhibits high diagnostic value compared with plasma exosomal lncUEGC2 and serum CEA, which may serve as a primary diagnostic biomarker for GC (Lin et al., 2018). The expression of the serum exosomal lncRNA PCSK2-2:1 is significantly downregulated in GC patients compared with that in healthy controls and is associated with tumor size, tumor stage, and venous invasion, suggesting that exosomal RNA PCSK2-2:1 may be a new prospective biomarker for GC diagnosis (Cai et al., 2019). Guo and his colleagues found that the expression levels of exosomal lncRNA-GC1 are closely associated with tumor burden, and they considerably accelerate from early to advanced stages with the progression of GC, showing that the expression levels of serum exosomal lncRNA-GC1 can serve as a potential early diagnostic biomarker and monitor the progression of GC (Guo et al., 2020). In the study of Piao’s group, the ROC curve and AUC value of plasma exosomal lncRNA CEBPA-AS1 are significantly higher than those of traditional markers with better sensitivity and specificity, suggesting that CEBPA-AS1 may be used as a novel diagnostic biomarker for GC (Piao et al., 2020). Xu et al. found that the high expression levels of serum exosomal lncRNA MIAT were significantly correlated with differentiation, lymphatic metastasis, and TNM stages of GC patients. In addition, in the serum of treated GC patients, the expression levels of exosomal MIAT were significantly reduced, indicating that the serum exosomal lncRNA MIAT may be a potential biomarker for monitoring GC progression (Xu et al., 2020b). In recent studies, the expression levels of exosomal lncRNA SLC2A12-10:1 were found to be dramatically associated with size, differentiation, TNM stages, and lymph node metastasis of GC tumors. The aberrantly expressed exosomal SLC2A12-10:1 may have a great potential to be a new biomarker for cancer diagnosis and prognosis (Zheng et al., 2020). Zhou and his colleagues found that the AUC curve of exosomal lncRNA H19 is much higher than that of any other traditional biomarker in GC, which may serve as an appropriate diagnostic marker for GC (Zhou et al., 2020). In general, exosomal lncRNAs show enormous potential to become ideal biomarkers for diagnosis and prognosis of gastrointestinal cancers.
Exosomal lncRNAs as Therapeutic Targets
CAF-derived exosomal lncRNA LINC00659 can downregulate miR-342-3p and increase ANXA2 expression, which accelerates EMT and the progression of CRC cells (Zhou et al., 2021), and it may be targeted as a novel strategy for CRC treatment. Liu et al. found that the expression of lncRNA GAS5 in CRC patients is significantly downregulated, but miR-221 increases both in tissue, plasma exosomes, suggesting that the overexpression of lncRNA GAS5 may restrain the expression of miR22 (Liu et al., 2018). It deserves further study whether cancer growth can be inhibited by exosome-transferred GAS5. The research into the mechanism of exosomal lncRNAs in gastrointestinal cancer progression may have great significance for targeted therapies.
Conclusion
The burden of gastrointestinal cancer is increasing worldwide. How to make an early diagnosis of gastrointestinal cancers and provide early treatment for them is a great challenge. Finding novel treatment methods or biomarkers may be a prospective strategy. Exosomes play a vital role in intercellular communication by releasing a wide variety of biological molecules, such as miRNAs, lncRNAs, proteins, and their complexes (Thakur et al., 2022). In recent years, the studies of lncRNAs have shown that lncRNAs play a crucial role in occurrence and progression of cancers. Since the structure of exosomes can protect lncRNAs from degradation, exosomal lncRNAs display great potential to become emerging non-invasion biomarkers for cancer diagnosis, prognosis, and treatment. Recent studies have also showed that exosomal lncRNAs have better sensitivity and specificity than traditional markers. However, the detailed mechanism and biological functions of most exosomal lncRNAs remain unclear. In addition, we found that some exosomal lncRNAs are similarly expressed in different cancers, which will bring challenges to the clinical application of exosomal lncRNAs. It may become a significant research direction to find more specific exosomal lncRNAs and further study their underlying molecular mechanisms, aiming to assist in diagnosis and serve as targets for targeted therapies.
Author Contributions
Conception and design: FK, FJ, LO, SW, CF, YL, and ZL. Writing, review, and/or revision of the manuscript: XW and QH. Administrative, technical, or material support: YT and XC.
