The Iron-Inflammation Axis in Early-Stage Triple-Negative Breast Cancer
- 1Department of Medical Oncology, The State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
- 2Department of Breast Oncology, Dongguan People’s Hospital, Dongguan, China
- 3Department of Breast Oncology, The First People’s Hospital of Zhaoqing, Zhaoqing, China
The iron-related homeostasis and inflammatory biomarker have been identified as prognostic factors for cancers. We aimed to explore the prognostic value of a novel comprehensive biomarker, the iron-monocyte-to-lymphocyte ratio (IronMLR) score, in patients with early-stage triple-negative breast cancer (TNBC) in this study. We retrospectively analysed a total of 257 early-stage TNBC patients treated at Sun Yat-sen University Cancer Center (SYSUCC) between March 2006 and October 2016. Their clinicopathological information and haematological data tested within 1 week of the diagnosis were collected. According to the IronMLR score cutoff value of 6.07 μmol/L determined by maximally selected rank statistics, patients were stratified into the low- and high-IronMLR groups, after a median follow-up of 92.3 months (95% confidence interval [CI] 76.0–119.3 months), significant differences in 5-years disease-free survival (DFS) rate (81.2%, 95% CI 76.2%–86.5% vs. 65.5%, 95% CI 50.3%–85.3%, p = 0.012) and 5-years overall survival (OS) rate (86.0%, 95% CI 81.6%–90.7% vs. 65.5%, 95% CI 50.3%–85.3%, p = 0.011) were seen between two groups. Further multivariate Cox regression analysis revealed the IronMLR score as an independent predictor for DFS and OS, respectively, we then established a prognostic nomogram integrating the IronMLR score, T stage and N stage for individualized survival predictions. The prognostic model showed good predictive performance with a C-index of DFS 0.725 (95% CI 0.662–0.788) and OS 0.758 (95% CI 0.689–0.826), respectively. Besides, calibration curves for 1-, 3-, 5-DFS, and OS represented satisfactory consistency between actual and nomogram predicted survival. In conclusion, the Iron-inflammation axis might be a potential prognostic biomarker of survival outcomes for patients with early-stage TNBC, prognostic nomograms based on it with good predictive performance might improve individualized survival predictions.
Introduction
Triple-negative breast cancer (TNBC), characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2) expression, accounts for approximately 15% of all breast cancers (Ferlay et al., 2021; Wang et al., 2021). Compared to other subtypes of breast cancers, patients with TNBC usually experience worse survival outcomes due to its increased aggressiveness, heterogeneity, and risks of recurrence and metastasis (Li et al., 2017). Over the past few decades, despite great therapeutic advances in TNBC, TNBC remains a considerable challenge for women worldwide due to its relatively high mortality (Dent et al., 2007; Bianchini et al., 2016; Garrido-Castro et al., 2019). Therefore, the discovery of novel, precise biomarkers, or individualized therapeutic targets for patients with TNBC is urgently needed.
As an essential trace element, iron is involved in activating many proteins, enzymes, and biological processes, such as cell respiration and various signalling pathways (Torti et al., 2018). Increasing studies have demonstrated that the dysregulation of systemic iron homeostasis is related to tumor initiation, growth, development, and progression (Chen et al., 2015; Radulescu et al., 2016). The depletion of iron using iron chelators or targeting increased serum iron has been explored as a novel therapeutic strategy for some cancers (Nutting et al., 2009; Yamasaki et al., 2011; List et al., 2012; Neufeld et al., 2012; Kalinowski et al., 2016; Stockwell et al., 2017; von Hagens et al., 2017). Moreover, ferroptosis, an iron-dependent programmed cell death, has been classified as a type of regulated necrosis and is also recognized as a new therapeutic target for tumours (Dixon et al., 2012). Given its immunogenicity, ferroptosis can induce cells to release damage-associated molecular patterns (DAMPs) and alarmins, which might enhance cell death and facilitate a series of inflammation-related responses (Martin-Sanchez et al., 2017; Proneth and Conrad, 2019; Sun et al., 2020). However, the role of ferroptosis in inflammation is multifaceted, it not only induces inflammatory activity but also suppresses inflammatory cell infiltration (Umemura et al., 2017; Tsurusaki et al., 2019; Sun et al., 2020; Zhou et al., 2020).
Monocytes have been explored for the suppression of lymphocyte activation, which is associated with aggressive tumors and metastasis (Tiainen et al., 2021). Peripheral lymphocytes or lymphocytes infiltrating in the tumour microenvironment (TME) play an important role in antitumour immunity by T cell-mediated cellular cytotoxicity (Andre et al., 2013). Therefore, the serum monocyte-to-lymphocyte ratio (MLR) might serve as a predictor of systemic inflammatory status, and an increased MLR might reflect poor antitumour immunity (Miklikova et al., 2020). The prognostic value of the MLR has been previously explored in breast cancer (De Giorgi et al., 2019). Thus, we hypothesized that a comprehensive biomarker based on iron homeostasis and systemic inflammation status might also show potential prognostic value. However, to date, no study has examined this aspect.
We aimed to explore the prognostic value of the novel comprehensive biomarker, the IronMLR score, calculated based on the serum iron level and MLR, for female patients with early-stage TNBC. Subsequently, we attempted to establish a prognostic model based on this Iron-inflammation axis for individualized survival predictions.
Methods
Eligible Patients
We explored the predictive value of a novel biomarker, the IronMLR score, in female patients newly diagnosed with early-stage TNBC at Sun Yat-sen University Cancer Center (SYSUCC) between March 2006 and October 2016. Our study protocol was approved by the Ethics Committee of SYSUCC (registration number: B2021-282-01). Given the retrospective nature of the current study, the requirement of written informed consent from patients was waived. In addition, we anonymously analysed all personal data in line with the Declaration of Helsinki.
Patients were included if they met the following key inclusion criteria: 1) 18 ≤ age <75 years old; 2) pathological diagnosis of breast cancer; 3) absence of ER, PR, and HER2 expression (scored as 0, 1+, or 2+ by immunohistochemistry [IHC] without amplification of the ERBB2 gene on fluorescence in situ hybridization); 4) no local relapse or distant metastasis at the diagnosis; and 5) complete clinicopathological data and available haematological parameters assessed within 1 week of the date of diagnosis. All patients in this study were restaged according to the American Joint Committee on Cancer (AJCC 2010, seventh version).
Key exclusion criteria included 1) pregnancy or lactation; 2) a history of malignancy, including breast cancer; 3) medication affecting the patient’s inflammatory status before diagnosis; and 4) the presence of any severe or uncontrolled complications.
Information Collection and Measurement of Serum Iron Levels
Clinicopathological data were hand-retrieved from the electronic medical records system of our hospital and included age, menstrual status, histological type, T stage, N stage, histological grade, lymphovascular invasion, and Ki-67 index. Haematological parameters were assessed within 1 week of initiating any anti-tumor therapy, and the MLR was calculated as: MLR = monocyte count (109/L)/lymphocyte count (109/L). We acquired patient blood samples obtained within 1 week of diagnosis from the Tumor Resource Library of SYSUCC. The serum iron levels of patients were analysed using the Iron (Fe) Assay Kit (PAESA Chromogenic Method) with a Cobas 8,000 system (Roche Diagnostics, Basel, Switzerland). The IronMLR score was calculated as following: IronMLR score = serum iron level * MLR.
