Use of Rapid Drug Desensitization in Delayed Hypersensitivity Reactions to Chemotherapy and Monoclonal Antibodies
- 1Department of Allergy, University Hospital of Guadalajara, Guadalajara, Spain
- 2ARADyAL Spanish Thematic Network and Co-operative Research Centre RD16/0006/0023, Instituto de Salud Carlos III (ISCIII), Fundación Española para la Ciencia y la Tecnología (FECyT), Madrid, Spain
Background: Rapid drug desensitization (RDD) allows first-line therapies in patients with immediate drug hypersensitivity reactions (DHR) to chemotherapeutic drugs (ChD) and monoclonal antibodies (mAb). Desensitization in delayed drug reactions has traditionally used slow protocols extending up to several weeks; RDD protocols have been scarcely reported.
Patients and Method: We retrospectively analyzed the patients referred to the Allergy Department, who had experienced a delayed DHR (> 6 h) related to a ChD or mAb and underwent an RDD protocol. The rate of successful administration of the offending drug and the presence of adverse reactions were evaluated.
Results: A total of 93 RDDs were performed in 11 patients (including 6 men and 5 women, with a median age of 61 years). The primary DHR were maculopapular exanthema (MPE) (8), generalized delayed urticaria (1), MPE with pustulosis and facial edema (1), and facial edema with desquamative eczema (1). The meantime for the onset of symptoms was 3 days (range 1–16 days). RDD was performed using a protocol involving 8–13 steps, with temozolomide (25), bendamustine (4), rituximab (9), infliximab (24), gemcitabine (23), and docetaxel (8), within 4.6–6.5 h. Sixteen breakthrough reactions were reported during the RDD (17.2 %) in 5 patients; all were mild reactions including 11 delayed and 5 immediate reactions. All patients completed their treatment.
Conclusions: RDD is a potentially safe and effective procedure in patients suffering from delayed reactions to ChD and mAb. It allows them to receive full treatment in a short period, thereby reducing time and hospital visits.
Introduction
Rapid drug desensitization (RDD) has shown to be a safe and effective procedure for patients presenting with an immediate drug hypersensitivity reaction (DHR) (1). Chemotherapeutic drugs (ChD) and monoclonal antibodies (mAb) have been administered by means of this procedure for many years, allowing patients to get their first-line therapies (2–4). Several protocols have been proposed and some modifications have been applied (5–7). However, the one established at the Brigham and Women's Hospital, which is a three-solution, 12-step protocol, has been widely accepted (8, 9). The drug is delivered in consecutive steps at increasing doses by raising the infusion rate and the solution concentration, at fixed 15 min intervals, with the exception of the last step, in a total time of 5–6 h. Dose increments are 2–2.5 times that of the previous step. The number of solutions and steps can be modified depending on the patient's stratification risk and RDD tolerance.
Traditionally, delayed drug reactions have been treated with slow desensitization procedures that could extend for days or even weeks (10–14). There is a broad literature describing RDD for delayed reactions, including co-trimoxazole, betalactam antibiotics, sulfonamides and other antibiotics, such as ciprofloxacin, clindamycin, and tetracyclines, among others (15–18). However, there is little experience, with less extensive and more controversial literature, on RDD for chemotherapeutic agents and monoclonal antibodies (2, 19), although the European Academy of Allergy and Clinical Immunology (EAACI) position paper of the Drug Allergy Interest Group recommends performing an RDD on immediate DHR and on uncomplicated and non-serious, mild delayed drug reactions (20).
This study describes the use of RDDs with chemotherapeutic agents and monoclonal antibodies in patients with delayed DHR.
Patients and Method
A retrospective study was performed on patients who received desensitization as part of the standard care at the Allergy Department of University Hospital of Guadalajara, Spain. The study protocol was approved by the local ethics committee.
All patients had suffered a delayed hypersensitivity reaction related to ChD or mAb administration. The primary endpoints were the rate of successful administration of the offending drug and the presence of breakthrough reactions (BTR) using a rapid desensitization protocol.
Patients
This is a single institution-based retrospective study. Patients referred to the Allergy Department, who fulfilled the following criteria: (1) those who experienced DHR related to a ChD or mAb; (2) symptoms that started more than 6 h after drug administration; (3) those who needed to take the treatment with the same drug; and (4) those who accepted to undergo an RDD and signed informed consent.
Patients with severe delayed reactions, such as serum sickness, skin reactions with eosinophilia and systemic symptoms, Stevens–Johnson syndrome, or toxic epidermal necrolysis were excluded.