Funding
This work was supported by the Key Scientific Research Project of Xizang Minzu University and Xizang Autonomous Region (Grant Nos: 20 MDT02, XZ202101ZR0074G), the Scientific Research Program Funded by Shaanxi Provincial Education Department (Grant No: 18JS031), the Scientific Research Project of Shaanxi provincial Administration of Traditional Chinese Medicine (Grant Nos: 15-SCJH001, JCPT001), and the Natural Science Basic Research Plan in Shaanxi Province of China (Grant Nos: 2016JM8023, 2020JM590).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ajani, J. A., D'Amico, T. A., Almhanna, K., Bentrem, D. J., Chao, J., Das, P., et al. (2016). Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 14 (10), 1286–1312. Epub 2016/10/05PubMed PMID: 27697982. doi:10.6004/jnccn.2016.0137
Behera, J., Kumar, A., Voor, M. J., and Tyagi, N. (2021). Exosomal lncRNA-H19 Promotes Osteogenesis and Angiogenesis through Mediating Angpt1/Tie2-NO Signaling in CBS-Heterozygous Mice. Theranostics 11 (16), 7715–7734. Epub 2021/08/03PubMed PMID: 34335960; PubMed Central PMCID: PMCPMC8315071. doi:10.7150/thno.58410
Benson, A. B., Venook, A. P., Al-Hawary, M. M., Arain, M. A., Chen, Y.-J., Ciombor, K. K., et al. (2020). NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J. Natl. Compr. Canc Netw. 18 (7), 806–815. Epub 2020/07/08PubMed PMID: 32634771. doi:10.6004/jnccn.2020.0032
Benson, A. B., Venook, A. P., Al-Hawary, M. M., Cederquist, L., Chen, Y.-J., Ciombor, K. K., et al. (2018). NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J. Natl. Compr. Canc Netw. 16 (4), 359–369. Epub 2018/04/11PubMed PMID: 29632055. doi:10.6004/jnccn.2018.0021
Cai, C., Zhang, H., Zhu, Y., Zheng, P., Xu, Y., Sun, J., et al. (2019). Serum Exosomal Long Noncoding RNA Pcsk2-2:1 as A Potential Novel Diagnostic Biomarker for Gastric Cancer. Ott 12, 10035–10041. Epub 2019/12/11PubMed PMID: 31819499; PubMed Central PMCID: PMCPMC6883939. doi:10.2147/OTT.S229033
Chen, C., Luo, Y., He, W., Zhao, Y., Kong, Y., Liu, H., et al. (2019). Exosomal Long Noncoding RNA LNMAT2 Promotes Lymphatic Metastasis in Bladder Cancer. J. Clin. Invest. 130 (1), 404–421. Epub 2019/10/09PubMed PMID: 31593555; PubMed Central PMCID: PMCPMC6934220. doi:10.1172/jci130892
Cheng, J., Meng, J., Zhu, L., and Peng, Y. (2020). Exosomal Noncoding RNAs in Glioma: Biological Functions and Potential Clinical Applications. Mol. Cancer 19 (1), 66. Epub 2020/03/28PubMed PMID: 32213181; PubMed Central PMCID: PMCPMC7098115. doi:10.1186/s12943-020-01189-3
Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M., and Wallace, M. B. (2019). Colorectal Cancer. The Lancet 394 (10207), 1467–1480. Epub 2019/10/22PubMed PMID: 31631858. doi:10.1016/s0140-6736(19)32319-0
Deng, D., Luo, X., Zhang, S., and Xu, Z. (2021). Immune Cell Infiltration-Associated Signature in colon Cancer and its Prognostic Implications. Aging 13 (15), 19696–19709. PubMed PMID: 34349038; PubMed Central PMCID: PMC8386549. doi:10.18632/aging.203380
Deng, X., Ruan, H., Zhang, X., Xu, X., Zhu, Y., Peng, H., et al. (2020). Long Noncoding RNA CCAL Transferred from Fibroblasts by Exosomes Promotes Chemoresistance of Colorectal Cancer Cells. Int. J. Cancer 146 (6), 1700–1716. Epub 2019/08/06PubMed PMID: 31381140. doi:10.1002/ijc.32608