Follow-up and Endpoints
We retrieved patient follow-up information from the outpatient electronic records of our center or telephone interviews. Patients were monitored every 3 months during 2 years of the diagnosis, subsequently every 6 months to 5 years, and then once every year thereafter. The main follow-up items included routine laboratory tests, menstrual status, ultrasound (breast and abdomen), or computed tomography (CT). Patients underwent bone scintigraphy annually.
The primary endpoint of the current study was disease-free survival (DFS), which was defined as the time from the date of diagnosis to the date of disease progression or death due to any cause. The second endpoint was overall survival (OS), which was defined as the time from the date of diagnosis to the date of death due to any cause.
Statistical Analysis
Categorical variables are presented as frequencies with percentages, and continuous variables are listed as medians with interquartile ranges (IQRs). Chi-square tests and Mann-Whitney U tests were performed to compare the association between patient clinicopathological characteristics and the baseline IronMLR score. Patients with early-stage TNBC were stratified into high and low IronMLR groups according to the cut-off value of the IronMLR score determined by maximally selected rank statistics. The Kaplan-Meier method was performed to estimate survival curves of two different IronMLR score groups, and the log-rank test was used to compare their survival differences. Only variables with p values less than 0.05 in the univariate Cox analysis were included in the multivariate Cox regression model. Factors were tested according to the Schoenfeld residuals (Wileyto et al., 2013), and their corresponding hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated. Then, we developed a prognostic model integrating the IronMLR score with other independent clinicopathological indicators from the multivariate Cox analysis, and we evaluated the prognostic accuracy and discriminative ability of the predictive model by means of the concordance index (C-index), calibration curves, and time-dependent receiver operating characteristic (ROC) curves. A two-sided p < 0.05 was regarded as significant, and all statistical analyses were performed using R 4.0.1.
Results
Patient Clinicopathologic Characteristics According to the IronMLR Score
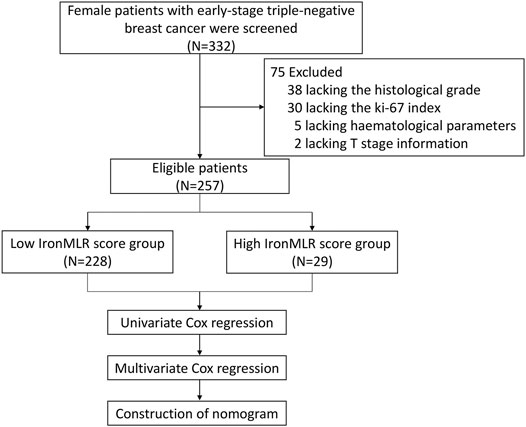
As presented in the flow chart in Figure 1, between March 2006 and October 2016, a total of 257 female patients with early-stage TNBC were eligible for inclusion in this study. A total of 75 patients were excluded due to a lack of complete information, i.e., 38 patients did not have complete histological grade data, 30 patients did not have Ki-67 index data, 5 patients lacked data on haematological parameters, and 2 patients had missing T stage information.
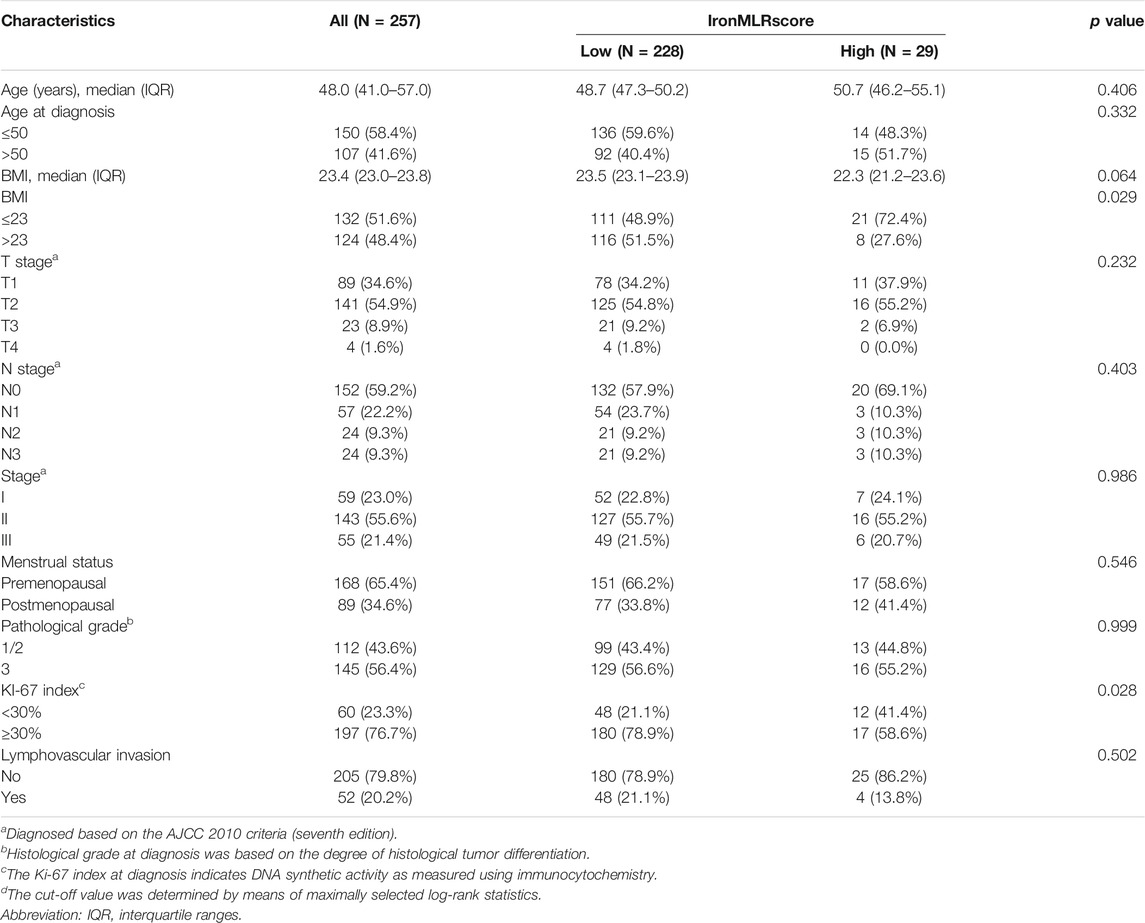
The baseline clinicopathological features are shown in Table 1. The median age at the diagnosis of all eligible patients was 48.0 (IQR: 41.0–57.0) years old, and 150 (58.4%) patients were ≤50 years old. The median BMI was 23.4 (IQR: 23.0–23.8) kg/m2, and 132 (51.6%) patients had a value of BMI ≤23.0 kg/m2. There were 168 (65.4%) patients in the premenopausal period and 197 (76.7%) with a Ki-67 index value ≥30%. According to the seventh AJCC staging criteria, stages I, II, and III accounted for 23.0, 55.6, and 21.4%, respectively.
