Possible Implications for Improved Osteogenesis? The Combination of Platelet-Rich Fibrin With Different Bone Substitute Materials
- 1Department of Oral and Maxillofacial Surgery, University Medical Center, Johannes Gutenberg University Mainz, Mainz, Germany
- 2Platform for Biomaterial Research, BiomaTiCS Group, University Medical Center, Johannes Gutenberg University Mainz, Mainz, Germany
- 3Department of Oral and Maxillofacial Surgery, Federal Armed Forces Hospital, Koblenz, Germany
Bone substitute materials (BSM) are widely used in oral regeneration, but sufficient angiogenesis is crucial for osteogenesis. The combination of BSM with autologous thrombocyte concentrations such as platelet-rich fibrin (PRF) may represent a clinical approach to overcome this limitation. This study analyzes the early influence on osteoblast (HOB) in vitro. Here, four different BSM (allogeneic, alloplastic, and two of xenogeneic origin) were combined with PRF. After the incubation with osteoblasts for 24 h, cell viability, migration, and proliferation were assessed. Next, marker of proliferation, migration, and differentiation were evaluated on gene and protein levels in comparison to the native BSM and osteoblast alone. Addition of PRF increased viability for both the xenogeneic BSM (p = 0.0008, p = 0.032, respectively) in comparison to HOB and vs. native BSM (p = 0.008), and led to a tendency for increased cell proliferation and migration for all BSM (each p > 0.05). On gene basis, allogeneic and alloplastic BSM displayed a significantly increased RUNX2 expression (each p = 0.050). Expression of alkaline phosphatase for alloplastic (p = 0.050) and collagen-1 for xenogeneic BSM (p = 0.05) were significantly increased in combination with PRF. In addition, bone morphogenic protein was expressed significantly higher when xenogeneic material was combined with PRF in comparison to HOB alone (each p = 0.05). In summary, the combination of PRF with different BSM increases initial viability and may influence early proliferation and migration potential of osteoblast via RUNX2, alkaline phosphatase, collagen, and BMP2 especially in combination with alloplastic and xenogeneic BSM. Biofunctionalization of BSM using PRF might improve osteogenesis and extend the range of indications.
Introduction
Autologous bone augmentation remains the treatment therapy of choice for regenerative craniomaxillofacial surgery in case of facial bone loss due to trauma, cancer, or other pathologies (Tatullo et al., 2012). However, disadvantages may be seen in the limited offer and enhanced morbidity with respect to the donor site especially in multimorbid patients (von Arx et al., 2001). Here, bone substitute materials (BSM) of allogeneic, xenogeneic, or alloplastic origins represent a suitable and promising therapy option with specific indications: in opposite to the osteoinductive capacity of autologous bone, BSM shows functional deficits due to their osteoconductive properties (Khosropanah et al., 2018). Only for allogeneic BSM, an osteoinductive potential could be demonstrated (Miron et al., 2016). Therefore, allogeneic materials in particular are frequently used for “bone engineering” where, e.g., via co-culture experiments, stem cell therapy or the addition of growth factors BSM were edited in order to improve bone regeneration procedures (Hinze et al., 2010).
As a key role in initial osteogenesis, a sufficient blood vessel supply and angiogenesis, the formation of new blood vessels from existing lumina, is mandatory (Rather et al., 2019). On the one hand, capillary structures supply the regenerated bony defect area with nutrients and minerals for homeostasis. In addition, they support and regulate diverse functions of the bone marrow and bone in osteogenesis processes, structurally and via paracrine pathways on different cellular levels (Grosso et al., 2017). Here, new engineering strategies may overcome the current limitations of an insufficient initial blood supply of BSM that, with an increased angiogenic potential, may lead the way to an optimized osseous regeneration.
Autologous platelet concentrate (PC) such as platelet-rich fibrin (PRF) are now broadly used in dental and craniomaxillofacial regenerative medicine (Dohan et al., 2006). Via the complex interplay of different cytokines and growth factors, the proliferation and differentiation of different cell lines is thrived (Miron et al., 2017). So far, a significant pro-angiogenic effect of the PRF could be shown especially for soft tissue regeneration procedures (Ghanaati et al., 2018; Blatt et al., 2020). Up to date, there is inconsistent data if PRF may also support bony regeneration (Miron et al., 2017). Still, raising evidence emerges that PRF may also support differentiation and proliferation of osteoblasts (Dohle et al., 2018). Lately, our working group demonstrated a positive effect after 3–10 days of co-incubation, especially in combination with an allograft in comparison to BSM alone or in combination with xenogeneic materials (Kyyak et al., 2020). However, data for a possible initial and early interaction remain spares.
Controversially, some studies and case reports report conflicting data if PRF may influence osteogenesis (Pripatnanont et al., 2013; Yoon et al., 2014). A possible explanation for the ambivalent data may be seen in the diversity of the analyzed BSM and their different biophysical properties. Furthermore, different time points of evaluation were chosen that counteract time points of the physiological wound healing phase. Therefore, the aim of this study was to investigate the early effect on viability, migration, proliferation, and differentiation of osteoblasts of the PRF when combined with BSM in vitro after 24 h. This way, a comprehensive understanding of the possible initial mechanism of PRF in comparison to the well-studied later time points in osteogenesis should be provided to detect intercellular implications and provide basic scientific evidence for potential clinical translation.
Materials and Methods
Bone Substitute Materials
Four commercially available BSM were tested: allogeneic (AKM: maxgràft®, botiss biomaterials GmbH, Zossen, Germany, granularity <2 mm), alloplastic (APKM: maxresob®, botiss biomaterials GmbH, Zossen, Germany, granularity 0.8–1.5 mm), and xenogeneic BSM (XKM1: cerabone®, botiss biomaterials GmbH, Zossen, Germany, granularity 1.0–2.0 mm, XKM2: BioOss®, Geistlich Pharma AG, Wolhusen, Switzerland, granularity 1−2 mm) were used for the further experiments.
PRF Protocol
For the PRF protocol, blood was collected from three healthy volunteers who gave their informed consent to this study in accordance with the ethical standards of the National Research Committee (Ärztekammer Rheinland-Pfalz, no. “2019-14705_1”) and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ten milliliters of peripheral venous blood per sample were collected after puncturing the cephalic or the median cubital vein with the vacutainer system and specific sterile plain vacuum tubes with additional silicone within their coating surface for solid (A-PRF+, Mectron, Carasco, Italy) and liquid PRF, respectively (iPRF, Mectron, Carasco, Italy). Next, PRF was directly manufactured (1,200 rpm for 8 min, relative centrifugal force 177 g at a fixed angle rotor with a radius of 110 mm, Duo centrifuge, Mectron, Carasco, Italy), as previously described (Blatt et al., 2020).
Cell Culture
Before the incubation with osteoblast, PRF was pressed to a stable membrane with the “PRF Box” (Mectron, Carasco, Italy) as indicated by the manufacturer. Next, PRF was cut into small pieces of 10–20 mm2, 0.3–0.5 ml of liquid PRF was added and mixed manually with an equal quantity of the respective BSM (100 mg) to obtain a sticky clot. Next, a commercially available human osteoblast cell line (HOB, PromoCell, Heidelberg, Germany) was used and cultivated with a standard HOB medium with an additive fetal calf serum (FCS, Gibco Invitrogen, Karlsruhe, Germany), Dulbecco’s modified Eagle’s medium (DMEM, Gibco Invitrogen), dexamethasone (100 nmol/l, Serva Bioproducts, Heidelberg, Germany), L-glutamine (Gibco Invitrogen), and streptomycin (100 mg/ml, Gibco Invitrogen). Cultivation was done at 37°C in a constant, humidified atmosphere with 95% room air and 5% CO2 until a confluence of approximately 70% was reached. Next, HOB were passaged using 0.25% trypsin (Seromed Biochrom KG, Berlin, Germany). HOB at passage five were used and seeded in a 24 well plate (Merck, Darmstadt, Germany) in a density of 5 × 104 cells per well. Now, 100 mg of the respective BSM were added in combination with (prepared as mentioned above) or without PRF and further incubated for 24 h at 37°C with 95% room air and 5% CO2. HOB alone served as control.
Cell Viability Analysis
Next, cell viability was analyzed after 24 h by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as previously described (Pabst et al., 2015). In brief, MTT (200 μL, 2 mg/mL) was added to the wells and incubated for 4 h at 37°C before the culture medium was discarded, and 10 ml of lysis buffer was added per well. Finally, a fluorescence microplate reader (Versamax, Molecular Devices, San Jose, CA, United States) was used at 570 nm to detect metabolic activity that reflects viability.
Cell Proliferation Analysis
Fluorescence red was applied after 24 h with CellTracker (Life Technologies, Thermo Fisher Scientific, Darmstadt, Germany) according to the manufacturer’s instructions to track cell number and therefore, proliferation rate. After the removal of the culture media, warmed Red dye was added and incubated for 30 min. Afterward, the dye was removed, washed with serum-free medium, and incubated for 30 min. Finally, Red fluorescence was analyzed with a fluorescence microscope (BZ-9000, Keyence, Osaka, Japan). Automatic thresholding was applied to extract cell structures and the area fraction (%) was calculated as previously described (Kyyak et al., 2020).
Cell Migration Assay
A scratch test was used to detect migration ability, as previously described (Kyyak et al., 2020). HOB were incubated with BSM in combination with and without PRF in a special scratch assay plate (ibidi GmbH, Gräfelfing, Germany) for 24 h at the above mentioned conditions. Here, red cell tracker was applied as mentioned above. Quantification of the migrated cells and visualization of cell viability was done with the ImageJ software (ACTREC, Navi Mumbai, India), as previously described (Kyyak et al., 2020). In brief, images at a 10× fold magnification were first converted to grayscale before image subtraction was used to correct background staining. Next, automatic thresholding was applied to extract cell structures, and cells migrated in the gap were evaluated and the area fraction (%) was calculated.
ELISA Quantification
Growth factor release on protein basis was analyzed after co-incubation with 1.4 ml of the cell supernatant, which was extracted after incubation for 24 h with HOB and the respective native and bio-activated BSM samples, as previously described (Blatt et al., 2020). Antibodies for alkaline phosphatase (AP), collagen (COL), bone morphogenic protein 2 (BMP), osteocalcin (OCN), and Runt-related transcription factor-2 (RUNX, all R&D Systems, Minneapolis, MN, United States) were evaluated according to the manufacturer’s protocol and analyzed via an ELISA plate reader and the specific software (SoftMax Pro 5.4, Molecular Devices, San Jose, CA, United States). In brief, after diluting the capture antibody in a coating buffer according to the manufacturer’s dilution protocol, a 96-well-plate was coated with 100 μL per well of coating solution and incubated overnight at 2–8°C. Afterward, wells were washed with a wash buffer and the excess liquid was removed. Two hundred microliters of blocking buffer was added and incubated for 1 h at room temperature and then removed. Next, 100 μl of standards and samples were added into the designated wells and incubated for 1 h at room temperature. The sample was then aspirated, the plate was then washed three times, and the excess liquid was removed. According to the manufacturer’s instructions, detection body was diluted in the blocking buffer and 100 μl was added to each well. After incubating for 2 h at room temperature, the plate was washed and the excess liquid was removed. Next, 100 μl of streptavidin-HRP diluted in the blocking buffer was added and incubated for 30 min at room temperature. After washing and removing the excess liquid, 100 μl of TMB substrate solution was added to each well and incubated for 30 min, then, 100 μl of stop solution was added and absorbance at 450 nm was measured with the ELISA plate reader and the specific software.
