An Opinion on How Nanobiotechnology is Assisting Humankind to Overcome the Coronavirus Disease 2019
- Department of Applied Science and Technology, Politecnico di Torino, Turin, Italy
Introduction
Analyzing results from a PubMed search done at the end of March 2022, more than 550,000 scientific publications relating to “Biotechnology” have been published. At the same time, if searching for “Nanobiotechnology,” it can be seen that more than 7,100 publications have been written in the last 20 years, with an ever-increasing annual publication rate. Nanobiotechnology, a relatively young branch of knowledge, is the crossing point between bio and nano technology, and comprises a wide range of nanotechnological applications in the life sciences field. In the last 2 years, just one hundred years after the last Pandemic Flu, humanity has sadly been hit by one of the worst infectious disease emergences of modern times. We believe that only in conjunction with this terrible health crisis have the real power of what are commonly recognized as Nanobiotechnologies been realized. Since the onset of the pandemic, these technologies have been the subject of heated discussions for scientists, clinicians, journalists, politicians, and ordinary people. For instance, next to the most accredited zoonotic transfer origin of SARS-CoV-2 (Andersen et al., 2020), it was even speculated (Casadevall et al., 2021) to have a nanobiotechnological origin in one of the biosafety level-4 biological laboratories of the Wuhan Institute of Virology.
Although there is still no way to reach unquestionable conclusions on the origins of this pandemic, allowing us to establish how much was due to chance, nature, or to the fast development of human technologies, in this opinion we will prove how much nanobiotechnology is doing in assisting the whole scientific community in quickly and effectively resolving this global health crisis. We want to highlight how much nanobiotechnology is contributing in such a short time with nanomaterials and technology to the development of safe and efficient solutions to prevent, diagnose, and treat COVID-19 and any infections of the same nature.
Why and How to Use “Nano” to Assist “Bio”
For at least a couple of decades, technology and nanoscience have demonstrated an important role in the design of effective solutions for biomedical applications, offering new methods and materials. Due to their dimensions ranging from a few to hundreds of nanometers, nanomaterials, whether of biological, inorganic, or synthetic origin, demonstrate exclusive performances (Xiao et al., 2022) as core elements or components of a wide range of nanotechnological solutions to various kinds of diseases.
Nanobioengineered solutions, including nanomaterials and nanovehicles, are ever more frequently used for medical microbiology, tissue repair and regeneration, drug delivery, diagnosis, and treatment of inflammatory and cancer diseases (Susa et al., 2019; Contera et al., 2020). Furthermore, a wide range of micro and nanofabrication processes allows the design and fabrication of highly sensitive sensors and transistor-based biosensors able to detect and analyze multicomponent mixtures from human samples for early diagnosis or real-time continuous monitoring (Coluccio et al., 2015; Nagamine and Tokito, 2021; Park et al., 2021; Sung et al., 2021; Finnegan et al., 2022; Kaloumenou et al., 2022).
The impact of the use of nanotechnologies and of their related and waste products must be assessed based on both people and the environment. In agreement with most of our colleagues, we believe that nanobiotechnology-based solutions deserve special administrative rules (Osman, 2019; Allan et al., 2021; Carlander and Skentelbery, 2021). Although regulatory organizations, such as the Global Coalition for Regulatory Science Research, the European Commission’s Joint Research Centre, the United States Environmental Protection Agency and the U.S. Food and Drug Administration, have begun to regulate the potential problems arising from the use of nanotechnologies, there is still an urgent need to define universally shared toxicity standards and updated regulatory guidelines (Subhan and Subhan, 2022).
Has the Nanotechnological Contribution Made a Difference in the Fight Against COVID-19 Compared to Other Epidemics?
Among the various pathologies, infectious ones involve a very high incidence rate and, obviously, actions against these diseases, such as human immunodeficiency virus, malaria, and tuberculosis, are particularly challenging, considering their unending spread and high mortality rate (Kirtane et al., 2021).