The optimal cut-off value of the IronMLR score defined by the maximally selected rank statistics for OS was 6.07 umol/L (Figure 2A). Overall, 228 (88.7%) patients had a low IronMLR score, and 29 (11.3%) patients had a high IronMLR score. The baseline clinicopathological characteristics between these two IronMLR groups were much more balanced (Table 1). Compared to TNBC patients in the low IronMLR score group, a higher proportion of patients in the high IronMLR score group had a BMI ≤23 kg/m2 (p = 0.029) and Ki-67 index <30% (p = 0.028).
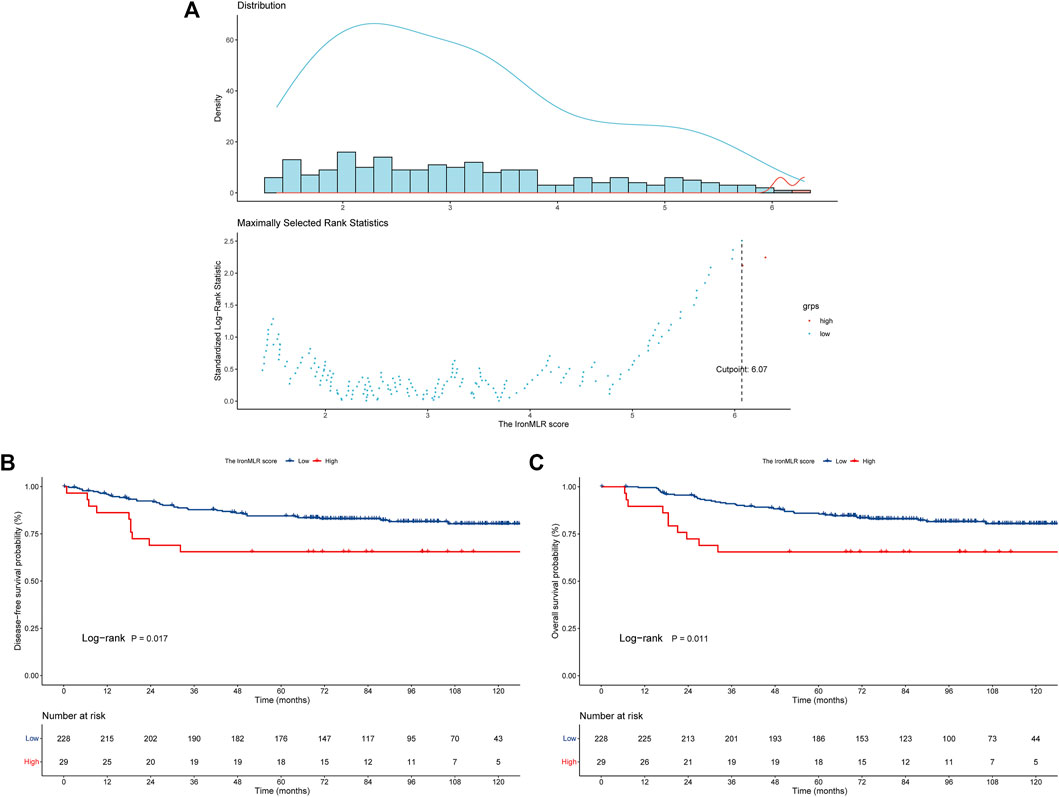
FIGURE 2. Determination of the cutoff value of the IronMLR score and Survival risk stratification of patients. (A) A cutoff value of 6.07 μmol/L defined by maximally selected rank statistics. (B) Survival curves for disease-free survival (DFS) between different IronMLR score groups. (C) Survival curves for overall survival (OS) between different IronMLR score groups.
Survival Outcomes
The median follow-up time was 92.3 months (95% CI 76.0–119.3 months). Compared to patients in the low IronMLR score group, early-stage TNBC patients in the high IronMLR score group had a significantly worse 5-years DFS rate (81.2%, 95% CI 76.2%–86.5% vs. 65.5%, 95% CI 50.3%–85.3%, p = 0.012) (Figure 2B) and 5-years OS rate (86.0%, 95% CI 81.6%–90.7% vs. 65.5%, 95% CI 50.3%–85.3%, p = 0.011) (Figure 2C).
Independent Indicators for Survival
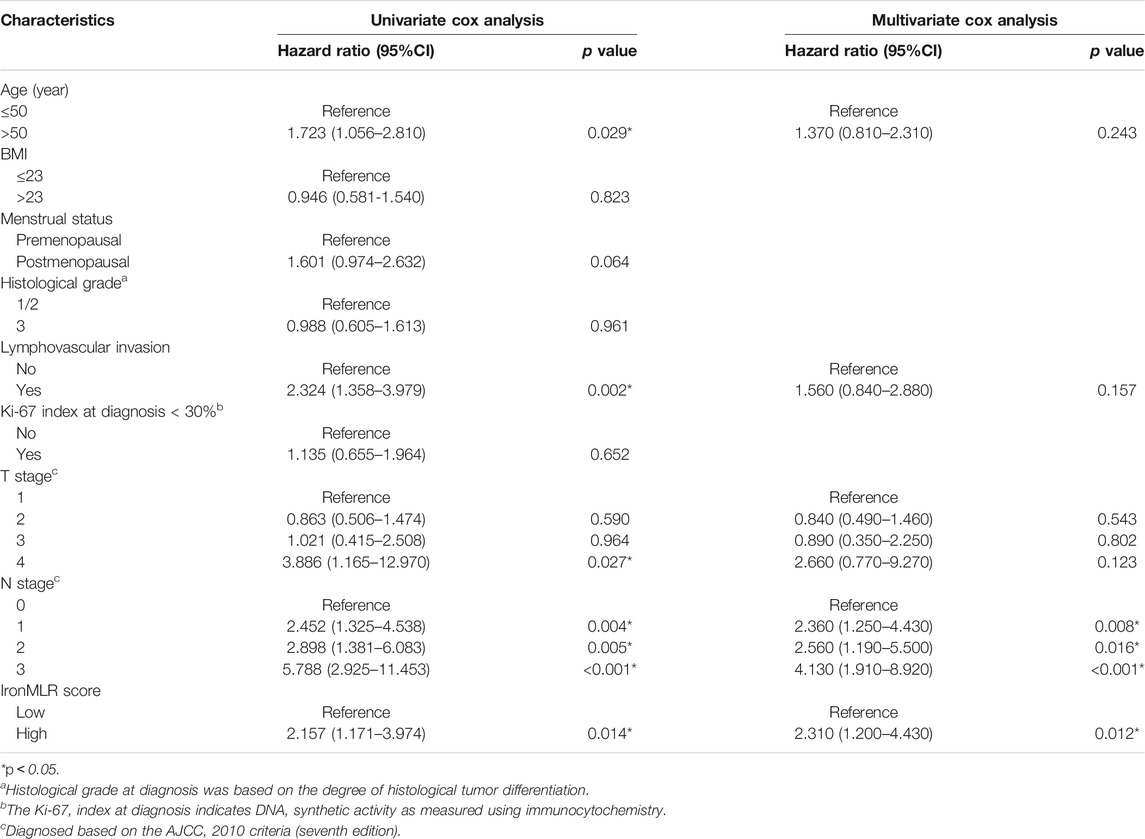
As shown in Table 2, five factors were considered independent indicators for DFS according to the univariate Cox regression model, including age, lymphovascular invasion, T stage, N stage, and the IronMLR score. Further multivariate Cox analysis demonstrated that N stage and the IronMLR score remained independent predictors of DFS for patients with early-stage TNBC.