Allergological Study
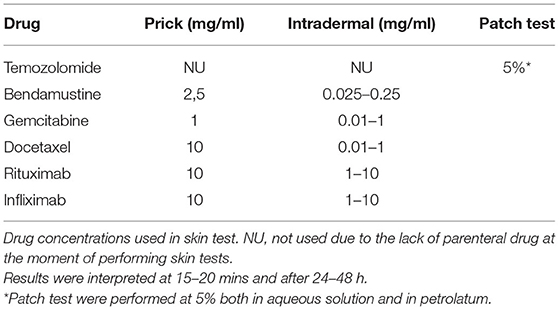
Signs and symptoms of the hypersensitivity reaction were recorded. Skin prick tests with ChD and mAb were run at full strength of the drug, and if negative, were followed by intradermal tests using serial dilutions (21) with non-irritating concentrations. Results were interpreted in 15–20 mins, and after 24 and 72 h (19, 22). Serum saline and histamine were used as negative and positive controls, respectively. Patch tests were run when the parenteral form of the drug was not available. Hypersensitivity to other drugs was ruled out with skin and challenge tests, if there was a concomitant administration and the patients had not subsequently received the drug (23).
Desensitization Protocol
Depending on the administered drug, the patients were pretreated 20–30 min before the desensitization protocol with corticoids, antiemetics, antihistamines, or acetaminophen, as dictated by the oncology standards or product information.
A standard 12-step protocol described by Brigham and Women's Hospital group was used with the intravenous drugs (4, 8, 24, 25). For oral drugs, a 13-step protocol adapted from the previous one was elaborated (26). The total time for desensitization ranged from 4 to 6.5 h.
All desensitizations were carried out in an outpatient basis at the allergy day care unit, and performed by allergists and specially trained nurses. Resuscitation personnel and resources were easily available. Beta-blockers were held for 24 h before desensitization.
Breakthrough Reactions During and After Desensitization
If an adverse reaction appeared during desensitization, the drug infusion was immediately stopped. Depending on the patient's symptoms (27), if required, treatment with intramuscular epinephrine, intravenous dexchlorpheniramine, hydrocortisone or 6-metilprednisolone, acetaminophen, ondansetron, or nebulized salbutamol was available. Once the reaction subsided, the protocol was restarted from the step at which it had been paused (4, 8). If a delayed reaction took place, the patient was evaluated and treated at emergency department of the hospital and reevaluated at the allergy department before the next desensitization.
Depending on the immediate and delayed tolerance to the RDD, the protocol was modified in subsequent desensitizations by adding or reducing steps and/or administering prophylactic medication before and/or after the RDD (4, 28).
Statistical Analysis
The analysis was descriptive and no hypothesis tests were performed. Quantitative variables were described as the mean and standard deviation (SD) or as medians and interquartile range (IQR, defined as percentiles 25 and 75), whereas categorical variables were presented as frequency and percentage. All analyses were performed using the SPSS package (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp).
Results
Characteristics of the Patients and the Initial Reactions
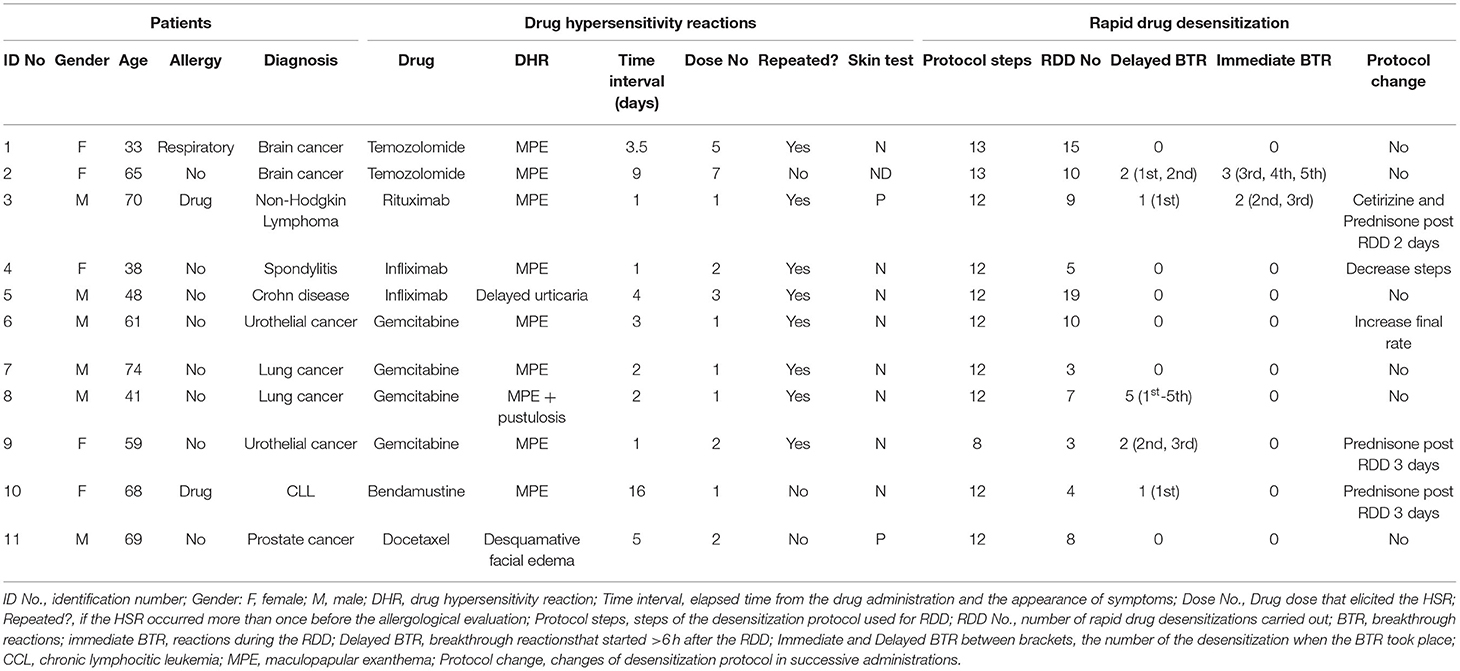
A total of 11 patients who fulfilled the inclusion criteria of the study, including 6 men and 5 women with a median age of 61 years (IQR 44.5–68.5 years), were admitted in the desensitization unit between January 2009 and March 2021. Nine patients were receiving oncologic treatment, while the other 2 were treated for Crohn's disease and ankylosing spondylitis, respectively. Table 1 summarizes the demographic and clinical characteristics of the patients in the study. Only one patient was atopic and 2 had been previously diagnosed with hypersensitivity to other drugs.