More than 2 years ago, humanity was confronted by the SARS-CoV-2 virus that caused COVID-19 disease. There is no question that this tragic event has radically changed the lives of billions of people, since it rapidly spread everywhere, leading to the closure of public and private facilities, filling hospitals, and shutting down worldwide economies and social activities. Professionals from different backgrounds joined forces to try to stem this pandemic with all the losses in human and socio-economic life that it entailed. The massive investigation effort made for COVID-19 prevention, diagnosis, and treatment started 2 years ago, is still ongoing, and should be recognized as a prodigious success since it is substantially contributing to this pandemic disease becoming endemic.
Many solutions, such as vaccine production and medication repurposing, have been adopted and, among the various strategies used, nanotechnology undoubtedly has provided better solutions in the implementation of valid preventive and diagnostic actions, such as through the design of faster and more effective treatments (Weiss et al., 2020).
A very recent (March 2022) query, done in the Web of Science Core Collection, for the string “COVID-19 nanotechnology” provided more than 400 papers published between 2020 and 2022. By refining the search, adding respectively the words “prevention,” “diagnostics,” and “treatments,” we noticed that about 10% concern prevention application, 40% diagnostics, and 50% treatments. This bibliographic research had clearly shown how, in just 2 years, a remarkable variety of nanotechnological approaches assisted scientific and medical communities in developing nanomaterials for the implementation of disinfection systems, molecular or antigenic diagnosis kits, antiviral drugs, and nanoarchitectured vaccines (Ruiz-Hitzky et al., 2020).
Have Patents, During the COVID-19 Emergency, Promoted DISCLOSURE OF INVENTIONS, REDUCING DUPLICATION OF RESEARCH, COSTS OF RESEARCh, and bargaining?
Given the exceptional number of scientific publications produced on the just introduced topic by colleagues in these pandemic years, in this opinion article for the Nanobiotechnology section of the journal Frontiers in Bioengineering and Biotechnology, we focused on one of the most important engineering and technological aspects of the discussion, patents.
One of the first works on this aspect of the pandemic is the one in which Ruiz-Hitzky and others provided an indication on patented work related to nanotechnology applied to coronaviruses (Ruiz-Hitzky et al., 2020). They carried out a systematic search on the distribution of patents dealing with SARS-CoV viruses (comprising SARS-CoV and SARS-CoV-2) by using the “coronavirus and nano” topics to refine the query in the ESPACENET patent website. Since their article was published in September 2020 and their ESPACENET database query focused generically on the terms “coronavirus and nano,” on April 2022, we did a new search on both VaxPal and ESPACENET websites, looking for patents related to the applications of nanobiotechnologies for COVID-19 prevention, vaccines, diagnosis, and treatments. The results confirmed that, in all the application areas of our interest such as prevention, diagnostic, and therapeutic, nanotechnologies have given and are greatly assisting in the successful management of a sudden and unexpected global emergency like the COVID-19 pandemic. More in detail, as summarized in Table 1, considering the 45 resulting patents, four refer to prevention, 20 to vaccines development, 14 to diagnostics, and seven to therapeutic applications. Most of them were filed by companies and academic institutes (18 and 16 patents, respectively), eight by private organizations, and three by governmental entities.