Ghafouri-Fard, S., Hajiesmaeili, M., Shoorei, H., Bahroudi, Z., Taheri, M., and Sharifi, G. (2021). The Impact of lncRNAs and miRNAs in Regulation of Function of Cancer Stem Cells and Progression of Cancer. Front. Cell Dev. Biol. 9, 696820. PubMed PMID: 34368145; PubMed Central PMCID: PMC8339916. doi:10.3389/fcell.2021.696820
Global Burden of Disease Cancer, C., Fitzmaurice, C., Abate, D., Abbasi, N., Abbastabar, H., Abd-Allah, F., et al. (2019). Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 5 (12), 1749–1768. Epub 2019/09/29PubMed PMID: 31560378; PubMed Central PMCID: PMCPMC6777271. doi:10.1001/jamaoncol.2019.2996
Guo, X., Lv, X., Ru, Y., Zhou, F., Wang, N., Xi, H., et al. (2020). Circulating Exosomal Gastric Cancer-Associated Long Noncoding RNA1 as a Biomarker for Early Detection and Monitoring Progression of Gastric Cancer. JAMA Surg. 155 (7), 572–579. Epub 2020/06/11PubMed PMID: 32520332; PubMed Central PMCID: PMCPMC7287948. doi:10.1001/jamasurg.2020.1133
Hu, D., Zhan, Y., Zhu, K., Bai, M., Han, J., Si, Y., et al. (2018). Plasma Exosomal Long Non-coding RNAs Serve as Biomarkers for Early Detection of Colorectal Cancer. Cell Physiol Biochem 51 (6), 2704–2715. Epub 2018/12/19PubMed PMID: 30562751. doi:10.1159/000495961
Huang, Y., Luo, Y., Ou, W., Wang, Y., Dong, D., Peng, X., et al. (2021). Exosomal lncRNA SNHG10 Derived from Colorectal Cancer Cells Suppresses Natural Killer Cell Cytotoxicity by Upregulating INHBC. Cancer Cell Int 21 (1), 528. Epub 2021/10/14PubMed PMID: 34641864; PubMed Central PMCID: PMCPMC8507338. doi:10.1186/s12935-021-02221-2
Iyer, M. K., Niknafs, Y. S., Malik, R., Singhal, U., Sahu, A., Hosono, Y., et al. (2015). The Landscape of Long Noncoding RNAs in the Human Transcriptome. Nat. Genet. 47 (3), 199–208. PubMed PMID: 25599403. doi:10.1038/ng.3192
Jacob, R., Zander, S., and Gutschner, T. (2017). The Dark Side of the Epitranscriptome: Chemical Modifications in Long Non-coding RNAs. Ijms 18 (11), 2387. PubMed PMID: 29125541. doi:10.3390/ijms18112387
Kalluri, R., and LeBleu, V. S. (2020). The Biology , Function , and Biomedical Applications of Exosomes. Science 367 (6478). Epub 2020/02/08PubMed PMID: 32029601; PubMed Central PMCID: PMCPMC7717626. doi:10.1126/science.aau6977
Kok, V. C., and Yu, C.-C. (2020). Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Ijn 15, 8019–8036. Epub 2020/10/30PubMed PMID: 33116515; PubMed Central PMCID: PMCPMC7585279. doi:10.2147/ijn.S272378
Kong, H., Sun, M.-L., Zhang, X.-A., and Wang, X.-Q. (2021). Crosstalk Among circRNA/lncRNA, miRNA, and mRNA in Osteoarthritis. Front. Cell Dev. Biol. 9, 774370. PubMed PMID: 34977024; PubMed Central PMCID: PMC8714905. doi:10.3389/fcell.2021.774370
Kumar, P., Becker, J. C., Gao, K., Carney, R. P., Lankford, L., Keller, B. A., et al. (2019). Neuroprotective Effect of Placenta‐derived Mesenchymal Stromal Cells: Role of Exosomes. FASEB j. 33 (5), 5836–5849. PubMed PMID: 30753093; PubMed Central PMCID: PMC6463921. doi:10.1096/fj.201800972R
