Transglutaminase-Catalyzed Bottom-Up Synthesis of Polymer Hydrogel
- Guangxi Key Laboratory of Green Processing of Sugar Resources, College of Biological and Chemical Engineering, Guangxi University of Science and Technology, Liuzhou, China
Enzyme catalysis has attracted increasing attention for application in the synthesis of polymer hydrogel due to the eco-friendly process and the devisable catalytic reaction. Moreover, bottom-up approaches combining enzyme catalysts and molecular self-assembly have been explored for synthesizing hydrogel with complex architectures. An enzyme widely distributed in nature, transglutaminase (TGase) has been confirmed to catalyze the formation of isopeptide bonds between proteins, which can effectively improve the gelation of proteins. In this mini-review, TGase-catalyzed synthesis of polymer hydrogels, including fibrin hydrogels, polyethylene glycol hydrogels, soy protein hydrogels, collagen hydrogels, gelatin hydrogels and hyaluronan hydrogels, has been reviewed in detail. The catalytic process and gel formation mechanism by TGase have also been considered. Furthermore, future perspectives and challenges in the preparation of polymer hydrogels by TGase are also highlighted.
Introduction
Enzymes are biological catalysts essential for life processes and catalyze many vital reactions selectively in nature and living cells (Behabtu and Kralj, 2020). For materials synthesis, natural polymer materials such as proteins and polysaccharides are synthesized utilizing enzymes in nature. Similarly, synthetic polymer materials including polyesters, polycarbonates, and polyphosphates have also been synthesized using enzyme-catalyzed processes (Varma et al., 2005). For biomedical application, natural and synthetic polymer materials with desired molecular architecture and biocompatibility are required to meet the specific needs (Yan et al., 2020). Since enzyme catalysis has proven to be an effective synthetic method for polymers with complex architectures and mimicking extracellular matrices, increasing interest in the enzyme-catalyzed synthesis strategy has been developed.
As a biomedical polymer material, hydrogels with a three-dimensional (3D) network structure can mimic cell and tissue culture environments, leading to application in tissue engineering, wound healing, cartilage repair and other fields (Sharma and Tiwari, 2020). Additionally, hydrogels can provide multidimensional ion or biologically active molecules transport pathways for the delivery and controlled release (Lai et al., 2019; Cui et al., 2021). Although hydrogels have been traditionally synthesized using chemical and physical methods, rapid developments in enzyme-catalyzed synthesis technology are emerging. Compared with physical and chemical crosslinking methods, enzyme-catalyzed reactions can react with target molecules directly in mild condition and mimic in vivo biosynthetic processes, making the active molecules safer during the hydrogel synthesis process (Li et al., 2020).
TGase have received increasing attention due to the ability to catalyse the coupling of a free lysine amine group from a protein or peptide bond to a deamidated glutamine protein or peptide-bound γ-carboxamide group (Guebitz and Nyanhongo, 2018). These are important catalysts for the formation of protein-based hydrogels with varying tunable properties, and remarkable achievements of hydrogels synthesized by TGase based on natural and synthetic molecules have been made. The present paper reviews the TGase-catalyzed reactions for the synthesis of polymer hydrogel with a bottom-up strategy. The catalytic crosslinking and gelation mechanism of TGase is discussed, and future perspectives and challenges in the preparation of polymer hydrogel by TGase-catalytic reaction are also provided.