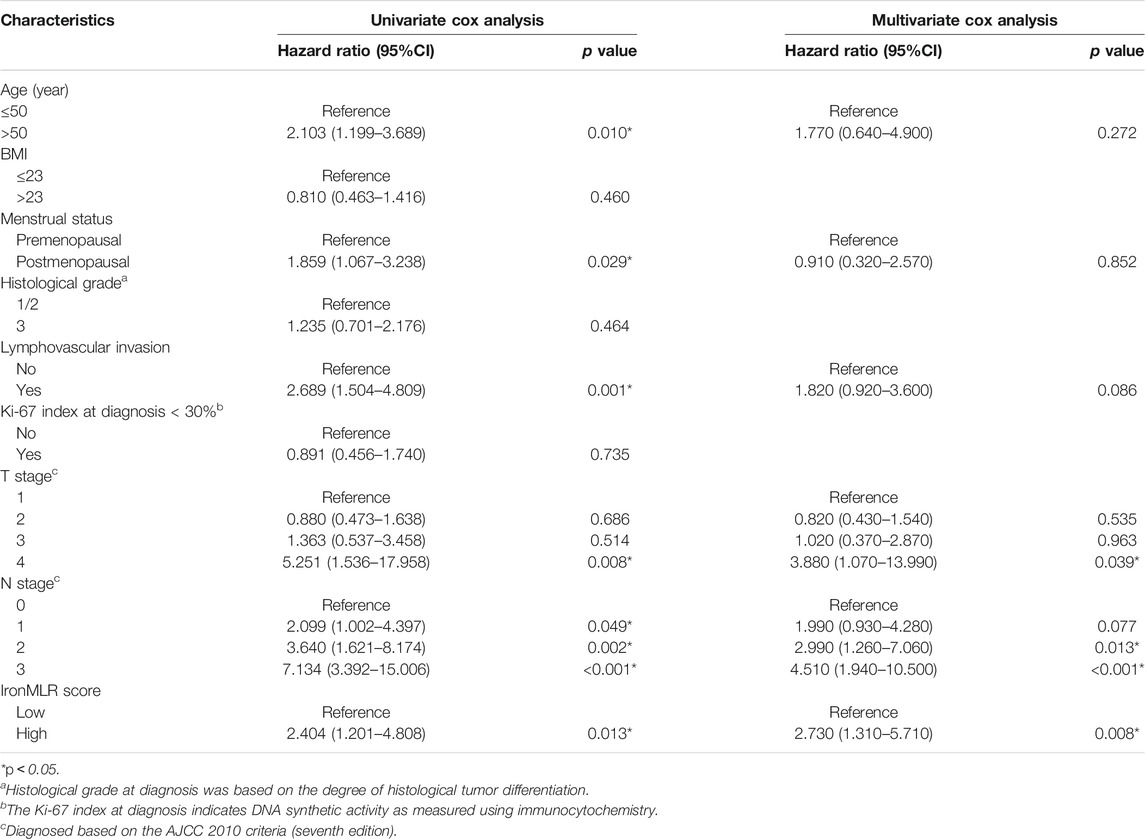
Table 3 presents that age, menstrual status, lymphovascular invasion, T stage, N stage, and the IronMLR score of patients achieved the predominate threshold of OS (p < 0.05) for early-stage TNBC female patients in the univariate Cox analysis. Then, these variables were further analysed in the multivariate Cox regression model, which revealed that T stage, N stage, and the IronMLR score continued to be significantly associated with OS.
Establishment and Evaluation of the Prognostic Model
Based on independent indicators for DFS identified in the above multivariate Cox analysis, i.e., N stage and the IronMLR score, we established a prognostic model for individualized DFS prediction for patients with early-stage TNBC. Given that T stage is commonly used in clinical practice, we also integrated it into the prognostic model (Figure 3A). The model showed perfect predictive performance with a good C-index of 0.725 (95% CI 0.662–0.788). Satisfactory consistency between observational 1-, 3-, and 5-years DFS rates and nomogram-predicted DFS rates was found in calibration curves (Figure 3B). Time-dependent ROC curves showed that the area under the curve (AUC) of our nomogram for DFS was higher than the AUCs for T stage, N stage, and the traditional tumor-node-metastasis (TNM) staging system (Figure 3C).
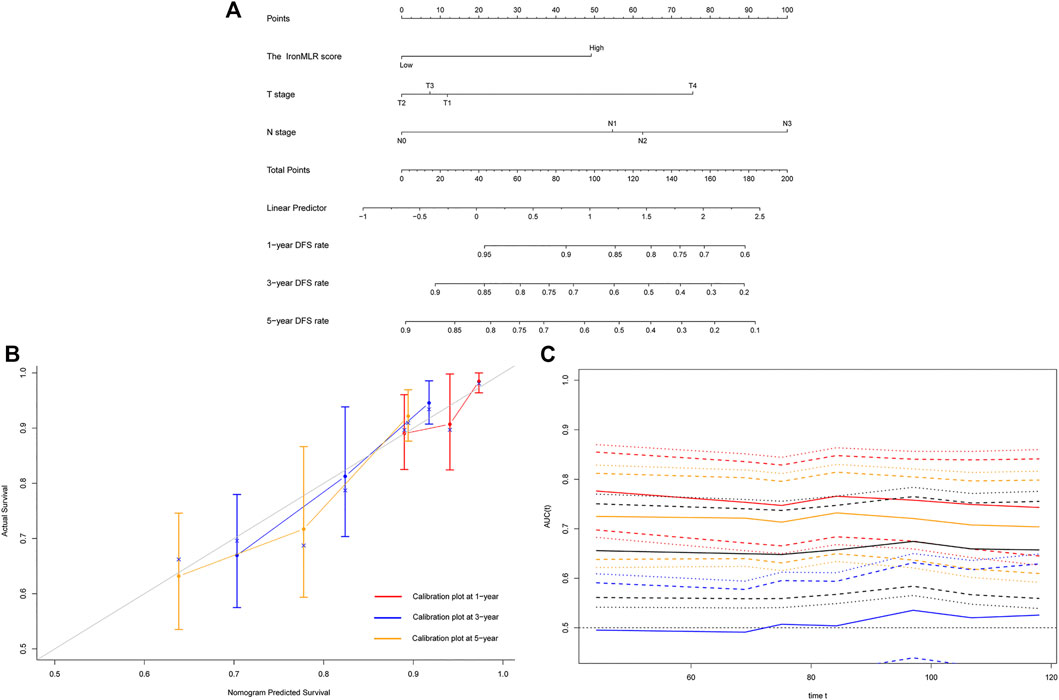
FIGURE 3. Development and evaluation of a model for individualized prediction of disease-free survival (DFS). (A) Nomogram of prognostic model for patients with early-stage triple-negative breast cancer. (B) Calibration plots of 1-, 3-, and 5-years DFS predictions. (C) Time-dependent receiver operating characteristic (ROC) curves (Nomogram [red], T stage [blue], N stage [orange], TNM stage [black]).