Lee, J. Y., Seo, E.-H., Oh, C.-S., Paik, J.-H., Hwang, D.-Y., Lee, S. H., et al. (2017). Impact of Circulating T Helper 1 and 17 Cells in the Blood on Regional Lymph Node Invasion in Colorectal Cancer. J. Cancer 8 (7), 1249–1254. Epub 2017/06/14PubMed PMID: 28607600; PubMed Central PMCID: PMCPMC5463440. doi:10.7150/jca.18230
Li, N., Li, J., Mi, Q., Xie, Y., Li, P., Wang, L., et al. (2020). Long Non‐coding RNA ADAMTS9‐AS1 Suppresses Colorectal Cancer by Inhibiting the Wnt/β‐catenin Signalling Pathway and Is a Potential Diagnostic Biomarker. J. Cel. Mol. Med. 24 (19), 11318–11329. Epub 2020/09/06PubMed PMID: 32889785; PubMed Central PMCID: PMCPMC7576284. doi:10.1111/jcmm.15713
Li, S., Zhang, M., Zhang, H., Hu, K., Cai, C., Wang, J., et al. (2020). Exosomal Long Noncoding RNA Lnc-GNAQ-6:1 May Serve as a Diagnostic Marker for Gastric Cancer. Clinica Chim. Acta 501, 252–257. Epub 2019/11/16PubMed PMID: 31730812. doi:10.1016/j.cca.2019.10.047
Li, W., Zhang, L., Guo, B., Deng, J., Wu, S., Li, F., et al. (2019). Exosomal FMR1-AS1 Facilitates Maintaining Cancer Stem-like Cell Dynamic Equilibrium via TLR7/NFκB/c-Myc Signaling in Female Esophageal Carcinoma. Mol. Cancer 18 (1), 22. PubMed PMID: 30736860. doi:10.1186/s12943-019-0949-7
Li, Y., Yin, Z., Fan, J., Zhang, S., and Yang, W. (2019). The Roles of Exosomal miRNAs and lncRNAs in Lung Diseases. Sig Transduct Target. Ther. 4, 47. Epub 2019/11/16PubMed PMID: 31728212; PubMed Central PMCID: PMCPMC6851157. doi:10.1038/s41392-019-0080-7
Liang, Z.-x., Liu, H.-s., Wang, F.-w., Xiong, L., Zhou, C., Hu, T., et al. (2019). LncRNA RPPH1 Promotes Colorectal Cancer Metastasis by Interacting with TUBB3 and by Promoting Exosomes-Mediated Macrophage M2 Polarization. Cell Death Dis 10 (11), 829. Epub 2019/11/07PubMed PMID: 31685807; PubMed Central PMCID: PMCPMC6828701. doi:10.1038/s41419-019-2077-0
Lin, L.-Y., Yang, L., Zeng, Q., Wang, L., Chen, M.-L., Zhao, Z.-H., et al. (2018). Tumor-originated Exosomal lncUEGC1 as a Circulating Biomarker for Early-Stage Gastric Cancer. Mol. Cancer 17 (1), 84. Epub 2018/04/25PubMed PMID: 29690888; PubMed Central PMCID: PMCPMC5978993. doi:10.1186/s12943-018-0834-9
Liu, L., Meng, T., Yang, X.-H., Sayim, P., Lei, C., Jin, B., et al. (2018). Prognostic and Predictive Value of Long Non-coding RNA GAS5 and mircoRNA-221 in Colorectal Cancer and Their Effects on Colorectal Cancer Cell Proliferation, Migration and Invasion. Cbm 22 (2), 283–299. Epub 2018/04/10PubMed PMID: 29630521. doi:10.3233/cbm-171011
Liu, X., Gan, W., Zou, Y., Yang, B., Su, Z., Deng, J., et al. (2016). Elevated Levels of Urinary Markers of Oxidative DNA and RNA Damage in Type 2 Diabetes with Complications. Oxidative Med. Cell. longevity 2016, 4323198. PubMed PMID: 26770653; PubMed Central PMCID: PMC4685146. doi:10.1155/2016/4323198
Lu, S., Ding, X., Wang, Y., Hu, X., Sun, T., Wei, M., et al. (2021). The Relationship between the Network of Non-coding RNAs-Molecular Targets and N6-Methyladenosine Modification in Colorectal Cancer. Front. Cell Dev. Biol. 9, 772542. PubMed PMID: 34938735; PubMed Central PMCID: PMC8685436. doi:10.3389/fcell.2021.772542
