Allergen immunotherapy in China
- 1Department of Allergy, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China
- 2Institute of Allergy and Clinical Immunology, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China
Allergen immunotherapy (AIT) is an etiological treatment strategy that involves administering escalating doses of clinically relevant allergens to desensitize the immune system. It has shown encouraging results in reducing allergy symptoms and enhancing patients' quality of life. In this review, we offer a thorough overview of AIT in China, examining its efficacy, safety, current practices, and prospects. We further underscore the progress made in AIT research and clinical applications, as well as the distinct challenges and opportunities that China faces in this area.
Introduction
Allergic diseases have become a significant global health concern, with a growing number of individuals being affected by various allergic conditions (1). In recent decades, the prevalence of allergic diseases in China has consistently been on the rise. A study conductedas part of the EuroPrevall-INCO project in China revealed that urban children have higher prevalence rates of self-reported allergic diseases compared to their rural counterparts. Specifically, the study found higher rates of allergic rhinitis (AR) (23.2% vs. 5.3%), asthma (6.6% vs. 2.5%), and eczema (34.1% vs. 25.9%) among urban children (2). A multi-center epidemiological survey conducted in 2011 revealed that the prevalence of adult AR was 17.6% in 18 major cities across China (3). Additionally, a nationwide cross-sectional study reported an adult asthma prevalence in China of 4.2% (4). According to the Global Burden of Disease Study 2019, there was a substantial increase of 25.65% in the number of patients diagnosed with atopic dermatitis (AD) between 1990 and 2019. The prevalence of AD in China has been observed to increase at a faster rate than the global average (5). In the Jiangxi province, around 4% of the adult population reported experiencing food allergies (6). Notably, the prevalence of this condition among children experienced a significant increase, rising from 3.5% in 1999 to 11.1% in 2019 (7). Allergic diseases significantly impact the overall well-being and quality of life of individuals affected by them, as well as their families. Allergen avoidance plays a crucial role in managing allergic diseases. However, it is often challenging to completely avoid these triggers, and conventional pharmacological interventions consistently fall short in meeting the patient's need for symptom relief.
Allergen immunotherapy (AIT) can induce immune tolerance towards allergens, exert a disease-modifying effect on immunoglobulin E (IgE)-mediated allergic diseases, and potentially influence the natural progression of allergic diseases (8, 9). The World Allergy Organization (WAO) suggests that AIT may be considered the only etiological treatment for IgE-mediated allergic diseases (10). Over the years, AIT, including subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT), has proven to be an effective treatment for patients with AR, allergic conjunctivitis, and allergic asthma due to inhaled allergens (11, 12). SCIT can be administered to individuals diagnosed with Hymenoptera venom allergy (13). Oral immunotherapy (OIT) has emerged as a promising treatment for IgE-mediated food allergy. Several studies have suggested that OIT could be a safe and effective approach for managing peanut, cow's milk, and hen's egg allergies (14).
In China, the use of AIT for the managing allergic conditions began in 1956. Since then, there has been a significant rise in the number of allergic patients in China, accompanied by increased awareness and attention from Chinese doctors towards allergic diseases. Consequently, AIT has gained considerable popularity and has been extensively researched and utilized. In this review, we explore the historical background of AIT in China and summarize its efficacy, safety, current implementation, and future prospects.
Brief history of AIT in China
In 1956, the establishment of the first Department of Allergy at Peking Union Medical College Hospital (PUMCH) signaled the formal introduction of allergology in China. This development facilitated the provision of medical care to patients affected by allergic diseases. Initially, medical practitioners faced a significant influx of individuals presenting typical symptoms of hay fever. However, the skin prick test (SPT) conducted using allergen preparations procured from American vendors, yielded negative results. This observation prompted doctors to consider that the allergens causing hay fever in Chinese patients might differ from those abroad. Following several years of extensive investigation, Ye and his colleagues identified Artemisia pollen and Humulus pollen as the primary allergenic pollens in North China (15, 16). From then on, the pioneering group of Chinese allergists developed a comprehensive array of nearly 100 in-house allergen extracts specifically designed for SPT and AIT, catering to the unique characteristics of domestic individuals suffering from allergies. However, all the allergen extracts employed in the study were in the form of crude extracts, including house dust mites (HDMs), pollens, fungi, animal dander, and insect extracts.
After 2001, the National Medical Products Administration (NMPA) undertook efforts to enhance the regulation of allergen preparations, leading to restrictions on the use of in-house crude allergen extracts in certain medical facilities. Currently, the NMPA in China has approved only three standardized allergen extracts of dust mites for AIT. Novo Helisen-Depot (Allergopharma Joachim Ganzer KG, Germany) received NMPA approval in 1999, making it one of the first commercial products available. Alutard-SQ (ALK-Abelló, Denmark), which received NMPA approval in 2004, has gained substantial popularity for SCIT in China. Challergen-Dermatophagoides farinae Drops (Wolwo Bio-Pharmaceutical China) is the only HDM-SLIT product that received approval from the China Food and Drug Administration (CFDA) in 2006 (17). In 2012, the Beijing Municipal Medical Products Administration approved the commercial use of nine types of in-house crude allergen extracts (including dust mite, Artemisia, Humulus, Oleaceae, Cypress, Alternaria, cat, and dog dander) provided by PUMCH in Beijing and several provinces. In 2021, China approved the marketing of the first standardized drops of Artemisia annua (Wolwo Bio-Pharmaceutical China). In January 2023, thanks to the licensed healthcare policy of Boao LeCheng Medical Advance Zone in Hainan, HDM allergen sublingual tablets (ACARIZAX®, ALK-Abelló, Denmark) were introduced at Ruijin Hainan Hospital in LeCheng, with the first prescription issued on January 13th. The NMPA formally accepted the Biologics Listing License Application (BLA) for ACARIZAX® in 2023. Review and approval are anticipated to be completed in 2024.
Allergen sensitization profile in China
Inhalant allergens primarily contribute to the etiology of AR and asthma. Similar to other countries, HDMs, such as Dermatophagoides pteronyssinus (Der p) and Dermatophagoides farina (Der f), are the main indoor allergens in China (18–20). However, the complex geographical features, diverse climate patterns, and varied levels of industrial development across China have led to significant variations in allergenic substances across different regions. In China, there is a noted decreasing trend of sensitization to HDM from the southeast to the northwest region (20). For instance, in central China, HDM was the dominant allergen, with a sensitization rate exceeding 90% (18). In contrast, in Lhasa, HDM was ranked as the fourth most common inhalant allergen (21). Overall, the prevalence of sensitization to HDM in China continues to rise (22).
Pollen allergens display regional variations influenced by the dominant plant species in different geographical locations. Artemisia pollen is widely recognized as the leading allergen in the northern region of the Yangtze River in China (20). From 2008 to 2018, there was a significant increase in the prevalence of pollen sensitization, particularly to Artemisia vulgaris, in the northern region of China (22). During a study conducted in Central China, it was noted that more patients exhibited sensitization to tree pollens, specifically Platanus, compared to Artemisia (18).
Sensitization to animal allergens has seen a notable increase, especially among children and adolescents. Cat dander is the most common allergen of animal origin (23). In Central China, there has been a significant rise in the overall positive rates of cat and dog dander from 1.3% to 15.5% and 0.8% to 10.5%, respectively, between 2016 and 2021 (24).
Fungi are also significant inhalant allergens in China. The prevalence of mold sensitization was reported to be higher in children diagnosed with rhinitis and asthma (22). In a study conducted in Wuhan, SPT data from 1,365 patients with respiratory allergies revealed that 14.8% of them exhibited sensitization to fungi. The most commonly detected fungi allergens were Cladosporium (11.72%), Penicillium (4.76%), and Alternaria (4.69%) (25). Similar to other allergens, there has been an observed increase in sensitization rates to Cladosporium and Alternaria over the past five years (24). In Taiwan, a study conducted revealed that the fungal species most likely to induce allergic reactions were Candida, Aspergillus, and Penicillium (26).
Allergen sensitization profile in other countries
Asian countries, with their diverse climate conditions, foster a variety of plants and animals. In Asia, Japanese cedar (27) and Japanese hop (28) are the two most distinct sources of pollen allergens. Oak (29), mugwort, and grass are also common, but their species differ from those found in Western countries (30).
A multicenter study conducted across 14 European countries revealed that grass pollen, HDM, birch pollen, cat dander, olive pollen, mugwort, German cockroach, and Alternaria are the most common allergens in the majority of subjects across these countries (31). Allergy sensitization in Southern Europe is relatively straightforward, with grass and olive allergies being the most common throughout most of the region. Conversely, Northern and Central Europe present a more complex sensitization profile, with allergies to grasses and birch pollen being dominant. Moving towards Central and especially Eastern Europe, allergies to Ambrosia, Artemisia, and ash tree pollen may appear (9).
In the United States (US), the population is most commonly sensitized to grass pollen, dust mites, and ragweed pollen (32). The sensitization rate to fungi in the US ranges from 7.4% to 18.6% with the highest rates for Candida albicans (18.6%), Alternaria alternata (16.6%), Stemphylium herbarum (14.9%), and Aspergillus fumigatus (14.2%) (33).
AIT in China
Efficacy of SCIT
In China, numerous non-standardized crude extracts have been employed for a long duration and have demonstrated significant efficacy (34–40). In 1987, a one-year controlled trial was carried out to evaluate the efficacy of SCIT in 50 patients with hay fever who were sensitive to Artemisia. The trial results showed a significant improvement in symptoms among the treatment group, with an overall effective rate of 78% (34). A cross-sectional, real-world study was conducted at multiple centers to assess the effects of SCIT using allergen crude extracts on 246 patients with AR, with or without asthma. The study found that 96.7% of the patients experienced an improvement in clinical symptoms following SCIT. Additionally, the use of concomitant medications, such as antihistamines and nasal corticosteroids was reduced after SCIT (35). Du et al. conducted a retrospective evaluation to assess the effectiveness and safety of SCIT using mixed allergens for the treatment of allergic asthma (36). The study revealed an increase in the percentage of forced expiratory volume in the first second to the predicted value (FEV1%) in all patients, in addition to symptom relief and a reduction in concomitant medication. Furthermore, a retrospective study spanning three years and involving a total of 1,640 patients was conducted to examine the impact of SCIT on the occurrence of new sensitization in individuals with respiratory allergies (37). In this study, the crude allergen extracts utilized included dust mites, weed pollens, grass pollens, molds, and animal dander. The study revealed that patients who were mono-sensitized had a lower likelihood of developing new sensitization compared to those who were multi-sensitized. New sensitization is observed during the initial phases of SCIT, with a subsequent decline in the rate of new sensitization over time.
Standardized HDM extracts have been utilized in SCIT for patients with AR and asthma in China for over 20 years. The utilization of these extracts has consistently demonstrated favorable efficacy (41–68).