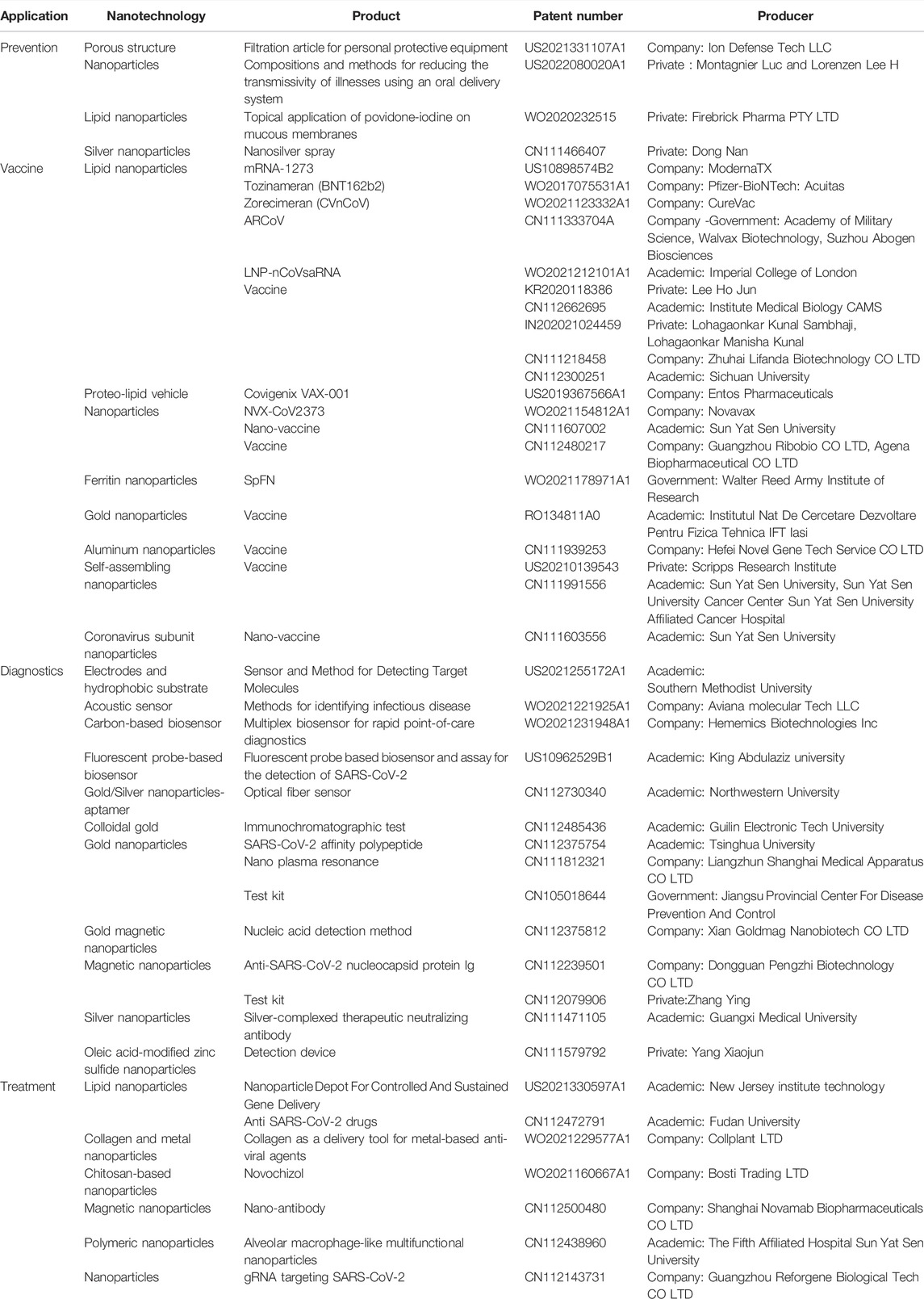
TABLE 1. Patents related to the applications of nanobiotechnologies for COVID-19 prevention, vaccines, diagnosis, and treatments. Data from Tavares et al. (2022) and from a query on VaxPal and ESPACENET websites carried out on April 2022.
Can a Fluid and Transversal Network Between Different Skills Favor a Faster and More Effective Management of Health Emergencies Such as the One We Have Been Suffering Worldwide in Recent Years?
In this opinion piece we tried to highlight how, in nanobiotechnology, the intercommunication of skills has allowed technology to design and apply nanoscale solutions for biological applications. Coronavirus prevention, recognition, and handling technologies represent the central weaponries in the fight against Covid diseases. Patent worldwide status mirrors the innovation’s grade in the nanobiotechnological field. According to our opinion, in addition to what has already been designed and implemented, the most ambitious objectives will certainly be achieved with the development of integrated hybrid systems. Recent progresses in nanoscience, smart materials, wireless expertise, and wearable integrated circuit technology allowed Collins and others to engineer a face mask capable of both functioning as an individual protection device and, at the same time, sensing possible viral particles in the wearer’s breath (Nguyen et al., 2021).