PCR Quantification
The evaluation of proliferation and migration marker on gene basis were done with real-time quantitative PCR (qRT-PCR, CFX Connect Real-Time PCR Detection System, Bio-Rad, Germany) using SYBR Green Supermix (BioRad, Hercules, CA, United States), as previously described (Kyyak et al., 2020), for the following genes: alkaline phosphatase (ALPL), bone morphogenic protein 2 (BMP2), collagen type 1 alpha 1 chain (COL1A1), bone gamma-carboxyglutamate protein (alias: osteocalcin, OCN), and RUNX family transcription factor 2 (RUNX2). For internal control, housekeeping genes actin alpha 1, skeletal muscle (ACTA1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were ran (primer sequences: Table 1). Briefly, the total RNA was extracted after 24 h of co-incubation using a commercial kit (Qiagen, Hilden, Germany) before RNA was converted to cDNA by the iScript cDNA synthesis kit (BioRad, Hercules, CA, United States) according to the manufacturers’ instructions. Eleven microliters of SYBR, 1 μl of primer sense, 1 μl of primer antisense, and 5 μl of RNA-free water were used with the thermal cycler at the first step −95°C for 3 min; second Step (repeated 39 times) −95°C for 10 s, then 58°C for 30 s, and finally 72°C for 20 s; final step −65°C for 0.5 s and then 95°C for 5 s. Quantification of gene expression was evaluated via the ΔΔCT method.
Statistical Analysis
The results were interpreted in mean values with its standard error and rounded to the first decimal place. For normal distribution, the Shapiro–Wilk test was used. In case of normally distributed values, the Student’s t-test for paired samples was applied. For non-normal distributions, the Mann–Whitney test was used. In order to compare all the groups, the Kruskal–Wallis rank sum test was applied. A p-value of ≤0.05 was considered to be statistically significant. Finally, bar charts with error bars were used for data illustration.
Results
Combination of PRF With BSM Increases Initial Viability and Tent to Improve Early Proliferation and Migration Potential of Osteoblast
First, viability of HOB after 24 h of incubation with the respective BSM with or without PRF was analyzed via 3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (five samples in triplets each, n = 60, Table 2A). Here, all samples leveled over the negative control of HOB except for XKM1. There was no statistical significance between the groups (p = 0.467). In comparison to HOB alone, xenogeneic BSM did reveal a statistically significant increased viability (XKM1: p = 0.016, XKM1+: p = 0.008, XKM2+: p = 0.032 all other tested samples: p > 0.05). Metabolic activity was significantly higher for xenogeneic material 1 only when combined with PRF vs. the native material (∗p = 0.008, all other tested samples: p > 0.05, Figure 1). The differences between the groups were statistically significant (p = 0.007, Figure 1).
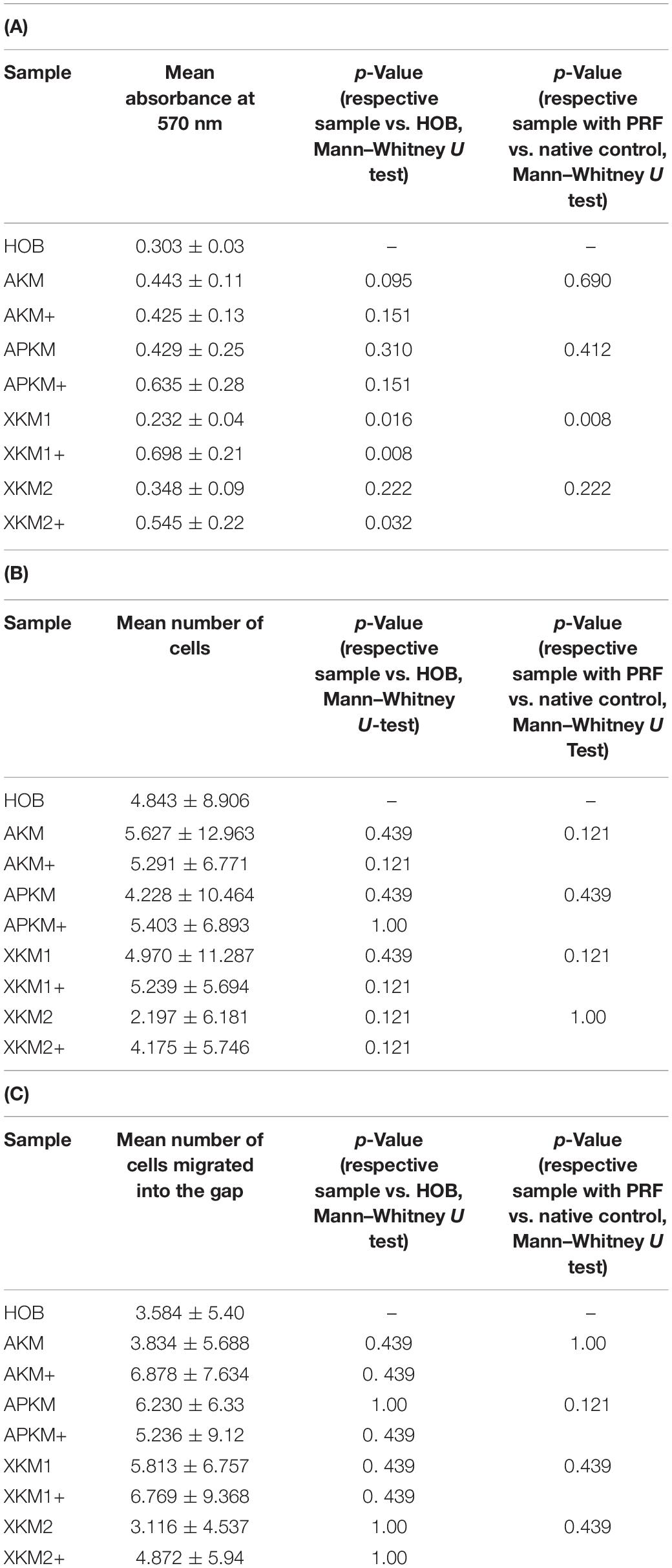
Table 2. (A) MTT assay: Mean absorbances found at 570 nm for the respective samples with (+)/without PRF and respective p-values vs. HOB alone and native BSM, (B) Cell proliferation assessed with cell tracker red: Mean number of cells with its standard error and respective p-values vs. HOB alone and native BSM, (C) Scratch assay: Mean number of cells migrated into the gap for the respective samples with (+)/without PRF and respective p-values vs. HOB alone and native BSM.
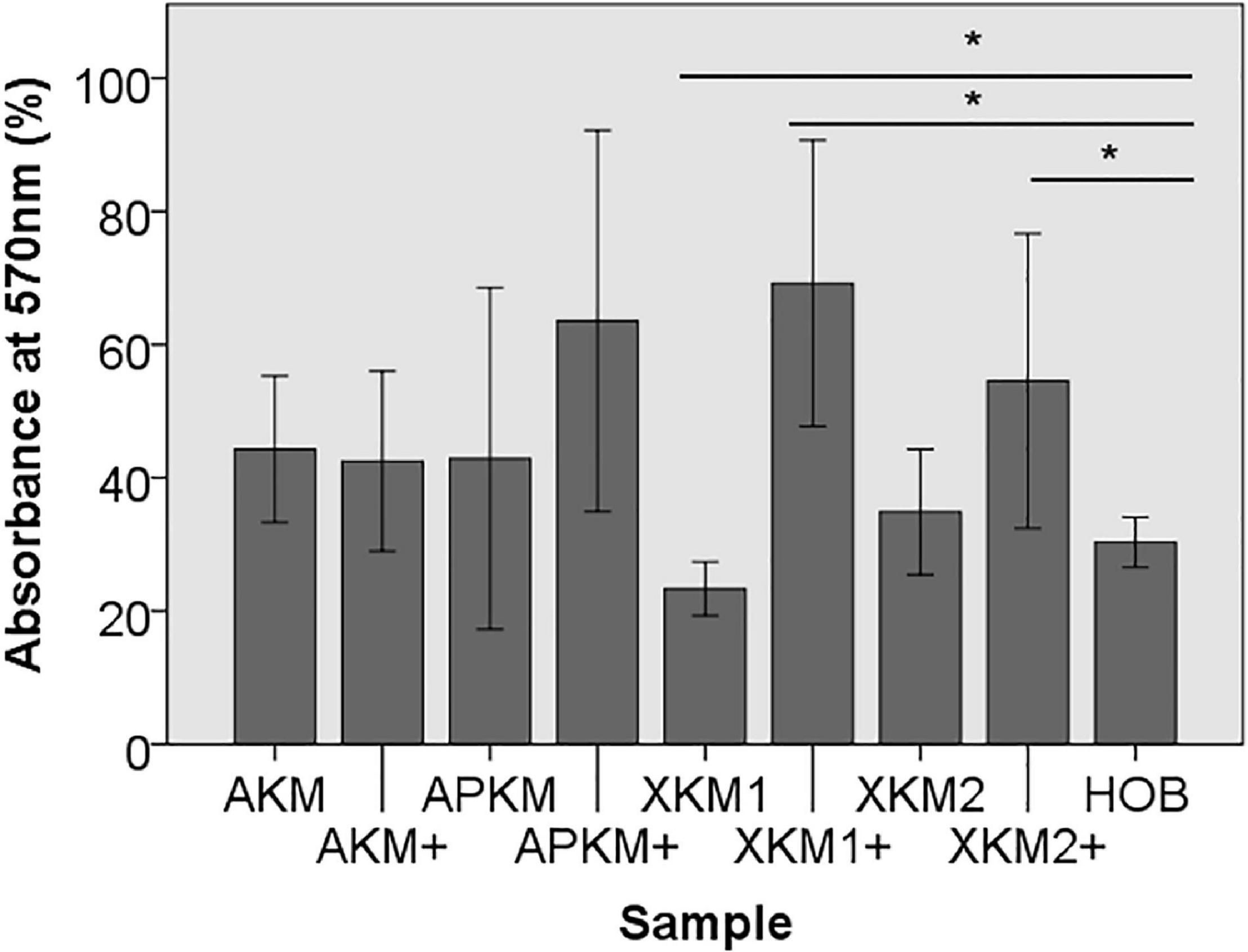
Figure 1. MTT assay to evaluate viability at absorbance of 570 nm of HOB after co-incubation with the respective samples with (+)/without PRF (*p < 0.05 Mann–Whitney U testing vs. HOB, XKM1: p = 0.016, XKM1+: p = 0.008, XKM2+: p = 0.032).