Similarly, we developed a prognostic model with independent indicators according to the multivariate Cox regression model, i.e., T stage, N stage, and IronMLR score, for individualized OS prediction (Figure 4A). The predictive accuracy of our nomogram for OS was very good, with a satisfactory C-index of 0.758 (95% CI 0.689–0.826). The calibration plots for 1-, 3-, and 5-years OS rates demonstrated good consistency between actual and nomogram-predicted OS rates (Figure 4B). Compared to traditional T stage, N stage, and TNM stage, our prognostic nomogram for OS achieved a higher AUC according to time-dependent ROC curves (Figure 4C).
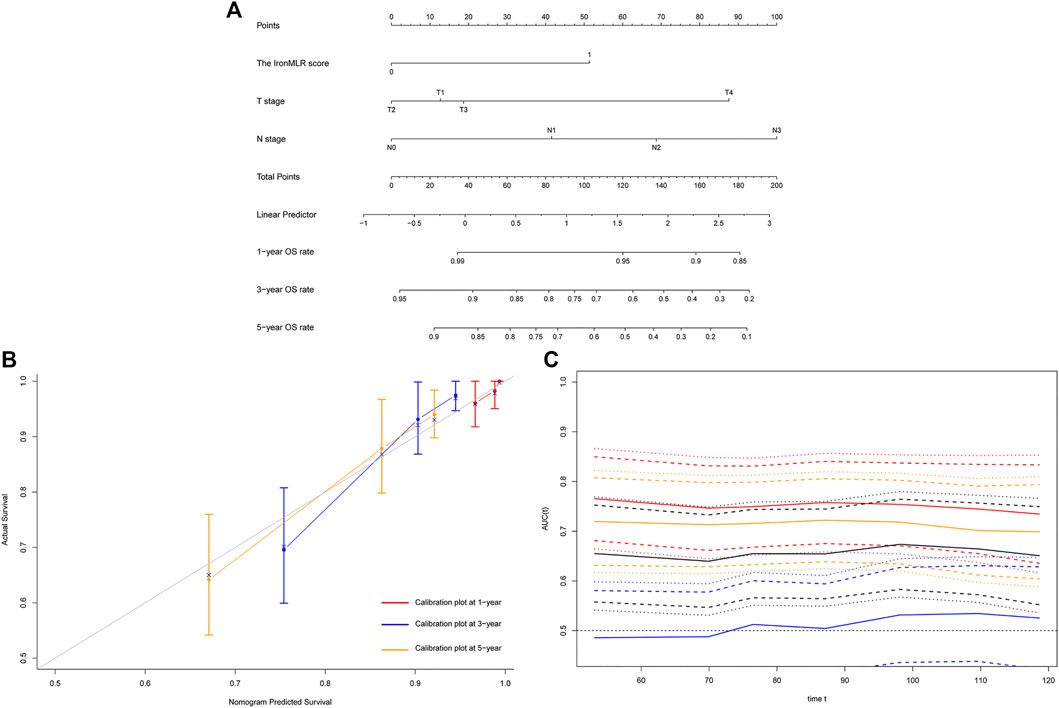
FIGURE 4. Development and evaluation of a model for individualized prediction of overall survival (OS). (A) Nomogram of prognostic model for patients with early-stage triple-negative breast cancer. (B) Calibration plots for predicting OS at 1-, 3-, and 5-years. (C) Time-dependent receiver operating characteristic (ROC) curves (Nomogram [red], T stage [blue], N stage [orange], TNM stage [black]).
Discussion
In this study, we constructed a novel comprehensive biomarker based on the serum iron level and systemic inflammation status, the IronMLR score. According to a cut-off value of 6.07 determined by maximally selected rank statistics, heterogeneous patients with early-stage TNBC were divided into groups based on a low or high IronMLR score. Significant differences in DFS and OS were found between these two groups. Further univariate and multivariate Cox regression analyses demonstrated that a high IronMLR score was a negatively independent predictor of poor survival for early-stage TNBC patients. Subsequently, prognostic models integrating the IronMLR score and two clinicopathological features (T stage and N stage) for DFS and OS were established and graphically depicted as nomograms. These prognostic nomograms presented good discriminative ability and satisfactory predictive agreement between observed clinical outcomes and the nomogram-predicted survival probability.
Although iron is indispensable for many proteins and enzymes, playing an important role in various biological processes (Hider and Maret, 2015; Torti et al., 2018), it also attributes to oxidative stress and DNA damage. Excess iron has been found to be significantly associated with tumor initiation, progression, aggressiveness, and metastasis (Chen et al., 2015; Guo et al., 2015; Radulescu et al., 2016). Increasing studies have found iron metabolic dysregulation, iron homeostatic disorder, and distribution changes in peripheral iron in patients with various malignancies, including breast cancer (Torti et al., 2018; Galaris et al., 2019). Meanwhile, iron accumulation could induce lipid peroxidation and facilitate lethal injury to tumor cells, which subsequently could contribute to inhibiting tumor initiation and progression (Chang et al., 2019; Forciniti et al., 2020; Yang et al., 2021). Iron accumulation is involved in some programmed cell death pathways, such as apoptosis, necroptosis, ferroptosis, and so on (Dixon et al., 2012; Torti et al., 2018). Thus, iron-related metabolism or iron chelators might be potential novel therapeutic targets and strategies for antitumor treatment (Nutting et al., 2009; Yamasaki et al., 2011; List et al., 2012; Neufeld et al., 2012; Kalinowski et al., 2016). Hence, the measurement of iron levels is necessary. Many previous studies have used iron-bound proteins such as transferrin and ferritin to reflect body iron levels (Fonseca-Nunes et al., 2014; Torti et al., 2018; Morales and Xue, 2021), but this indirect test might produce errors in reflecting actual iron levels, direct detection of serum iron would be better (Torti et al., 2018; El Hout et al., 2018). Thus, we adopted the peripheral iron level to construct the IronMLR score in this study.
Inflammation, a hallmark feature of tumors, has shown a significant association with tumorigenesis, progression, development, and metastasis (Diakos, et al., 2014). Cancer-associated inflammation refers to complicated connections between tumors and inflammatory responses, and it might show a significant relation to poor prognosis and therapeutic failure (Zitvogel et al., 2017; Li et al., 2021). As vital inflammatory mediators, peripheral inflammatory cells or inflammatory cells infiltrating in the TME are potential prognostic biomarkers for breast cancer (Diakos et al., 2014; van der Willik et al., 2018). Among inflammatory cells, monocytes have been identified as inhibitors of lymphocyte activation and play an important role in tumor aggressiveness and metastasis (Tiainen et al., 2021). Circulating lymphocytes or lymphocytes infiltrating in the TME are both involved in antitumor immune responses (Andre et al., 2013). Therefore, the circulating marker MLR might reflect systemic inflammatory status, an elevated MLR indicates an increased monocyte count or a reduced lymphocyte count, which suggests poor antitumor immunity (Miklikova et al., 2020). The negative association between the MLR and survival outcomes of breast cancer patients has also been explored (De Giorgi et al., 2019).
