1
Department of Biological and Medical Psychology, University of Bergen, Bergen, Norway
2
Division of Psychiatry, Haukeland University Hospital, Bergen, Norway
People with schizophrenia frequently report cannabis use, and cannabis may be a risk factor for schizophrenia, mediated through effects on brain function and biochemistry. Thus, it is conceivable that cannabis may also influence cognitive functioning in this patient group. We report data from our own laboratory on the use of cannabis by schizophrenia patients, and review the existing literature on the effects of cannabis on cognition in schizophrenia and related psychosis. Of the 23 studies that were found, 14 reported that the cannabis users had better cognitive performance than the schizophrenia non-users. Eight studies reported no or minimal differences in cognitive performance in the two groups, but only one study reported better cognitive performance in the schizophrenia non-user group. Our own results confirm the overall impression from the literature review of better cognitive performance in the cannabis user group. These paradoxical findings may have several explanations, which are discussed. We suggest that cannabis causes a transient cognitive breakdown enabling the development of psychosis, imitating the typical cognitive vulnerability seen in schizophrenia. This is further supported by an earlier age of onset and fewer neurological soft signs in the cannabis-related schizophrenia group, suggesting an alternative pathway to psychosis.
A history of cannabis use is more common in schizophrenia than in the normal population (Regier et al., 1990
; Arseneault et al., 2004b
; Barnes et al., 2006
). Life-time cannabis use has been reported to be as high as 64.4% in patients with schizophrenia (Barnes et al., 2006
), and Løberg et al. (2003)
found that 45% of schizophrenia patients participating in research studies had a history of previous cannabis use. Since cannabis may be a risk factor for schizophrenia, mediated through changes in brain functioning and biochemistry, cannabis may also have an effect on cognitive functioning in this patients group. In a preliminary study in our laboratory we were struck by apparent paradoxical positive effects of cannabis on cognition in patients with schizophrenia (Løberg et al., 2003
, 2008
). These preliminary findings prompted a review of the existing literature on the relationship between cannabis use and cognitive functioning in schizophrenia. For this purpose, we found 23 studies (see Table 1
) that have looked at the relationship between cannabis use and cognitive impairments in schizophrenia. The results from the review are discussed and possible explanations suggested.
Longitudinal studies have reported an increased risk for schizophrenia and other psychoses after cannabis use. In two large-scale Swedish studies, the same cohort of about 50 000 military conscripts were for followed longitudinally over 15 and 26 years. Dose-dependent relationships were found between cannabis use at 18 years of age and a later diagnosis of schizophrenia (Andreasson et al., 1987
; Zammit et al., 2002
). Cannabis have also been shown to increase the rate of conversion to psychosis in individuals at risk for psychosis (Kristensen and Cadenhead, 2007
). Furthermore, several large-scale longitudinal studies have reported a relationship between cannabis use in adolescence and later symptoms of psychosis in the normal population (Tien and Anthony, 1990
; Arseneault et al., 2002
; van Os et al., 2002
; Fergusson et al., 2003
; Stefanis et al., 2004
; Ferdinand et al., 2005
; Henquet et al., 2005a
). In one study, cannabis use at age 18 and 21 led to 3.7 and 2.3 higher rates of psychotic symptoms, respectively (Fergusson et al., 2003
). The relationship between cannabis and schizophrenia seems fairly specific to schizophrenia, as compared to other mental disorders (Chambers et al., 2001
; Degenhardt et al., 2007
; Di Forti et al., 2007
; Moore et al., 2007
), and cannot be explained by potentially confounding factors, like premorbid disorders, drug use, intoxication, personality traits, sosiodemographic markers and intellectual ability (Smit et al., 2004
; Moore et al., 2007
). Accordingly, five recent reviews concluded with an increased risk for schizophrenia and psychosis in individuals who have used cannabis (Arseneault et al., 2004b
; Macleod et al., 2004
; Smit et al., 2004
; Henquet et al., 2005b
; Semple et al., 2005
; Moore et al., 2007
).