Next, cell proliferation was investigated via cell tracker (Table 2B, three samples in duplets for each, n = 54). After 24 h of incubation, no significant differences between the groups could be revealed (p = 0.098). However, the addition of PRF led to a tendency for increased viability for APKM in comparison to HOB (all tested samples: p > 0.05). Viability was increased when alloplastic and xenogeneic materials where combined with PRF in comparison to their native control, however without reaching statistical significance (all tested samples: p > 0.05, Figures 2–4).
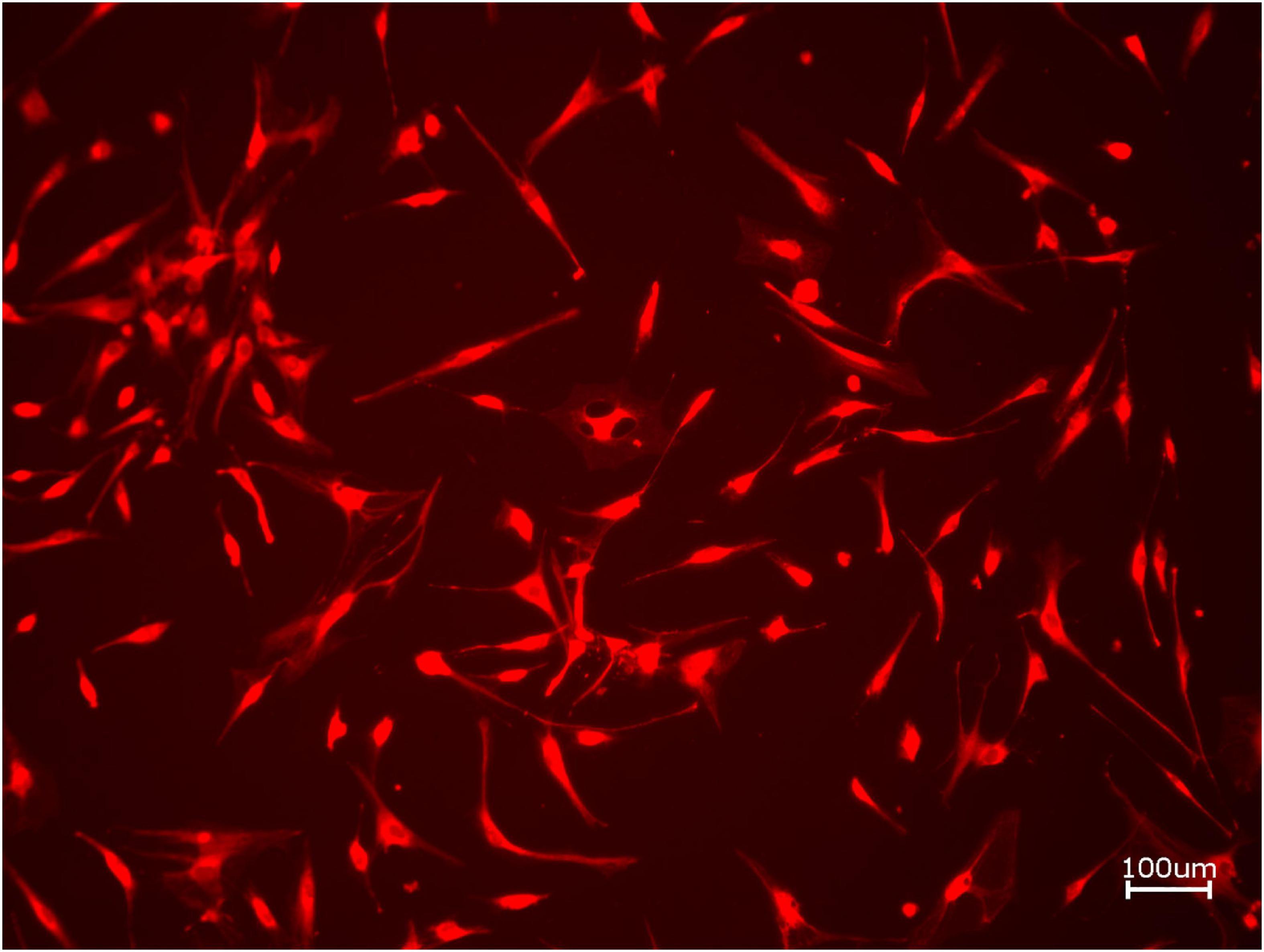
Figure 2. Exemplary micrograph of cell tracker red for allogeneic BSM without the addition of PRF (10× magnification).
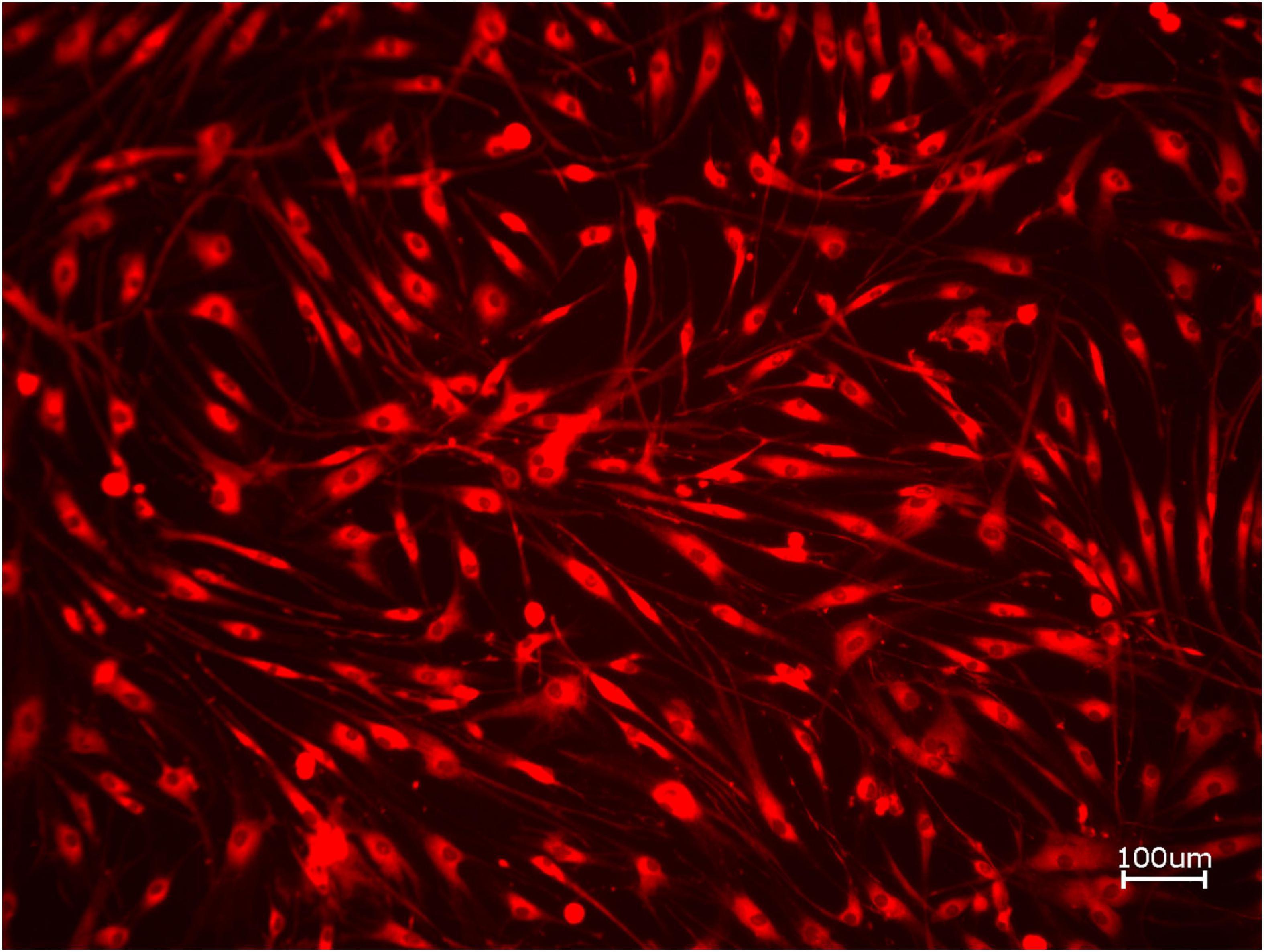
Figure 3. Exemplary micrograph of cell tracker red for allogeneic BSM with the addition of PRF (10× magnification).
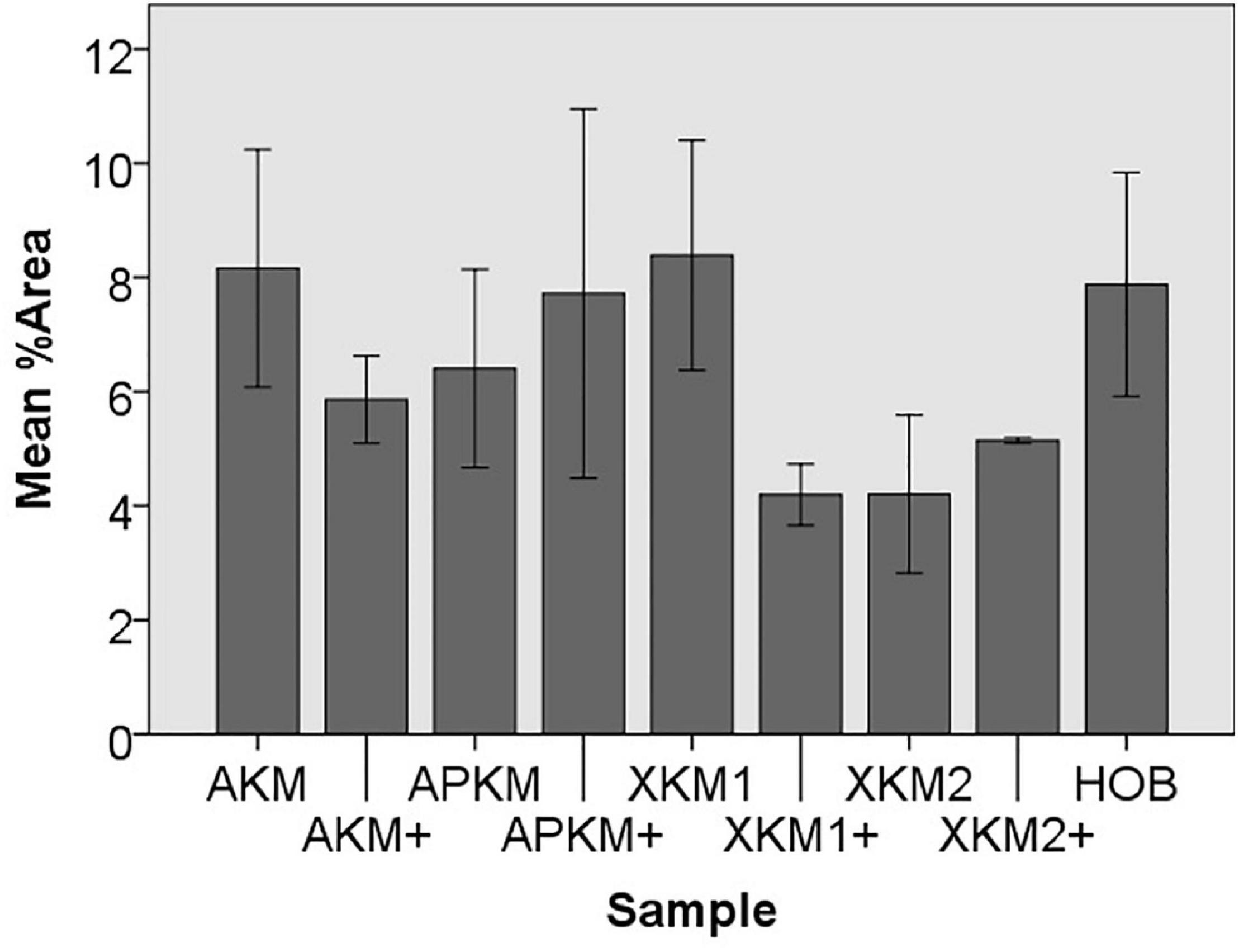
Figure 4. Mean percentage of cells assessed via cell tracker assay of HOB after co-incubation with the respective samples with (+)/without PRF to detect proliferation potential (each p > 0.05, Mann–Whitney U testing in comparison to HOB and native BSM).