In a historical cohort study conducted in Guangzhou, a total of 158 patients with persistent AR were included. Out of these, 114 patients received treatment with a standardized mite depot-allergen extract (NovoHelisen Depot, Germany), which consisted of a 50% mixture of Der p and Der f allergens (59). This study presented findings that support the effectiveness of SCIT in treating patients with AR caused by HDM allergens. The study utilized a standardized allergen product and observed improvements in clinical symptoms both after the termination of SCIT and during the 2-year follow-up period. Importantly, SCIT demonstrated a significant reduction in the likelihood of asthma development among patients with AR even after discontinuation of SCIT for 2 years. Furthermore, there was a head-to-head study that compared the efficacy and safety of Der p extracts (Alutard SQ) and Der p/Der f extracts (Novo Helisen Depot) in AR patients (60). This study has confirmed that both HDM extracts exhibit equal efficacy and safety profiles. A randomized trial conducted in Mainland China aimed to investigate the efficacy of SCIT with Alutard-SQ in mild to moderate allergic asthma. The study involved 132 asthmatic patients aged 6–45 years, who were recruited from three different regions of Mainland China. This study was the first of its kind in China and employed a double-blind, placebo-controlled design (61). The findings indicated that the scores for symptoms started to decrease at week 29 and persisted until week 48 in the immunotherapy group. They also observed a decrease in SPT response in the immunotherapy group, while the levels of Der p-sIgE remained unchanged. Another study, which followed a randomized, double-blind, placebo-controlled design, examined the effectiveness of SCIT with Alutard-SQ over three years. The study included a sample of 90 children diagnosed with AR and asthma (62). The findings indicate that a 3-year course of SCIT has the potential to decrease both day-time and night-time asthmatic symptom scores, improve peak expiratory flow (PEF) values, lower serum IgE levels, and most notably, reduce the requirement of inhaled corticosteroids (ICSs). Zhang et al. conducted a study in which they recruited 51 children with allergic asthma to compare the effectiveness and safety of long-term HDM-SCIT in mono- and polysensitized children (63). In terms of clinical effectiveness and safety of HDM-SCIT, there was no significant difference between mono-sensitized and poly-sensitized children with allergic asthma. However, Huang et al. conducted a comparative analysis to assess the effectiveness and safety of HDM-SCIT in patients with HDM mono-sensitized AR and individuals sensitized to multiple allergens. Their study yielded contrasting outcomes (64). The findings suggested that HDM-SCIT was safe and effective for AR patients with only HDM and multiple allergens including HDM, but the profile of allergen sensitization could potentially influence the efficacy of SCIT, notably, the efficacy of SCIT was more pronounced in AR patients who were sensitized to three or fewer allergens, excluding HDM. The difference in the results of the two studies may be related to the selected diseases, sample size, and there were fewer patients with pollen allergy in Zhang's trial.
Overall, numerous studies provide evidence supporting the effectiveness of SCIT in the management of AR and/or asthma among Chinese patients. These studies have primarily concentrated on the following areas: (1) the alleviation of allergic symptoms and enhancement of quality of life, (2) the reduction of drugs used for symptomatic treatment, (3) the durability of effects even after the cessation of treatment, (4) the prevention of AR from progressing into asthma, (5) the prevention of new sensitization.
Furthermore, Zhou et al. conducted a retrospective analysis to evaluate the long-term effectiveness and safety of SCIT in patients with AD who were sensitized to HDM (69). In this study, a total of 164 patients were administered SCIT plus pharmacotherapy for 3 years. Additionally, a separate group of 214 patients with AD solely received pharmacotherapy. The findings of this study revealed a significant decrease in both the symptoms of AD and the scores on the pruritus visual analog scale (VAS) in the SCIT group compared to the non-SCIT group. This decrease was observed after 3 years of treatment.
Safety of SCIT
The safety profile of SCIT has been demonstrated to be favorable in both adults and children, as evidenced by data from Randomized Controlled Trials (RCTs) and clinical practice in China (70–78).
Wen et al. conducted a review of adverse events (AEs) observed in patients who underwent SCIT using crude pollen allergen extracts at their department between December 1993 and September 2013 (70). A total of 70 AEs were observed in 35 patients. The study found that a significant proportion (97.1% or 68/70)) of systemic reactions (SRs) occurred when the maximal concentration was administered. Among these SRs, the majority were classified as mild to moderate, with 58.6% being grade 1, 15.7% grade 2, 17.1% grade 3, and 8.6% grade 4. Several risk factors were identified, including the administration of large doses (0.6–1.0 ml), increasing doses during the pollen season, administering higher doses without considering obvious local reactions (LRs), and suspected incorrect injection techniques.
Yang et al. conducted a study to investigate the safety of HDM-SCIT (Alutard SQ, ALK) in preschool children diagnosed with respiratory allergic diseases (71). A total of 3,109 injections were recorded in 91 patients. Out of these injections, 186 (5.98%) resulted in immediate LRs in 62 (68.13%) patients. Additionally, 6 injections (0.19%) led to delayed LRs in 4 patients (4.4%), while 44 injections (1.42%) caused immediate SRs in 11 patients (12.09%). This study revealed that body mass index (BMI) and HDM-sIgE were identified as risk factors for LRs. A multicenter study was conducted to investigate the safety of semi-depot HDM allergen extract (Novo-Helisen Depot) in children and adolescents diagnosed with AR and asthma (74). A total of 3,600 injections were administered to 250 patients. Among these injections, 361 (10%) were associated with SCIT-related AEs occurred in 96 (38.4%) of the patients. Additionally, 321 injections (8.9%) resulted in LRs occurring in 89 (35.6%) patients, while 40 injections (1.1%) led to SRs occurring in 23 (9.2%) patients.
SLIT
Efficacy of SLIT
Numerous studies have demonstrated the short- and long-term efficacy of SLIT in AR and/or asthma in both adult and pediatric patients in China (79–98). Individualized treatment is essential to improve response rates to SLIT, as there are variations in efficacy and side effects among individuals. Gao et al. enrolled 157 AR patients aged 4–60 years, and categorized patients into high response (HR) and low response (LR) groups based on reductions in combined symptom and medication scores (CSMS) after 6 months of SLIT treatment (83). HR groups were the patients with CSMS reduced by over 50% and continued the original dose, while the LR groups were the patients with CSMS down 20%–50% and received an increased dose (the percentage of dose increase was 33.33% for patients younger than 14 years of age and 50% for patients older than 14 years of age). They found a significant difference in CSMS and VAS between the two groups at 6 months and 1 year, but not in later follow-ups. They concluded that dosage enhancement within a certain range may improve the efficacy of SLIT.
SLIT of Artemisia annua is currently being conducted in China and has shown promising efficacy (34, 99–103). A randomized, double-blind, placebo-controlled phase 3 clinical trial involving 71 seasonal AR investigated the efficacy and mechanisms underlying SLIT of Artemisia annua (100). The results revealed that SLIT with Artemisia annua consistently improved patients' nasal symptom scores during peak pollen season in years one and two, decreased Th2 cells, increased nTreg and Tr1 cells in blood after 16 weeks, increased Cystatin 1 in nasal secretion after 16 and 32 weeks. Another randomized, double-blind, placebo-controlled, multicenter, phase III clinical trial was conducted to assess the efficacy and safety of SLIT in 702 patients with Artemisia annua-induced AR (101). The findings of this study indicate that SLIT had a significant positive impact on the severity of rhino-conjunctivitis and total nasal symptoms experienced daily. Additionally, SLIT was found to effectively reduce the need for daily rescue medication during the peak pollen period. Yang et al. conducted a study to investigate the efficacy and safety of Artemisia annua-SLIT in seasonal AR patients, focusing on the impact of different intervention times (102). This study has provided evidence to support the equivalent efficacy and safety of Artemisia annua-SLIT in the treatment of seasonal AR patients. The study found that both 8–9 and 12–13 weeks of pre-season therapy with Artemisia annua-SLIT resulted in comparable outcomes in terms of efficacy and safety. This was observed in both mono-sensitized and poly-sensitized groups. The investigation of the long-term efficacy and safety of Artemisia annua-SLIT is necessary.
The SLIT has also demonstrated effectiveness in treating AD (104–106). A multicenter, randomized, double-blind, placebo-controlled clinical trial was conducted over 36 weeks. The trial involved 239 patients diagnosed with AD and aimed to evaluate the efficacy and safety of SLIT using Der f Drops (105). This study reported that significant decreases in Scoring Atopic Dermatitis (SCORAD) indexes, skin lesion area scores, dermatology life quality indexes, and total medication scores were seen in both the medium- and high-dose groups.
Safety of SLIT
Both SLIT with Der f drops and SLIT with Artemisia annua drops have demonstrated a satisfactory safety profile in both children and adults (93, 100–102, 107–110).
Shao et al. conducted a study that aimed to investigate the effectiveness and safety of SLIT in young children (264 children aged 3–13 years old, including 133 children aged 3–5 years old) (93). There were no significant differences in clinical efficacy, time to onset, immunologic parameters, or safety between children younger and older than 5 years of age in the SLIT group. No serious systemic AEs were reported.
In the study of Artemisia annua-SLIT, Lou, et al. reported that 17/47 patients experienced mild local AEs and 2 patients experienced mild systemic AEs. The most common AEs observed were oral paresthesia, nasopharyngitis, sneezing, nasal pruritus, rhinorrhea, eye pruritus, nasal congestion, throat irritation, oropharyngeal pain, cough, upper respiratory tract infection, ear pruritus, headache, throat-clearing, diarrhea, tongue itching, and swollen tongue, listed in the descending order of frequency (100). In a multicenter randomized trial on Artemisia annua-SLIT trial (101), no serious SLIT-related AEs were reported.
SCIT vs. SLIT
After many years of clinical practice, both SCIT and SLIT have exhibited favorable outcomes. In general, SLIT has been observed to have fewer and milder adverse effects compared to SCIT, while SCIT typically demonstrates greater effectiveness and has a faster onset of action (17, 111–115). A prospective, open-label, and single-center study was conducted to compare the efficacy, safety, and compliance of SCIT and SLIT in HDM-induced AR children (116). Their results suggested SCIT had a higher compliance rate than SLIT, whereas SLIT had fewer adverse events than SCIT. The total nasal symptom score, rescue medication score, and symptom medication score were all lower in the SCIT group than that in the SLIT group. However, in other studies SLIT has same clinical effect compared with SCIT (117–121). Xian et al. compared clinical effectiveness and immune responses between SLIT and SCIT in AR sensitized to HDM (120). They found that both SLIT and SCIT have similar rates of clinical improvement. In both groups, there was a trend towards upregulation of CD4 + CD25 + FoxP3+ Tregs, but this was only found to be inversely correlated with total rhinitis score in SLIT. Furthermore, the levels of Der p specific immunoglobulin G4 (Der-p-sIgG4) increased significantly in both SCIT and SLIT group, but it was found to be 30 times higher in SCIT than SLIT after the treatment.
AIT in other countries
The efficacy and safety of AIT for AR and asthma have been confirmed in other Asian countries (122–129). The effect of AIT on AD has also been reported (130–133).
In Korea, the commercial allergens used for SCIT include HDM, pollens, mold and animal epithelia, while SLIT was prescribed only for HDM (134). SCIT prescription is more popular than SLIT in Korea (125).
In Japan, SCIT was introduced in the early 1960s as a treatment for AR and/or asthma. Nowadays, SCIT and SLIT are both permitted for patients allergic to Japanes Cedar Pollen (JCP) and HDM (135–137), and dual SLIT for JCP and HDM is also safe (138). SLIT in form of liquid formulations and tablets are both available in Japan (137). Sales of SCIT products in Japan declined steadily from the 1980s, possibly related to the disadvantages of SCIT (137). The first Japanese cedar SLIT drop product, Cedartolen, was registered in October 2014, and has been shown to significantly reduce the total nasal symptom and medication scores in Phase II and III clinical trials (139, 140). HDM SLIT tablets (Miticure and Actair) were launched in 2015 (141–143). A JCP SLIT tablet was developed in 2018 based on the same efficient freeze-dried formulation as Miticure (144). This JCP SLIT tablet now approved for market in Japan as named Cedarcure.