An alternative explanation is what can be called reversed causality, namely that schizophrenia patients use cannabis as a form of self-medication, although existing data does not seem to support this hypothesis (Chambers et al., 2001
). An important argument against reversed causality is an “order-effect”; i.e. cannabis use seems to occur before the outbreak of psychosis, and not the other way around (Linszen et al., 1994
; Degenhardt et al., 2007
; Corcoran et al., 2008
). Furthermore, in contrast to a self-medication hypothesis, the psychoactive substance in cannabis, delta-(9)-tetrahydrocannabinol (THC), increases, and not decreases, anxiety (Fusar-Poli et al., 2009
; Morrison et al., 2009
). Several studies have also shown that THC increases symptoms of psychosis and cognitive impairments (D’Souza et al., 2005
; Morrison et al., 2009
), with a possible increased sensitivity in schizophrenia to the adverse effects of THC (D’Souza et al., 2004
). Moreover, cannabis has been shown to have clinical significance. Cannabis use in schizophrenia can lead to worsened illness prognoses; worsened clinical outcome, longer psychotic episodes, more relapse and re-hospitalizations, poorer social functioning, more frequent relapses, poorer compliance, and increased treatment needs (Linszen et al., 1994
; Caspari, 1999
; Grech et al., 2005
). Thus, taken together, the available data seem to point to cannabis use as increasing psychotic symptoms, and increasing the vulnerability for a psychotic outbreak. For example, Moore and colleagues have argued that we now know enough to warn young people about the risk for psychosis after cannabis use (Moore et al., 2007
).
However, most individuals do not develop schizophrenia after cannabis use, suggesting that a heightened risk for a development of psychosis must be related to other vulnerability factors. Verdoux (2004)
found that subjects with established vulnerability for psychoses showed a stronger risk of follow-up psychosis after cannabis use than individuals without such vulnerability. The relationship between cannabis use and psychosis may also be genetically mediated. In a longitudinal study of 803 individuals, an interaction between the Val allele of the Catechol-O-methyltransferase (COMT) gene and adolescent cannabis use significantly increased the likelihood of exhibiting psychotic symptoms and the development of schizophreniform disorders (Caspi et al., 2005
). In accordance with this, an interaction between the COMT Val allele and sensitivity for psychosis and cognitive effects of the psychoactive substance in cannabis has been found in individuals with psychosis and their relatives (Henquet et al., 2006
, 2009
).
The relationship between cannabis and schizophrenia may be attributed to effects of cannabis on brain functioning and biochemistry. The endogenous cannabinoid system may directly or indirectly be involved in the development of the effects of cannabis on symptoms of psychosis and cognition (Solowij and Michie, 2007
). THC affects cannabinoid receptors, which are distributed with high density in the cerebral cortex, including brain regions implicated in schizophrenia (D’Souza et al., 2005
). The endogenous cannabinoid system interacts with the dopaminergic system of the brain, and THC influence dopamine synthesis and uptake (D’Souza et al., 2005
). Abnormalities of the endogenous cannabinoid system in schizophrenia, not caused by cannabis use, have also been reported. Increased levels of endogenous cannabinoids have been found in the frontal cortex (Dean et al., 2001
), in addition to elevated levels of endogenous cannabinoids in the cerebral spinal fluids (Leweke et al., 1999
).
A stronger relationship between adolescent cannabis use and psychosis or schizophrenia, as compared to adult use, has been reported in several studies (Caspi et al., 2005
; Konings et al., 2008
). Even though some of these findings can be explained by an increased cumulative exposure to cannabis with earlier onset of cannabis use, it may also suggest that a developing brain is more vulnerable to the effects of cannabis than a matured brain.
However although there seems to be ample evidence for the influence of cannabis on the development and outbreak of psychosis or schizophrenia (Moore et al., 2007
), possibly mediated by adverse effects on brain functioning acting on the dopaminergic system (D’Souza et al., 2005
), much less is known regarding the effects of cannabis use on cognitive functioning in schizophrenia. If cannabis influences schizophrenia neurodevelopment and brain functioning, it could be expected that cannabis use may impact on cognitive functioning in this patients group. The aim of the present review was therefore to examine the relationship between cannabis use and cognitive functioning in schizophrenia.
Cognitive impairment is now universally recognized as a core feature of schizophrenia, and clinically relevant cognitive impairments are observed in a majority of patients with schizophrenia (Green, 1996
; Palmer et al., 1997
, 2009
). Cognitive impairment is often observed before the development of psychosis and in close relatives, and cognitive symptoms may also reside after clinical symptoms have been reduced or are no longer seen (Neuchterlein et al., 1994
; Weinberger, 1995
; Heaton et al., 2001
; Gschwandtner et al., 2003
), Thus, it is clear that eventual effects of cannabis use on cognitive functioning in schizophrenia would be of both theoretical value for the understanding of the disorder, and of clinical relevance for the diagnosis and treatment of the disorder. Intuitively, a worsened outcome on cognitive functioning would be expected after cannabis use, since cannabis has negative effects on psychosis in general, and from findings that cannabis use impairs illness prognosis (Linszen et al., 1994
; Caspari, 1999
; Grech et al., 2005
), in addition to the adverse effects of cannabis on brain functioning (D’Souza et al., 2004
).