To assess the differences between the groups concerning cell migration, the scratch assay was assessed (three samples in duplets for each, n = 54, Figures 5–7). Here, comparisons between all samples did not reveal any statistically significant differences (p = 0.467). However, the percentage of HOB migrated into the gap after 24 h was slightly higher for all groups when PRF was added and was the highest for APKM (almost doubled in comparison to APKM alone) but failed to show statistical significance when compared to HOB alone (all tested samples: p > 0.05). In comparison to their native BSM, PRF tended to increase cell migration for alloplastic material but no statistical significance differences where found (all tested samples: p > 0.05, Table 2C).
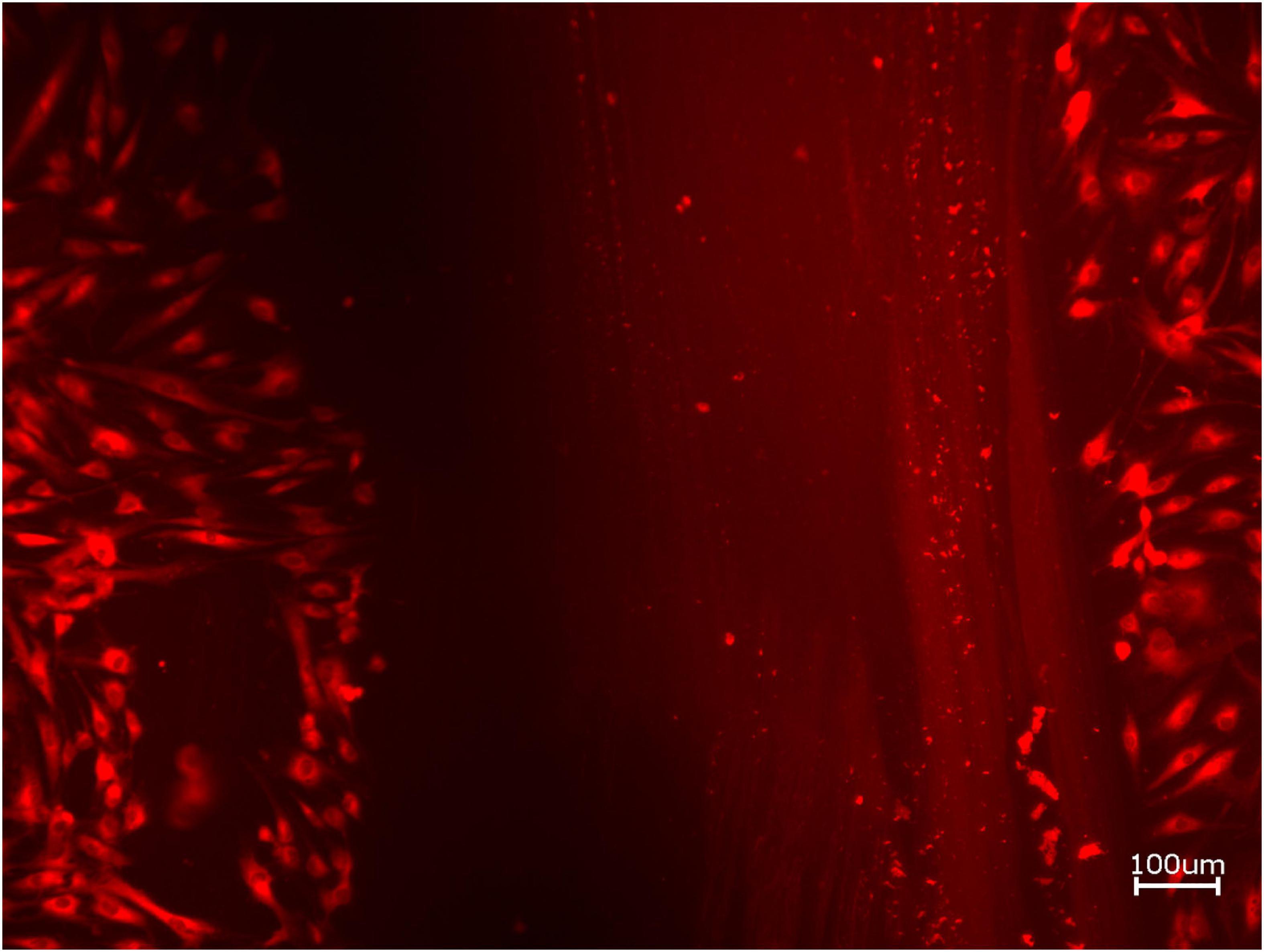
Figure 5. Exemplary micrographs of migrated HOBs assessed via scratch test assay for allogeneic BSM without the addition of PRF (10× magnification).
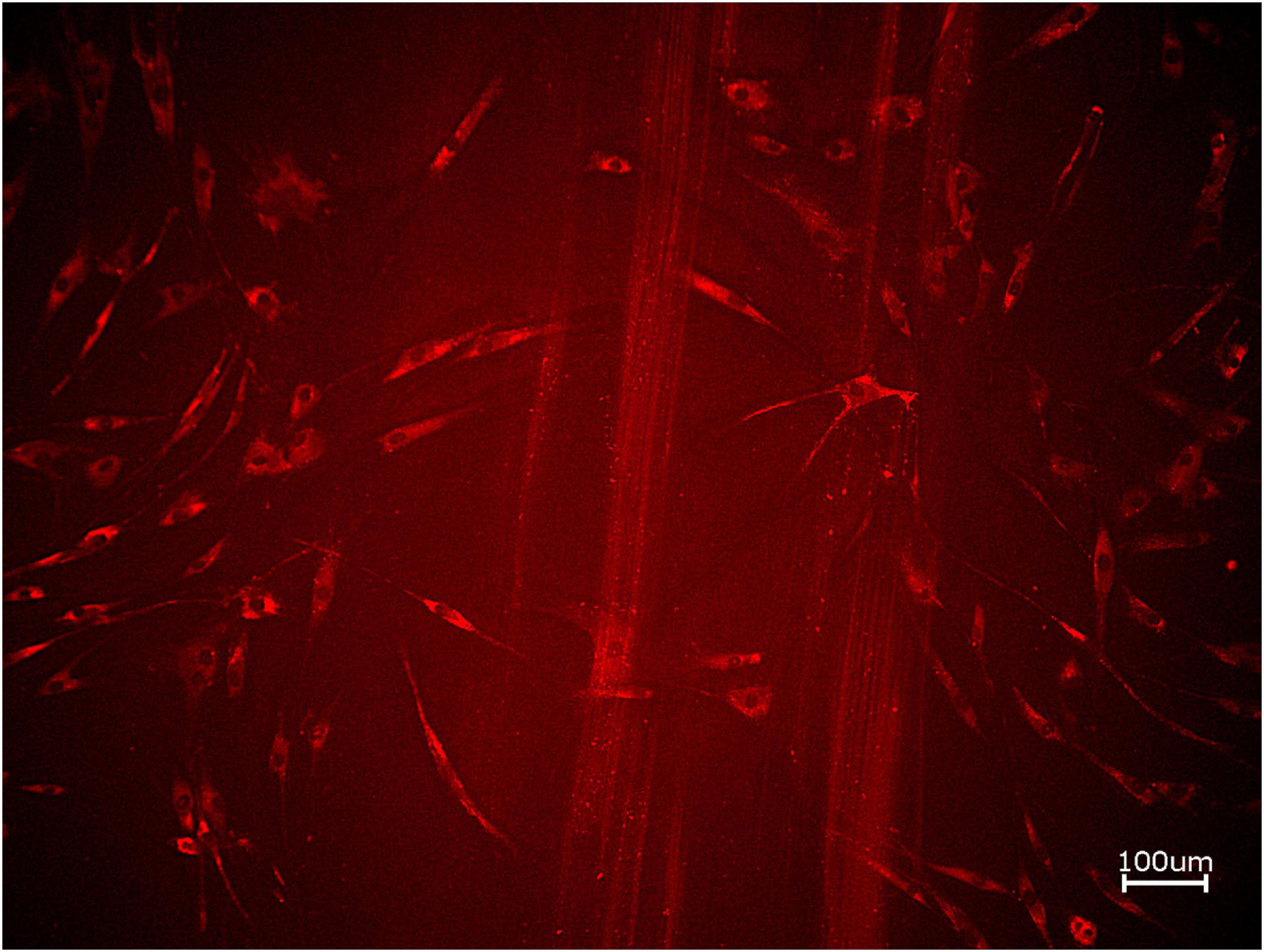
Figure 6. Exemplary micrographs of migrated HOBs assessed via scratch test assay for allogeneic BSM with the addition of PRF (10× magnification).