In the US, there are currently 4 companies that manufacture and market allergen extracts for clinical use, and allow standardization of 19 SCIT products for HDM, molds, pollens, Hymenoptera venom, mammalian epithelia and feathers, whole body insect and miscellaneous items. US Food and Drug Administration (FDA) approved tablet products standardized for allergenic potency include grass, ragweed, HDM and a grass mix. However, it's worth noting that most commercially available allergen extracts are not standardized (145, 146).
In Europe, the first SCIT products were authorized in 1976, whereas the first SLIT product was authorized in Germany in 2004, and most currently available SCIT products were authorized in the 1990s, more product options are available in Europe, including adsorbed allergens, chemically modified allergens, or both. Both tablets and liquid extracts are approved for SLIT (9). There are major differences in the clinical approach to SCIT in polysensitized patients, European allergists suggested preferably do not mix more than 3 components in a single vaccine, whereas in US mixed extracts containing multiple aeroallergens are used (145).
Modified regimen and novel routes of administration of AIT
Rush and cluster immunotherapy schedules
Rush immunotherapy (RIT) offers the most expedited build-up time, reducing up-dosing treatment from the traditional 4 months to less than 1 week. A prospective, open-label phase IV clinical trial compared the efficacy and safety of RIT and conventional immunotherapy in AR patients (147). The study showed a reduced incidence of adverse effects and a decreasing trend in leukotriene levels among both the RIT and conventional immunotherapy groups, suggesting comparable safety profiles. Notably, the VAS scores showed a decrease in the RIT group at the end of the second week. Additionally, the levels of IgG4 were found to be higher after the completion of the RIT dose accrual, one week later. Moreover, the weekly drug dosage scores were comparatively higher in the conventional immunotherapy group, suggesting that RIT exhibits a more accelerated onset of action when compared to conventional immunotherapy.
Cluster immunotherapy typically takes 4–8 weeks to reach a maintenance dose and requires patients to receive multiple allergen injections (generally two to four injections) sequentially in a single day of treatment on non-consecutive days. A randomized and open-label trial enrolled 149 AR patients to compare the efficacy of conventional and cluster immunotherapy during the build-up phase in adults and children (148). After the completion of the build-up phase of immunotherapy, a significant decrease in symptom scores was observed among the majority of patients, regardless of whether they followed the conventional or accelerated cluster schedules. However, there was no difference in efficacy between conventional and cluster SCIT, nor between adults and children. Similar results have demonstrated the safety of RIT and cluster immunotherapy in other studies (149–160).
Intralymphatic immunotherapy (ILIT)
ILIT is a novel route of immunotherapy for patients. Several studies on ILIT in China have shown promising results (161–165). A pilot study was conducted to assess the clinical effectiveness and safety of cervical ILIT in HDM-induced AR adult patients (163). This trial demonstrated that ILIT significantly improved both symptoms and quality of life, reduced administration of rescue medication, and no moderate or severe adverse events. Another prospective randomized controlled trial, spanning over 3 years, assessed the long-term efficacy and safety of cervical ILIT in 50 children with HDM-induced allergic rhino-conjunctivitis (164). The trial showed that compared with SCIT, cervical ILIT could improve allergic symptoms more rapidly, shorten the period of treatment, and lower pain perception. However, the long-term effects were found to be better in the SCIT group. The cervical ILIT group was safer, as evidenced by the occurrence of only 3 mild local adverse reactions and the absence of any systemic adverse reactions in the cervical ILIT group. In contrast, the SCIT group experienced 14 systemic adverse reactions. Wang et al. conducted a study on adult AR patients to evaluate the long-term effectiveness of cervical ILIT (165). They found that the cervical ILIT had long-term efficacy, high safety, and high compliance, but its long-term efficacy was inferior to that in the SCIT group.
Epicutaneous immunotherapy (EPIT)
In China, there have been no clinical studies of EPIT, but several studies in mice model have reported its efficacy and safety (166, 167). Zhang et al. used composite microneedles (MNs) to deliver sustained antigens for EPIT (167). They found that this novel EPIT is more effective at a lower dose compared to conventional SCIT. However, clinical data is needed to demonstrate its efficacy and safety before it can be approved for routine clinical use.
Biologicals in AIT
In recent years, there has been a significant increase in the inclusion of biologicals in medical insurance, leading to their widespread utilization in China. Several studies have substantiated the efficacy of omalizumab in RIT (168–171) or cluster immunotherapy (172). For instance, Zhang et al. designed a real-world retrospective study to investigate the efficacy, safety, compliance, and cost of combination treatment with RIT plus one dose of pretreatment omalizumab in Chinese children with respiratory allergies (169). The findings of this study indicate that RIT plus one dose of pretreatment omalizumab had comparable safety, better adherence, and potentially faster onset of efficacy at no additional cost compared to conventional immunotherapy. Huang et al. conducted a comparative study to evaluate the short-term efficacy and safety of conventional SCIT, RIT, and RIT plus one dose of pretreatment omalizumab. The findings revealed that the addition of omalizumab to RIT resulted in a significant improvement in early-stage efficacy. Furthermore, this therapy exhibited the advantages of effectiveness, safety, and convenience (170).
In addition, several studies have shown that omalizumab combined with SCIT can enhance the efficacy and safety of SCIT, while decreasing adverse event (173–178). For instance, Long found that omalizumab combined with SCIT can achieve complete asthma control faster, with a reduction in the amount of asthma medication used, and a better improvement in lung function. Compared with SCIT alone, omalizumab combined with SCIT had a lower incidence of adverse events (174).
Some hospitals are currently implementing a combined treatment approach involving Dupilumab and AIT. Deng et al. retrospectively observed the efficacy and safety of 10 patients with moderate-to-severe AD, treated with a combination of Dupilumab and SCIT (179). This study indicated that dupilumab and SCIT combination therapy was safe and effective for treating moderate to severe AD patients who are resistant to either dupilumab or SCIT monotherapy. However, further clinical research is required to fully understand the role of Dupilumab in AIT.
Adjuvants
Adjuvants encompass a diverse range of complexes that serve as depot foundations, enhancing the stimulation and modulation of protective responses (180). Several animal studies conducted in China have investigated the use of adjuvants for the treatment of allergic diseases. These studies have demonstrated promising potential for development, as outlined in Table 1. Chitosan is one of the most explored polysaccharide for mucosal vaccine delivery. Li et al. entrapped Der f 2-47-67 in chitosan to obtain Derf 2-47-67 loaded chitosan microparticles, which were injected intraperitoneally into asthma mice (181). The results showed Der f 2-47-67-loaded chitosan microparticle inhibited airway allergic inflammation. Similar to Li et al, Yu et al. prepare Der f chitosan nanoparticle vaccine to treat asthma mice by sublingual administration (182). Der f chitosan nanoparticle vaccine could reduce airway hyperresponsiveness (AHR) and lung inflammation. Xu et al. investigated the immunological adjuvant effect of silver nanoparticles (AgNPs) in vitro and vivo (183). The results indicated that AgNPs elicited Th2-biased immune responses in vivo and could recruit and active local leukocytes and especially macrophages in vitro. Ma et al. prepared a new versatile Toll-like receptor 7 agonist (TLR7a) conjugate to Der f 1 (184). They found the course of AHR and eosinophilia of the TLR7a vaccine-treated mice were limited. The levels of specific IgG1, IgG2a, and IgE antibodies in these mice exhibited significant changes compared to those in the model mice. Following treatment with the TLR7a vaccine, there was a notable decrease in the expression of Th2 cytokine interleukin (IL)-4 production in bronchoalveolar lavage fluid (BALF) and splenocytes, while the levels of interferon-γ (IFN-γ), IL-12, and IL-10 were significantly increased.
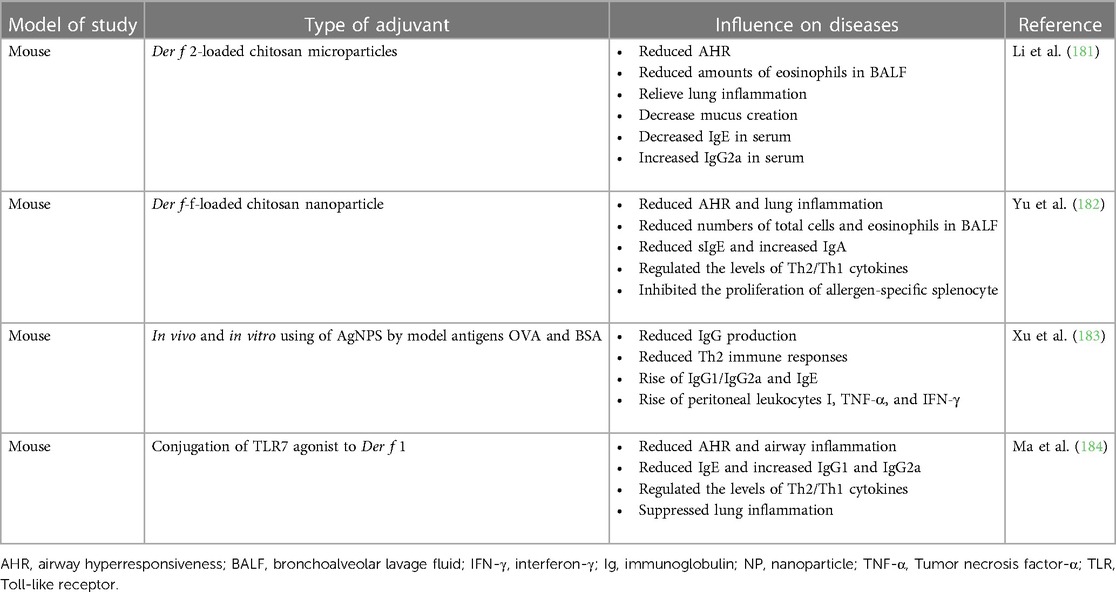
Table 1. Animal studies of adjuvants in allergic diseases in China (180).
DNA vaccines
DNA vaccines are not only immunogenic and safe, but they also offer greater flexibility than previous protein vaccines, as they can be easily modified and constructed. Various DNA vaccines have been studied for their effects in mice in China (185–191). Recently, Hu et al. developed a DNA vaccine that co-expressing Der p2 and A20 protein (Pvax1-Der p2-A20). This vaccine was encapsulated into poly (L-lactide-co-glycolide) (PLGA) nanoparticles, and its effect was investigated through intranasal administration in mice with AR (191). The results indicated that this DNA vaccine could alleviate nasal allergic inflammation, and inhibit serum Der p2-sIgE, IL-4, and IL-13 expression. Concurrently, it increased Der p2-sIgG1, IgG2a and IFN-γ expression in serum and splenic CD4+CD25+Fox3+Treg population.
Recombinant allergens
Lactic acid bacteria (LAB), considered safe for consumption and possessing probiotic properties, have gained attention as potential carriers for mucosal vaccines due to their safety profile and probiotic nature. LAB have also been recognized for their anti-allergic effects. An increasing number of studies have used LAB to express a variety of heterologous antigens as oral vaccines, with several of these studies conducted in China (186, 192–194). Ren et al. used transgenic LAB to produce the peanut allergen Ara h2 through various protein-targeting systems and investigated the immune-modulatory efficacy of these systems on allergic immune responses in mice (194). The results demonstrated that oral administration of recombinant LAB could induce sIgA and regulatory T cells at the local levels. Charng et al. designed recombinant LAB containing a plasmid-encoded Der p5, and found that these recombinant LAB could suppress allergen-induced airway inflammation (192).