The initial DHRs included 8 cases (6 of them had associated eyelid angioedema) of maculopapular exanthema (MPE); one patient had generalized delayed urticaria with facial angioedema; another presented MPE with pustulosis and facial edema (acute generalized exanthematous pustulosis was ruled out by Dermatology); and another showed facial edema with desquamative eczema.
Eight patients had presented the same reaction with the offending drug in two or more consecutive administrations before being referred for an allergy evaluation.
Implied drugs were temozolomide (2), bendamustine, rituximab, infliximab (2), gemcitabine (4), and docetaxel. The mean time between the drug administration and the onset of the DHR was 3 days, with an average between 1 to 16 days, and a mean time of 3 days (IQR 1.5–4.5).
Allergological Study
Skin tests were performed on 10 patients, in which 9 patients underwent prick and intradermal tests and one patient underwent patch test with temozolomide. Drugs and concentrations used are detailed in Table 2. Positive intradermal tests were obtained in 2 patients (one immediate reading for rituximab and one 24 h delayed reading for docetaxel). At the time when the allergological study was performed, the patients with ID Numbers 1, 2, 5, 8, and 11 were on steroid treatment.
Hypersensitivity to concomitantly administered drugs was ruled out. In some cases, the patients had tolerated them before the allergy evaluation (dexamethasone, carboplatine, ondasentron, metroclopramide, and difenhydramine); while in 4 patients, the skin and drug challenge tests were carried out with negative results (alopurinol, ondansetron, cotrimoxazole, acyclovir, levofloxacin, and erythropoietin).
The diagnosis of drug hypersensitivity was established by the recurrence of DHR in 2 or more successive administrations with the same drug in 7 patients and by skin testing in 2 other patients. In the case of the two remaining patients, there was suspicion about the implied drug, which was later confirmed when both of them experienced BTR reactions during RDD.
Rapid Drug Desensitizations
A total of 93 RDDs were performed in the 11 patients, which included 25 with temozolomide, 4 with bendamustine, 9 with rituximab, 24 with infliximab, 23 with gemcitabine, and 8 with docetaxel. Nine patients received pretreatment before RDD, prescribed by an oncologist or the patient's specialist, which involved metoclopramide or dexamethasone and ondansetron with gemcitabine; 6-metyl-prednisolone and acetaminophen with infliximab; 6-metyl-prednisolone and diphenhydramine with rituximab; dexamethasone, diphenhydramine, ranitidine, acetaminophen, and ondansetron with bendamustine; dexamethasone, diphenhydramine, and ranitidine with docetaxel. Additionally, we added diphenhydramine in the treatment of two patients receiving Infliximab (patient No. 4 and 5), and added deflazacort and cetirizine to a third patient (Patient No. 2).
The RDD was performed intravenously in 9 patients, with a 12-step protocol in 8 patients (63 RDDs) lasting around 4.6–5.6 h depending on the final infusion rate, and an 8-step protocol in 2 patients (5 RDDs) lasting 4 h, while 2 patients received a 13-step oral protocol (25 RDDs) lasting 6.5 h. The first desensitization was performed in all the patients in <4 weeks after the delayed hypersensitivity reaction.