Transglutaminase-Catalytic Process and Gel Formation Mechanism
Sources and Characteristics of Transglutaminase
Transglutaminase (TGase) is an acyltransferase, which catalyzes the amide-transferase reaction between the γ-amyl group of glutamine residue and the ε-amino group of lysine in protein, resulting in form the ε- (γ-glutamine) -lysine heteromorphic peptide bond (Fisher et al., 2017). It can also make polymers containing amino and glutamate functional groups undergo cross-linking reactions (Maddock et al., 2020). TGase is widely distributed in nature and can be isolated and extracted from animals, plants and microorganisms. Notably, the coagulation factor XIII in human blood is also a kind of TGase. With the enzyme, fibrin stabilizing molecules were formed by the cross-linking reaction between fibrin molecules, leading to blood coagulation (Schmitz et al., 2020). Therefore, TGase has a unique effect in catalyzing cross-linking of protein molecules. TGase from animal tissue has few sources, low yield, complex separation and purification process. More importantly, the TGase from animal tissue cannot show its catalytic activity without calcium ions (Savoca et al., 2018). Compared with the TGase from animal tissue, TGase derived from microorganisms (microbial transglutaminase, MTGase) can be directly secreted into the culture medium, which is easy to separate and purify with cheap raw materials and a short production cycle (Xue et al., 2020). For use, MTGases can show their activities without calcium ions and have a wide range of optimum temperatures and pH values, which have advantages over the TGase from animal issues.
Transglutaminase-Induced Gel Formation Mechanism
Due to the excellent properties of TGase, it can be used to modify the protein. The catalytic cross-linking effect is beneficial to the gelation of protein molecules, leading to improving elasticity, adhesion, water retention and other qualities of the protein (Gharibzahedi et al., 2018). For protein molecules, the catalytic effect of TGase is mainly by three ways, including amine import, cross-linking and deamination. TGase catalyses the acyl transfer reaction between glutamine residues γ-carbonyl and primary amine in the peptide bond, introducing lysine into the protein (Fatima and Khare, 2018). When the lysine residue γ-amino is used as the acyl receptor, the covalent bond of ε- (γ-glutamyl) lysine is formed between protein molecules, which means the protein molecules are crosslinked. In addition, water can become the acyl receptor in the absence of primary amines, and the γ-amino group is deaminated into glutamate residues (Miwa, 2020).
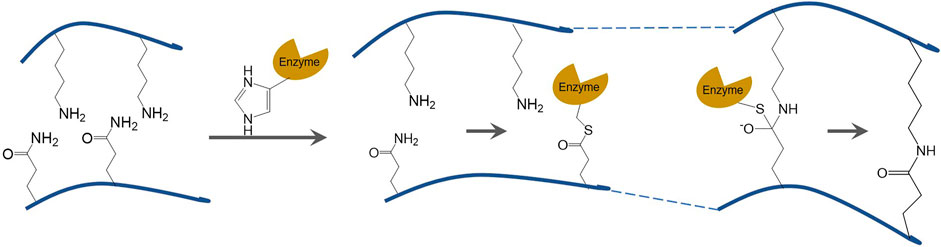
A gel is a substance with a specific spatial structure formed by the aggregation of denatured protein molecules. At the microscopic level, the gel is represented by the equilibrium of attraction and repulsion between protein molecules. It is represented by the formation of semi-solid inverted colloidal substances without flow macroscopically (Wang J. et al., 2021). In general, the covalent crosslinking reaction catalyzed by TGase occurs faster than the acyl transfer and deamidation reactions. Therefore, TGase can induce the formation of highly elastic and irreversible gel structures even at low protein concentrations (Duarte et al., 2020). Typically, TGase catalyzes the formation of ε-(γ-glutamyl) lysine covalent bond between the γ-hydroxylamine group of glutamine residues in soybean protein and the ε-amino group on lysine in wheat protein (Qin et al., 2017). Figure 1 depicts the schematic diagram of TGase catalyzed crosslinking reaction, and the formation of covalent bonds between a free amine group and the γ-carboxamide group. These covalent bonds are highly resistant to proteolytic degradation and more stable than the hydrogen bond and disulfide bond, resulting in the formation of stable polymeric networks.