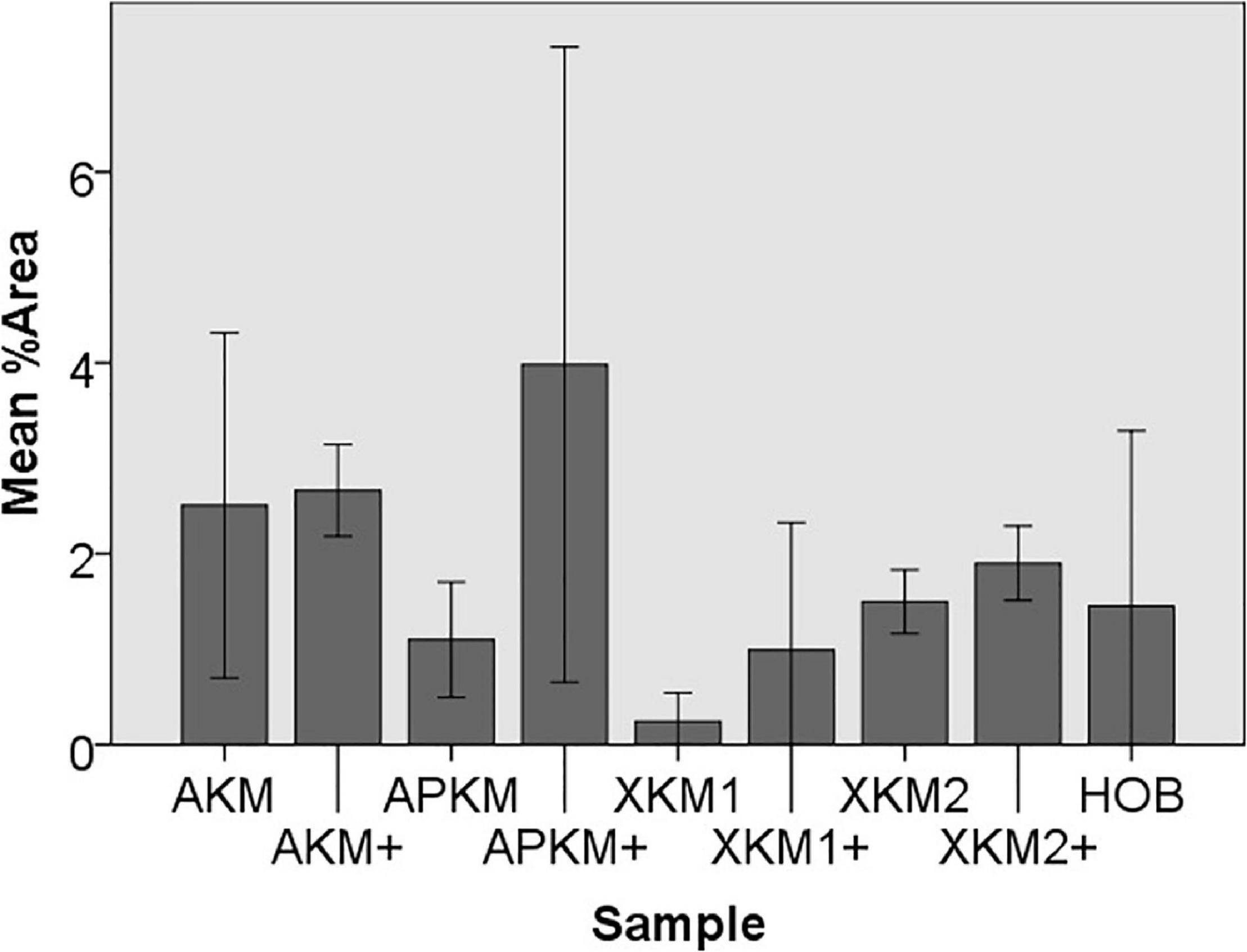
Figure 7. Mean percentage of migrated cells assessed via scratch assay of HOB after co-incubation with the respective samples with (+)/without PRF (each p > 0.05, Mann–Whitney U testing in comparison to HOB and native BSM).
PRF in Combination With Different BSM Triggers Early Release of Marker for Osteoblast Proliferation and Differentiation
To further characterize the early interaction of PRF with the respective BSM and their influence on osteoblasts, the evident marker of proliferation and differentiation on gene and protein level via PCR and ELISA quantification, respectively, were analyzed.
Gene Expression
The PCR results (three samples in triplets each per gene, n = 36, Figures 8, 9) showed no significant differences between the groups (p = 0.069, Tables 3A–E).
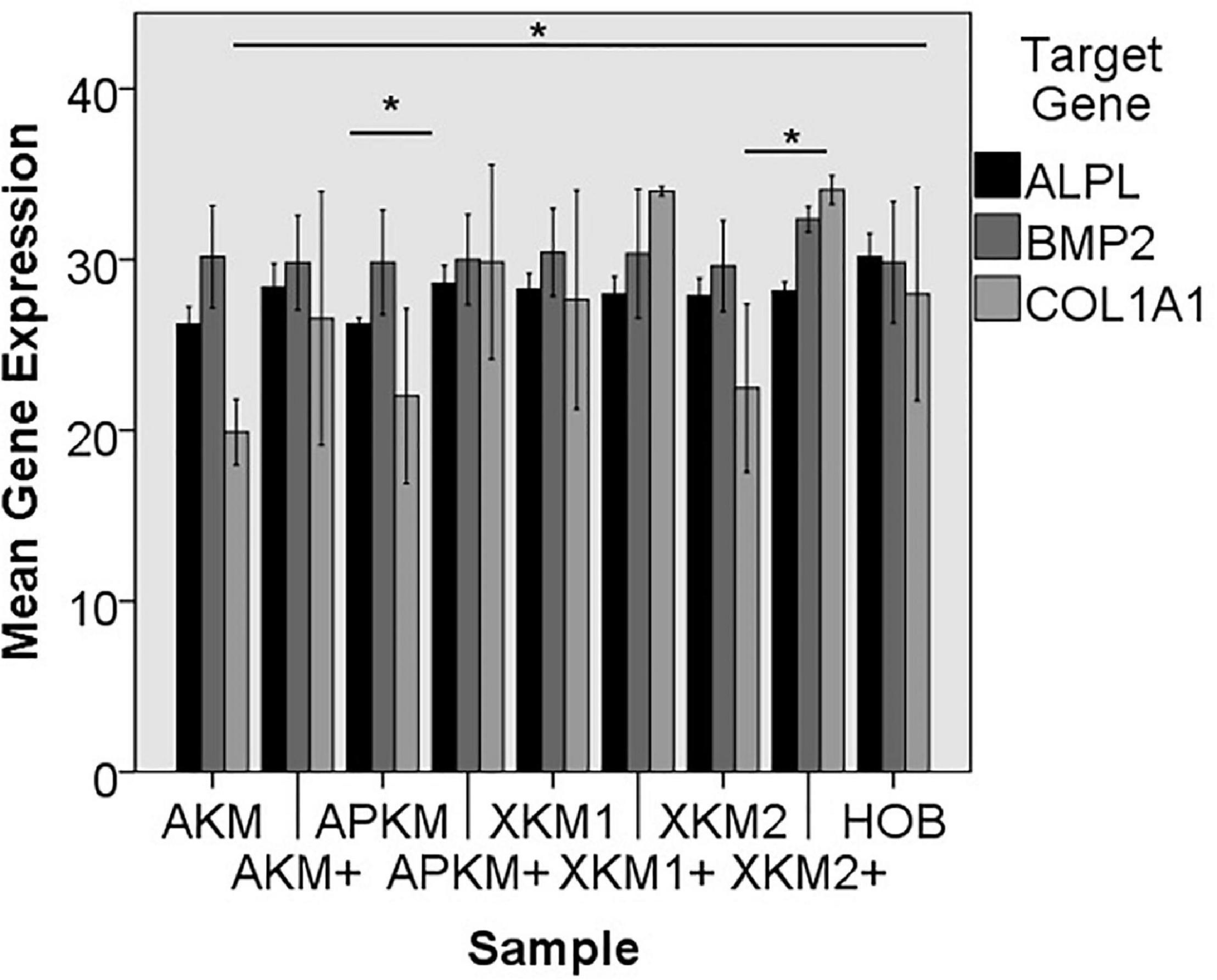
Figure 8. Mean gene expression of ALPL, BMP2, and COL1A1 after co-incubation of HOB with the respective samples with (+)/without PRF (*p < 0.05, Mann–Whitney U testing in comparison to HOB and native BSM, ALPL: APKM vs. APKM+: p = 0.050, COL1A1:AKM vs. HOB: p = 0.050, XKM2 vs. XKM2+: p = 0.050).
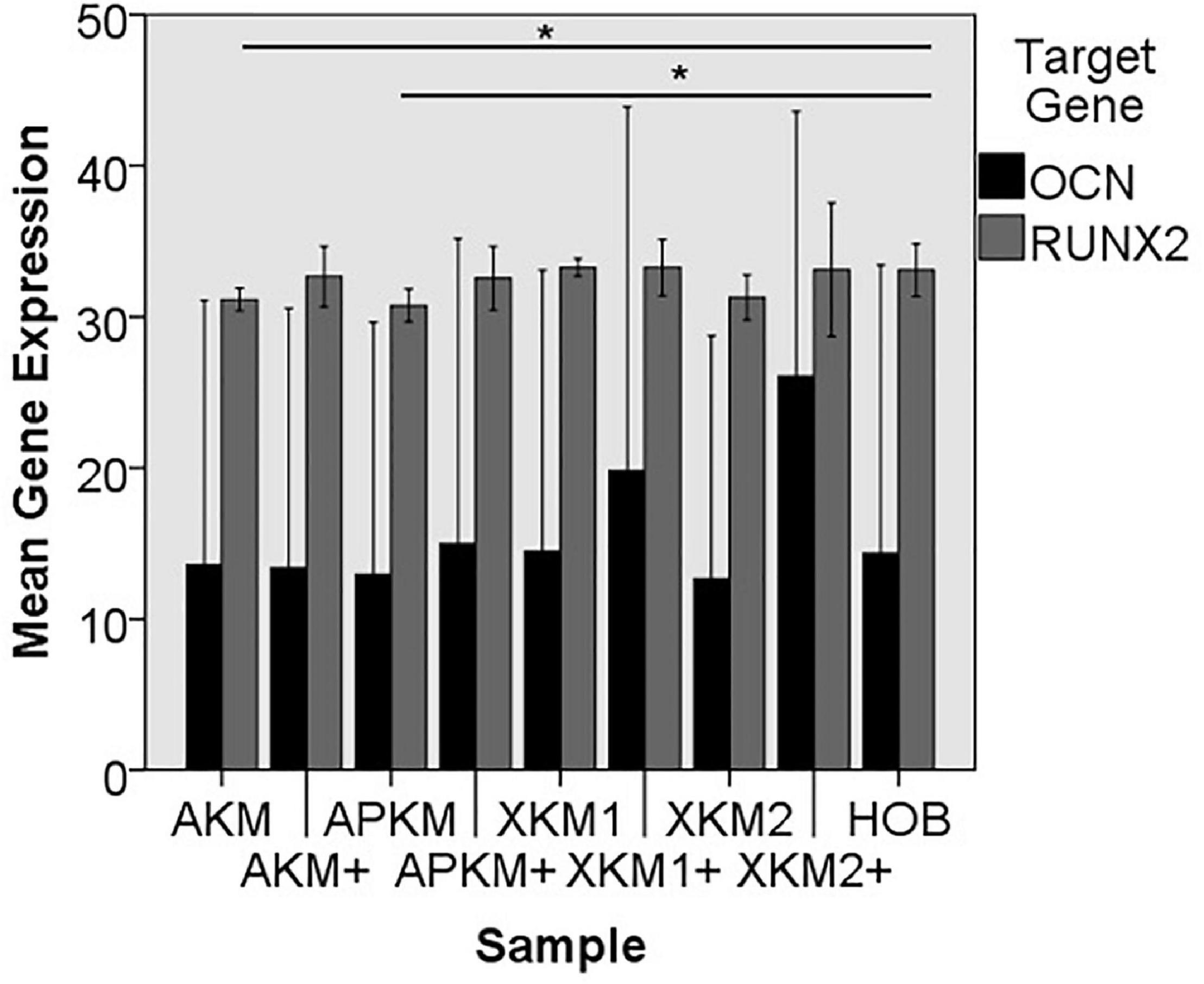
Figure 9. Mean gene expression of OCN and RUNX2 after co-incubation of HOB with the respective samples with (+)/without PRF (*p < 0.05, Mann–Whitney U testing in comparison to HOB and native BSM, RUNX2: AKM vs. HOB: p = 0.050, APKM vs. HOB: p = 0.050).
