Meropenem Administered via Intravenous Regional Limb Perfusion for Orthopedic Sepsis in Horses: A Clinical Retrospective Study
- 1College of Veterinary Medicine, Iowa State University, Ames, IA, United States
- 2Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA, United States
- 3Biomedical Sciences, College of Veterinary Medicine, Iowa State University, Ames, IA, United States
- 4Veterinary Clinical Sciences, College of Veterinary Medicine, Iowa State University, Ames, IA, United States
- 5Veterinary Microbiology and Preventive Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA, United States
Septic synovitis is a critical orthopedic condition in horses. Early intervention is key, with antibiotic therapy typically initiated prior to culture and susceptibility reports becoming available. The pharmacokinetics of several antibiotics have been studied in horses for use in intravenous regional limb perfusion (IVRLP) for septic synovitis, including the carbapenem antibiotic, meropenem. For a variety of factors, some veterinary clinicians may select IVRLP meropenem as therapy for these cases. Meropenem is a vital antibiotic in human medicine, making veterinary use divisive. However, verifying the efficacy of meropenem contrasted to other IVRLP antibiotics is essential for appropriate antimicrobial stewardship. To investigate this, equine patient medical records at a single veterinary teaching hospital were examined. Cases treated with meropenem or gentamicin via IVRLP for septic synovitis were retrospectively analyzed for demographics, diagnostics, treatments, outcomes, and adverse effects. Twenty-three meropenem and 37 gentamicin treated horses were analyzed; demographic information was similar between groups. In the meropenem group, nine horses received meropenem only; the remainder received another antibiotic initially then changed to meropenem. Structures infected included joints (meropenem = 13, gentamicin = 17), tendon sheaths (meropenem = 5, gentamicin = 8) and navicular bursae (meropenem = 2, gentamicin = 6). Overall survival to discharge was 86% (52/60), with meropenem 91% (21/23) and gentamicin 84% (31/37), with no statistically significant differences noted between meropenem or gentamicin groups for overall survival to discharge or outcome after discharge. Twenty-four of 26 bacterial isolates obtained from culture were reported as sensitive to imipenem, a carbapenem antibiotic similar to meropenem. Reported susceptibility to other antibiotics such as ceftiofur (n = 22/26), ampicillin (n = 18/26), amikacin (n = 15/26), or gentamicin (n = 12/26) was also frequently present. In the population of this study, antimicrobial activity augmented with IVRLP using either meropenem or gentamicin both appear to be an effective treatment for septic synovial structures, therefore, less critical antimicrobials may be a viable and more judicious treatment option.
Introduction
Meropenem, a carbapenem antibiotic, is included in the beta-lactam family of antibiotics. The mechanism of action of beta-lactam antibiotics is to inhibit cell wall synthesis leading to cell lysis in a time dependent manner. Carbapenem antibiotics are effective against gram positive or negative bacteria, however, when used parenterally, they require frequent (multiple administrations per day) dosing due to a short half-life (1–4). According to the World Health Organization (WHO), meropenem is a critically important antibiotic in human medicine for multi-drug resistant (MDR) infections (5). Thus, it has been recommended to be used sparingly in veterinary medicine to avoid selecting for antimicrobial resistant organisms (5, 6). Many veterinary institutions, including the institution represented in this study, have protocols in place which restrict the use of carbapenems to the rare use for severe, life threatening conditions in individual animals when culture and susceptibility testing indicates that it is the only suitable option.
Septic synovitis is an orthopedic emergency with high morbidity, with discharge to hospital survival ranging from 38.5 to 86% (7, 8). Early, aggressive treatment with lavage and antibiotics has been associated with higher survival rates (7). Multi-drug resistant or gram-negative synovial infections have been associated with reduced survival (8). Intravenous regional limb perfusions (IVRLP), a common procedure used for treating septic synovial structures in horses, allow for higher concentrations of antibiotics targeting the affected structures while decreasing the adverse effects of systemic antibiotic treatment (9). Additionally, IVRLP may increase survival rates when used in conjunction with other septic synovitis therapy (10). For distal limb perfusions in horses a tourniquet or pressure cuff is placed proximal to the affected joint for approximately 15–30 min and a catheter is placed into the palmar/plantar digital vein to administer the selected antibiotic (9). The pharmacokinetic parameters of multiple drugs, including amikacin, penicillin, gentamicin, vancomycin, enrofloxacin, chloramphenicol, ceftiofur, imipenem and meropenem have been evaluated for IVRLP use in horses (11, 11–19). The most common bacteria associated with equine septic synovitis have been shown to have susceptibility to antibiotics other than meropenem (7, 8, 20–22). However, due to the perceived risk of MDR infection some veterinarians use meropenem in contrast to WHO guidelines.