Transglutaminase Catalysed Formation of Polymer Hydrogel
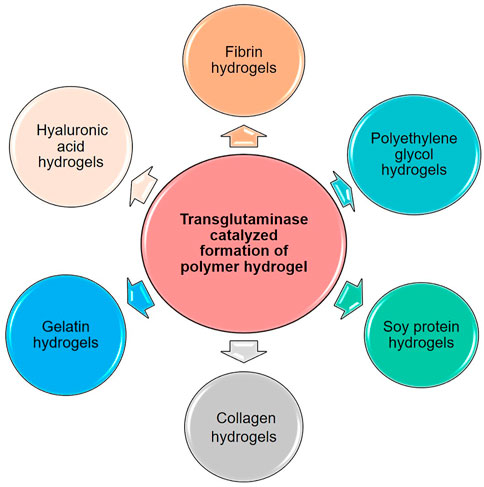
Based on the characteristics of the transglutaminase, various macromolecules with glutamine and lysine have been fabricated into polymer hydrogels via transglutaminase catalyzed reaction. Here, the main polymer hydrogels prepared by TGase catalyzed crosslinking are discussed in detail, as shown in Figure 2.
Fibrin Hydrogels
Fibrin is a biopolymer that the end product of the physiological blood coagulation cascade, which plays a pivotal role in wound healing (Heher et al., 2018). Under physiological conditions, thrombin activates the transglutaminase Factor XIII (FXIIIa) and can further stabilizes the clot by cross-linking of fibrin polymers (Pieters and Wolberg, 2019). Therefore, fibrin hydrogels can be formed via the combination of fibrinogen and thrombin mixtures (Nelson and Gilbert, 2021). Abrego et al. (2022) compared the elastic properties of fibrin hydrogels and polyethylene glycol (PEG) hydrogels, and found an increase in the elasticity of the hydrogel with higher concentrations of fibrin and PEG, respectively. However, in vitro showed fast biodegradability and moderate mechanical properties, which limited the application in tissue engineering. Development of fibrin based composite hydrogels supplemented with different types polymer materials could be an effective strategy. Recently, Wang K. et al. (2021) combined cellulose nanocrystals (CNC) with fibrinogen and obtained CNC reinforced fibrin nanocomposite hydrogel, and the obtained hydrogel with improved mechanical stability showed an improvement in the formation of long myotubes (up to 800 μm).
Polyethylene Glycol Hydrogels
PEG is a water-soluble and nontoxic synthetic polymer that has been broadly explored for biomedical applications. The coagulation factor XIII can prepare PEG hydrogel under physiological conditions without the addition of chemical initiators, one PEG precursor with a glutamine-containing sequence and the other one with a lysine-containing sequence can be coupled by factor XIII (Anjum et al., 2016). To overcome limitations of cell adhesion in the PEG hydrogel, cell-adhesion peptides (RGD) was utilized to conjugate onto the PEG polymer. The modified PEG hydrogels can alter adult dermal progenitor cells behavior and phenotype (Sparks et al., 2019). To advance the translation of human tissue analogues to the clinics, a hybrid hydrogel composed of gelatin and PEG were fabricated via factor XIII, and the hybrid hydrogel can be tailored by tuning the ratio between gelatin and PEG, the total polymer concentration and so on (Klotz et al., 2019).
Soy Protein Hydrogels
As a kind of plant protein, soybean protein contains a complete variety of amino acids, especially lysine, essential for the human body. Hydrogels prepared from soybean protein have a broad application prospect in biomedicine, food and other fields. Tian and Liang (2005) used MTGase to promote soy protein isolate solution to form soy protein isolate gel and analyzed the effect of MTGase on soy protein isolate gel by changing enzyme dosage, pH value, reaction temperature, substrate protein concentration and reaction time. Song and Zhang (2008) hydrolyzed soybean protein isolate using MTGase and obtained protein-based hydrogels with adjustable gel time and mechanical strength. The hydrogel has further been applied for the controlled release of 5-amino salicylic acid. Guo et al. (2012) introduced glucan sulfate into soybean globulin to form a high-charge complex with glycine in protein and prepared transparent hydrogel with a stable network structure by MTGase. Comparably, layered montmorillonite nanoclay was intercalated into soy protein to enhance elastic properties of the hydrogel via TGase crosslinking (Jin and Zhong, 2013).