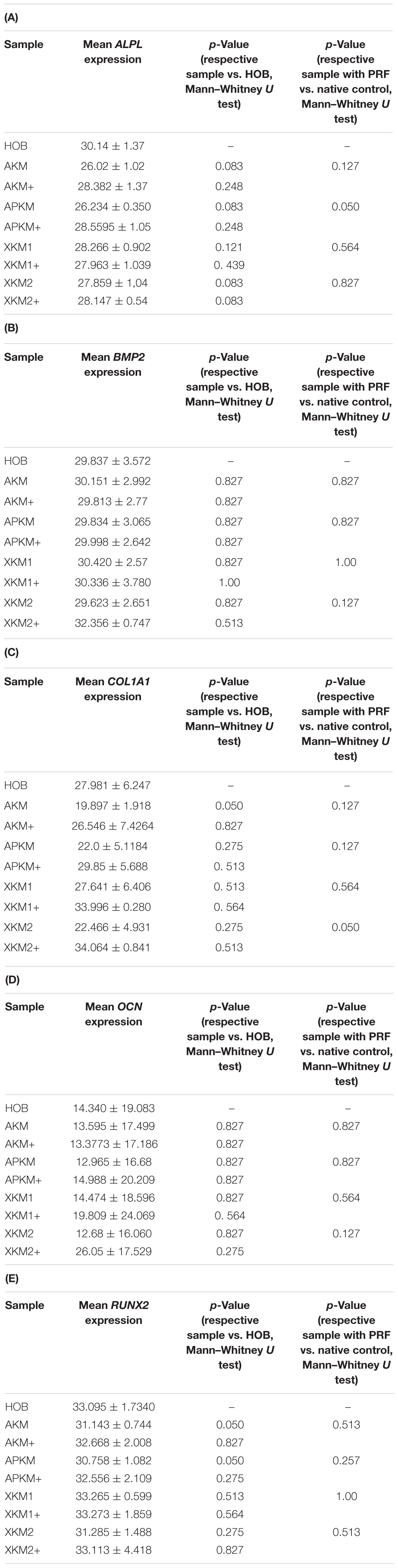
Table 3. Gene expression of (A) ALPL, (B) BMP2, (C) COL1A1, (D) OCN, (E) RUNX2 assessed via PCR for the respective samples and respective p-values vs. HOB alone and native BSM.
The ALPL expression was highest for HOB alone in comparison to other samples (all tested samples: p > 0.05). In comparison to the native BSM, the mean expression for ALPL was higher for each BSM when PRF was added with a significant increase for alloplastic material (APKM vs. APKM+: p = 0.050, all other tested samples: p > 0.05). BMP2 gene expression tended to be increased for all the tested samples in comparison to HOB alone (all tested samples: p > 0.05) and for the combination of PRF and the respective material in comparison to the native BSM (all tested samples: p > 0.05). Allogeneic BSM significantly decreased the COL1A1 expression in comparison to HOB alone (p = 0.050), but other samples did not (all other tested samples: p > 0.05). In comparison to the native BSM, the COL1A1 expression was significantly increased for PRF in combination with the combination of xenogeneic material 2 with PRF (p = 0.050, all other tested samples: p > 0.05). For OCN expression, no significant difference for the tested material in comparison to HOB alone (all tested samples: p > 0.05) and between bio-activated and native BSM (all tested samples: p > 0.05) was found. Allogeneic and alloplastic BSM displayed a significant increase in the RUNX2 expression, whereas the other analyzed BSM did not show noteworthy differences (AKM: p = 0.050, APKM: p = 0.050, all other tested samples: p > 0.05). Combination of the respective sample with PRF did not significantly increase the RUNX-2 expression vs. the native BSM (all tested samples: p > 0.05).
Protein Expression
Next, ELISA quantification (five samples in triplets each per antibody, n = 60, Figures 10, 11) was done to analyze the differences on protein basis. There was no significant difference between all the tested samples (p = 0.069, Tables 4A–D).
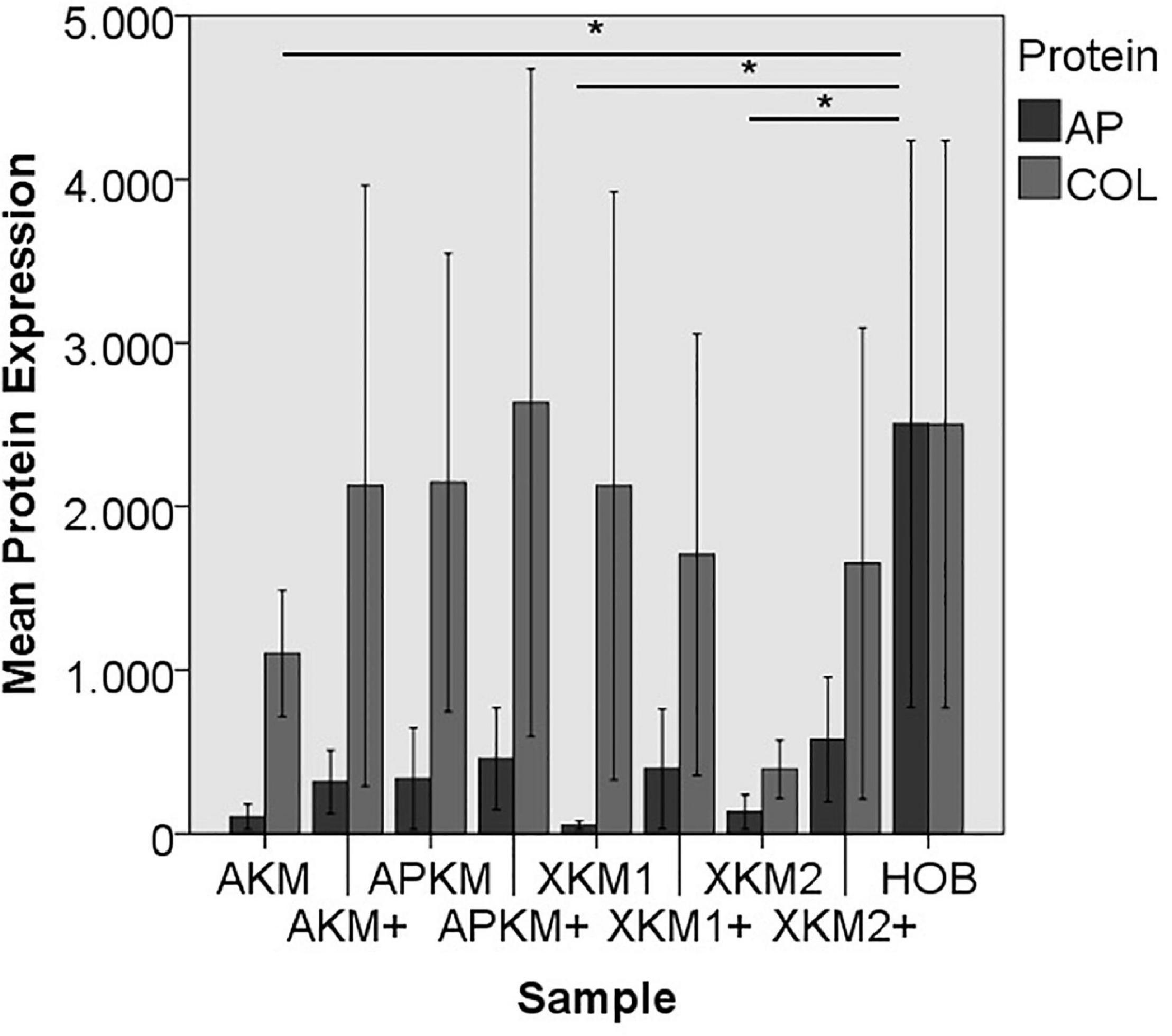
Figure 10. Mean protein expression of AP and COL after co-incubation of HOB with the respective samples with (+)/without PRF (*p < 0.05, Mann–Whitney U testing in comparison to HOB and native BSM, AP: AKM vs. HOB: p = 0.050, XKM1 vs. HOB: p = 0.050, XKM2 vs. HOB: p = 0.050).