Lu, Y., Hou, K., Li, M., Wu, X., and Yuan, S. (2020). Exosome-Delivered LncHEIH Promotes Gastric Cancer Progression by Upregulating EZH2 and Stimulating Methylation of the GSDME Promoter. Front. Cell Dev. Biol. 8, 571297. Epub 2020/11/10PubMed PMID: 33163491; PubMed Central PMCID: PMCPMC7591465. doi:10.3389/fcell.2020.571297
Luan, Y., Li, X., Luan, Y., Zhao, R., Li, Y., Liu, L., et al. (2020). Circulating lncRNA UCA1 Promotes Malignancy of Colorectal Cancer via the miR-143/MYO6 Axis. Mol. Ther. - Nucleic Acids 19, 790–803. Epub 2020/01/20PubMed PMID: 31955010; PubMed Central PMCID: PMCPMC6970172. doi:10.1016/j.omtn.2019.12.009
Luo, M., and Li, L. (2019). Clinical Utility of Miniprobe Endoscopic Ultrasonography for Prediction of Invasion Depth of Early Gastric Cancer. Medicine 98 (6), e14430. PubMed PMID: 30732202. doi:10.1097/MD.0000000000014430
Oehme, F., Krahl, S., Gyorffy, B., Muessle, B., Rao, V., Greif, H., et al. (2019). Low Level of Exosomal Long Non-coding RNA HOTTIP Is a Prognostic Biomarker in Colorectal Cancer. RNA Biol. 16 (10), 1339–1345. Epub 2019/06/30PubMed PMID: 31251124; PubMed Central PMCID: PMCPMC6779381. doi:10.1080/15476286.2019.1637697
Pan, L., Liang, W., Fu, M., Huang, Z.-h., Li, X., Zhang, W., et al. (2017). Exosomes-mediated Transfer of Long Noncoding RNA ZFAS1 Promotes Gastric Cancer Progression. J. Cancer Res. Clin. Oncol. 143 (6), 991–1004. Epub 2017/03/13PubMed PMID: 28285404. doi:10.1007/s00432-017-2361-2
Phan, J., Kumar, P., Hao, D., Gao, K., Farmer, D., and Wang, A. (2018). Engineering Mesenchymal Stem Cells to Improve Their Exosome Efficacy and Yield for Cell-free Therapy. J. extracellular vesicles 7 (1), 1522236. PubMed PMID: 30275938; PubMed Central PMCID: PMC6161586. doi:10.1080/20013078.2018.1522236
Piao, H.-y., Guo, S., Wang, Y., and Zhang, J. (2020). Exosomal Long Non-coding RNA CEBPA-AS1 Inhibits Tumor Apoptosis and Functions as a Non-invasive Biomarker for Diagnosis of Gastric Cancer. Ott 13, 1365–1374. Epub 2020/02/29PubMed PMID: 32110038; PubMed Central PMCID: PMCPMC7034294. doi:10.2147/ott.S238706
Piao, H.-y., Guo, S., Wang, Y., and Zhang, J. (2021). Exosome-transmitted lncRNA PCGEM1 Promotes Invasive and Metastasis in Gastric Cancer by Maintaining the Stability of SNAI1. Clin. Transl Oncol. 23 (2), 246–256. Epub 2020/06/11PubMed PMID: 32519176. doi:10.1007/s12094-020-02412-9
Qiu, G., Thakur, A., Xu, C., Ng, S.-P., Lee, Y., and Wu, C.-M. L. (2019). Detection of Glioma-Derived Exosomes with the Biotinylated Antibody-Functionalized Titanium Nitride Plasmonic Biosensor. Adv. Funct. Mater. 29 (9), 1806761. doi:10.1002/adfm.201806761
Ren, J., Ding, L., Zhang, D., Shi, G., Xu, Q., Shen, S., et al. (2018). Carcinoma-associated Fibroblasts Promote the Stemness and Chemoresistance of Colorectal Cancer by Transferring Exosomal lncRNA H19. Theranostics 8 (14), 3932–3948. Epub 2018/08/08PubMed PMID: 30083271; PubMed Central PMCID: PMCPMC6071523. doi:10.7150/thno.25541
Richtig, G., Ehall, B., Richtig, E., Aigelsreiter, A., Gutschner, T., and Pichler, M. (2017). Function and Clinical Implications of Long Non-coding RNAs in Melanoma. Ijms 18 (4), 715. PubMed PMID: 28350340. doi:10.3390/ijms18040715