Collagen Hydrogels
Collagen has excellent biocompatibility and biodegradability, and it is the main component of the extracellular matrix (ECM) of interstitial tissues (including skin, bone, cartilage, tendon and ligament). Orban et al. (2004) selectively mediated the chemical reaction between glutamine and lysine residues on protein fibers by MTGase, resulting in bovine collagen type I gel formation by the crosslinking reaction. Temperature-responsive and enzyme-responsive collagen hydrogels by MTGase were synthesized, and the enzyme concentrations could tune the degradation time and biocompatibility of the hydrogels (Zhao et al., 2016). Using 50 U/g MTGase, the prepared hydrogels had a high cross-linking degree and good resistance to collagenase I and collagenase II degradation. Lee et al. (2013) prepared collagen hydrogel with increased stiffness to promote more angiogenic sprouts that invade deeper, and the stiffness independent of ECM collagen concentration can be modulated by MTGase. Humanoid collagen hydrogels and fishbone collagen hydrogels based on MTGase and 1-ethyl-3-methylimidazolium acetate crosslinking were prepared, and the hydrogels could promote the proliferation of fibroblasts and inhibit the proliferation of cancer cells (Li and Fan, 2019).
Gelatin Hydrogels
Gelatin is a protein derived from the hydrolysis of collagen with excellent biocompatibility and biodegradability. MTGase and gelatin can be dissolved in PBS (Phosphate Buffered Saline) solution and prepared gelatin hydrogel by the cross-linked reaction (Ren et al., 2016). Two types of gelatin (type-A and type-B) crosslinked by MTGase with different concentration were compared in physical and mechanical properties, and the type-A gelatin showed the superiority in crosslinking efficiency than type-B (Liu et al., 2020). Gelatin hydrogel encapsulated human adipocyte globules can increase the cell proliferation significantly with low cytotoxicity (Tsai et al., 2020). A sustained-release absorbable hydrogel by combining TGase with gelatin, alginate and antibiotics has developed for local delivery of antibiotics in orthopedic surgery (Sun et al., 2021). Gupta et al. (2021) used gelatin hydrogel prepared by MTGase crosslinking as an engineered skeletal muscle matrix, and the hydrogel was stable after 2 weeks under similar culture conditions of natural skeletal muscle. Tannic acid modified gelatin also can be crosslinked by TGase to form hydrogel, and the hydrogel exhibited comparable elasticity and flexibility, and therapeutic healing effects in the mouse skin incision and wound model in vivo (Zhou et al., 2021).
Hyaluronic Acid Hydrogels
Hyaluronic acid (HA) is a natural polysaccharide in the extracellular matrix of numerous soft connective tissues, and best known for its intrinsic hydrating properties (Zamboni et al., 2021). Broguiere et al. (2016) introduced a hyaluronan hydrogel encapsulated neurons based on high molecular weight hyaluronic acid using activated transglutaminase factor XIII. At the same time, hyaluronic acid moieties was covalently linked to poly (ethylene glycol) macromer by activated transglutaminase factor XIII, leading to the formation of hybrid hydrogels (Ranga et al., 2016). In comparison to pure PEG hydrogel or HA hydrogel, the hybrid hydrogel can afford ideal attributes of both materials with minimizing macrophage infiltration in vivo, which was suited for bone marrow organoid formation (Vallmajo-Martin et al., 2020). Similarly, hyaluronic acid-fibrin hydrogel loaded with lipophilic anti-inflammatory drugs has been formed in situ by factor XIII, and the hydrogel can be a drug delivery system for intra-articluar administration (Storozhylova et al., 2020).