Breakthrough Reactions During and After RDD
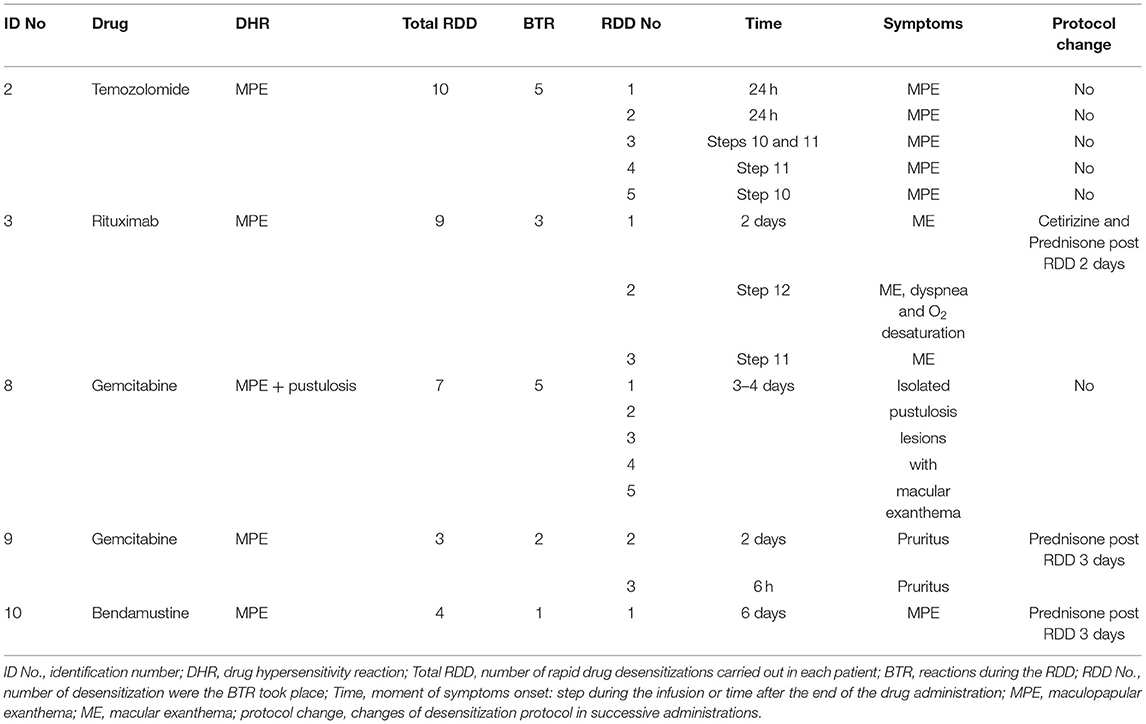
Five patients experienced a total number of 16 hypersensitivity reactions during desensitization (17.2% of the total RDDs). They included 11 delayed reactions in 5 patients: 5 isolated pustulosis lesions with macular exanthema, 3 macular exanthemas, 1 MPE, and 2 delayed pruritus. All of them occurred after the first course of RDD (Table 3). Five immediate reactions were observed in 2 patients: 5 macular exanthemas, with dyspnea and oxygen desaturation, observed in one of them. Both patients had previously experienced delayed BTR with former RDDs. All delayed reactions subsided, with five subsiding spontaneously and 6 with the use of corticoids. Immediate reactions were subsided completely. They were handled at the allergy day care unit, one with corticoids and salbutamol, while the others subsided spontaneously.
In patients who presented several reactions, the time interval for the onset of symptoms decreased with every desensitization, and so was the duration and severity (Table 3).
Three patients who suffered adverse delayed reactions were pretreated with prednisone after the RDD in some of the subsequent administrations. On the contrary, in some patients who showed a good tolerance to the desensitization, the protocol was modified to reduce the total time. All patients received the full target dose and all the administrations prescribed by their specialists.
Discussion
We present the results of 93 RDDs with ChD and mAb carried out in 11 patients who had suffered delayed, uncomplicated, and non-serious reactions with these drugs. In the initial DHR, the meantime from the onset of symptoms was 3 days; two patients experienced an interval longer than a week. In published large series, RDDs are performed only in immediate reactions (5, 29) or in delayed reactions starting within the first 48 h after drug administration (4–6). RDD has also shown effectiveness in delayed drug reactions to ChD and mAb in short series or case reports (26, 30–33).
Most of our patients had suffered 2 DHR with the same drug before being referred for an allergy evaluation. This shows the lack of suspicion when dealing with delayed reactions, especially when the interval of symptoms is longer than 3 days. Delayed cutaneous reactions to drugs generally become apparent 4–21 days after exposure, with reactions reaching their maximum after 24–72 h. Subsequent reactions may develop within 1 or 2 days, with initial symptoms appearing after only a few hours (34). This was observed in one of our patients, who presented a DHR 16 days after receiving bendamustine and developed a BTR with the first desensitization, around 6 days later. Delayed reactions may well appear after only 1 day or even earlier in case of presensitization or direct interaction of the drug with the receptor (pi-concept) (35).