Rinn, J. L., and Chang, H. Y. (2020). Long Noncoding RNAs: Molecular Modalities to Organismal Functions. Annu. Rev. Biochem. 89, 283–308. Epub 2020/06/23PubMed PMID: 32569523. doi:10.1146/annurev-biochem-062917-012708
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C., and Lordick, F. (2020). Gastric Cancer. The Lancet 396 (10251), 635–648. PubMed PMID: 32861308. doi:10.1016/S0140-6736(20)31288-5
Sun, J., Jia, H., Bao, X., Wu, Y., Zhu, T., Li, R., et al. (2021). Tumor Exosome Promotes Th17 Cell Differentiation by Transmitting the lncRNA CRNDE-H in Colorectal Cancer. Cell Death Dis 12 (1), 123. Epub 2021/01/27PubMed PMID: 33495437; PubMed Central PMCID: PMCPMC7835218. doi:10.1038/s41419-020-03376-y
Sun, Z., Yang, S., Zhou, Q., Wang, G., Song, J., Li, Z., et al. (2018). Emerging Role of Exosome-Derived Long Non-coding RNAs in Tumor Microenvironment. Mol. Cancer 17 (1), 82. Epub 2018/04/22PubMed PMID: 29678180; PubMed Central PMCID: PMCPMC5909226. doi:10.1186/s12943-018-0831-z
Thakur, A., Ke, X., Chen, Y.-W., Motallebnejad, P., Zhang, K., Lian, Q., et al. (2021). The Mini Player with Diverse Functions: Extracellular Vesicles in Cell Biology, Disease, and Therapeutics. Protein Cell. PubMed PMID: 34374936. doi:10.1007/s13238-021-00863-6
Thakur, A., Parra, D. C., Motallebnejad, P., Brocchi, M., and Chen, H. J. (2022). Exosomes: Small Vesicles with Big Roles in Cancer, Vaccine Development, and Therapeutics. Bioactive Mater. 10, 281–294. PubMed PMID: 34901546; PubMed Central PMCID: PMC8636666. doi:10.1016/j.bioactmat.2021.08.029
Thakur, A., Qiu, G., Xu, C., Han, X., Yang, T., Ng, S. P., et al. (2020). Label-free Sensing of Exosomal MCT1 and CD147 for Tracking Metabolic Reprogramming and Malignant Progression in Glioma. Sci. Adv. 6 (26), eaaz6119. PubMed PMID: 32637597; PubMed Central PMCID: PMC7319757. doi:10.1126/sciadv.aaz6119
Wang, F.-W., Cao, C.-H., Han, K., Zhao, Y.-X., Cai, M.-Y., Xiang, Z.-C., et al. (2019). APC-activated Long Noncoding RNA Inhibits Colorectal Carcinoma Pathogenesis through Reduction of Exosome Production. J. Clin. Invest. 129 (2), 727–743. Epub 2018/12/05PubMed PMID: 30511962; PubMed Central PMCID: PMCPMC6355227. doi:10.1172/jci122478
Wang, J., Huang, F., Shi, Y., Zhang, Q., Xu, S., Yao, Y., et al. (2021). RP11-323N12.5 Promotes the Malignancy and Immunosuppression of Human Gastric Cancer by Increasing YAP1 Transcription. Gastric Cancer 24 (1), 85–102. PubMed PMID: 32623586. doi:10.1007/s10120-020-01099-9
Wang, J., Lv, B., Su, Y., Wang, X., Bu, J., and Yao, L. (2019). Exosome-Mediated Transfer of lncRNA HOTTIP Promotes Cisplatin Resistance in Gastric Cancer Cells by Regulating HMGA1/miR-218 Axis. Ott 12, 11325–11338. Epub 2020/01/08PubMed PMID: 31908497; PubMed Central PMCID: PMCPMC6930390. doi:10.2147/ott.S231846
Wang, L., Bo, X., Yi, X., Xiao, X., Zheng, Q., Ma, L., et al. (2020). Exosome-transferred LINC01559 Promotes the Progression of Gastric Cancer via PI3K/AKT Signaling Pathway. Cell Death Dis 11 (9), 723. Epub 2020/09/09PubMed PMID: 32895368; PubMed Central PMCID: PMCPMC7477231. doi:10.1038/s41419-020-02810-5