Concluding Remarks
In this work, TGase catalyzed reactions for the synthesis of polymer hydrogels were reviewed. As outlined, various polymer materials including fibrin, polyethylene glycol, soybean protein, collagen, gelatin and hyaluronic acid have been successfully catalyzed to form hydrogels by the TGase. More importantly, the TGase can offer tight integration between the formed hydrogel and the native tissue, making it feasible to improve their biocompatibility of the hydrogel. Despite the significant advances of using the TGase catalyzed strategy, there is still much room for improvement. For example, the mechanical properties of some hydrogels by TGase are poor or limited for medical application. In particular, large-scale production of hydrogel with high intensity and strength is still a challenge. For the diverse biomedical fields, the polymer hydrogel with functional groups and higher biocompatibility prepared by TGase is deficient. In practical clinical application, the safety and functionality of the hydrogel materials is extremely crucial. It is believed that a massive effort in this exciting field will be made, and continuous breakthroughs will be made with further research. The enzyme catalysis provides a powerful pool to synthesize polymer hydrogel with selective bioprocess, and the enzyme-catalyzed hydrogels are expected to be the next generation of biomaterials for tissue engineering and regenerative medicine.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the Natural Science Foundation of Guangxi Province (No. 2018GXNSFBA281084), the Middle-aged and Young Teachers Basic Ability Promotion Project of Guangxi (No. 2019KY0371) and Science Foundation project of Guangxi University of Science and Technology (No. 19Z01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrego, C. J. G., Dedroog, L., Deschaume, O., Wellens, J., Vananroye, A., Lettinga, M. P., et al. (2022). Multiscale Characterization of the Mechanical Properties of Fibrin and Polyethylene Glycol (PEG) Hydrogels for Tissue Engineering Applications. Macro Chem. Phys. 223 (1), 2100366. doi:10.1002/macp.202100366
Anjum, F., Lienemann, P. S., Metzger, S., Biernaskie, J., Kallos, M. S., and Ehrbar, M. (2016). Enzyme Responsive GAG-Based Natural-Synthetic Hybrid Hydrogel for Tunable Growth Factor Delivery and Stem Cell Differentiation. Biomaterials 87, 104–117. doi:10.1016/j.biomaterials.2016.01.050
Behabtu, N., and Kralj, S. (2020). Enzymatic Polymerization Routes to Synthetic-Natural Materials: A Review. ACS Sustain. Chem. Eng. 8, 9947–9954. doi:10.1021/acssuschemeng.0c01664
Broguiere, N., Isenmann, L., and Zenobi-Wong, M. (2016). Novel Enzymatically Cross-Linked Hyaluronan Hydrogels Support the Formation of 3D Neuronal Networks. Biomaterials 99, 47–55. doi:10.1016/j.biomaterials.2016.04.036
Cui, R., Wu, Q., Wang, J., Zheng, X., Ou, R., Xu, Y., et al. (2021). Hydrogel-By-Design: Smart Delivery System for Cancer Immunotherapy. Front. Bioeng. Biotechnol. 9, 723490. doi:10.3389/fbioe.2021.723490
Duarte, L., Matte, C. R., Bizarro, C. V., and Ayub, M. A. Z. (2020). Review Transglutaminases: Part II-Industrial Applications in Food, Biotechnology, Textiles and Leather Products. World J. Microbiol. Biotechnol. 36, 11. doi:10.1007/s11274-019-2792-9