Summary
Allergic diseases have imposed a substantial burden on public health in China, with a significant increase in the proportion of the population affected by such diseases. This trend has necessitated effective treatment options like AIT. In China, AIT has gained significant recognition and is widely used in clinical practice. While AIT is available in major cities and specialized allergy clinics, its accessibility in rural areas and smaller cities remains limited.
China is actively working towards creating standardized allergen extracts and treatment protocols to ensure quality and efficacy, researching novel allergens, personalized immunotherapy, and adjuvants to enhance the effectiveness of AIT. However, raising awareness among patients and healthcare providers about the benefits and safety of AIT remains a challenge.
In the future, it will be necessary to expand the range of allergen extracts available for immunotherapy to cover a broader spectrum of allergens specific to the Chinese population. Future directions also include personalized immunotherapy, tailored to an individual's specific allergens and immune response. Meanwhile, enhancing the knowledge and skills of healthcare professionals through training programs and continuing medical education can improve the implementation and effectiveness of AIT. Providing support and education to patients, including information about AIT, managing expectations, and addressing concerns, can improve patient adherence and satisfaction. Efforts should be made to increase the accessibility of AIT in rural areas and smaller cities through training programs for healthcare professionals and the establishment of more specialized clinics.
In conclusion, while AIT is already being used in China, further development, standardization, and accessibility are still needed. With advancements in technology and increasing research efforts, AIT has the potential to become a widely available and personalized treatment option for allergic diseases in China.
Author contributions
YY: Writing – original draft. WL: Writing – review & editing. RZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work is supported by Fundamental Research Project of Wuhan Science and Technology Bureau (No. 2023020201010157).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Genuneit J, Standl M. Epidemiology of allergy: natural course and risk factors of allergic diseases. Handb Exp Pharmacol. (2022) 268:21–7. doi: 10.1007/164_2021_507
2. Fu W, Zheng Z, Zhao J, Feng M, Xian M, Wei N, et al. Allergic disease and sensitization disparity in urban and rural China: AEuroPrevall-INCO study. Pediatr Allergy Immunol. (2022) 33:e13903. doi: 10.1111/pai.13903
3. Wang XD, Zheng M, Lou HF, Wang CS, Zhang Y, Bo MY, et al. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. (2016) 71:1170–80. doi: 10.1111/all.1287426
4. Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. (2019) 394:407–18. doi: 10.1016/S0140-6736(19)31147-X
5. Dong WL, An J, Yu M, Yin P, Xu TL, Liu B, et al. The prevalence and year lived with disability of atopic dermatitis in China: findings from the global burden of disease study 2019. World Allergy Organ J. (2021) 14:100604. doi: 10.1016/j.waojou.2021.100604
6. Feng H, Zhou J, Lu Y, Zhao Q, Yang Z, Xiong X, et al. Prevalence of self-reported food allergy among adults in Jiangxi, China. World Allergy Organ J. (2023) 16:100773. doi: 10.1016/j.waojou.2023.100773
7. Ma Z, Chen L, Xian R, Fang H, Wang J, Hu Y. Time trends of childhood food allergy in China: three cross-sectional surveys in 1999, 2009, and 2019. Pediatr Allergy Immunol. (2021) 32:1073–79. doi: 10.1111/pai.13490
8. Alvarez-Cuesta E, Bousquet J, Canonica GW, Durham SR, Malling HJ, Valovirta E. Standards for practical allergen-specific immunotherapy. Allergy. (2006) 61(Suppl 82):1–20. doi: 10.1111/j.1398-9995.2006.01219_1.x
9. Alvaro-Lozano M, Akdis CA, Akdis M, Alviani C, Angier E, Arasi S, et al. EAACI allergen immunotherapy user’s guide. Pediatr Allergy Immunol. (2020) 31(Suppl 25):1–101. doi: 10.1111/pai.13189
10. Canonica GW, Bachert C, Hellings P, Ryan D, Valovirta E, Wickman M, et al. Allergen immunotherapy (AIT): a prototype of precision medicine. World Allergy Organ J. (2015) 8:31. doi: 10.1186/s40413-015-0079-7
11. Roberts G, Pfaar O, Akdis CA, Ansotegui IJ, Durham SR, Gerth VWR, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. (2018) 73:765–98. doi: 10.1111/all.13317
12. Bousquet J, Pfaar O, Agache I, Bedbrook A, Akdis CA, Canonica GW, et al. ARIA-EAACI care pathways for allergen immunotherapy in respiratory allergy. Clin Transl Allergy. (2021) 11:e12014. doi: 10.1002/clt2.12014
13. Sturm GJ, Varga EM, Roberts G, Mosbech H, Bilo MB, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: hymenoptera venom allergy. Allergy. (2018) 73:744–64. doi: 10.1111/all.13262
14. de Silva D, Rodriguez DRP, de Jong NW, Khaleva E, Singh C, Nowak-Wegrzyn A, et al. Allergen immunotherapy and/or biologicals for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. (2022) 77:1852–62. doi: 10.1111/all.15211
15. Ye S. Creation and development of the department of allergy in Peking Union Medical College Hospital, China. Chin J Allergy Clin Immunol. (2011) 5:160–64. CNKI:SUN:OZHL.0.2011-02-020
16. Tang R, Sun JL, Yin J, Li Z. Artemisia allergy research in China. Biomed Res Int. (2015) 2015:179426. doi: 10.1155/2015/179426
17. Guan K, Liu B, Wang M, Li Z, Chang C, Cui L, et al. Principles of allergen immunotherapy and its clinical application in China: contrasts and comparisons with the USA. Clin Rev Allergy Immunol. (2019) 57:128–43. doi: 10.1007/s12016-019-08751-y
18. Wang J, Wu Y, Li J, Huang X, Zhu R. Eight aeroallergen skin extracts may be the optimal panel for allergic rhinitis patients in Central China. Int Arch Allergy Immunol. (2017) 173:193–98. doi: 10.1159/000479429
19. Zeng G, Luo W, Wu Z, Li L, Zheng P, Huang H, et al. A cross-sectional observational study on allergen-specific IgE positivity in a southeast coastal versus a southwest inland region of China. Sci Rep. (2017) 7:9593. doi: 10.1038/s41598-017-10109-3
20. Cheng L, Chen J, Fu Q, He S, Li H, Liu Z, et al. Chinese society of allergy guidelines for diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. (2018) 10:300–53. doi: 10.4168/aair.2018.10.4.300
21. Nima D, Zhizhang Z, Bian Z, Yixi C, Zhaxi Y, Zhan M, et al. An analysis of 735 cases of allergen specific IgE detection results in Lhasa area. Labeled Immunoassays Clin Med. (2023) 30:409–15. doi: 10.11748/bjmy.issn.1006-1703.2023.03.009
22. Wang W, Wang J, Song G, Xie H, Lin X, Chai R, et al. Environmental and sensitization variations among asthma and/or rhinitis patients between 2008 and 2018 in China. Clin Transl Allergy. (2022) 12:e12116. doi: 10.1002/clt2.12116
23. Li WJ, Huang ZF, Zhu HQ, Liu Y, Zhang RF, Li GP, et al. Epidemiological investigation on allergic diseases related to animal dander of cats, dogs and horses. Zhonghua Yu Fang Yi Xue Za Zhi. (2022) 56:1279–88. doi: 10.3760/cma.j.cn112150-20220529-00542
24. Yin W, Xiaoli Z, Wenjin D, Lin Y, Xiaofei Y, Qing J, et al. Sensitization profiles of aeroallergens among allergic rhinitis patients in China: a 13-year multicenter retrospective study. Allergy. (2023). doi: 10.1111/all.15784
25. Yang L, Li W, Qi S, Jiang Q, Huang N, Yang Y, et al. A survey of airborne fungi and their sensitization profile in Wuhan, China. Int Arch Allergy Immunol. (2023) 184(11):1153–63. doi: 10.1159/000531245
26. Chang FY, Lee JH, Yang YH, Yu HH, Wang LC, Lin YT, et al. Analysis of the serum levels of fungi-specific immunoglobulin E in patients with allergic diseases. Int Arch Allergy Immunol. (2011) 154:49–56. doi: 10.1159/000319208
27. Ohashi-Doi K, Utsumi D, Mitobe Y, Fujinami K. Japanese Cedar pollen allergens in Japan. Curr Protein Pept Sci. (2022) 23:837–50. doi: 10.2174/1389203723666220930155719
28. Jung CG, Park HS. Emerging hop Japanese pollinosis in Asia. Curr Protein Pept Sci. (2022) 23:714–20. doi: 10.2174/1389203723666220603155320
29. Jeong KY, Park JW. Oak pollen allergy in Korea. Curr Protein Pept Sci. (2022) 23:721–30. doi: 10.2174/1389203723666220624141550
30. Jeong KY. Outdoor allergens of regional importance in Asia. Curr Protein Pept Sci. (2022) 23:713. doi: 10.2174/138920372311221123113637
31. Bousquet PJ, Burbach G, Heinzerling LM, Edenharter G, Bachert C, Bindslev-Jensen C, et al. GA2LEN skin test study III: minimum battery of test inhalent allergens needed in epidemiological studies in patients. Allergy. (2009) 64:1656–62. doi: 10.1111/j.1398-9995.2009.02169.x
32. Salo PM, Calatroni A, Gergen PJ, Hoppin JA, Sever ML, Renee J, et al. Allergy related outcomes in relation to serum IgE: results from the national health and nutrition examination survey 2005–2006. J Allergy Clin Immunol. (2011) 127:1226–35.e7. doi: 10.1016/j.jaci.2010.12.1106
33. Kwong K, Robinson M, Sullivan A, Letovsky S, Liu AH, Valcour A. Fungal allergen sensitization: prevalence, risk factors, and geographic variation in the United States. J Allergy Clin Immunol. (2023) 152:1658–68. doi: 10.1016/j.jaci.2023.09.010
34. Leng X, Ye ST. One year observation of immunotherapy for Artemisia hay fever in China: a clinical and immunological study. Asian Pac J Allergy Immunol. (1987) 5:167–72. doi: 10.1016/S0335-7457(88)80016-9
35. Li L, Yin J, Du ZR, Liu ML, Yu YM, Wang XY, et al. Efficacy and safety of subcutaneous immunotheapy for allergic rhinitis and allergic asthma using allergen products of peking union medical college hospital: a real-world study. Chin J Allergy Clin Immunol. (2021) 15:489–99.42. doi: 10.3969/j.issn.1673-8705.2021.05.002
36. Du ZR, Yin J, Li L, Li JD, Liu J. Efficacy and safety of subcutaneous immunotherapy for allergic asthma with multiple summer and autumn pollen allergen extract and dermatophagoides pteronyssinus allergen extract. Chin J Allergy Clin Immunol. (2020) 14:325–34. doi: 10.3969/j.issn.1673-8705.2020.04.006
37. Nie JD, Xu QX, Li WJ, Huang N, Wang Y, Wang XL, et al. Influence of allergen-specific immunotherapy on new sensitization in respiratory allergic diseases: a 3 years retrospective study. Chin J Allergy Clin Immunol. (2020) 14:434–40. doi: 10.3969/j.issn.1673-8705.2020.05.004
38. Liu J. tfh and breg cells in immunotherapy with alternaria alternata and analysis of the endotypes in alternaria-induced asthma patients (dissertation/doctoral thesis). Peking Union Medical College, Beijing (2022).