We believe that, in the wake of what is already in progress in the nanobiotechnological field with the development of advanced lab on chip and smart nanofeatured devices, it will be possible to develop solutions capable of quickly giving reliable and reproducible results even in personalized medicine, both in diagnostics and in therapy.
We would like to give our opinion on how it could be possible to integrate, on a single microchip, some nano-technologies, nano-features, and nanosystems including thin films and 3D printing for fluidics, separations as molecular and cellular sorting, nucleic acids sequencing, manipulation and genomics, proteomics, or metabolomics for targeted analysis.
Referring to infectious diseases, such as COVID-19, through a simple prick on a finger, using a nanofeatured chip, it could be possible to carry out both the diagnosis and the eventually related post-exposure prophylaxis since testing the presence of the viral RNA or protein simultaneously and estimating how long before the infection started, some therapies, like the one based on monoclonal antibodies, which are efficient only if started before the recommended terms (Taylor et al., 2021), can be administered.
To conclude, although much progress has been made in biotechnological development towards the nanotechnological approach, much still needs to be done in regards to the safety, regulatory, and policy perspective. In our opinion, this could be done by trying to fill the lack of global standardization in the application of robust methods and in the characterization of reference materials through the combination of the needs of consumers/patients and of the skills of scientists, clinics, engineers, jurists, standardization bodies, and companies.
Author Contributions
TL: conceptualization, hypothesis, discussion, writing, and revision. FS: discussion, writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the starting grant of the Politecnico of Torino to new fixed term researchers in strategic research fields and by the Frontiers Fee Support program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allan, J., Belz, S., Hoeveler, A., Hugas, M., Okuda, H., Patri, A., et al. (2021). Regulatory Landscape of Nanotechnology and Nanoplastics from a Global Perspective. Regul. Toxicol. Pharmacol. 122, 104885. doi:10.1016/j.yrtph.2021.104885
Andersen, K. G., Rambaut, A., Lipkin, W. I., Holmes, E. C., and Garry, R. F. (2020). The Proximal Origin of SARS-CoV-2. Nat. Med. 26 (4), 450–452. doi:10.1038/s41591-020-0820-9
Carlander, D., and Skentelbery, C. (2021). “EU Regulations and Nanotechnology Innovation,” in Nanotoxicology in Humans and the Environment (Springer), 229–248. doi:10.1007/978-3-030-79808-6_8
Casadevall, A., Weiss, S. R., and Imperiale, M. J. (2021). Can Science Help Resolve the Controversy on the Origins of the SARS-CoV-2 Pandemic? mBio 12 (4), e01948–01921. doi:10.1128/mBio.01948-21
Coluccio, M. L., Gentile, F., Das, G., Nicastri, A., Perri, A. M., Candeloro, P., et al. (2015). Detection of Single Amino Acid Mutation in Human Breast Cancer by Disordered Plasmonic Self-Similar Chain. Sci. Adv. 1 (8), e1500487. doi:10.1126/sciadv.1500487
Contera, S., Bernardino de la Serna, J., and Tetley, T. D. (2020). Biotechnology, Nanotechnology and Medicine. Emerg. Top. Life Sci. 4 (6), 551–554. doi:10.1042/etls20200350
Finnegan, M., Duffy, E., and Morrin, A. (2022). The Determination of Skin Surface pH via the Skin Volatile Emission Using Wearable Colorimetric Sensors. Sens. Bio-Sensing Res. 35, 100473. doi:10.1016/j.sbsr.2022.100473