Fatima, S. W., and Khare, S. K. (2018). Current Insight and Futuristic Vistas of Microbial Transglutaminase in Nutraceutical Industry. Microbiol. Res. 215, 7–14. doi:10.1016/j.micres.2018.06.001
Fisher, S. A., Baker, A. E. G., and Shoichet, M. S. (2017). Designing Peptide and Protein Modified Hydrogels: Selecting the Optimal Conjugation Strategy. J. Am. Chem. Soc. 139 (22), 7416–7427. doi:10.1021/jacs.7b00513
Gharibzahedi, S. M. T., Roohinejad, S., George, S., Barba, F. J., Greiner, R., Barbosa-Cánovas, G. V., et al. (2018). Innovative Food Processing Technologies on the Transglutaminase Functionality in Protein-Based Food Products: Trends, Opportunities and Drawbacks. Trends Food Sci. Techn. 75, 194–205. doi:10.1016/j.tifs.2018.03.014
Guebitz, G. M., and Nyanhongo, G. S. (2018). Enzymes as green Catalysts and Interactive Biomolecules in Wound Dressing Hydrogels. Trends Biotechnol. 36, 1040–1053. doi:10.1016/j.tibtech.2018.05.006
Guo, J., Zhang, Y., and Yang, X.-Q. (2012). A Novel Enzyme Cross-Linked Gelation Method for Preparing Food Globular Protein-Based Transparent Hydrogel. Food Hydrocolloids 26 (1), 277–285. doi:10.1016/j.foodhyd.2011.06.005
Gupta, D., Santoso, J. W., and McCain, M. L. (2021). Characterization of Gelatin Hydrogels Cross-Linked with Microbial Transglutaminase as Engineered Skeletal Muscle Substrates. Bioengineering 8 (1), 6. doi:10.3390/bioengineering8010006
Heher, P., Mühleder, S., Mittermayr, R., Redl, H., and Slezak, P. (2018). Fibrin-based Delivery Strategies for Acute and Chronic Wound Healing. Adv. Drug Deliv. Rev. 129, 134–147. doi:10.1016/j.addr.2017.12.007
Jin, M., and Zhong, Q. (2013). Transglutaminase Cross-Linking to Enhance Elastic Properties of Soy Protein Hydrogels with Intercalated Montmorillonite Nanoclay. J. Food Eng. 115, 33–40. doi:10.1016/j.jfoodeng.2012.09.016
Klotz, B. J., Oosterhoff, L. A., Utomo, L., Lim, K. S., Vallmajo‐Martin, Q., Clevers, H., et al. (2019). A Versatile Biosynthetic Hydrogel Platform for Engineering of Tissue Analogues. Adv. Healthc. Mater. 8 (19), 1900979. doi:10.1002/adhm.201900979
Lai, E., Yue, X., Ning, W. e., Huang, J., Ling, X., and Lin, H. (2019). Three-dimensional Graphene-Based Composite Hydrogel Materials for Flexible Supercapacitor Electrodes. Front. Chem. 7, 660. doi:10.3389/fchem.2019.00660
Lee, P.-F., Bai, Y., Smith, R. L., Bayless, K. J., and Yeh, A. T. (2013). Angiogenic Responses Are Enhanced in Mechanically and Microscopically Characterized, Microbial Transglutaminase Crosslinked Collagen Matrices with Increased Stiffness. Acta Biomater. 9 (7), 7178–7190. doi:10.1016/j.actbio.2013.04.001
Li, P., Zhong, Y., Wang, X., and Hao, J. (2020). Enzyme-regulated Healable Polymeric Hydrogels. ACS Cent. Sci. 6, 1507–1522. doi:10.1021/acscentsci.0c00768
Li, X., and Fan, D. (2019). Smart Collagen Hydrogels Based on 1-Ethyl-3-Methylimidazolium Acetate and Microbial Transglutaminase for Potential Applications in Tissue Engineering and Cancer Therapy. ACS Biomater. Sci. Eng. 5 (7), 3523–3536. doi:10.1021/acsbiomaterials.9b00393
Liu, Y., Weng, R., Wang, W., Wei, X., Li, J., Chen, X., et al. (2020). Tunable Physical and Mechanical Properties of Gelatin Hydrogel after Transglutaminase Crosslinking on Two Gelatin Types. Int. J. Biol. Macromolecules 162, 405–413. doi:10.1016/j.ijbiomac.2020.06.185