Nowadays, the underlying association between iron and inflammation is still poorly understood. As previously mentioned, ferroptosis plays multifaceted functions in cancer-related inflammation, activation, and infiltration of inflammatory cells (Umemura et al., 2017; Tsurusaki et al., 2019; Sun et al., 2020; Zhou et al., 2020). In addition, IL-6, an inflammatory cytokine, is secreted locally in the TME of breast cancer such as monocytes (Masjedi et al., 2018), and it has been demonstrated to directly regulate systemic iron homeostasis through IL6/IL6R/Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathway (Hentze et al., 2010; Ganz and Nemeth, 2011; Guo et al., 2015). Moreover, IL-6 also likely contributes to the dysregulated hepcidin/ferroportin signaling in breast cancer and mediates further iron homeostasis (Masjedi et al., 2018). So, the blockade of IL-6 inflammatory signaling cascade has been investigated as a potential therapeutic strategy to suppress hepcidin in breast cancer and for anaemic cancer patients (Jiang et al., 2011; Guo et al., 2015). In recent years, researchers have taken great interest in developing prognostic models for risk stratification and clinical outcomes predictions. Predictive models incorporating multiple biomarkers rather than a single marker have higher prognostic accuracy (Li et al., 2021). Thus, we comprehensively developed a new biomarker integrating serum iron with systemic inflammatory markers. Our analyses showed that the novel IronMLR score is an independent indicator of the survival of patients with early-stage TNBC, and patients stratified according to its cut-off value experience significantly different survival outcomes.
Based on the classification of the IronMLR score, prognostic nomogram incorporating it and the commonly used T stage and N stage were established. In clinical practice, 21-gene tests, 70-gene assays, and PAM50 predictive models are widely applied for risk stratification or therapeutic recommendations, but these genetic models are restricted to patients with specific subtypes, lymph node-negative patients or women at high clinical risk of recurrence from breast cancer, with limited prognostic accuracy (Dixon et al., 2012; Gnant et al., 2015; Ibraheem et al., 2020; Poorvu et al., 2020). Comparatively speaking, the predictive nomogram developed in our study were more accurate with a high C-index, economic, convenient, and easier to be applied in primary hospitals. Clinicians and patients usually use the TNM staging system to stratify recurrence or death risks and to guide therapeutic strategies, however, the TNM criteria just incorporate a limited number of clinical features, so its predictive accuracy is limited due to the heterogeneity observed among patients (Bareche et al., 2018; Grosselin et al., 2019). Our time-dependent ROC curves showed that compared to the common TNM staging system, higher AUCs for DFS and OS were achieved with our prognostic nomograms, which suggested that our predictive models have greater predictive accuracy and might be a strong supplement to traditional TNM criteria. For example, TNBC patients with high IronMLR scores tended to have poorer prognosis, for them, more intensive care, closer follow-up, and more precise routine imaging monitor such as CT or magnetic resonance imaging (MRI) instead of breast/abdominal ultrasound would be much meaningful to monitor their tumor condition and improve their survival outcomes as much as possible.
Our study had several limitations, which should be acknowledged. First, because we retrospectively explored the prognostic value of the IronMLR score, selection bias is inevitable. Second, we only evaluated the baseline serum iron level and MLR before the initiation of any anti-tumor therapy, but we failed to explore the dynamic changes of the serum iron level and inflammatory status of patients during subsequent treatment. Monitoring changes in these parameters might help clinicians both to adjust the therapeutic strategy in time and to guide personalized treatment. Third, this study was a preliminary research, underlying mechanisms of the IronMLR on TNBC, and the relationship between MLR and iron require more investigations. Finally, patients with early-stage TNBC included in this study were from only a single centre in China. Further large or multicentre cohort studies are warrant to enhance the power of our results. Although we actively sought help and cooperation from other hospitals to validate our prognostic models, unfortunately, we failed to obtain complete haematological parameters and follow-up information. Thus, the prognostic accuracy of our nomograms for early-stage TNBC patients from other geographic backgrounds requires further evaluation in future studies.
Conclusion
In conclusion, we propose the Iron-inflammation axis, the IronMLR score, as a novel prognostic biomarker for trace element and systemic inflammatory status in female patients with early-stage TNBC. Prognostic models based on the IronMLR score for individualized survival predictions showed good predictive performance and discriminative accuracy, but validation of the predictive role of the IronMLR score is needed in a large or multicentre cohort. Additional studies, especially those exploring the IronMLR score as a potential dynamic biomarker related to survival outcomes during treatment, are warranted in future.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Sun Yat-sen University Cancer Center. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
JH and XB designed this study. FD, MZ, and JY collected, primarily analysed, and interpreted data. FD, MZ, JY, LW, and CJ participated in the drafting of the manuscript. FD, MZ, JY, ZY, XB, and JH contributed to administrative, technical, or material support. All authors revised this manuscript and approved the final summitted version.
Funding
This study was funded by the Natural Science Foundation of Guangdong Province (No. 2019A151011781), the Sci-Tech Project Foundation of Guangzhou City (No. 202002020033), and the cultivation foundation for the junior teachers in Sun Yat-sen University (No. 20ykpy164).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We gratefully acknowledge patients and their family for all their help in enabling completion of this study.