39. Xue L, Yang LP, Chen YH, Li JY, Wang HY, Liu B, et al. Application research on specific immunotherapy in the treatment of allergic rhinitis sensitized by dust mites and pollen. J Med Pest Control. (2020) 36:418–22. CNKI:SUN:YXDZ.0.2020-05-003
40. Guan K, Wei QY, Yin J. Efficacy and safety of specific immunotherapy with Humulus pollen extract. Chin J Allergy Clin Immunol. (2012) 6:279–84.47. doi: 10.3969/j.issn.1673-8705.2012.04.007
41. Chen XY, Li F, Feng GP, Huang YL, Zhang S, Tang Y, et al. Effect of specific dust mite subcutaneous immunotherapy on allergic rhinitis in children. Shandong Med J. (2021) 61:63–65.48. doi: 10.3969/j.issn.1002-266X.2021.30.016
42. Liu J. Research on subcutaneous injection of standardized dust mite allergen in the treatment of allergic rhinitis (dissertation/master's thesis). Hebei Medical University, Hebei (Shi JZ) (2022).
43. Ma HY, Wang DM, Ding L, Guo JX, Zheng SB, Wang JY. Effect of subcutaneous immunotherapy with specific dust mites on allergic rhinitis in children. J Binzhou Med Univer. (2022) 45:273–76. doi: 10.19739/j.cnki.issn1001-9510.2022.04.007
44. Yu LJ. Allergen-specific immunotherapy induced the change of specific immunoglobulin G4 in blood and saliva toward allergens of mite (dissertation/master's thesis). Ningbo University, Zhejiang (NB) (2021).
45. Pan ZH, Liu HQ, Xie K, Hua XZ. Effect and clinical significance of standardized house dust mite subcutaneous immunotherapy on serum house dust mite sIgG4 in children with allergic rhinitis and asthma. Matern Child Health Care China. (2020) 35:3039–42. doi: 10.19829/j.zgfybj.issn.1001-4411.2020.16.034
46. Liu YT. Long term effect of standardized dust mite allergen subcutaneous immunotherapy on allergic rhinitis (dissertation/master's thesis). Jilin University, Jilin (CC) (2020).
47. Di YY. Effects of specific subcutaneous immunotherapy on airway inflammation and HMGB1/TLR4/NF-κB signaling pathway in asthma (dissertation/doctoral thesis). Southern Medical University, Guangdong (GZ) (2020).
48. Qin GH, Fan SF. Clinical curative effects of specific immunotherapy for children with allergic asthma. China Med Pharm. (2019) 9:79–81. doi: 10.3969/j.issn.2095-0616.2019.14.024
49. Lou LF, Huang YR, Wang CS, Wang XD, Zhao Y, Cao FF, et al. Long-term efficacy of house dust mite subcutaneous immunotherapy in allergic rhinitis patients. J Clin Otorhinolaryngol Head Neck Surg. (2018) 32:1627–31. doi: 10.13201/j.issn.1001-1781.2018.21.006
50. Cai Q, Wang Y. Effect of house dust mite allergen preparation on treating children with allergic rhinitis and its impact on total IgE and Th17 levels. Chin J Clin Rational Drug Use. (2023) 16:27–30. doi: 10.15887/j.cnki.13-1389/r.2023.22.008
51. Li W, Yin LL, Zhao QY. Clinical analysis of house dust Mite allergen preparation desensitization in children with bronchial asthma. China Foreign Med Treat. (2023) 42:122–25. doi: 10.16662/j.cnki.1674-0742.2023.11.122
52. Yang JY. Study on the efficacy of subcutaneous specific immunotherapy with standardized allergens in children with single and multiple allergic asthma (dissertation/doctoral thesis). Anhui Medical University, Anhui (HF) (2023).
53. Liu L, Xu C, Huang HH, Chai RL. Long-term efficacy of standardized house dust mite allergen subcutaneous specific immunotherapy for allergic rhinitis with asthma in children. Chin J Otolaryngol Integr Med. (2021) 29:440–45. doi: 10.16542/j.cnki.issn.1007-4856.2021.06.010
54. Rong JY, Huang J, Chen A, Liu XT, Wang BJ, Lin JB. Prognosis analysis of dust mite specific subcutaneous immunotherapy for dust mite allergic asthma with allergic rhinitis in children. China Practical Village Doctor J. (2021) 28:52–5. doi: 10.3969/j.issn.1672-7185.2021.06.013
55. Zhu L. Clinical study of house dust Mite standardized immunotherapy in children with asthma. China Standardization. (2020) 13:142–44. doi: 10.3969/j.issn.1002-5944.2020.13.033
56. Pan ZH, Chen J, Xie F. Curative effect of desensitization with house dust mite allergen preparation on children with allergic asthma and its influences on serum IgE and inflammatory factors. Clin Educ Gen Pract. (2021) 19:627–30. doi: 10.13558/j.cnki.issn1672-3686.2021.007.014
57. Lei LJ, Chen L. Analysis of the efficacy, safety and compliance of house dust mite allergen preparation for immunotherapy of 260 cases of allergic rhinitis. Contemp Med. (2020) 26:87–90. doi: 10.3969/j.issn.1009-4393.2020.21.035
58. Yu QQ, Tang J, Wang YJ, Liu MH, Wang K. A clinical study of dermatophagoides pteronyssinus specific subcutaneous immunotherapy against local allergic rhinitis. China Med Pharm. (2020) 10:227–31. doi: 10.3969/j.issn.2095-0616.2020.12.060
59. Peng H, Li CW, Lin ZB, Li TY. Long-term efficacy of specific immunotherapy on house dust mite-induced allergic rhinitis in China. Otolaryngol Head Neck Surg. (2013) 149:40–6. doi: 10.1177/0194599813485222
60. Li J, Wu Y, Yang Y, Huang N, Li W, Zhang S, et al. The efficacy and safety of two commercial house dust mite extracts for allergic rhinitis: a head-to-head study. Int Forum Allergy Rhinol. (2019) 9:876–82. doi: 10.1002/alr.22343
61. Wang H, Lin X, Hao C, Zhang C, Sun B, Zheng J, et al. A double-blind, placebo-controlled study of house dust mite immunotherapy in Chinese asthmatic patients. Allergy. (2006) 61:191–97. doi: 10.1111/j.1398-9995.2005.00913.x
62. Hui Y, Li L, Qian J, Guo Y, Zhang X, Zhang X. Efficacy analysis of three-year subcutaneous SQ-standardized specific immunotherapy in house dust mite-allergic children with asthma. Exp Ther Med. (2014) 7:630–34. doi: 10.3892/etm.2014.1469
63. Zhang P, Jia Y, Jing Z, Huang J, Wu H, Sun X. Efficacy and safety of house dust mite subcutaneous immunotherapy in polysensitized children with allergic asthma. Pulm Pharmacol Ther. (2023) 78:102187. doi: 10.1016/j.pupt.2022.102187
64. Huang HH, Xu C, Liu L, Chai RN. Efficacy comparison and safety analysis of subcutaneous specific immunotherapy with standardized house dust mite allergen in patients with single and multiple allergic rhinitis. Zhonghua Yu Fang Yi Xue Za Zhi. (2022) 56:774–83. doi: 10.3760/cma.j.cn112150-20220120-00071
65. Li L, Hui Y, Qian J, Guo Y, Zhang XL, Zhang XJ. Therapeutic efficacy of 3-year subcutaneous immunotherapy in asthmatic children allergic to mite. Zhongguo Dang Dai Er Ke Za Zhi. (2013) 15:368–71. doi: 10.7499/j.issn.1008-8830.2013.05.012
66. Song W, Lin X, Chai R. Evaluation of long-term effect for house dust mite subcutaneous immunotherapy for patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2015) 50:632–35. doi: 10.3760/cma.j.issn.1673-0860.2015.08.005
67. Ye Z, Huang Y, Wang Y, Gong C, Jiang Y. Effect of house dust mite vaccine on pulmonary function and inhaled corticosteroid doses in children with allergic asthma. Nan Fang Yi Ke Da Xue Xue Bao. (2012) 32:1632–35. doi: 10.3969/j.issn.1673-4254.2012.11.024
68. Wang CS, Wang XD, Zhang W, She WY, Xi L, Ouyang YH, et al. Long-term efficacy of dermatophagoides pteronyssinus immunotherapy in patients with allergic rhinitis: a 3-year prospective study. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2012) 47:804–08. doi: 10.3760/cma.j.issn.1673-0860.2012.10.003
69. Zhou J, Chen S, Song Z. Analysis of the long-term efficacy and safety of subcutaneous immunotherapy for atopic dermatitis. Allergy Asthma Proc. (2021) 42:e47–54. doi: 10.2500/aap.2021.42.200126
70. Wen L, Wen Z, Yang N. Risk factors and management of systemic reactions induced by subcutaneous immunotherapy. Zhonghua Yi Xue Za Zhi. (2014) 94:3001–04. doi: 10.3760/cma.j.issn.0376-2491.2014.38.011
71. Yang Y, Ma D, Huang N, Li W, Jiang Q, Wang Y, et al. Safety of house dust mite subcutaneous immunotherapy in preschool children with respiratory allergic diseases. Ital J Pediatr. (2021) 47:101. doi: 10.1186/s13052-021-01046-z
72. Shen Y, Hong SL, Zhang M, Ke X. Observation of systemic adverse reactions by specific immunotherapy and analysis of risk factors in allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2017) 52:801–05. doi: 10.3760/cma.j.issn.1673-0860.2017.11.001
73. Xue JR, Ma J, Qiu CY, Hu ZB, Jiang X, Pan M, et al. Observation and analysis of systemic reactions to house dust mite subcutaneous immunotherapy in 362 patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2020) 55:445–51. doi: 10.3760/cma.j.cn115330-20200426-00333
74. Xiang L, Liu F, Zhi L, Jiang W, Liu C, Xie H, et al. Safety of semi-depot house dust mite allergen extract in children and adolescents with allergic rhinitis and asthma. Immunotherapy. (2021) 13:227–39. doi: 10.2217/imt-2020-0232
75. Yu QQ, Tang J, Wang YJ, Xu YX, Liu MH. Observation on the adverse reactions of specific subcutaneous immunotherapy for allergic rhinitis. China Med Pharm. (2020) 10:235–38. doi: 10.3969/j.issn.2095-0616.2020.10.067
76. Rong JY, Huang DM, Wang GL, Zhang L, Wang BJ, Huang J, et al. Systemic adverse reactions induced by house dust mite specific immunotherapy among children with asthma and/or allergic rhinitis. Chin J Allergy Clin Immunol. (2020) 14:233–38. doi: 10.3969/j.issn.1673-8705.2020.03.010
77. Me W, Li JY, Lin X. Observation of systemic adverse reactions caused by specific subcutaneous immunotherapy in patients with allergic rhinitis and analysis of risk factors. Guizhou Med J. (2018) 42:1323–24. doi: 10.3969/j.issn.1000-744X.2018.11.017
78. Wang T, Wu K, Li LQ, Gong LL. Factors associated with systemic adverse reactions after subcutaneous immunotherapy injections and treatment options. J Otolaryngol Ophthalmol Shandong Univer. (2018) 32:71–4. doi: 10.6040/j.issn.1673-3770.0.2018.158
79. Wang L, Yin J, Fadel R, Montagut A, de Beaumont O, Devillier P. House dust mite sublingual immunotherapy is safe and appears to be effective in moderate, persistent asthma. Allergy. (2014) 69:1181–88. doi: 10.1111/all.12188
80. Chen S, Zeng X, Wang L, Chen B, Chen L, Wu S, et al. Effects of house dust mite sublingual immunotherapy in children with allergic rhinitis and asthma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2015) 50:627–31. doi: 10.3760/cma.j.issn.1673-0860.2015.08.004
81. Lin Z, Liu Q, Li T, Chen D, Chen D, Xu R. The effects of house dust mite sublingual immunotherapy in patients with allergic rhinitis according to duration. Int Forum Allergy Rhinol. (2016) 6:82–7. doi: 10.1002/alr.21657
82. Lin X, Lin H, Wei X, Huang Q. The efficacy and safety of sublingual immunotherapy in children and adult patients with allergic rhinitis. Allergol Immunopathol (Madr). (2017) 45:457–62. doi: 10.1016/j.aller.2016.10.016
83. Gao Y, Lin X, Ma J, Wei X, Wang Q, Wang M. Enhanced efficacy of dust Mite sublingual immunotherapy in low-response allergic rhinitis patients after dose increment at 6 months: a prospective study. Int Arch Allergy Immunol. (2020) 181:311–19. doi: 10.1159/000505746
84. Huang HJ, Wang YR, Hou XL, Yang SQ, Ren YX, Liu YG, et al. Observation on the effect of sublingual specific immunotherapy of dust mite on children with bronchial asthma. Beijing Med J. (2021) 43:761–66. doi: 10.15932/j.0253-9713.2021.08.013
85. Chen LF. Analysis of dermatophagoides farina drops in the treatment of allergic rhinitis in children sensitized by dust mites combined with different allergens (dissertation/doctoral thesis). Shantou University, Guangdong (ST) (2022).