Cutaneous tests were not of value for diagnosis in our study, as opposed to other authors (2, 4). Only 2 patients showed a positive result. The sensitivity and specificity of delayed intradermal skin test readings have not been established. The tests were helpful in the diagnosis of non-severe delayed reactions induced by beta-lactam antibiotics, radiocontrast media, heparins, and biological agents (23). Moreover, some authors did not perform skin tests with certain drugs because validation of these clinical tests does not exist or the negative predictive value is unknown (4, 36). It is also important to remark that half of the patients were under a chronic corticoid treatment that could not be suspended. They were already receiving it when they suffered the DHR. Even though the corticosteroids did not avoid the DHR, they could influence the results of the allergological study. This could lead to false-negative results in the delayed reading of skin tests. Moreover, the recommendation to perform the allergological study 4–6 weeks after the hypersensitivity reaction had subsided (19, 37) appeared to be not possible in most of our patients due to the characteristics of some reactions, lasting for several days or weeks, and the patients' need for urgent treatment which could not be postponed. In clinical practice, it is difficult to wait for the recommended period to perform skin tests for ChD due to the oncologic strict administration schemes that must not be changed to preserve efficacy and avoid toxicity (38). So, it is important to have in mind that a false-negative result is possible (23). Even so, the diagnosis of delayed DHR was well established in our patients: 8 patients experienced the same reaction to the same drug at least twice (one of these cases also had a positive skin test); another patient showed a positive intradermal test; and the remaining 2 suffered from BTR during the RDD. Some patients were in need of skin and challenge tests to rule out hypersensitivity to other concomitant drugs, as previously described (28, 39, 40).
Desensitization procedures for delayed drug reactions have been traditionally treated with slow desensitization protocols lasting over many days or even weeks (10–14). The introduction of rapid protocols represents a better approach to achieve therapeutic doses, thereby reducing patient visits and resources to a few hours, and maintaining patients on their most effective treatments (3, 4, 41).
In published series breakthrough reactions during RDD to ChD and mAB are up to 25% (2, 4–6, 19, 29). In our study, BTR were present in 17% of cases, i.e., in 5 patients. The BTR were initially delayed and they took place after the first desensitization, in concordance with previous publications (6, 8, 29). We want to emphasize how the time-to-onset of BTR was shorter with the subsequent desensitization procedures, and became immediate in the last steps of the RDD in 2 patients. BTR severity also decreased. In a sensitized individual, the initial symptoms with subsequent reactions could appear within few hours (6, 34). Some studies also report the change from a delayed to an immediate DHR. In one such study, Picard et al. describe that among patients with hypersensitivity to taxanes, 4 patients with an initial delayed DHR had a recurrent reaction that was immediate with RDD (19). Jiménez-Rodríguez described the “converter phenotype” in patients treated with taxanes, who presented delayed DHR and in subsequent exposures developed immediate HSR, generally type I reactions (42). Other authors suggest that type IV reactions may predispose the development of IgE-mediated type I reactions (2). All reactions related to the desensitization, both immediate and delayed, were easily solved. Epinephrine was not used. RDD was a safe procedure in these patients when performed by a trained allergy team. As shown in Table 3, we continued with a corticosteroid treatment after the RDD, for 3 days in 3 patients. This treatment did not prevent the appearance of new reactions in 2 of them. There is no clear evidence about the use of premedication before or after RDD (20, 28) and there is also a considerable variation regarding diagnostic procedures and therapeutic approaches (43).
This study has some limitations. There are a variety of diseases and drugs implied in the DHR in our patients. The allergological study is difficult in oncologic patients with DHR due to the short time available to perform the skin tests, the treatment with corticoids in many of them, and the lack of validation of skin test for delayed reactions with these drugs.
Drug desensitization is an effective and safe option that allows patients with DHR to continue their first-line treatments. It has proved to be a safe procedure when performed by experienced allergists. It has no additional costs and survival outcomes with no differences from those via standard administration (3, 4, 43, 44).
RDD in delayed reactions can achieve the target dose in a short time, which is a matter of great importance in this type of patients. The RDD used in our patients is a flexible protocol that can be lengthened or shortened depending on their tolerance (4, 8, 19, 28, 45). It has allowed to receive full treatment to all of them.
Conclusions
Rapid drug desensitization could be a safe and effective procedure for patients suffering from delayed reactions to ChD and mAb, allowing them to continue the optimal treatment for their diseases. Full target doses are achieved in a few hours, reducing patients' visits to the hospital.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the University Hospital of Guadalajara. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
AV designed the study. All authors have participated in the review, written, and approved the manuscript.
Funding
Part of this work was supported by the Instituto de Salud Carlos III (ISCIII) co-funded by Fondo Europeo de Desarrollo Regional—FEDER for the Thematic Networks and Co-operative Research Centres: ARADyAL (RD16/0006/0023).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank all the nurses from the allergy department for their contribution to the desensitization procedure, and the pharmacy department of the Hospital of Guadalajara, especially Paula de Juan, for the drug skin tests and infusion preparations.