For this purpose we reanalyzed previously collected data in our laboratory on cognitive performance in schizophrenia patients, including cannabis use as an explanatory variable (data from Løberg et al., 2003
, 2008
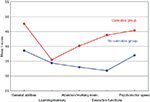
). Information on the history of cannabis use was based on the patients’ clinical records and therapist questionnaires, and was further validated through SCID-interviews. Surprisingly, we found that patients with schizophrenia who had a history of cannabis use scored significantly above their fellow counterparts without a history of cannabis use (see Figure 1
). This was found for almost all cognitive functions investigated, such as general intellectual ability, executive functions, attention, working memory and psychomotor speed. These results did not change when other illegal drugs where controlled for, and there were no differences in the two groups with regard to clinical variables (Løberg et al., 2003
).
Figure 1. Mean T-scores for the cannabis and no-cannabis group for the five cognitive functions. General abilities = general verbal and visuospatial abilities = WAIS (Information, Vocabulary, Block Design), Verbal Fluency (FAS), Rey-Osterrieth Complex Figure test, Wisconsin Card Sorting Test (WCST). Learning/memory = California Verbal Learning Test (CVLT) II, Rey-Osterrieth Complex Figure Test. Attention/working mem. = attention/working memory = Digit Vigilance Test, Calcap Continuous Performance Test (CPT), Trail Making Test B. Execute functions = Wisconsin Card Sorting Test (WCST), Stroop Test. Psychomotor speed = Trail Making Test A, Grooved Pegboard Test, Fingertapping Test.
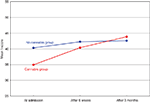
In a second, prospective, study of patients with acute psychosis we assessed cognitive function at admission to a psychiatric emergency ward, after 6 weeks, and after 3 months. Information on the history of cannabis use was based on patient’s clinical records and the Clinician Drug Use Scale (Drake et al., 1990
), and was further validated through urine samples. The patients with both cannabis use and psychosis showed a significantly larger improvement in their cognitive performance in the three months after admission, as compared to the psychotic patients with no cannabis use. Both groups showed cognitive impairments at admission, but these were more prevalent in the non-cannabis psychosis group (see Figure 2
; Løberg et al., 2008
).
Figure 2. Mean neuropsychological T-scores for the cannabis and no-cannabis group at admission, after 6 weeks and 3 months.
The paradoxical results reported by Løberg et al. (2003
, 2008
) seem to be consistent with several other studies on the relationship between cognition, cannabis and/or illegal drugs and schizophrenia (Joyal et al., 2003
; Jockers-Scherubl et al., 2007
). We performed a PubMed search on all combinations of the following search words: cannabis, substance, schizophr*, psychos*, cognit* and neuropsych*, and searched the reference lists for all included papers of other studies covering this topic. This resulted in 23 studies comparing schizophrenia and related psychoses with and without cannabis use (alone or in combination with other substances) on cognitive performance (see Table 1
for further details).
Fourteen of the studies listed in Table 1
reported that the cannabis groups showed better cognitive performance than the no-cannabis groups (Sevy et al., 2001
; Carey et al., 2003
; Joyal et al., 2003
; Løberg et al., 2003
, 2008
; Herman, 2004
; Kumra et al., 2005
; Potvin et al., 2005
; Stirling et al., 2005
; McCleery et al., 2006
; Coulston et al., 2007a
; Jockers-Scherubl et al., 2007
; Thoma et al., 2007
; Schnell et al., 2009
). Eight of the studies in Table 1
reported no or minimal differences in cognitive performance in the two groups (Cleghorn et al., 1991
; Addington and Addington, 1997
; Liraud and Verdoux, 2002
; Pencer and Addington, 2003
; Barnes et al., 2006
; Sevy et al., 2007
; Wobrock et al., 2007
; Thoma and Daum, 2008
), and one study reported better cognitive performance in the no-cannabis compared to the drug group (Mata et al., 2008
).