86. Jin M, Zhang L, Zhou G, Zhang S, Li X, Hu S. The effect of the standard length of the first prescription on the adherence to sublingual immunotherapy for patients with allergic rhinitis. Int Forum Allergy Rhinol. (2020) 10:768–72. doi: 10.1002/alr.22553
87. Huang LF, Xiao JG, Huang XY. Meta-analysis of the therapeutic effect and course of subolingual dust mite drops in the treatment of children with allergic asthma. Strait Pharm J. (2020) 32:115–21. doi: 10.3969/j.issn.1006-3765.2020.09.040
88. Qian Y, Huang GF, Gao XJ, Cai MJ, Xiao M, Xie JQ. Effect of sublingual specific immunotherapy on the levels of inflammatory factors and serum immunological indexes in dust mite allergic asthmatic children. Clin Med. (2023) 43:56–8. doi: 10.19528/j.issn.1003-3548.2023.07.017
89. Qin H, Liu T, He M. Evaluation of the treatment effect of subelingual single dust mite preparation on allergic rhinitis in children induced by multiple allergens. Shandong Med J. (2020) 60:80–2. doi: 10.3969/j.issn.1002-266X.2020.18.022
90. Wang F, Fu HM, Dai JY, Yang J. Clinical effect and safety of sublingual immunotherapy on allergic cough in children caused by dust mites allergy. Pract J Cardiac Cerebral Pneumal Vasc Dis. (2020) 28:85–8. doi: 10.3969/j.issn.1008-5971.2020.06.016
91. Wang F, Yang J, Zhang O, Zhang LF, Zhang MP. Clinical effect of sublingual immunotherapy on allergic nasopharyngitis in children with dust mite allergy. China Pract Med. (2020) 15:148–50. doi: 10.14163/j.cnki.11-5547/r.2020.25.069
92. Wang QY. Clinical efficacy of sublingual immunotherapy in treatment course of allergic rhinitis (dissertation/master's thesis). China Medical University, Liaoning (SY) (2021).
93. Shao J, Cui YX, Zheng YF, Peng HF, Zheng ZL, Chen JY, et al. Efficacy and safety of sublingual immunotherapy in children aged 3–13 years with allergic rhinitis. Am J Rhinol Allergy. (2014) 28:131–39. doi: 10.2500/ajra.2014.28.4006
94. Li L, Yan H, Chen Q. Clinical efficacy of sublingual immunotherapy for elderly patients with allergic rhinitis and its influence on immune function and inflammatory factors. J Hunan Normal Univer (Medical Edition). (2022) 19:41–4. doi: 10.3969/j.issn.1673-016X.2022.03.012
95. Chi SS, Zhang C, Yu QQ, Bai J, Wang K. Objective evaluation of improvement of nasal ventilation function in patients with allergic rhinitis treated by sublingual immunization. Med Innov China. (2020) 17:153–56. doi: 10.3969/j.issn.1674-4985.2020.08.038
96. Sun ZH, Gao ZH, Xue JM, Wang XS, Song MT. The efficacy of sublingual immunotherapy in the treatment of allergic rhinitis and the prediction of cytokine effect. J Clin Otolaryngol Head Neck Surg. (2019) 33:332–36. doi: 10.13201/j.issn.1001-1781.2019.04.011
97. Shi HY, Wang XJ. Effect of sublingual immunity of dust mite allergen on Th2 immune response in allergic rhinitis. J Clin Exp Med. (2019) 18:174–78. doi: 10.3969/j.issn.1671-4695.2019.02.018
98. Lin Y, Zhang DW. Efficacy of personalized sublingual immunotherapy versus conventional sublingual immunotherapy in patients with moderate to severe allergic rhinitis. Jiangsu Med. (2023) 49:621–23. doi: 10.19460/j.cnki.0253-3685.2023.06.019
99. Leng X, Fu YX, Ye ST, Duan SQ. A double-blind trial of oral immunotherapy for Artemisia pollen asthma with evaluation of bronchial response to the pollen allergen and serum-specific IgE antibody. Ann Allergy. (1990) 64:27–31. doi: 10.2500/108854190778999537
100. Lou H, Huang Y, Ouyang Y, Zhang Y, Xi L, Chu X, et al. Artemisia annua-sublingual immunotherapy for seasonal allergic rhinitis: a randomized controlled trial. Allergy. (2020) 75:2026–36. doi: 10.1111/all.14218
101. Lou H, Wang X, Wei Q, Zhao C, Xing Z, Zhang Q, et al. Artemisia Annua sublingual immunotherapy for seasonal allergic rhinitis: a multicenter, randomized trial. World Allergy Organ J. (2020) 13:100458. doi: 10.1016/j.waojou.2020.100458
102. Yang J, Shen Z, Liu L, Kang W, Shao Y, Zhang P, et al. Clinical efficacy and safety of artesimia annua-sublingual immunotherapy in seasonal allergic rhinitis patients based on different intervention time. Int Arch Allergy Immunol. (2022) 183:852–59. doi: 10.1159/000524108
103. Cao YZ, Feng Y, Wang Q, Han SF. Investigation on compliance of sublingual immunotherapy for allergic rhinitis caused by Artemisia pollen. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2023) 37:448–52. doi: 10.13201/j.issn.2096-7993.2023.06.008
104. Qin YE, Mao JR, Sang YC, Li WX. Clinical efficacy and compliance of sublingual immunotherapy with dermatophagoides farinae drops in patients with atopic dermatitis. Int J Dermatol. (2014) 53:650–55. doi: 10.1111/ijd.12302
105. Liu L, Chen J, Xu J, Yang Q, Gu C, Ni C, et al. Sublingual immunotherapy of atopic dermatitis in mite-sensitized patients: a multi-centre, randomized, double-blind, placebo-controlled study. Artif Cells Nanomed Biotechnol. (2019) 47:3540–47. doi: 10.1080/21691401.2019.1640709
106. Huang CD. Clinical study of sublingual immunotherapy for atopic dermatitis in children treated with dust mite drops (dissertation/doctoral thesis). University of South China, Henan (HY) (2020).
107. Mao CG, Wang LJ, Zhou XC, Zhang XQ, Liu H. Efficacy and safety evaluation of sublingual immunotherapy for allergic rhinitis. Chin J Otolaryngol Skull Base Surg. (2011) 17:116–20. CNKI:SUN:ZEBY.0.2011-02-011
108. Zhao H, Jiang H, Long Y, Luo YJ. Meta-analysis of efficacy and safety of sublingual immunotherapy for adult seasonal allergic rhinitis. J Local Surg. (2016) 25:292–97. doi: 10.11659/j.jssx.01E016035
109. Liu MZ, Liu CS. Clinical efficacy and safety of dust mite drops sublingual immunotherapy for children with asthma and allergic rhinitis. Tianjin Med Univer. (2018) 24:344–47.