Previous Presentation
Part of the data included in this manuscript was presented in oral communication format during the Spanish Society of Allergy and Clinical Immunology (SEAIC) Annual Congress in 2017.
Abbreviations
BTR, breakthrough reactions; ChD, chemotherapeutic drugs; DHR, drug hypersensitivity reactions; mAb, monoclonal antibodies; MPE, maculopapular exanthema; RDD, rapid drug desensitization.
References
1. Cernadas JR, Brockow K, Romano A, Aberer W, Torres MJ, Bircher A, et al. General considerations on rapid desensitization for drug hypersensitivity—a consensus statement. Allergy. (2010) 65:1357–66. doi: 10.1111/j.1398-9995.2010.02441.x
2. Isabwe GAC, Garcia Neuer M. de las Vecillas Sanchez L, Lynch DM, Marquis K, Castells M. Hypersensitivity reactions to therapeutic monoclonal antibodies: phenotypes and endotypes. J Allergy Clin Immunol. (2018) 142:159–70. doi: 10.1016/j.jaci.2018.02.018
3. Caiado J, Castells MC. Drug desensitizations for chemotherapy: safety and efficacy in preventing anaphylaxis. Curr Allergy Asthma Rep. (2021) 21:1–13. doi: 10.1007/s11882-021-01014-x
4. Sloane D, Govindarajulu U, Harrow-Mortelliti J, Barry W, Hsu FI, Hong D, et al. Safety, costs, and efficacy of rapid drug desensitizations to chemotherapy and monoclonal antibodies. J Allergy Clin Immunol Pract. (2016) 4:497–504. doi: 10.1016/j.jaip.2015.12.019
5. Pérez-Rodríguez E, Martínez-Tadeo JA, Pérez-Rodríguez N, Hernández-Santana G, Callero-Viera A, Rodríguez-Plata E, et al. Outcome of 490 desensitizations to chemotherapy drugs with a rapid one-solution protocol. J Allergy Clin Immunol Pract. (2018) 6:1621–7. doi: 10.1016/j.jaip.2017.11.033
6. Madrigal-Burgaleta R, Bernal-Rubio L, Berges-Gimeno MP, Carpio-Escalona LV, Gehlhaar P, Alvarez-Cuesta E, et al. Large Single-hospital experience using drug provocation testing and rapid drug desensitization in hypersensitivity to antineoplastic and biological agents. J Allergy Clin Immunol Pract. (2019) 7:618–32. doi: 10.1016/j.jaip.2018.07.031
7. Vidal C, Méndez-Brea P, López-Freire S, Bernárdez B, Lamas M-J, Armisén M, et al. A modified protocol for rapid desensitization to chemotherapy agents. J Allergy Clin Immunol Pract. (2016) 4:1003–5. doi: 10.1016/j.jaip.2016.05.015
8. Castells MC, Tennant NM, Sloane DE, Ida Hsu F, Barrett NA, Hong DI, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. (2008) 122:574–80. doi: 10.1016/j.jaci.2008.02.044
9. Brennan PJ, Bouza TR, Hsu FI, Sloane DE, Castells MC. Hypersensitivity reactions to mAbs: 105 desensitizations in 23 patients, from evaluation to treatment. J Allergy Clin Immunol. (2009) 124:1259–66. doi: 10.1016/j.jaci.2009.09.009
10. Lee B, Yu HJ, Kang ES, Lee M, Lee J. Human leukocyte antigen genotypes and trial of desensitization in patients with oxcarbazepine-induced skin rash: A pilot study. Pediatr Neurol. (2014) 51:207–14. doi: 10.1016/j.pediatrneurol.2014.03.021
11. Lee MJ, Wickner P, Fanning L, Schlossman R, Richardson P, Laubach J, et al. Lenalidomide desensitization for delayed hypersensitivity reactions in 5 patients with multiple myeloma. Br J Haematol. (2014) 167:127–31. doi: 10.1111/bjh.12925
12. Galvan-Blasco P, Guilarte M, Cardona V, Labrador-Horrillo M, Sala-Cunill A, Gil-Serrano IJ, et al. Delayed drug hypersensitivity to bortezomib: Desensitization and tolerance to its analogue carfilzomib. Allergy. (2019) 74:1384–6. doi: 10.1111/all.13735
13. Caumes E, Guermonprez G, Lecomte C, Katlama C, Bricaire F. Efficacy and safety of desensitization with sulfamethoxazole and trimethoprim in 48 previously hypersensitive patients infected with human immunodeficiency virus. Arch Dermatol. (1997) 133:465–9. doi: 10.1001/archderm.1997.03890400065009
14. Fam AG, Dunne SM, Iazzetta J, Paton TW. Efficacy and safety of desensitization to allopurinol following cutaneous reactions. Arthritis Rheum. (2001) 44:231–8. doi: 10.1002/1529-0131(200101)44:1<231::AID-ANR30>3.0.CO;2-7