References
Andre, F., Dieci, M. V., Dubsky, P., Sotiriou, C., Curigliano, G., Denkert, C., et al. (2013). Molecular Pathways: Involvement of Immune Pathways in the Therapeutic Response and Outcome in Breast Cancer. Clin. Cancer Res. 19 (1), 28–33. doi:10.1158/1078-0432.CCR-11-2701
Bareche, Y., Venet, D., Ignatiadis, M., Aftimos, P., Piccart, M., Rothe, F., et al. (2018). Unravelling Triple-Negative Breast Cancer Molecular Heterogeneity Using an Integrative Multiomic Analysis. Ann. Oncol. 29 (4), 895–902. doi:10.1093/annonc/mdy024
Bianchini, G., Balko, J. M., Mayer, I. A., Sanders, M. E., and Gianni, L. (2016). Triple-negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat. Rev. Clin. Oncol. 13 (11), 674–690. doi:10.1038/nrclinonc.2016.66
Chang, V. C., Cotterchio, M., and Khoo, E. (2019). Iron Intake, Body Iron Status, and Risk of Breast Cancer: a Systematic Review and Meta-Analysis. BMC Cancer 19 (1), 543. doi:10.1186/s12885-019-5642-0
Chen, Y., Zhang, S., Wang, X., Guo, W., Wang, L., Zhang, D., et al. (2015). Disordered Signaling Governing Ferroportin Transcription Favors Breast Cancer Growth. Cell Signal. 27 (1), 168–176. doi:10.1016/j.cellsig.2014.11.002
De Giorgi, U., Mego, M., Scarpi, E., Giordano, A., Giuliano, M., Valero, V., et al. (2019). Association between Circulating Tumor Cells and Peripheral Blood Monocytes in Metastatic Breast Cancer. Ther. Adv. Med. Oncol. 11, 175883591986606. doi:10.1177/1758835919866065
Dent, R., Trudeau, M., Pritchard, K. I., Hanna, W. M., Kahn, H. K., Sawka, C. A., et al. (2007). Triple-negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 13 (15 Pt 1), 4429–4434. doi:10.1158/1078-0432.CCR-06-3045
Diakos, C. I., Charles, K. A., McMillan, D. C., and Clarke, S. J. (2014). Cancer-related Inflammation and Treatment Effectiveness. Lancet Oncol. 15 (11), e493–e503. doi:10.1016/S1470-2045(14)70263-3
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an Iron-dependent Form of Nonapoptotic Cell Death. Cell 149 (5), 1060–1072. doi:10.1016/j.cell.2012.03.042
El Hout, M., Dos Santos, L., Hamaï, A., and Mehrpour, M. (2018). A Promising New Approach to Cancer Therapy: Targeting Iron Metabolism in Cancer Stem Cells. Semin. Cancer Biol. 53, 125–138. doi:10.1016/j.semcancer.2018.07.009
Ferlay, J., Colombet, M., Soerjomataram, I., Parkin, D. M., Piñeros, M., Znaor, A., et al. (2021). Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 149, 778–789. doi:10.1002/ijc.33588
Fonseca-Nunes, A., Jakszyn, P., and Agudo, A. (2014). Iron and Cancer Risk-A Systematic Review and Meta-Analysis of the Epidemiological Evidence. Cancer Epidemiol. Biomarkers Prev. 23 (1), 12–31. doi:10.1158/1055-9965.EPI-13-0733
Forciniti, S., Greco, L., Grizzi, F., Malesci, A., and Laghi, L. (2020). Iron Metabolism in Cancer Progression. Ijms 21 (6), 2257. doi:10.3390/ijms21062257
Galaris, D., Barbouti, A., and Pantopoulos, K. (2019). Iron Homeostasis and Oxidative Stress: An Intimate Relationship. Biochim. Biophys. Acta (Bba) - Mol. Cel Res. 1866 (12), 118535. doi:10.1016/j.bbamcr.2019.118535
Ganz, T., and Nemeth, E. (2011). The Hepcidin-Ferroportin System as a Therapeutic Target in Anemias and Iron Overload Disorders. Hematol. Am Soc Hematol Educ Program 2011, 538–542. doi:10.1182/asheducation-2011.1.538
Garrido-Castro, A. C., Lin, N. U., and Polyak, K. (2019). Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 9 (2), 176–198. doi:10.1158/2159-8290.CD-18-1177
Gnant, M., Sestak, I., Filipits, M., Dowsett, M., Balic, M., Lopez-Knowles, E., et al. (2015). Identifying Clinically Relevant Prognostic Subgroups of Postmenopausal Women with Node-Positive Hormone Receptor-Positive Early-Stage Breast Cancer Treated with Endocrine Therapy: a Combined Analysis of ABCSG-8 and ATAC Using the PAM50 Risk of Recurrence Score and Intrinsic Subtype. Ann. Oncol. 26 (8), 1685–1691. doi:10.1093/annonc/mdv215
Grosselin, K., Durand, A., Marsolier, J., Poitou, A., Marangoni, E., Nemati, F., et al. (2019). High-throughput Single-Cell ChIP-Seq Identifies Heterogeneity of Chromatin States in Breast Cancer. Nat. Genet. 51 (6), 1060–1066. doi:10.1038/s41588-019-0424-9
Guo, W., Zhang, S., Chen, Y., Zhang, D., Yuan, L., Cong, H., et al. (2015). An Important Role of the Hepcidin-Ferroportin Signaling in Affecting Tumor Growth and Metastasis. Acta Biochim. Biophys. Sin 47 (9), 703–715. doi:10.1093/abbs/gmv063
Hentze, M. W., Muckenthaler, M. U., Galy, B., and Camaschella, C. (2010). Two to Tango: Regulation of Mammalian Iron Metabolism. Cell 142 (1), 24–38. doi:10.1016/j.cell.2010.06.028
Hider, R. C., and Maret, W. (2015). Iron and Zinc Sensing in Cells and the Body. Metallomics 7 (2), 200–201. doi:10.1039/c4mt90051k
Ibraheem, A., Olopade, O. I., and Huo, D. (2020). Propensity Score Analysis of the Prognostic Value of Genomic Assays for Breast Cancer in Diverse Populations Using the National Cancer Data Base. Cancer 126 (17), 4013–4022. doi:10.1002/cncr.32956
Jiang, X. P., Yang, D. C., Elliott, R. L., and Head, J. F. (2011). Down-regulation of Expression of Interleukin-6 and its Receptor Results in Growth Inhibition of MCF-7 Breast Cancer Cells. Anticancer Res. 31 (9), 2899–2906.
Kalinowski, D. S., Stefani, C., Toyokuni, S., Ganz, T., Anderson, G. J., Subramaniam, N. V., et al. (2016). Redox Cycling Metals: Pedaling Their Roles in Metabolism and Their Use in the Development of Novel Therapeutics. Biochim. Biophys. Acta (Bba) - Mol. Cel Res. 1863 (4), 727–748. doi:10.1016/j.bbamcr.2016.01.026
Li, W.-Z., Hua, X., Lv, S.-H., Liang, H., Liu, G.-Y., Lu, N., et al. (2021). A Scoring System Based on Nutritional and Inflammatory Parameters to Predict the Efficacy of First-Line Chemotherapy and Survival Outcomes for De Novo Metastatic Nasopharyngeal Carcinoma. Jir 14, 817–828. doi:10.2147/JIR.S296710
Li, X., Yang, J., Peng, L., Sahin, A. A., Huo, L., Ward, K. C., et al. (2017). Triple-negative Breast Cancer Has Worse Overall Survival and Cause-specific Survival Than Non-triple-negative Breast Cancer. Breast Cancer Res. Treat. 161 (2), 279–287. doi:10.1007/s10549-016-4059-6
List, A. F., Baer, M. R., Steensma, D. P., Raza, A., Esposito, J., Martinez-Lopez, N., et al. (2012). Deferasirox Reduces Serum Ferritin and Labile Plasma Iron in RBC Transfusion-dependent Patients with Myelodysplastic Syndrome. Jco 30 (17), 2134–2139. doi:10.1200/JCO.2010.34.1222