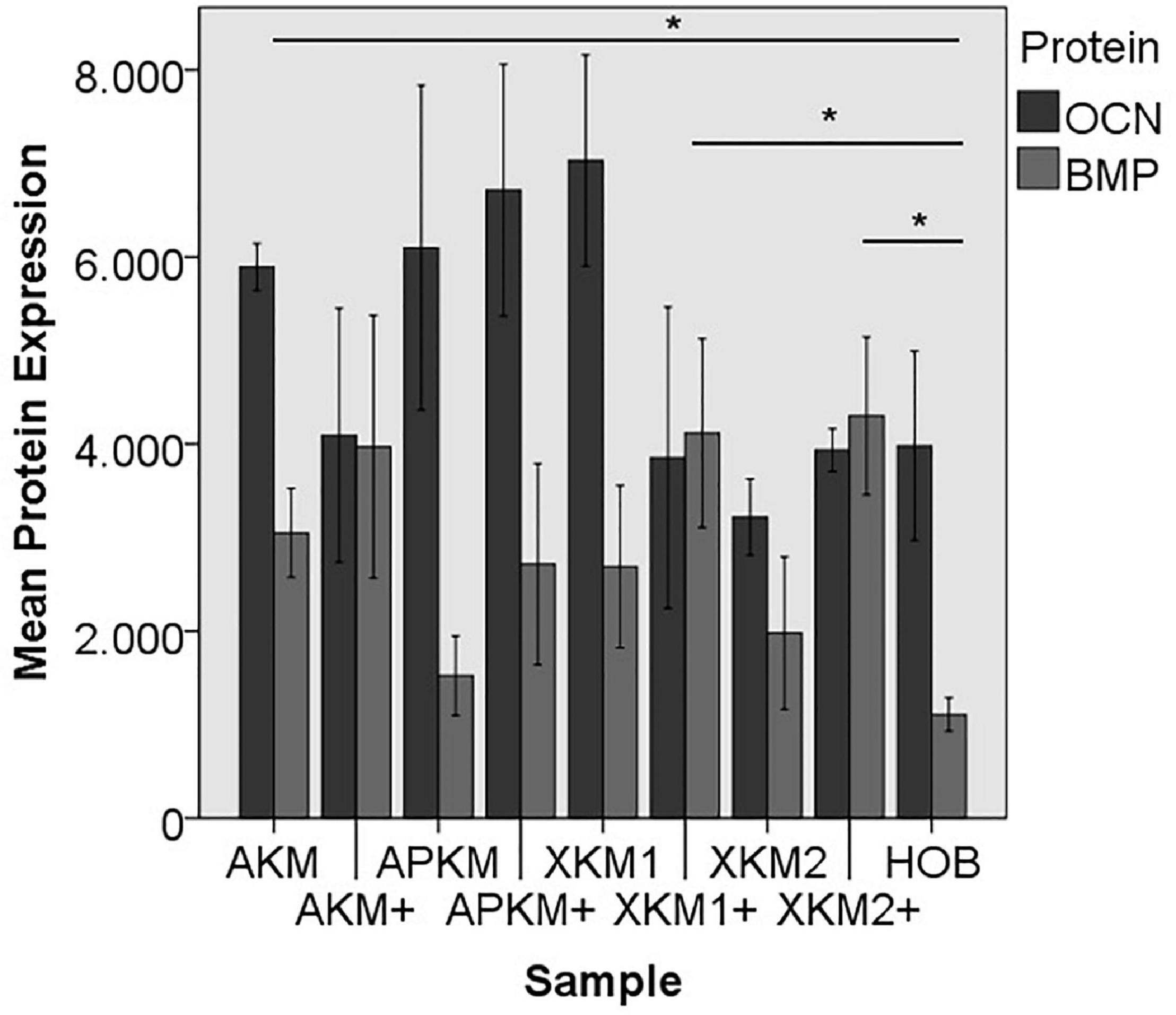
Figure 11. Mean protein expression of OCN and BMP after co-incubation of HOB with the respective samples with (+)/without PRF (*p < 0.05, BMP: AKM vs. HOB: p = 0.050, XKM1+ vs. HOB: p = 0.050, XKM2+ vs. HOB: p = 0.050).
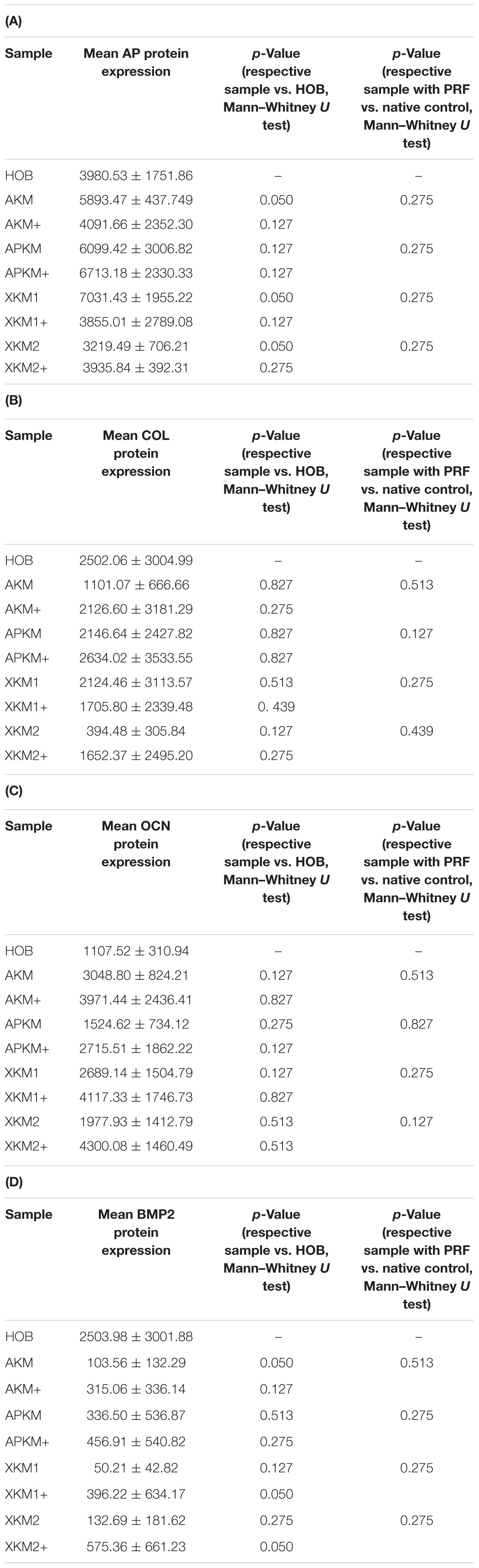
Table 4. textbf(A) AP, (B) COL, (C) OCN, (D) BMP protein expression assessed via ELISA for the respective samples and respective p-values vs. HOB alone and native BSM.
ALP expression was found to be highest for HOB alone with a significant decrease for allogeneic and xenogeneic samples (AKM: p = 0.050, XKM1: p = 0.050, XKM2: p = 0.050, all other samples: p > 0.05). Furthermore, all BSM in combination with PRF tended to increase ALP expression (all tested samples: p > 0.05). For COL expression, there were no statistical significant differences of the respective samples in comparison to HOB alone (all tested samples: p > 0.05) and the native BSM (all tested samples: p > 0.05). Similarly, OCN expression did not have a significant statistical difference in comparison to HOB alone (all tested samples: p > 0.05) and combination of PRF and the respective BSM vs. native material (all tested samples: p > 0.05). BMP expression was increased for allogeneic (p = 0.050) and the combination of PRF and xenogeneic materials in comparison to HOB alone (XKM1+: p = 0.050, XKM2+: p = 0.050). Furthermore, PRF addition tended to increase BMP expression for the respective BSM vs. native material, however without reaching statistical significance (all tested samples: p > 0.05).
Discussion
Within this study, a comparative analysis of the initial interaction of the combination of different BSM with PRF and its possible influence on early osteoblast viability, proliferation, and migration were performed in vitro.
As a major result, the combination of PRF with different BSM increases initial viability of HOBs. Furthermore, marker of proliferation and differentiation on gene and protein level, especially RUNX2, alkaline phosphatase, and collagen-1 demonstrated a noteworthy increase after co-incubation with BSM in addition to PRF and HOB in comparison to HOB alone for 24 h.
Other in vitro studies demonstrate ambivalent results where PRF did not significantly affect the expression of osteoblastic marker genes for differentiation, encoding ALP, RUNX2, or BMP2 (Sumida et al., 2019). Here, ALP mRNA levels were even decreased in comparison to premature osteoblasts alone. As a possible explanation, the authors state that ALP activity is high in mature osteoblasts and PRF did not inhibit, but rather delay the peak of osteoblast differentiation. This regulation may optimize bone remodeling to an osteogenic state during the early osteoblastic differentiation stages before ALP expression gradually increased over time (Sumida et al., 2019). This is in line with the presented results, where PRF led to an increase of the ALPL gene expression after 24 h. In addition, other studies found that TGF-β and PDGF, both growth factors released by PRF, may even reduce alkaline phosphatase and consequently delay differentiation (Strauss et al., 2020). Therefore, it can be discussed if PRF predominately assists in early stage osteogenesis by optimizing primarily osteoblast differentiation (Sumida et al., 2019). The increased collagen expression found in this study is also in accordance with the literature where other in vitro studies proved that PRF increased osteoblast attachment and proliferation via upregulating collagen-related protein production (Wu et al., 2012). Furthermore, the elevated BMP and RUNX2 expressions in the combination of PRF especially with allogeneic BSM may additionally induce osteoprotegerin and promote bone forming activity by increased collagen or osteocalcin production (Engler-Pinto et al., 2019; Sumida et al., 2019).
This is seen in the presented significant increased cell viability via MTT assay especially for xenogeneic BSM. In a recent analysis, the negative effect of zoledronic acid on the viability and proliferation of osteoblasts could partly be reversed by the application of PRF (Steller et al., 2019). In this study, differentiation and proliferation of osteoblasts tended to be increased when BSMs were combined with PRF but failed to show significant differences. Here, further immunological features should be addressed in subsequent studies to understand the cellular background. Using a first generation PC (Platelet Rich Plasma, PRP), the combination of PC and carbonated hydroxyapatite tended to decrease pro-inflammatory cell inflammation and subsequently showed a histologically increased bone formation (Oley et al., 2018).
This study suffers from some limitations. First and foremost, in vitro studies lack the general bias that results cannot reflect complex interactions in a biological system that may distort the effects. However, only in vitro analysis allows drawing conclusions about single cell-cell interaction. Next and in accordance with the literature, only one human osteoblast cell line was used for analysis. Surely, a multi cell line approach could strengthen the discussed hypothesis and should therefore be included in future studies. Additionally, this study solemnly focuses on the initial and early interaction of PRF and BSM and implications for HOB’s viability proliferation and differentiation. This way, new insights in the underlying intercellular processes and protein release kinetics may be gained in comparison to the complemented data in the literature. However, subsequent time points are not validated in this analysis. Finally, most of the given results did not reach statistical significance. However, since only small sample sizes (as a further limitation) were analyzed, statistical significance should be treated with caution and may reflect overall limited validity. Taken together, future in vivo studies are much in need to validate the found tendencies.
Within the named limitation of the presented approach, no recommendation can be given which BSM may best optimize bony regeneration in combination with PRF. However, without reaching statistical significance, alloplastic and especially xenogeneic BSM interacted strongly with PRF and did influence osteoblast features the most.
The possible underlying intercellular mechanism and early angiogenic interactions of the PRF with the respective BSM were evaluated in another study by our working group (Blatt et al., 2021). Here, it was demonstrated that PRF initially interacts with its respective BSM via platelet activation in vitro. Furthermore, PRF had a significant positive pro-angiogenic effect, especially in combination with alloplastic and xenogeneic materials in vivo. Here, validated by scanning electron microscopy, a “storage” of the respective growth factors of the PRF via the close spatial relationship between the fibrin network and the BSM and a consecutive slow release that triggers vasoformative responses was hypothesized (Blatt et al., 2021). This assumption may be transferred to the implications of bony regeneration and could explain the release kinetics and expression of the above investigated markers found in this study: initially, PRF boosts primary viability of HOBs and subsequently releases differentiation and migration marker. This hypothesis also explains the fact that migration assay did demonstrate a noteworthy influence of the PRF but failed to reach statistical significance at this early time point.