110. Li J, Teng YS, Han JH, Shen Q, Chen X, Li Y. Compliance and safety analysis of sublingual immunotherapy in allergic rhinitis. J Med Res. (2013) 42:105–08. doi: 10.3969/j.issn.1673-548X.2013.08.033
111. Wang C, Bao Y, Chen J, Chen X, Cheng L, Guo YS, et al. Chinese Guideline on allergen immunotherapy for allergic rhinitis: the 2022 update. Allergy Asthma Immunol Res. (2022) 14:604–52. doi: 10.4168/aair.2022.14.6.604
112. Zhao Y, He JP, Zhao XG, Deng PP. Efficacy and safety of subcutaneous and sublingual immunotherapy for allergic rhinitis. Chin Pract Med. (2020) 15:153–55. doi: 10.14163/j.cnki.11-5547/r.2020.03.074
113. Zhao GH, Liu J, Zong GC, Feng XB, Lu J, Wang Y. Clinical effect observation of different types of specific immune administration in the treatment of allergic rhinitis. Prog Modern Biomed. (2018) 18:2384–87. doi: 10.13241/j.cnki.pmb.2018.12.039
114. Ma Y, Liu YH, Li XH, Qiu JX, Fang P. Evaluation of long-term efficacy of sublingual and subcutaneous immunotherapy for multiple sensitized allergic rhinitis. ACTA Univer Med Anhui. (2018) 53:458–62. doi: 10.19405/j.cnki.issn1000-1492.2018.03.028
115. Chu X, Liu YX, Dang XW, Zhou YH, Zhao M. Efficacy and safety evaluation of subcutaneous and sublingual immunotherapy for dust mite allergic rhinitis. Chronic Pathematol J. (2018) 19:1015–18. doi: 10.16440/j.cnki.1674-8166.2018.08.008
116. Liu W, Zeng Q, He C, Chen R, Tang Y, Yan S, et al. Compliance, efficacy, and safety of subcutaneous and sublingual immunotherapy in children with allergic rhinitis. Pediatr Allergy Immunol. (2021) 32:86–91. doi: 10.1111/pai.13332
117. You SH, Qin XY, Xu C, Qiu X, Luan ZL, Jia HX, et al. Comparison study of subcutaneous immunotherapy and sublingual immunotherapy in patients with allergic rhinitis. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2016) 30:689–93. doi: 10.13201/j.issn.1001-1781.2016.09.005
118. Zhu L, Zhu LP, Chen RX, Tao QL, Lu JH, Cheng L. Clinical efficacy of subcutaneous and sublingual immunotherapy in mite-sensitized patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2011) 46:986–91. doi: 10.3760/cma.j.issn.1673-0860.2011.12.005
119. Wang ZX, Shi H. Single-allergen sublingual immunotherapy versus multi-allergen subcutaneous immunotherapy for children with allergic rhinitis. J Huazhong Univ Sci Technolog Med Sci. (2017) 37:407–11. doi: 10.1007/s11596-017-1748-2
120. Xian M, Feng M, Dong Y, Wei N, Su Q, Li J. Changes in CD4+CD25+FoxP3+ regulatory T cells and Serum cytokines in sublingual and subcutaneous immunotherapy in allergic rhinitis with or without asthma. Int Arch Allergy Immunol. (2020) 181:71–80. doi: 10.1159/000503143
121. Lv J, Liu JJ, Ke D, Chen Z, Deng YQ, Yu Y, et al. Clinical efficacy and safety analysis of subcutaneous and sublingual immunotherapy for atopic dermatitis. J Pract Dermatol. (2019) 12:143–47. doi: 10.11786/sypfbxzz.1674-1293.20190304
122. Okamoto Y, Ohta N, Okano M, Kamijo A, Gotoh M, Suzuki M, et al. Guiding principles of subcutaneous immunotherapy for allergic rhinitis in Japan. Auris Nasus Larynx. (2014) 41:1–05. doi: 10.1016/j.anl.2013.06.003
123. Fujisawa T, Shimoda T, Masuyama K, Okubo K, Honda K, Okano M, et al. Long-term safety of subcutaneous immunotherapy with TO-204 in Japanese patients with house dust mite-induced allergic rhinitis and allergic bronchial asthma: multicenter, open label clinical trial. Allergol Int. (2018) 67:347–56. doi: 10.1016/j.alit.2017.11.004
124. Nakagome K, Nagata M. Allergen immunotherapy in asthma. Pathogens. (2021) 10:1406. doi: 10.3390/pathogens10111406
125. Hur GY, Kim TB, Han MY, Nahm DH, Park JW. A survey of the prescription patterns of allergen immunotherapy in Korea. Allergy Asthma Immunol Res. (2013) 5:277–82. doi: 10.4168/aair.2013.5.5.277
126. Kim CK, Callaway Z, Park JS, Kwon E. Efficacy of subcutaneous immunotherapy for patients with asthma and allergic rhinitis in Korea: effect on eosinophilic inflammation. Asia Pac Allergy. (2021) 11:e43. doi: 10.5415/apallergy.2021.11.e43
127. Lee JH, Kim SC, Choi H, Jung CG, Ban GY, Shin YS, et al. Subcutaneous immunotherapy for allergic asthma in a single center of Korea: efficacy, safety, and clinical response predictors. J Korean Med Sci. (2017) 32:1124–30. doi: 10.3346/jkms.2017.32.7.1124
128. Liu X, Ng CL, Wang Y. The efficacy of sublingual immunotherapy for allergic diseases in Asia. Allergol Int. (2018) 67:309–19. doi: 10.1016/j.alit.2018.02.007
129. Jung JH, Kang TK, Kang IG, Kim ST. Comparison of sublingual immunotherapy in patients with allergic rhinitis sensitive to house dust mites in Korea. Ear Nose Throat J. (2021) 100:505S–12S. doi: 10.1177/0145561319882593
130. Lee SH, Kim ME, Shin YS, Ye YM, Park HS, Nahm DH. Safety of ultra-rush schedule of subcutaneous allergen immunotherapy with house dust Mite extract conducted in an outpatient clinic in patients with atopic dermatitis and allergic rhinitis. Allergy Asthma Immunol Res. (2019) 11:846–55. doi: 10.4168/aair.2019.11.6.846
131. Nahm DH, Kim ME, Kwon B, Cho SM, Ahn A. Clinical efficacy of subcutaneous allergen immunotherapy in patients with atopic dermatitis. Yonsei Med J. (2016) 57:1420–26. doi: 10.3349/ymj.2016.57.6.1420
132. Chu H, Park KH, Kim SM, Lee JH, Park JW, Lee KH, et al. Allergen-specific immunotherapy for patients with atopic dermatitis sensitized to animal dander. Immun Inflamm Dis. (2020) 8:165–69. doi: 10.1002/iid3.291
133. Nahm DH, Lee ES, Park HJ, Kim HA, Choi GS, Jeon SY. Treatment of atopic dermatitis with a combination of allergen-specific immunotherapy and a histamine-immunoglobulin complex. Int Arch Allergy Immunol. (2008) 146:235–40. doi: 10.1159/000115892
134. Lee HY, Lee SM, Kang SY, Kim K, Kim JH, Ryu G, et al. KAAACI Guidelines for allergen immunotherapy. Allergy Asthma Immunol Res. (2023) 15:725–56. doi: 10.4168/aair.2023.15.6.725
135. Okamoto Y, Fujieda S, Okano M, Hida H, Kakudo S, Masuyama K. Efficacy of house dust mite sublingual tablet in the treatment of allergic rhinoconjunctivitis: a randomized trial in a pediatric population. Pediatr Allergy Immunol. (2019) 30:66–73. doi: 10.1111/pai.12984
136. Yonekura S, Gotoh M, Kaneko S, Kanazawa K, Takeuji Y, Okubo K, et al. Treatment duration-dependent efficacy of Japanese cedar pollen sublingual immunotherapy: evaluation of a phase II/III trial over three pollen dispersal seasons. Allergol Int. (2019) 68:494–505. doi: 10.1016/j.alit.2019.05.002
137. Ohashi-Doi K, Lund K, Mitobe Y, Okamiya K. State of the art: development of a sublingual allergy immunotherapy tablet for allergic rhinitis in Japan. Biol Pharm Bull. (2020) 43:41–8. doi: 10.1248/bpb.b19-00093
138. Gotoh M, Okubo K, Yuta A, Ogawa Y, Nagakura H, Ueyama S, et al. Safety profile and immunological response of dual sublingual immunotherapy with house dust mite tablet and Japanese cedar pollen tablet. Allergol Int. (2020) 69:104–10. doi: 10.1016/j.alit.2019.07.007
139. Okamoto Y, Okubo K, Yonekura S, Hashiguchi K, Goto M, Otsuka T, et al. Efficacy and safety of sublingual immunotherapy for two seasons in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. (2015) 166:177–88. doi: 10.1159/000381059
140. Okubo K, Gotoh M, Fujieda S, Okano M, Yoshida H, Morikawa H, et al. A randomized double-blind comparative study of sublingual immunotherapy for cedar pollinosis. Allergol Int. (2008) 57:265–75. doi: 10.2332/allergolint.O-07-514
141. Masuyama K, Okamoto Y, Okamiya K, Azuma R, Fujinami T, Riis B, et al. Efficacy and safety of SQ house dust mite sublingual immunotherapy-tablet in Japanese children. Allergy. (2018) 73:2352–63. doi: 10.1111/all.13544
142. Okubo K, Masuyama K, Imai T, Okamiya K, Stage BS, Seitzberg D, et al. Efficacy and safety of the SQ house dust mite sublingual immunotherapy tablet in Japanese adults and adolescents with house dust mite-induced allergic rhinitis. J Allergy Clin Immunol. (2017) 139:1840–48. doi: 10.1016/j.jaci.2016.09.043
143. Tanaka A, Tohda Y, Okamiya K, Azuma R, Terada I, Adachi M. Efficacy and safety of HDM SLIT tablet in Japanese adults with allergic asthma. J Allergy Clin Immunol Pract. (2020) 8:710–20. doi: 10.1016/j.jaip.2019.09.002
144. Gotoh M, Yonekura S, Imai T, Kaneko S, Horikawa E, Konno A, et al. Long-Term efficacy and dose-finding trial of Japanese cedar pollen sublingual immunotherapy tablet. J Allergy Clin Immunol Pract. (2019) 7:1287–97. doi: 10.1016/j.jaip.2018.11.044
145. Mahler V, Esch RE, Kleine-Tebbe J, Lavery WJ, Plunkett G, Vieths S, et al. Understanding differences in allergen immunotherapy products and practices in North America and Europe. J Allergy Clin Immunol. (2019) 143:813–28. doi: 10.1016/j.jaci.2019.01.024
146. Zimmer J, Bridgewater J, Ferreira F, van Ree R, Rabin RL, Vieths S. The history, present and future of allergen standardization in the United States and Europe. Front Immunol. (2021) 12:725831. doi: 10.3389/fimmu.2021.725831
147. Qiu Q, Xu M, Lu C, Chen J, Chen S, Kong W, et al. Safety and efficacy of rush allergen-specific immunotherapy in Chinese allergic rhinitis patients. Int J Immunopathol Pharmacol. (2016) 29:720–25. doi: 10.1177/0394632016659301
148. Yu J, Zhong N, Luo Q, Liu Y, Yi H, Ye J, et al. Early efficacy analysis of cluster and conventional immunotherapy in patients with allergic rhinitis. Ear Nose Throat J. (2021) 100:378–85. doi: 10.1177/0145561319863370
149. Zhang B. Comparative study of subcutaneous cluster immunotherapy and conventional immunotherapy for house dust mite in allergic rhinitis (dissertation/master's thesis). Ningbo University, Zhejiang (NB) (2020).
150. Wang YF, Liu CH, Li YX, Lou XS, Shao MJ, Sha L, et al. Safety analysis of house dust mite cluster immunotherapy in children with asthma. Chin J Med. (2022) 57:919–23. doi: 10.3969/j.issn.1008-1070.2022.08.029
151. Cui L, Li LS, Wang ZX, Xu YY, Bian SN, Li L, et al. Safety of cluster immunotherapy with perennial allergens. Chin J Allergy Clin Immunol. (2022) 16:232–38. doi: 10.3969/j.issn.1673-8705.2022.03.002
152. He Y, Yu LJ, Zhang B, Xu BH. Application of subcutaneous specific cluster immunotherapy in allergic rhinitis. 2020 Zhejiang Medical Association Otorhinolaryngology Head and Neck Surgery Academic Conference Paper Compilation. doi: 10.26914/c.cnkihy.2020.071982
153. Li LS, Yang DM, Guan K, Yu CX, Liu M, Zhu YX. Safety of cluster immunotherapy in allergic rhinitis. Chin J Allergy Clin Immunol. (2019) 13:511–15. doi: 10.3969/j.issn.1673-8705.2019.06.017
154. Sun YM, Liu LP, Xing HY, He N, Tian FY, Ding J, et al. Safety of house dust mite allergen cluster immunotherapy versus conventional immunotherapy initiation in children with asthma. Chin J Allergy Clin Immunol. (2018) 12:14–8. doi: 10.3969/j.issn.1673-8705.2018.01.003
155. Zhang ZL. Study on tolerance of cluster immunotherapy in SIT for allergic rhinitis. Contemp Med. (2017) 23:92–4. doi: 10.3969/j.issn.1009-4393.2017.12.045
156. Fan QJ, Liu GJ, Liu XJ, Gao JJ, Huang SY, Ni LY. Clinical comparative study on efficacy and safety of cluster immunization and conventional immunotherapy for allergic rhinitis due to dust mite allergy. J Med Res. (2013) 42:123–27. doi: 10.3969/j.issn.1673-548X.2013.03.038
157. Zhou B, Chen XY, Lu ZJ, Cheng LL, He SH. Rapid immunotherapy for perennial allergic rhinitis. Otolaryngol Head Neck Surg. (2000) 7:333–35. doi: 10.16066/j.1672-7002.2000.06.009
158. Zhu ZC, Qiu QH, Chen Z, Huang HM, Han H, Chen JJ, et al. Analysis of the efficacy and compliance of conventional immunotherapy and rush immunotherapy in patients with allergic rhinitis. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2018) 32:81–6. doi: 10.13201/j.issn.1001-1781.2018.02.001
159. Shen Y, Ke X, Yang YC, Huang JJ, Liu J, Zhang M, et al. Clinical observation and preliminary economic study of rush immunotherapy in patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2022) 57:1491–96. doi: 10.3760/cma.j.cn115330-20220104-00003
160. Xie W, Zhang H, Chen J, Wang Y. Clinical efficacy and safety of rush immunotherapy in patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2015) 50:641–45. doi: 10.3760/cma.j.issn.1673-0860.2015.08.007
161. Cai ZM. Clinical study of lymph node injection immunotherapy for dust mite allergic rhinitis (dissertation/master's thesis). Southern Medical University, Guangdong (GZ) (2020).