Most of the studies in Table 1
have small “n”, and may therefore be influenced by Type-II statistical errors (false negatives), underestimating group differences due to lack of power. For instance, Thoma and Daum (2008)
suggested that this may have been a problem in their 2008 study, influencing their conclusion of no differences between the groups. Furthermore, some of the studies included diverse drug use in addition to cannabis use, for instance alcohol and opiates in clusters of stimulating and/or hallucinatory illegal drugs. These drugs may have different, and sometimes opposite effects on brain functioning and neurochemistry, and consequently on cognition. In the overview in Table 1
, all studies included cannabis; as a high frequent drug together with other drugs used, or as the only drug used. Thus, no study was included that did not include cannabis. Previous drug use versus current drug use is included in Table 1
as a separate factor since this may have influenced the results. Current drug use may influence cognition by means of persisting intoxication effects or more acute effects on brain functioning, thus creating a “false” cognitive impairment not otherwise present. Furthermore, the use of diagnostic criteria is noted, yielding a SUD, since this usually means that the patients meet criteria for abuse or addictive behavior, and that the drug use has negative consequences for everyday living. This may bias the drug groups to consist of quite heavy users due to the exclusion of patients without a SUD diagnosis who nevertheless may have a frequent drug problem. An example of this is the study by Addington and Addington (1997)
where it was reported that the no-drug group actually included 13 patients with previous drug use. Another problem when comparing the studies in Table 1
is the different diagnostic groups included in the studies, possibly with different levels of cognitive vulnerability.
Coulston et al. (2007b)
did not find consistent neuropsychological patterns of cannabis use on cognition when examining seven studies (one of these studies reported intoxications effects, though), and attributed this to methodological variability between and methodological limitations within the studies. Methodological and clinical heterogeneity is also a problem in studies comparing differences in brain structure and function between drug and no-drug groups by means of brain-imaging methods, and inconsistent results have been reported (Quickfall and Crockford, 2006
; Rais et al., 2008
; Wobrock et al., 2009
).
Table 1
show that a majority of the studies report better cognitive functioning in the cannabis-related schizophrenia and psychosis groups compared to non-drug groups. This conclusion is supported even when confounding factors, like age, years of education, premorbid IQ, medical history, substance use, and psychiatric symptoms (Coulston et al., 2007a
) are controlled for. Likewise, Potvin et al. (2008)
argued that most studies have shown superior neuropsychological functioning in cannabis use and schizophrenia combined, then in schizophrenia patients alone (Potvin et al., 2008
).
The seemingly paradoxical cognitive findings in cannabis-related schizophrenia could have several explanations. One explanation is that the group differences in cognition are attributed to superior social skills in the cannabis schizophrenia groups, making them “skillful” enough to get hold of illegal drugs. Superior social skills are however not consistent with the finding of poorer prognosis in this group. Few studies have, however, examined this directly, and the issue therefore remains unresolved. Two Norwegian studies reported poorer premorbid functioning in psychosis patients who also abused illegal drugs (Ringen et al., 2008
), and better premorbid social functioning and poorer premorbid academic functioning in this group (Larsen et al., 2006
), respectively. It has also been suggested that the group differences could be caused by cannabis having a protective or positive influence on brain functioning (Coulston et al., 2007a
). Based on the effects of cannabis on brain function and prognosis of the psychosis, this is not supported by the existing data.
A second explanation could be that cannabis imitates the typical cognitive vulnerability seen in schizophrenia. The major psychoactive component in cannabis, THC, creates transient negative effects on cognitive functioning and psychotic symptoms (D’Souza et al., 2005
; Semple et al., 2005
; Morrison et al., 2009
). Cannabis use of sufficient magnitude, or in individuals particularly vulnerable to the effects of cannabis, may lead to compromised brain functioning, causing a breakdown of reality testing. In addition, adolescent cannabis use seems to cause an especially strong risk for later psychosis (Caspi et al., 2005
; Konings et al., 2008
), consistent with a sensitive adolescent brain in the middle of important neurodevelopmental processes. Thus, cannabis would induce more transient cognitive changes that mimic the typical cognitive vulnerability. These changes can cause psychosis for some individuals, but will normally not cause the characteristic persistent cognitive impairments seen in schizophrenia. Consistent with this, fewer neurological soft signs have been shown in schizophrenia patients who also use cannabis (Bersani et al., 2002
; Ruiz-Veguilla et al., 2009
). Stirling et al. (2005)
also reported fewer neurological soft signs, and better cognitive functioning, in the drug group after 10–12 years, and suggested that the drug group followed a different path to schizophrenia with less negative events of early brain development.
Further support for the imitation of cognitive vulnerability hypothesis is findings regarding age of onset of the disorder. The development of schizophrenia is usually seen in late adolescence/early adulthood. This is in line with a neurodevelopmental model (Weinberger, 1995
), since the age of onset coincides with the late maturation of the prefrontal cortex through pruning of exuberant synapses and myelination of axons (Woo and Crowell, 2005
). Studies have reported earlier age of onset in schizophrenia patients who have used cannabis (Stirling et al., 2005
; Barnes et al., 2006
). Consistent with this, data from our own laboratory showed four years earlier debut of schizophrenia in cannabis users (Løberg et al., 2003
). Again, this suggests a different pathway to schizophrenia, and is consistent with a hypothesis stating that cannabis is an environmental factor imitating the effect of the typical cognitive vulnerability (Solowij and Michie, 2007
).