Maddock, R. M. A., Pollard, G. J., Moreau, N. G., Perry, J. J., and Race, P. R. (2020). Enzyme-catalysed Polymer Cross-Linking: Biocatalytic Tools for Chemical Biology, Materials Science and beyond. Biopolymers 111, e23390. doi:10.1002/bip.23390
Miwa, N. (2020). Innovation in the Food Industry Using Microbial Transglutaminase: Keys to success and Future Prospects. Anal. Biochem. 597, 113638. doi:10.1016/j.ab.2020.113638
Nelson, D. W., and Gilbert, R. J. (2021). Extracellular Matrix‐Mimetic Hydrogels for Treating Neural Tissue Injury: A Focus on Fibrin, Hyaluronic Acid, and Elastin‐Like Polypeptide Hydrogels. Adv. Healthc. Mater. 10 (22), 2101329. doi:10.1002/adhm.202101329
Orban, J. M., Wilson, L. B., Kofroth, J. A., El-Kurdi, M. S., Maul, T. M., and Vorp, D. A. (2004). Crosslinking of Collagen Gels by Transglutaminase. J. Biomed. Mater. Res. 68A (4), 756–762. doi:10.1002/jbm.a.20110
Pieters, M., and Wolberg, A. S. (2019). Fibrinogen and Fibrin: an Illustrated Review. Res. Pract. Thromb. Haemost. 3, 161–172. doi:10.1002/rth2.12191
Qin, X. S., Jiang, S. T., Zhao, Y. Y., and Zheng, Z. (2017). Effect of Wheat Gluten on Gelation Properties of Soy Protein Isolate Induced by Transglutaminase Crosslinking. Sci. Technol. Food Ind. 38 (8), 214–217+243. doi:10.13386/j.issn1002-0306.2017.08.033
Ranga, A., Lutolf, M. P., Hilborn, J., and Ossipov, D. A. (2016). Hyaluronic Acid Hydrogels Formed In Situ by Transglutaminase-Catalyzed Reaction. Biomacromolecules 17 (5), 1553–1560. doi:10.1021/acs.biomac.5b01587
Ren, X. M., Qian, H., Xiao, Z. H., Long, H. Y., Guo, Y. Q., and Yang, G. (2016). Three-dimensional Cultured Adipose-Derived Stem Cells Based on Microbial Transglutaminase Enzyme Crosslinked Gelatin Hydrogel. Chin. J. Rep. Reconst. Sur. 30 (12), 1532–1537. doi:10.7507/1002-1892.20160316
Savoca, M., Tonoli, E., Atobatele, A., and Verderio, E. (2018). Biocatalysis by Transglutaminases: a Review of Biotechnological Applications. Micromachines 9 (11), 562. doi:10.3390/mi9110562
Schmitz, T., Bäuml, C. A., and Imhof, D. (2020). Inhibitors of Blood Coagulation Factor XIII. Anal. Biochem. 605, 113708. doi:10.1016/j.ab.2020.113708
Sharma, S., and Tiwari, S. (2020). A Review on Biomacromolecular Hydrogel Classification and its Applications. Int. J. Biol. Macromolecules 162, 737–747. doi:10.1016/j.ijbiomac.2020.06.110
Song, F., and Zhang, L.-M. (2008). Enzyme-catalyzed Formation and Structure Characteristics of a Protein-Based Hydrogel. J. Phys. Chem. B 112 (44), 13749–13755. doi:10.1021/jp8041389
Sparks, H. D., Anjum, F., Vallmajo-Martin, Q., Ehrbar, M., Abbasi, S., Kallos, M. S., et al. (2019). Flowable Polyethylene Glycol Hydrogels Support the In Vitro Survival and Proliferation of Dermal Progenitor Cells in a Mechanically Dependent Manner. ACS Biomater. Sci. Eng. 5 (2), 950–958. doi:10.1021/acsbiomaterials.8b01294
Storozhylova, N., Crecente-Campo, J., Cabaleiro, D., Lugo, L., Dussouy, C., Simões, S., et al. (2020). An In Situ Hyaluronic Acid-Fibrin Hydrogel Containing Drug-Loaded Nanocapsules for Intra-articular Treatment of Inflammatory Joint Diseases. Regen. Eng. Transl. Med. 6, 201–216. doi:10.1007/s40883-020-00154-2