15. McNulty CMG, Park MA. Delayed cutaneous hypersensitivity reactions to antibiotics: management with desensitization. Immunol Allergy Clin North Am. (2017) 37:751–60. doi: 10.1016/j.iac.2017.07.002
16. Ban GY, Jeong YJ, Lee SH, Shin SS, Shin YS, Park HS, et al. Efficacy and tolerability of desensitization in the treatment of delayed drug hypersensitivities to anti-tuberculosis medications. Respir Med. (2019) 147:44–50. doi: 10.1016/j.rmed.2018.12.017
17. Karaatmaca B, Aytac S, Sahiner UM, Sekerel BE, Soyer O. Successful oral desensitization with dasatinib in delayed cutaneous hypersensitivity reactions. Ann Allergy Asthma Immunol. (2019) 123:216–7. doi: 10.1016/j.anai.2019.05.011
18. Whitaker P, Shaw N, Gooi J, Etherington C, Conway S, Peckham D. Rapid desensitization for non-immediate reactions in patients with cystic fibrosis. J Cyst Fibros. (2011) 10:282–5. doi: 10.1016/j.jcf.2011.02.002
19. Picard M, Pur L, Caiado J, Giavina-Bianchi P, Galvão VR, Berlin ST, et al. Risk stratification and skin testing to guide re-exposure in taxane-induced hypersensitivity reactions. J Allergy Clin Immunol. (2016) 137:1154–64. doi: 10.1016/j.jaci.2015.10.039
20. Scherer K, Brockow K, Aberer W, Gooi JHC, Demoly P, Romano A, et al. Desensitization in delayed drug hypersensitivity reactions—an EAACI position paper of the Drug Allergy Interest Group. Allergy. (2013) 68:844–52. doi: 10.1111/all.12161
21. Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, et al. Skin test concentrations for systemically administered drugs—an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. (2013) 68:702–12. doi: 10.1111/all.12142
22. Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. (2002) 57:45–51. doi: 10.1046/j.0105-4538.2001.00001.x-i8
23. Broyles AD, Banerji A, Barmettler S, Biggs CM, Blumenthal K, Brennan PJ, et al. Practical guidance for the evaluation and management of drug hypersensitivity: specific drugs. J Allergy Clin Immunol Pract. (2020) 8:S16–116. doi: 10.1016/j.jaip.2020.08.002
24. Lee CW, Matulonis UA, Castells MC. Carboplatin hypersensitivity: A 6-h 12-step protocol effective in 35 desensitizations in patients with gynecological malignancies and mast cell/IgE-mediated reactions. Gynecol Oncol. (2004) 95:370–6. doi: 10.1016/j.ygyno.2004.08.002
25. Hong DI, Dioun AF. Indications, protocols, and outcomes of drug desensitizations for chemotherapy and monoclonal antibodies in adults and children. J Allergy Clin Immunol Pract. (2014) 2:13–9. doi: 10.1016/j.jaip.2013.11.007
26. Alonso-Llamazares A, Vega-Castro A, Beitia-Mazuecos JM, Mateo-Borrega B, Cardenas-Contreras R. Rapid desensitization with temozolomide in patients with delayed maculopapular rash. J Investig Allergol Clin Immunol. (2012) 22:448–9.
27. Castells M. Diagnosis and management of anaphylaxis in precision medicine. J Allergy Clin Immunol. (2017) 140:321–33. doi: 10.1016/j.jaci.2017.06.012
28. Vega A, Jimenez-Rodriguez TW, Barranco R, Bartra J, Diéguez MC, Doña I, et al. Hypersensitivity reactions to cancer chemotherapy: practical recommendations of ARADyAL for diagnosis and desensitization. J Investig Allergol Clin Immunol. (2021) 31:364–84. doi: 10.18176/jiaci.0712
29. Caiado J, Brás R, Paulino M, Costa L, Castells M. Rapid desensitization to antineoplastic drugs in an outpatient immunoallergology clinic: outcomes and risk factors. Ann Allergy, Asthma Immunol. (2020) 125:325–33. doi: 10.1016/j.anai.2020.04.017
30. Garcia CM, Sierra Pacho M, Moreno Rodilla E, Callejo Melgosa AM, Martinez Alonso JC, Fernández Colino T, et al. Successful bendamustine desensitization for a delayed-type hypersensitivity reaction. J Investig Allergol Clin Immunol. (2019) 29:68–9. doi: 10.18176/jiaci.0335
31. Divekar R, Butterfield J, Maddox D. Successful rapid desensitization to temozolomide: a case series. J Allergy Clin Immunol Pract. (2016) 4:545–6. doi: 10.1016/j.jaip.2015.12.007