Kaloumenou, M., Skotadis, E., Lagopati, N., Efstathopoulos, E., and Tsoukalas, D. (2022). Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors. Sensors 22 (3), 1238. doi:10.3390/s22031238
Kirtane, A. R., Verma, M., Karandikar, P., Furin, J., Langer, R., and Traverso, G. (2021). Nanotechnology Approaches for Global Infectious Diseases. Nat. Nanotechnol. 16 (4), 369–384. doi:10.1038/s41565-021-00866-8
Nagamine, K., and Tokito, S. (2021). Organic-transistor-based Biosensors Interfaced with Human Skin for Non-invasive Perspiration Analysis. Sensors Actuators B Chem. 349, 130778. doi:10.1016/j.snb.2021.130778
Nguyen, P. Q., Soenksen, L. R., Donghia, N. M., Angenent-Mari, N. M., de Puig, H., Huang, A., et al. (2021). Wearable Materials with Embedded Synthetic Biology Sensors for Biomolecule Detection. Nat. Biotechnol. 39 (11), 1366–1374. doi:10.1038/s41587-021-00950-3
Osman, E. M. (2019). Environmental and Health Safety Considerations of Nanotechnology, Nano Safety. Biomed. J. Sci. Tech. Res. 19 (4), 14501–14515. doi:10.26717/bjstr.2019.19.003346
Park, H., Park, W., and Lee, C. H. (2021). Electrochemically Active Materials and Wearable Biosensors for the In Situ Analysis of Body Fluids for Human Healthcare. NPG Asia Mater 13 (1), 23. doi:10.1038/s41427-020-00280-x
Ruiz‐Hitzky, E., Darder, M., Wicklein, B., Ruiz‐Garcia, C., Martín‐Sampedro, R., Del Real, G., et al. (2020). Nanotechnology Responses to COVID‐19. Adv. Healthc. Mater. 9 (19), 2000979.
Subhan, M. A., and Subhan, T. (2022). “Safety and Global Regulations for Application of Nanomaterials,” in Chapter 5 - Safety and Global Regulations for Application of nanomaterialsNanomaterials Recycling. Editors M. Rai, and T. A. Nguyen (Elsevier), 83–107. doi:10.1016/b978-0-323-90982-2.00005-6
Sung, W.-H., Tsao, Y.-T., Shen, C.-J., Tsai, C.-Y., and Cheng, C.-M. (2021). Small-volume Detection: Platform Developments for Clinically-Relevant Applications. J. Nanobiotechnol 19 (1), 114. doi:10.1186/s12951-021-00852-1
Susa, F., Limongi, T., Dumontel, B., Vighetto, V., and Cauda, V. (2019). Engineered Extracellular Vesicles as a Reliable Tool in Cancer Nanomedicine. Cancers 11 (12), 1979. doi:10.3390/cancers11121979
Tavares, J. L., Cavalcanti, I. D. L., Santos Magalhães, N. S., and Lira Nogueira, M. C. d. B. (2022). Nanotechnology and COVID-19: Quo Vadis? J. Nanopart Res. 24 (3), 62. doi:10.1007/s11051-022-05452-0
Taylor, P. C., Adams, A. C., Hufford, M. M., de la Torre, I., Winthrop, K., and Gottlieb, R. L. (2021). Neutralizing Monoclonal Antibodies for Treatment of COVID-19. Nat. Rev. Immunol. 21 (6), 382–393. doi:10.1038/s41577-021-00542-x
Weiss, C., Carriere, M., Fusco, L., Capua, I., Regla-Nava, J. A., Pasquali, M., et al. (2020). Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 14 (6), 6383–6406. doi:10.1021/acsnano.0c03697
Keywords: COVID-19, SARS-CoV-2, nanobiotechnology, health emergencies, prevention, diagnostics, treatments, patents
Citation: Limongi T and Susa F (2022) An Opinion on How Nanobiotechnology is Assisting Humankind to Overcome the Coronavirus Disease 2019. Front. Bioeng. Biotechnol. 10:916165. doi: 10.3389/fbioe.2022.916165
Received: 08 April 2022; Accepted: 10 May 2022;
Published: 13 June 2022.
Edited by:
Gianni Ciofani, Italian Institute of Technology (IIT), ItalyReviewed by:
Fernando Soto, Stanford University, United StatesFlavia Vitale, University of Pennsylvania, United States
Copyright © 2022 Limongi and Susa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tania Limongi, tania.limongi@polito.it
 Tania Limongi
Tania Limongi Francesca Susa
Francesca Susa