This postulation is validated by another recent analysis by Kyyak et al. (2020) that investigated if the combination of an allogeneic or a xenogeneic BSM in combination with PRF may influence osteoblast activity after longer incubation time points (after 3, 7, and 14 days). It was shown that the addition of PRF to allogeneic and, to a minor content, to xenogeneic BSM revealed a significant increase of HOB viability, migration, proliferation, and differentiation (Kyyak et al., 2020). In a bone remodeling animal study, the incorporation of PRF into a carbonated hydroxyapatite loaded hydrogel demonstrated a higher number of osteoblasts and decreased osteoclast activity in comparison to BSM alone after 14 and 21 days (Alhasyimi et al., 2018). Therefore, it can be discussed if the combination of PRF and BSM predominately optimizes early stage osteogenesis whereas a significantly increased expression is seen at later time points after the passive release of the growth factors physically entrapped within the fibrin network. At this point in time, allogeneic BSMs that seem to bear osteoconductive properties may be in favor to increase angiogenesis and new vessel sprouting (Kyyak et al., 2020). In context with the above-mentioned hypothesis, future studies should investigate if biomechanical aspects of the investigated BSM may influence interactions with PRF to a greater extent than what was previously assumed. This may broaden the indications of bioceramics and other BSM in regenerative medicine (Ana et al., 2018).
Conclusion
To conclude, PRF in combination with different BSM led to a noteworthy early influence on osteoblast proliferation, differentiation, and viability in vitro. In contrast to other bone-engineering methods that are hardly integrated in clinical workflow (mostly due to regulatory and practically restrictions), PC and especially PRF are autologous materials that are easy to produce and use chair-side. As shown, they seem capable to enhance the features that optimize bony regeneration. Therefore, translation in the clinical pathway seems feasible.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ärztekammer Rheinland-Pfalz, vote no. “2019-14705_1.” The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SB and AP contributed to the conceptualization. PK and SK contributed to the methodology. SB, DT, and AP contributed to the validation. BA-N and PK contributed to the formal analysis and supervision. SB, DT, and AP contributed to the investigation. PK and SK contributed to the data curation. SB and PK contributed to the writing—original draft preparation. DT, AP, and BA-N contributed to the writing—review and editing. SK contributed to the visualization. AP contributed to the project administration. PK contributed to the funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by an intramural grant of the BiomaTiCS group for SB.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Jutta Goldschmidt and Christina Babel for their technical support as well as Mr. Wellbe Bartsma for the language editing. The data from this study are part of the dissertation work submitted to the Johannes-Gutenberg University, Mainz, as part of the medical doctoral thesis of SB.
References
Alhasyimi, A. A., Pudyani, P. P., Asmara, W., and Ana, I. D. (2018). Enhancement of post-orthodontic tooth stability by carbonated hydroxyapatite-incorporated advanced platelet-rich fibrin in rabbits. Orthod. Craniofac. Res. 21, 112–118. doi: 10.1111/ocr.12224
Ana, I. D., Satria, G. A. P., Dewi, A. H., and Ardhani, R. (2018). Bioceramics for clinical application in regenerative dentistry. Adv. Exp. Med. Biol. 1077, 309–316. doi: 10.1007/978-981-13-0947-2_16
Blatt, S., Burkhardt, V., Kämmerer, P. W., Pabst, A. M., Sagheb, K., Heller, M., et al. (2020). Biofunctionalization of porcine-derived collagen matrices with platelet rich fibrin: influence on angiogenesis in vitro and in vivo. Clin. Oral Investig. 24, 3425–3436. doi: 10.1007/s00784-020-03213-8
Blatt, S., Thiem, D. G. E., Pabst, A., Al-Nawas, B., and Kämmerer, P. W. (2021). Does platelet-rich fibrin enhance the early angiogenetic potential of different bone substitute materials? An in vitro and in vivo analysis. Biomedicines 9:61. doi: 10.3390/biomedicines9010061
Dohan, D. M., Choukroun, J., Diss, A., Dohan, S. L., Dohan, A. J., Mouhyi, J., et al. (2006). Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 101, e37–e44.
Dohle, E., El Bagdadi, K., Sader, R., Choukroun, J., James Kirkpatrick, C., and Ghanaati, S. (2018). Platelet-rich fibrin-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J. Tissue Eng. Regen. Med. 12, 598–610. doi: 10.1002/term.2475
Engler-Pinto, A., Siéssere, S., Calefi, A., Oliveira, L., Ervolino, E., de Souza, S., et al. (2019). Effects of leukocyte- and platelet-rich fibrin associated or not with bovine bone graft on the healing of bone defects in rats with osteoporosis induced by ovariectomy. Clin. Oral. Implants Res. 30, 962–976. doi: 10.1111/clr.13503
Ghanaati, S., Herrera-Vizcaino, C., Al-Maawi, S., Lorenz, J., Miron, R. J., Nelson, K., et al. (2018). Fifteen years of platelet rich fibrin in dentistry and oromaxillofacial surgery: how high is the level of scientific evidence? J. Oral Implantol. 44, 471–492. doi: 10.1563/aaid-joi-d-17-00179
Grosso, A., Burger, M. G., Lunger, A., Schaefer, D. J., Banfi, A., and Di Maggio, N. (2017). It takes two to tango: coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng Biotechnol. 5:68. doi: 10.3389/fbioe.2017.00068
Hinze, M. C., Wiedmann-Al-Ahmad, M., Glaum, R., Gutwald, R., Schmelzeisen, R., and Sauerbier, S. (2010). Bone engineering-vitalisation of alloplastic and allogenic bone grafts by human osteoblast-like cells. Br. J. Oral Maxillofac. Surg. 48, 369–373. doi: 10.1016/j.bjoms.2009.06.011
Khosropanah, H., Lashkarizadeh, N., Ayatollahi, M., Kaviani, M., and Mostafavipour, Z. (2018). The impact of calcium hydroxide on the osteoinductive capacity of demineralized freeze-dried bone allograft: an in-vitro study. J. Dent. (Shiraz) 19, 19–27.
Kyyak, S., Blatt, S., Pabst, A., Thiem, D., Al-Nawas, B., and Kämmerer, P. W. (2020). Combination of an allogenic and a xenogenic bone substitute material with injectable platelet-rich fibrin - A comparative in vitro study. J. Biomater. Appl. 35, 83–96. doi: 10.1177/0885328220914407
Miron, R. J., Zhang, Q., Sculean, A., Buser, D., Pippenger, B. E., Dard, M., et al. (2016). Osteoinductive potential of 4 commonly employed bone grafts. Clin. Oral Investig. 20, 2259–2265. doi: 10.1007/s00784-016-1724-4
Miron, R. J., Zucchelli, G., Pikos, M. A., Salama, M., Lee, S., Guillemette, V., et al. (2017). Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin. Oral Investig. 21, 1913–1927.
Oley, M. C., Islam, A. A., Hatta, M., Hardjo, M., Nirmalasari, L., Rendy, L., et al. (2018). Effects of platelet-rich plasma and carbonated hydroxyapatite combination on cranial defect Bone Regeneration: an animal study. Wound Med. 21, 12–15. doi: 10.1016/j.wndm.2018.05.001
Pabst, A. M., Kruger, M., Ziebart, T., Jacobs, C., Sagheb, K., and Walter, C. (2015). The influence of geranylgeraniol on human oral keratinocytes after bisphosphonate treatment: an in vitro study. J. Craniomaxillofac Surg. 43, 688–695. doi: 10.1016/j.jcms.2015.03.014
Pripatnanont, P., Nuntanaranont, T., Vongvatcharanon, S., and Phurisat, K. (2013). The primacy of platelet-rich fibrin on bone regeneration of various grafts in rabbit’s calvarial defects. J. Craniomaxillofac Surg. 41, e191–e200.
Rather, H. A., Jhala, D., and Vasita, R. (2019). Dual functional approaches for osteogenesis coupled angiogenesis in bone tissue engineering. Mater Sci. Eng. C Mater Biol. Appl. 103:109761. doi: 10.1016/j.msec.2019.109761
Steller, D., Herbst, N., Pries, R., Juhl, D., and Hakim, S. G. (2019). Positive impact of Platelet-rich plasma and Platelet-rich fibrin on viability, migration and proliferation of osteoblasts and fibroblasts treated with zoledronic acid. Sci. Rep. 9:8310.
Strauss, F. J., Nasirzade, J., Kargarpoor, Z., Stahli, A., and Gruber, R. (2020). Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: a systematic review of in vitro studies. Clin. Oral Investig. 24, 569–584. doi: 10.1007/s00784-019-03156-9
Sumida, R., Maeda, T., Kawahara, I., Yusa, J., and Kato, Y. (2019). Platelet-rich fibrin increases the osteoprotegerin/receptor activator of nuclear factor-kappaB ligand ratio in osteoblasts. Exp. Ther. Med. 18, 358–365.
Tatullo, M., Marrelli, M., Cassetta, M., Pacifici, A., Stefanelli, L. V., Scacco, S., et al. (2012). Platelet Rich Fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int. J. Med. Sci. 9, 872–880. doi: 10.7150/ijms.5119
von Arx, T., Cochran, D. L., Hermann, J. S., Schenk, R. K., and Buser, D. (2001). Lateral ridge augmentation using different bone fillers and barrier membrane application. A histologic and histomorphometric pilot study in the canine mandible. Clin. Oral Implants Res. 12, 260–269. doi: 10.1034/j.1600-0501.2001.012003260.x
Wu, C. L., Lee, S. S., Tsai, C. H., Lu, K. H., Zhao, J. H., and Chang, Y. C. (2012). Platelet-rich fibrin increases cell attachment, proliferation and collagen-related protein expression of human osteoblasts. Aust. Dent. J. 57, 207–212. doi: 10.1111/j.1834-7819.2012.01686.x
Keywords: bone substitute, oral regeneration, platelet-rich fibrin, tissue engineering, osteoblast, allograft, xenograft
Citation: Blatt S, Thiem DGE, Kyyak S, Pabst A, Al-Nawas B and Kämmerer PW (2021) Possible Implications for Improved Osteogenesis? The Combination of Platelet-Rich Fibrin With Different Bone Substitute Materials. Front. Bioeng. Biotechnol. 9:640053. doi: 10.3389/fbioe.2021.640053
Received: 10 December 2020; Accepted: 22 February 2021;
Published: 16 March 2021.
Edited by:
Zhilian Yue, University of Wollongong, AustraliaReviewed by:
Ika Dewi Ana, Gadjah Mada University, IndonesiaAlvaro Mata, University of Nottingham, United Kingdom
Copyright © 2021 Blatt, Thiem, Kyyak, Pabst, Al-Nawas and Kämmerer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastian Blatt, sebastian.blatt@unimedizin-mainz.de
 Sebastian Blatt
Sebastian Blatt Daniel G. E. Thiem1
Daniel G. E. Thiem1  Solomiya Kyyak
Solomiya Kyyak