Wang, M., Gu, J., Zhang, X., Yang, J., Zhang, X., and Fang, X. (2021). Long Non-coding RNA DANCR in Cancer: Roles, Mechanisms, and Implications. Front. Cell Dev. Biol. 9, 753706. PubMed PMID: 34722539; PubMed Central PMCID: PMC8554091. doi:10.3389/fcell.2021.753706
Wang, Y.-H., Ji, J., Wang, B.-C., Chen, H., Yang, Z.-H., Wang, K., et al. (2018). Tumor-Derived Exosomal Long Noncoding RNAs as Promising Diagnostic Biomarkers for Prostate Cancer. Cell Physiol Biochem 46 (2), 532–545. PubMed PMID: 29614511. doi:10.1159/000488620
Wei, L., Sun, J., Zhang, N., Shen, Y., Wang, T., Li, Z., et al. (2021). Novel Implications of MicroRNAs, Long Non-coding RNAs and Circular RNAs in Drug Resistance of Esophageal Cancer. Front. Cell Dev. Biol. 9, 764313. PubMed PMID: 34881242; PubMed Central PMCID: PMC8645845. doi:10.3389/fcell.2021.764313
Xian, D., Niu, L., Zeng, J., and Wang, L. (2021). LncRNA KCNQ1OT1 Secreted by Tumor Cell-Derived Exosomes Mediates Immune Escape in Colorectal Cancer by Regulating PD-L1 Ubiquitination via MiR-30a-5p/USP22. Front. Cell Dev. Biol. 9, 653808. Epub 2021/08/06PubMed PMID: 34350172; PubMed Central PMCID: PMCPMC8326752. doi:10.3389/fcell.2021.653808
Xin, L., Zhou, L. Q., Liu, C., Zeng, F., Yuan, Y. W., Zhou, Q., et al. (2021). Transfer of LncRNA CRNDE in TAM‐derived Exosomes Is Linked with Cisplatin Resistance in Gastric Cancer. EMBO Rep. 22 (12), e52124. Epub 2021/10/15PubMed PMID: 34647680; PubMed Central PMCID: PMCPMC8647143. doi:10.15252/embr.202052124
Xu, H., Zhou, J., Tang, J., Min, X., Yi, T., Zhao, J., et al. (2020). Identification of Serum Exosomal lncRNA MIAT as a Novel Diagnostic and Prognostic Biomarker for Gastric Cancer. J. Clin. Lab. Anal. 34 (8), e23323. Epub 2020/04/11PubMed PMID: 32274858; PubMed Central PMCID: PMCPMC7439433. doi:10.1002/jcla.23323
Xu, J., Xiao, Y., Liu, B., Pan, S., Liu, Q., Shan, Y., et al. (2020). Exosomal MALAT1 Sponges miR-26a/26b to Promote the Invasion and Metastasis of Colorectal Cancer via FUT4 Enhanced Fucosylation and PI3K/Akt Pathway. J. Exp. Clin. Cancer Res. 39 (1), 54. Epub 2020/03/27PubMed PMID: 32209115; PubMed Central PMCID: PMCPMC7092616. doi:10.1186/s13046-020-01562-6
Yan, Y., Xu, Z., Chen, X., Wang, X., Zeng, S., Zhao, Z., et al. (2019). Novel Function of lncRNA ADAMTS9-AS2 in Promoting Temozolomide Resistance in Glioblastoma via Upregulating the FUS/MDM2 Ubiquitination Axis. Front. Cell Dev. Biol. 7, 217. PubMed PMID: 31632968; PubMed Central PMCID: PMC6783494. doi:10.3389/fcell.2019.00217
Yan, Y., Xu, Z., Li, Z., Sun, L., and Gong, Z. (2017). An Insight into the Increasing Role of LncRNAs in the Pathogenesis of Gliomas. Front. Mol. Neurosci. 10, 53. PubMed PMID: 28293170; PubMed Central PMCID: PMC5328963. doi:10.3389/fnmol.2017.00053
Yang, Y.-n., Zhang, R., Du, J.-w., Yuan, H.-h., Li, Y.-j., Wei, X.-l., et al. (2018). Predictive Role of UCA1-containing Exosomes in Cetuximab-Resistant Colorectal Cancer. Cancer Cell Int 18, 164. Epub 2018/11/01PubMed PMID: 30377411; PubMed Central PMCID: PMCPMC6196422. doi:10.1186/s12935-018-0660-6