162. Sun CD, Hao J, Xiao M, Luo LH, Zhao LY, Zhang J. A preliminary study of bultrasound-guided intralymph node injection immunotherapy for allergic rhinitis. Labeled Immunoassays Clin Med. (2021) 28:1457–63. doi: 10.11748/bjmy.issn.1006-1703.2021.09.004
163. Wang K, Zheng R, Chen Y, Yu Q, Zhong H, Xiao P, et al. Clinical efficacy and safety of cervical intralymphatic immunotherapy for house dust mite allergic rhinitis: a pilot study. Am J Otolaryngol. (2019) 40:102280. doi: 10.1016/j.amjoto.2019.102280
164. Wang Q, Wang K, Qin Y, Huang W, Li Y, Yu Q, et al. Intra-cervical lymphatic immunotherapy for dust mite-induced allergic rhinoconjunctivitis in children: a 3-year prospective randomized controlled trial. Front Immunol. (2023) 14:1144813. doi: 10.3389/fimmu.2023.1144813
165. Wang K, Qin Y, Wang QX, Huang WJ, Yu QQ, Li Y, et al. A randomized controlled study on the long-term efficacy of intra-cervical lymphatic immunotherapy for adult allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2023) 58:871–77. doi: 10.3760/cma.j.cn115330-20230330-00142
166. Li W, Zhang Z, Saxon A, Zhang K. Prevention of oral food allergy sensitization via skin application of food allergen in a mouse model. Allergy. (2012) 67:622–29. doi: 10.1111/j.1398-9995.2012.02798.x
167. Zhang E, Zeng B, Song R, Yao L, Che H. Sustained antigens delivery using composite microneedles for effective epicutaneous immunotherapy. Drug Deliv Transl Res. (2023) 13:1828–41. doi: 10.1007/s13346-023-01298-8
168. Gao P, Yu W, Zhou Y, Zhu W, Zhu Z, Jiang Y, et al. Safety comparison of omalizumab and glucocorticoid in rush allergen immunotherapy. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2020) 34:610–14. doi: 10.13201/j.issn.2096-7993.2020.07.008
169. Zhang P, Bian S, Wang X, Chen Z, Yang L, Xiao F, et al. A real-world retrospective study of safety, efficacy, compliance and cost of combination treatment with rush immunotherapy plus one dose of pretreatment anti-IgE in Chinese children with respiratory allergies. Front Immunol. (2022) 13:1024319. doi: 10.3389/fimmu.2022.1024319
170. Huang JY, Zhang W, Xiang R, Deng YQ, Tao ZZ, Xu Y. Short-term efficacy and safety observation of standardized mite allergen extract rush subcutaneous immunotherapy for allergic rhinitis: a prospective study. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2023) 58:854–62. doi: 10.3760/cma.j.cn115330-20230401-00149
171. Zhang WC, Du WJ, Wang SQ, Jin YX, Zhang QX. Efficacy and safety of omalizumab optimized pulse desensitization regimen. J Clin Pulmonary Medicine. (2022) 27:1634–38. doi: 10.3969/j.issn.1009-6663.2022.11.002
172. Li LS, Zhu YX, Zhu YX, Yang DM, Guan K, Yu CX. One case of cluster immunotherapy combined with omalizumab completed dose escalation phase. Chin J Allergy Clin Immunol. (2019) 13:520–22. doi: 10.3969/j.issn.1673-8705.2019.06.019
173. Zhang YY, Zhang M, Zhang JQ, Li QQ, Lu MP, Cheng L. Combination of omalizumab with allergen immunotherapy versus immunotherapy alone for allergic diseases: a meta-analysis of randomized controlled trials. Int Forum Allergy Rhinol. (2023). doi: 10.1002/alr.23268
174. Long C. Efficacy and safety of anti-IGE monoclonal antibody combined with dust mite subcutaneous immunotherapy in children with bronchial asthma (dissertation/master's thesis). Qingdao University, Shandong (QD) (2020).
175. Liu JA, Li YP, Li TL, Cai YH, Zhang ZR, Chen L. Analysis of the intervention effect of omalizumab on adverse reactions during allergen immunotherapy for mite allergic asthma with or without allergic rhinitis. Chin J Mod Drug Appl. (2022) 16:55–8. doi: 10.14164/j.cnki.cn11-5581/r.2022.16.014
176. Xue XM, Mo YQ, Cai H, Song XX, Ye L, Jin ML. Omalizumab combined with specific immunotherapy for severe allergic asthma has a significant effect. One case of omalizumab combined with specific immunotherapy for severe allergic asthma has a significant effect. Chin J Allergy Clin Immunol. (2020) 14:243–45. doi: 10.3969/j.issn.1673-8705.2020.03.012
177. Wang XY, Liu CS, Wang XL, Li YF. Combined application of anti-IgE therapy and subcutaneous immunotherapy in children with allergic asthma. Chi J Applied Clin Pediatr. (2021) 36:941–45. doi: 10.3760/cma.j.cn101070-20201116-01761
178. Long C, Sun CH, Lin H, Gao X, Qu ZH. Clinical analysis of 8 cases of combined application of anti-IgE monoclonal antibody and subcutaneous immunotherapy of dust mites in children with bronchial asthma. Adv Clin Med. (2022) 12:3608–15. doi: 10.12677/ACM.2022.124523
179. Deng S, Wang H, Chen S, Kong M, Yang X, Song Z, et al. Dupilumab and subcutaneous immunotherapy for the treatment of refractory moderate to severe atopic dermatitis: a preliminary report. Int Immunopharmacol. (2023) 125:111137. doi: 10.1016/j.intimp.2023.111137
180. Feng Z, Yi X, Hajavi J. New and old adjuvants in allergen-specific immunotherapy: with a focus on nanoparticles. J Cell Physiol. (2021) 236:863–76. doi: 10.1002/jcp.29941
181. Li J, Liu Z, Wu Y, Wu H, Ran P. Chitosan microparticles loaded with mite group 2 allergen der f 2 alleviate asthma in mice. J Investig Allergol Clin Immunol. (2008) 18:454–60. doi: 10.1089/jir.2007.0142
182. Yu HQ, Liu ZG, Guo H, Zhou YP. Therapeutic effect on murine asthma with sublingual use of dermatophagoides farinae/chitosan nanoparticle vaccine. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. (2011) 29:4–09. doi: 10.1111/j.1600-0714.2011.01024.x
183. Xu Y, Tang H, Liu JH, Wang H, Liu Y. Evaluation of the adjuvant effect of silver nanoparticles both in vitro and in vivo. Toxicol Lett. (2013) 219:42–8. doi: 10.1016/j.toxlet.2013.02.010
184. Ma L, Xiao X, Ma Y, Wu H, Qiu S, Li J, et al. Effect of activation of toll-like receptor 7 in the inhibition of allergic asthma on a mouse model. Am J Transl Res. (2017) 9:2143–52. PMID: 28559967
185. Jin H, Kang Y, Zhao L, Xiao C, Hu Y, She R, et al. Induction of adaptive T regulatory cells that suppress the allergic response by coimmunization of DNA and protein vaccines. J Immunol. (2008) 180:5360–72. doi: 10.4049/jimmunol.180.8.5360
186. Li GP, Liu ZG, Liao B, Zhong NS. Induction of Th1-type immune response by chitosan nanoparticles containing plasmid DNA encoding house dust mite allergen der p 2 for oral vaccination in mice. Cell Mol Immunol. (2009) 6:45–50. doi: 10.1038/cmi.2009.6
187. Shi WD, Cao W, Liu Y, Xu Y, Tao ZZ, Dai Q. Construction of recombinant house dust mite group 1 allergen vaccine and study on immune response induced by nasal immunization. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2013) 48:26–31. doi: 10.1097/00003246-200205000-00026
188. Ou J, Shi W, Xu Y, Tao Z. Intranasal immunization with DNA vaccine coexpressing der p 1 and ubiquitin in an allergic rhinitis mouse model. Ann Allergy Asthma Immunol. (2014) 113:658–65. doi: 10.1016/j.anai.2014.08.015
189. Sun T, Yin K, Wu LY, Jin WJ, Li Y, Sheng B, et al. A DNA vaccine encoding a chimeric allergen derived from major group 1 allergens of dust mite can be used for specific immunotherapy. Int J Clin Exp Pathol. (2014) 7:5473–83. PMID: 25337189
190. Yu S, Han B, Liu S, Wang H, Zhuang W, Huang Y, et al. Derp1-modified dendritic cells attenuate allergic inflammation by regulating the development of T helper type1(Th1)/Th2 cells and regulatory T cells in a murine model of allergic rhinitis. Mol Immunol. (2017) 90:172–81. doi: 10.1016/j.molimm.2017.07.015
191. Hu W, Ma L, Yang G, Zeng X, Liu J, Cheng B, et al. Der p2-A20 DNA vaccine attenuates allergic inflammation in mice with allergic rhinitis. Mol Med Rep. (2019) 20:4925–32. doi: 10.3892/mmr.2019.10760
192. Charng YC, Lin CC, Hsu CH. Inhibition of allergen-induced airway inflammation and hyperreactivity by recombinant lactic-acid bacteria. Vaccine. (2006) 24:5931–36. doi: 10.1016/j.vaccine.2005.07.107
193. Hu MS. Preparation and evaluation of peanut allergy recombinant lactic acid bacteria oral vaccine (dissertation/master's thesis). Jiangnan University, Jiangsu (WX) (2013).
Keywords: allergen immunotherapy, efficacy, safety, allergen extracts, adjuvant
Citation: Yang Y, Li W and Zhu R (2024) Allergen immunotherapy in China. Front. Allergy 4:1324844. doi: 10.3389/falgy.2023.1324844
Received: 20 October 2023; Accepted: 26 December 2023;
Published: 8 January 2024.
Edited by:
Kyoung Yong Jeong, Yonsei University, Republic of KoreaReviewed by:
Huiying Wang, Second Affiliated Hospital of Zhejiang University School of Medicine, ChinaKai Guan, Peking Union Medical College Hospital (CAMS), China
© 2024 Yang, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjing Li annlwj@sina.com Rongfei Zhu zrf13092@163.com
†These authors have contributed equally to this work
 Yaqi Yang
Yaqi Yang Wenjing Li
Wenjing Li Rongfei Zhu
Rongfei Zhu