Cannabis seems to be a risk factor for the development of schizophrenia, mimicking the typical cognitive vulnerability. As an environmental factor, cannabis use has the potential for being influenced by interventions, thus indirectly having an effect on the development of schizophrenia. Accordingly, clinical implications (Moore et al., 2007
) and public health implications (Arseneault et al., 2004a
) have been suggested. A promising clinical intervention would be to monitor cannabis use in patients known to be vulnerable for psychosis, and help them to stay away from cannabis. Cannabis does not appear to create additive cognitive impairments, however, and cannabis-using patients may actually have better cognitive functioning. This could suggest that cannabis-related schizophrenia represents a different subtype, although few consistent clinical differences in regard to symptom profiles have been found (Boydell et al., 2007
). This necessitates a better understanding of the paradox of better cognitive functioning, similar clinical profiles, and worse prognosis in this group, through for instance longitudinal studies on the effect of previous and ongoing cannabis use on the fluctuations of cognitive and clinical functioning in schizophrenia.
Possibly cannabis mimics the typical cognitive vulnerability seen in schizophrenia. Solowij and Michie (2007)
suggested that cannabis leads to similar cognitive impairment as what is typically seen in schizophrenia, but of a lower magnitude. Several studies have shown cognitive impairment during THC-intoxication (D’Souza et al., 2005
; Morrison et al., 2009
). The preliminary data from our own laboratory suggest more transient cognitive impairments in the cannabis group (Løberg et al., 2008
). Perhaps cannabis causes a transient cognitive breakdown enabling the development of psychosis, in spite of the absence of proper cognitive vulnerability. Thus, the effects of cannabis on cognition and brain functioning model the cognitive vulnerability in schizophrenia, and understanding this cognitive breakdown may provide a unique window to understanding schizophrenia neurodevelopment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support for the research reported in this article was given by Research Council of Norway (RCN), Haukeland University Hospital Strategic Research Programme, and Health Authority for Western Norway.
Caspi, A., Moffitt, T. E., Cannon, M., McClay, J., Murray, R., Harrington, H., Taylor, A., Arseneault, L., Williams, B., Braithwaite, A., Poulton, R., and Craig, I. W. (2005). Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol. Psychiatry 57, 1117–1127.
Corcoran, C. M., Kimhy, D., Stanford, A., Khan, S., Walsh, J., Thompson, J., Schobel, S., Harkavy-Friedman, J., Goetz, R., Colibazzi, T., Cressman, V., and Malaspina, D. (2008). Temporal association of cannabis use with symptoms in individuals at clinical high risk for psychosis. Schizophr. Res. 106, 286–293.
Fusar-Poli, P., Crippa, J. A., Bhattacharyya, S., Borgwardt, S. J., Allen, P., Martin-Santos, R., Seal, M., Surguladze, S. A., O’Carrol, C., Atakan, Z., Zuardi, A. W., and McGuire, P. K. (2009). Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch. Gen. Psychiatry 66, 95–105.
Gschwandtner, U., Aston, J., Borgwardt, S., Drewe, M., Feinendegen, C., Lacher, D., Lanzarone, A., Stieglitz, R.-D., and Riecher-Rössler, A. (2003). Neuropsychological and neurophysiological findings in individuals suspected to be at risk for schizophrenia: preliminary results from the Basel early detection of psychosis study – Fruherkennung von Psychosen (FEPSY). Acta Psychiatr. Scand. 108, 152–155.
Henquet, C., Rosa, A., Krabbendam, L., Papiol, S., Fananas, L., Drukker, M., Ramaekers, J. G., and van Os, J. (2006). An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology 31, 2748–2757.
Macleod, J., Oakes, R., Copello, A., Crome, I., Egger, M., Hickman, M., Oppenkowski, T., Stokes-Lampard, H., and Davey Smith, G. (2004). Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet 363, 1579–1588.
Mata, I., Rodriguez-Sanchez, J. M., Pelayo-Teran, J. M., Perez-Iglesias, R., Gonzalez-Blanch, C., Ramirez-Bonilla, M., Martinez-Garcia, O., Vazquez-Barquero, J. L., and Crespo-Facorro, B. (2008). Cannabis abuse is associated with decision-making impairment among first-episode patients with schizophrenia-spectrum psychosis. Psychol. Med. 38, 1257–1266.