Sun, C.-K., Ke, C.-J., Lin, Y.-W., Lin, F.-H., Tsai, T.-H., and Sun, J.-S. (2021). Transglutaminase Cross-Linked Gelatin-Alginate-Antibacterial Hydrogel as the Drug Delivery-Coatings for Implant-Related Infections. Polymers 13 (3), 414. doi:10.3390/polym13030414
Tian, S. J., and Liang, H. M. (2005). Effect of Glutaminase on Gelatabiligy of Soybean Protein Isolatezhon. Chin. Oil. Fat. 30 (8), 43–46. doi:10.3321/j.issn:1003-7969.2005.08.014
Tsai, C.-C., Kuo, S.-H., Lu, T.-Y., Cheng, N.-C., Shie, M.-Y., and Yu, J. (2020). Enzyme-Cross-linked Gelatin Hydrogel Enriched with an Articular Cartilage Extracellular Matrix and Human Adipose-Derived Stem Cells for Hyaline Cartilage Regeneration of Rabbits. ACS Biomater. Sci. Eng. 6 (9), 5110–5119. doi:10.1021/acsbiomaterials.9b01756
Vallmajo-Martin, Q., Broguiere, N., Millan, C., Zenobi-Wong, M., and Ehrbar, M. (2020). PEG/HA Hybrid Hydrogels for Biologically and Mechanically Tailorable Bone Marrow Organoids. Adv. Funct. Mater. 30 (48), 1910282. doi:10.1002/adfm.201910282
Varma, I. K., Albertsson, A.-C., Rajkhowa, R., and Srivastava, R. K. (2005). Enzyme Catalyzed Synthesis of Polyesters. Prog. Polym. Sci. 30, 949–981. doi:10.1016/j.progpolymsci.2005.06.010
Wang, J., Ding, Y., Jiang, Y., Dong, H., Wang, Q., and Cheng, J. (2021). Progress in Understanding the Effect of Enzymatic Modification on Gel Properties of Soy Protein Isolate. Food Sci. 15, 329–336. doi:10.7506/spkx1002-6630-20200602-023
Wang, K., Mosser, G., Haye, B., Baccile, N., Le Griel, P., Pernot, P., et al. (2021). Cellulose Nanocrystal-Fibrin Nanocomposite Hydrogels Promoting Myotube Formation. Biomacromolecules 22 (6), 2740–2753. doi:10.1021/acs.biomac.1c00422
Xue, T., Zheng, X., Su, X., Chen, D., Liu, K., Yuan, X., et al. (2020). Directed Evolution of the Transglutaminase from Streptomyces Mobaraensis and its Enhanced Expression in Escherichia coli. Food Biotechnol. 34 (1), 42–61. doi:10.1080/08905436.2019.1711112
Yan, J., Bockstaller, M. R., and Matyjaszewski, K. (2020). Brush-modified Materials: Control of Molecular Architecture, Assembly Behavior, Properties and Applications. Prog. Polym. Sci. 100, 101180. doi:10.1016/j.progpolymsci.2019.101180
Zamboni, F., Okoroafor, C., Ryan, M. P., Pembroke, J. T., Strozyk, M., Culebras, M., et al. (2021). On the Bacteriostatic Activity of Hyaluronic Acid Composite Films. Carbohydr. Polym. 260, 117803. doi:10.1016/j.carbpol.2021.117803
Zhao, L., Li, X., Zhao, J., Ma, S., Ma, X., Fan, D., et al. (2016). A Novel Smart Injectable Hydrogel Prepared by Microbial Transglutaminase and Human-like Collagen: Its Characterization and Biocompatibility. Mater. Sci. Eng. C 68, 317–326. doi:10.1016/j.msec.2016.05.108
Keywords: biocatalytic synthesis, transglutaminase, polymer hydrogel, bottom-up approaches, enzyme catalyzed reactions
Citation: Lai E, Bao B, Zhu Y and Lin H (2022) Transglutaminase-Catalyzed Bottom-Up Synthesis of Polymer Hydrogel. Front. Bioeng. Biotechnol. 10:824747. doi: 10.3389/fbioe.2022.824747
Received: 29 November 2021; Accepted: 04 March 2022;
Published: 22 March 2022.
Edited by:
Jennifer Ann Littlechild, University of Exeter, United KingdomReviewed by:
Ashley Carson Brown, North Carolina State University, United StatesCopyright © 2022 Lai, Bao, Zhu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enping Lai, nemodhu@163.com
 Enping Lai
Enping Lai Binyu Bao
Binyu Bao Yifei Zhu
Yifei Zhu Haitao Lin
Haitao Lin