Martin-Sanchez, D., Ruiz-Andres, O., Poveda, J., Carrasco, S., Cannata-Ortiz, P., Sanchez-Niño, M. D., et al. (2017). Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid-Induced AKI. Jasn 28 (1), 218–229. doi:10.1681/asn.2015121376
Masjedi, A., Hashemi, V., Hojjat-Farsangi, M., Ghalamfarsa, G., Azizi, G., Yousefi, M., et al. (2018). The Significant Role of Interleukin-6 and its Signaling Pathway in the Immunopathogenesis and Treatment of Breast Cancer. Biomed. Pharmacother. 108, 1415–1424. doi:10.1016/j.biopha.2018.09.177
Miklikova, S., Minarik, G., Sedlackova, T., Plava, J., Cihova, M., Jurisova, S., et al. (2020). Inflammation-Based Scores Increase the Prognostic Value of Circulating Tumor Cells in Primary Breast Cancer. Cancers (Basel) 12 (5), 1134. doi:10.3390/cancers12051134
Morales, M., and Xue, X. (2021). Targeting Iron Metabolism in Cancer Therapy. Theranostics 11 (17), 8412–8429. doi:10.7150/thno.59092
Neufeld, E. J., Galanello, R., Viprakasit, V., Aydinok, Y., Piga, A., Harmatz, P., et al. (2012). A Phase 2 Study of the Safety, Tolerability, and Pharmacodynamics of FBS0701, a Novel Oral Iron Chelator, in Transfusional Iron Overload. Blood 119 (14), 3263–3268. doi:10.1182/blood-2011-10-386268
Nutting, C. M., van Herpen, C. M. L., Miah, A. B., Bhide, S. A., Machiels, J.-P., Buter, J., et al. (2009). Phase II Study of 3-AP Triapine in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Ann. Oncol. 20 (7), 1275–1279. doi:10.1093/annonc/mdn775
Poorvu, P. D., Gelber, S. I., Rosenberg, S. M., Ruddy, K. J., Tamimi, R. M., Collins, L. C., et al. (2020). Prognostic Impact of the 21-Gene Recurrence Score Assay Among Young Women with Node-Negative and Node-Positive ER-Positive/HER2-Negative Breast Cancer. Jco 38 (7), 725–733. doi:10.1200/JCO.19.01959
Proneth, B., and Conrad, M. (2019). Ferroptosis and Necroinflammation, a yet Poorly Explored Link. Cell Death Differ 26 (1), 14–24. doi:10.1038/s41418-018-0173-9
Radulescu, S., Brookes, M. J., Salgueiro, P., Ridgway, R. A., McGhee, E., Anderson, K., et al. (2016). Luminal Iron Levels Govern Intestinal Tumorigenesis after Apc Loss In Vivo. Cel Rep. 17 (10), 2805–2807. doi:10.1016/j.celrep.2016.10.028
Stockwell, B. R., Friedmann Angeli, J. P., Bayir, H., Bush, A. I., Conrad, M., Dixon, S. J., et al. (2017). Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171 (2), 273–285. doi:10.1016/j.cell.2017.09.021
Sun, Y., Chen, P., Zhai, B., Zhang, M., Xiang, Y., Fang, J., et al. (2020). The Emerging Role of Ferroptosis in Inflammation. Biomed. Pharmacother. 127, 110108. doi:10.1016/j.biopha.2020.110108
Tiainen, S., Rilla, K., Hämäläinen, K., Oikari, S., and Auvinen, P. (2021). The Prognostic and Predictive Role of the Neutrophil-To-Lymphocyte Ratio and the Monocyte-To-Lymphocyte Ratio in Early Breast Cancer, Especially in the HER2+ Subtype. Breast Cancer Res. Treat. 185 (1), 63–72. doi:10.1007/s10549-020-05925-7
Torti, S. V., Manz, D. H., Paul, B. T., Blanchette-Farra, N., and Torti, F. M. (2018). Iron and Cancer. Annu. Rev. Nutr. 38, 97–125. doi:10.1146/annurev-nutr-082117-051732
Tsurusaki, S., Tsuchiya, Y., Koumura, T., Nakasone, M., Sakamoto, T., Matsuoka, M., et al. (2019). Hepatic Ferroptosis Plays an Important Role as the Trigger for Initiating Inflammation in Nonalcoholic Steatohepatitis. Cell Death Dis 10 (6), 449. doi:10.1038/s41419-019-1678-y
Umemura, M., Kim, J.-H., Aoyama, H., Hoshino, Y., Fukumura, H., Nakakaji, R., et al. (2017). The Iron Chelating Agent, Deferoxamine Detoxifies Fe(Salen)-Induced Cytotoxicity. J. Pharmacol. Sci. 134 (4), 203–210. doi:10.1016/j.jphs.2017.07.002
van der Willik, K. D., Koppelmans, V., Hauptmann, M., Compter, A., Ikram, M. A., and Schagen, S. B. (2018). Inflammation Markers and Cognitive Performance in Breast Cancer Survivors 20 Years after Completion of Chemotherapy: a Cohort Study. Breast Cancer Res. 20 (1), 135. doi:10.1186/s13058-018-1062-3
von Hagens, C., Walter-Sack, I., Goeckenjan, M., Osburg, J., Storch-Hagenlocher, B., Sertel, S., et al. (2017). Prospective Open Uncontrolled Phase I Study to Define a Well-Tolerated Dose of Oral Artesunate as Add-On Therapy in Patients with Metastatic Breast Cancer (ARTIC M33/2). Breast Cancer Res. Treat. 164 (2), 359–369. doi:10.1007/s10549-017-4261-1
Wang, X., Wang, S.-S., Huang, H., Cai, L., Zhao, L., Peng, R.-J., et al. (2021). Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs Observation on Disease-free Survival Among Patients with Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment. Jama 325 (1), 50–58. doi:10.1001/jama.2020.23370
Wileyto, E. P., Li, Y., Chen, J., and Heitjan, D. F. (2013). Assessing the Fit of Parametric Cure Models. Biostatistics 14 (2), 340–350. doi:10.1093/biostatistics/kxs043
Yamasaki, T., Terai, S., and Sakaida, I. (2011). Deferoxamine for Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 365 (6), 576–578. doi:10.1056/nejmc1105726
Yang, Y.-w., Dai, C.-m., Chen, X.-h., and Feng, J.-f. (2021). The Relationship between Serum Trace Elements and Oxidative Stress of Patients with Different Types of Cancer. Oxidative Med. Cell Longevity 2021, 1–13. doi:10.1155/2021/4846951
Zhou, Z., Ye, T. J., DeCaro, E., Buehler, B., Stahl, Z., Bonavita, G., et al. (2020). Intestinal SIRT1 Deficiency Protects Mice from Ethanol-Induced Liver Injury by Mitigating Ferroptosis. Am. J. Pathol. 190 (1), 82–92. doi:10.1016/j.ajpath.2019.09.012
Keywords: early-stage triple-negative breast cancer, serum iron level, monocyte-tolymphocyte ratio, predictive nomogram, survival
Citation: Duan F, Zhong M, Ye J, Wang L, Jiang C, Yuan Z, Bi X and Huang J (2022) The Iron-Inflammation Axis in Early-Stage Triple-Negative Breast Cancer. Front. Cell Dev. Biol. 10:784179. doi: 10.3389/fcell.2022.784179
Received: 27 September 2021; Accepted: 09 February 2022;
Published: 23 February 2022.
Edited by:
Chang Gong, Sun Yat-sen University, ChinaReviewed by:
Margarida Barroso, Albany Medical College, United StatesTira Tan, National Cancer Centre Singapore, Singapore
Copyright © 2022 Duan, Zhong, Ye, Wang, Jiang, Yuan, Bi and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiwen Bi, bixw@sysucc.org.cn; Jiajia Huang, huangjiaj@sysucc.org.cn
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Fangfang Duan
Fangfang Duan Muyi Zhong2†
Muyi Zhong2†  Xiwen Bi
Xiwen Bi