32. Nelson RP, Cornetta K, Ward KE, Ramanuja S, Fausel C, Cripe LD. Desensitization to imatinib in patients with leukemia. Ann allergy, asthma Immunol. (2006) 97:216–22. doi: 10.1016/S1081-1206(10)60016-6
33. Bar-Sela G, Kedem E, Hadad S, Pollack S, Haim N, Atrash F, et al. Successful desensitization protocol for hypersensitivity reaction caused by sunitinib in a patient with a gastrointestinal stromal tumor. Jpn J Clin Oncol. (2010) 40:163–5. doi: 10.1093/jjco/hyp118
34. Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy focus on hypersensitivity responses. Oncoimmunology. (2013) 2:26333. doi: 10.4161/onci.26333
35. Pichler WJ. Pharmacological interaction of drugs with antigen-specific immune receptors: the p-i concept. Curr Opin Allergy Clin Immunol. (2002) 2:301–5. doi: 10.1097/00130832-200208000-00003
36. Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International Consensus on drug allergy. Allergy. (2014) 69:420–37. doi: 10.1111/all.12350
37. Hong DI, Madrigal-Burgaleta R, Banerji A, Castells M, Alvarez-Cuesta E. Controversies in allergy: chemotherapy reactions, desensitize, or delabel? J Allergy Clin Immunol Pract. (2020) 8:2907–2915.e1. doi: 10.1016/j.jaip.2020.08.005
38. Madrigal-Burgaleta R, Vazquez-Revuelta P, Marti-Garrido J, Lleonart R, Ali FR, Alvarez-Cuesta E. Importance of diagnostics prior to desensitization in new drug hypersensitivity: chemotherapeutics and biologicals. Curr Treat Options Allergy. (2020) 7:1–13. doi: 10.1007/s40521-020-00238-y
39. Ureña-Tavera A, Zamora-Verduga M, Madrigal-Burgaleta R, Angel-Pereira D, Berges-Gimeno MP, Alvarez-Cuesta E. Hypersensitivity reactions to racemic calcium folinate (leucovorin) during FOLFOX and FOLFIRI chemotherapy administrations. J Allergy Clin Immunol. (2015) 135:1066–7. doi: 10.1016/j.jaci.2014.09.045
40. Alvarez-Cuesta E, Madrigal-Burgaleta R, Angel-Pereira D, Ureña-Tavera A, Zamora-Verduga M, Lopez-Gonzalez P, et al. Delving into cornerstones of hypersensitivity to antineoplastic and biological agents: value of diagnostic tools prior to desensitization. Allergy. (2015) 70:784–94. doi: 10.1111/all.12620
41. Khan DA. Hypersensitivity and immunologic reactions to biologics: opportunities for the allergist. Ann Allergy Asthma Immunol. (2016) 117:115–20. doi: 10.1016/j.anai.2016.05.013
42. Jimenez-Rodriguez TW, Alvarez Labella M, Garcia-Neuer M, Lynch D-MM, Castells M. Abstract 1769. Delayed hypersensitivity reactions to taxane can progress to Type I reactions: management with desensitization. Allergy. (2018) 73:811. doi: 10.26226/morressier.5afac509d0241f0023ccbcc3
43. Berges-Gimeno M, Carpio-Escalona L, Longo-Muñoz F, Bernal-Rubio L, Lopez-Gonzalez P, Gehlhaar P, et al. Does rapid drug desensitization to chemotherapy affect survival outcomes? J Investig Allergol Clin Immunol. (2020) 30:254–63. doi: 10.18176/jiaci.0425
44. Altwerger G, Florsheim EB, Menderes G, Black J, Schwab C, Gressel GM, et al. Impact of carboplatin hypersensitivity and desensitization on patients with recurrent ovarian cancer. J Cancer Res Clin Oncol. (2018) 144:2449–56. doi: 10.1007/s00432-018-2753-y
Keywords: rapid drug desensitization, desensitization, chemotherapeutic drugs, monoclonal antibodies, delayed drug reactions, no-immediate drug reactions
Citation: Vega A, Peña MI and Torrado I (2022) Use of Rapid Drug Desensitization in Delayed Hypersensitivity Reactions to Chemotherapy and Monoclonal Antibodies. Front. Allergy 2:786863. doi: 10.3389/falgy.2021.786863
Received: 30 September 2021; Accepted: 08 December 2021;
Published: 14 January 2022.
Edited by:
Ricardo Madrigal-Burgaleta, Barts Health NHS Trust, United KingdomReviewed by:
Kathrin Scherer Hofmeier, Aarau Cantonal Hospital, SwitzerlandGerald Wayne Volcheck, Mayo Clinic, United States
Copyright © 2022 Vega, Peña and Torrado. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arantza Vega, arantza.vega@gmail.com
 Arantza Vega
Arantza Vega M. Isabel Peña1,2
M. Isabel Peña1,2  Inés Torrado
Inés Torrado