Yue, Y., Lin, X., Qiu, X., Yang, L., and Wang, R. (2021). The Molecular Roles and Clinical Implications of Non-coding RNAs in Gastric Cancer. Front. Cell Dev. Biol. 9, 802745. PubMed PMID: 34966746; PubMed Central PMCID: PMC8711095. doi:10.3389/fcell.2021.802745
Zhang, L., and Yu, D. (2019). Exosomes in Cancer Development, Metastasis, and Immunity. Biochim. Biophys. Acta (Bba) - Rev. Cancer 1871 (2), 455–468. Epub 2019/05/03PubMed PMID: 31047959; PubMed Central PMCID: PMCPMC6542596. doi:10.1016/j.bbcan.2019.04.004
Zhang, Y., Chen, L., Ye, X., Wu, Z., Zhang, Z., Sun, B., et al. (2021). Expression and Mechanism of Exosome-Mediated A FOXM1 Related Long Noncoding RNA in Gastric Cancer. J. Nanobiotechnol 19 (1), 133. Epub 2021/05/12PubMed PMID: 33971889; PubMed Central PMCID: PMCPMC8111998. doi:10.1186/s12951-021-00873-w
Zhao, R., Zhang, Y., Zhang, X., Yang, Y., Zheng, X., Li, X., et al. (2018). Exosomal Long Noncoding RNA HOTTIP as Potential Novel Diagnostic and Prognostic Biomarker Test for Gastric Cancer. Mol. Cancer 17 (1), 68. Epub 2018/03/01PubMed PMID: 29486794; PubMed Central PMCID: PMCPMC6389063. doi:10.1186/s12943-018-0817-x
Zheng, P., Zhang, H., Gao, H., Sun, J., Li, J., Zhang, X., et al. (2020). Plasma Exosomal Long Noncoding RNA Lnc-Slc2a12-10:1 as a Novel Diagnostic Biomarker for Gastric Cancer. Ott 13, 4009–4018. Epub 2020/06/05PubMed PMID: 32494155; PubMed Central PMCID: PMCPMC7227815. doi:10.2147/ott.S253600
Zhi, J., Jia, X.-J., Yan, J., Wang, H.-C., Feng, B., Xing, H.-Y., et al. (2021). BRAFV600E Mutant Colorectal Cancer Cells Mediate Local Immunosuppressive Microenvironment through Exosomal Long Noncoding RNAs. Wjgo 13 (12), 2129–2148. PubMed PMID: 35070047. doi:10.4251/wjgo.v13.i12.2129
Zhou, H., Shen, W., Zou, H., Lv, Q., and Shao, P. (2020). Circulating Exosomal Long Non-coding RNA H19 as a Potential Novel Diagnostic and Prognostic Biomarker for Gastric Cancer. J. Int. Med. Res. 48 (7), 030006052093429. Epub 2020/07/15PubMed PMID: 32660285; PubMed Central PMCID: PMCPMC7361491. doi:10.1177/0300060520934297
Zhou, L., Li, J., Tang, Y., and Yang, M. (2021). Exosomal LncRNA LINC00659 Transferred from Cancer-Associated Fibroblasts Promotes Colorectal Cancer Cell Progression via miR-342-3p/ANXA2 axis. J. Transl Med. 19 (1), 8. Epub 2021/01/08PubMed PMID: 33407563; PubMed Central PMCID: PMCPMC7789760. doi:10.1186/s12967-020-02648-7
Keywords: gastrointestinal cancer, exosome, lncRNAs, pathogenesis, treatment
Citation: Kang F, Jiang F, Ouyang L, Wu S, Fu C, Liu Y, Li Z, Tian Y, Cao X, Wang X and He Q (2022) Potential Biological Roles of Exosomal Long Non-Coding RNAs in Gastrointestinal Cancer. Front. Cell Dev. Biol. 10:886191. doi: 10.3389/fcell.2022.886191
Received: 28 February 2022; Accepted: 30 March 2022;
Published: 04 May 2022.
Edited by:
Jinzhou Huang, Mayo Clinic, United StatesCopyright © 2022 Kang, Jiang, Ouyang, Wu, Fu, Liu, Li, Tian, Cao, Wang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingchun He, 404113@csu.edu.cn; Xiaoping Wang, wxpphd@aliyun.com
†These authors have contributed equally to this work and shared first authorship
 Fanhua Kang1†
Fanhua Kang1†  Feng Jiang
Feng Jiang Xiaoping Wang
Xiaoping Wang