If a critically important antibiotic, like meropenem, is to be used in veterinary species, it is imperative to evaluate its efficacy and clinical outcomes (6). There is concern meropenem use in IVRLP could lead to subtherapeutic concentration and increased antimicrobial resistance to this vital antibiotic (11, 23). Human patients treated parenterally with meropenem may experience adverse effects such as nausea, diarrhea, pain at injection site, seizures, and changes in hepatic biochemistry (19, 24). IVRLP in humans and other species has been associated with adverse effects such as thrombosis, venous scarring, and bruising, however, previous studies have found no adverse effects when used for IVRLP in healthy equine patients, (25–27). As such, IVRLP presents the chance to reduce adverse effects by significantly less drug exposure than by parental administration. The primary objective of this retrospective study of clinical cases was to compare clinical parameters, short term survival, and long term survival in equine orthopedic sepsis cases treated with antimicrobial therapy augmented with IVRLP using either meropenem or gentamicin.
Materials and Methods
Medical records were obtained from the Lloyd Veterinary Medical Center at Iowa State University's College of Veterinary Medicine. Records were screened for use of meropenem or gentamicin via IVRLP for orthopedic sepsis in equine patients treated in hospital from December 1, 2016 to March 1, 2020. Orthopedic sepsis was defined as an infection of an orthopedic structure as diagnosed by at least one of the following being consistent with infection: bacterial culture, cytology (28), diagnostic imaging results consistent with infection. Horses that received IVRLP with both meropenem and gentamicin were excluded from the gentamicin group. Any cases that used meropenem via a different route of administration, such as systemically or intra-articular, were excluded from the study. Information recorded included clinical parameters (age, breed, weight, sex, and body condition score), synovial structure involved, reported cause for synovial sepsis (hematogenous, intra-synovial injection, penetrating wound), IVRLP technique, previous/current systemic medications, surgical (arthroscopic lavage under general anesthesia) vs. non-surgical lavage, diagnostics performed, adverse effects, and survival to hospital discharge.
Diagnostic reports for cytology, culture and susceptibility, blood work, and various imaging methods were recorded. From cytology data, group cell count and protein concentrations were compared for normality, and then with an appropriate parametric or non-parametric test via commercial statistical software (GraphPad Prism 8.0, GraphPad Software, San Diego, CA) with a p < 0.05 considered statistically significant.
Owners of cases that survived to hospital discharge were contacted by phone and or email, from June 6 to July 10, 2020 to answer a script of standardized follow-up questions regarding survival outcome data (Supplementary File 1). Clients were asked what the patient's activity level was before hospitalization, if they returned to a prior level of activity after discharge, if they had additional treatments, and if the patient experienced any adverse effects. Outcome information between groups was compared with a commercial software program (GraphPad Prism 8.0, GraphPad Software, San Diego, CA) using Fisher's exact test with a p < 0.05 considered statistically significant.
Results
Signalment
The signalment demographics for both meropenem and gentamicin IVRLP groups are displayed in Table 1. The signalment, including age, sex, and breed, is similar between the meropenem and gentamicin groups.

Table 1. Demographic information for horses administered either meropenem or gentamicin via intravenous regional limb perfusion (IVRLP).
Diagnosis
Meropenem
The diagnoses in the meropenem group are displayed in Table 2. These diagnoses were due to trauma (n = 9), iatrogenic causes (n = 8), unknown reasons (n = 4), or presumptive hematogenous bacterial spread (n = 2). These conditions affected the horses' left hind (n = 13), right hind (n = 6), left front (n = 2), and right front (n = 2) limbs. Of the 15 horses that had infected joints, the metatarsal/metacarpal-phalangeal joint (n = 5), the distal interphalangeal joint (n = 4), the proximal interphalangeal joint (n = 3), and the tarsocrural joint (n = 3) were affected. Nineteen horses were hospitalized for their diagnosis once, but others were admitted for a second (n = 3) and third (n = 1) hospitalization due to re-occurrence of infection.
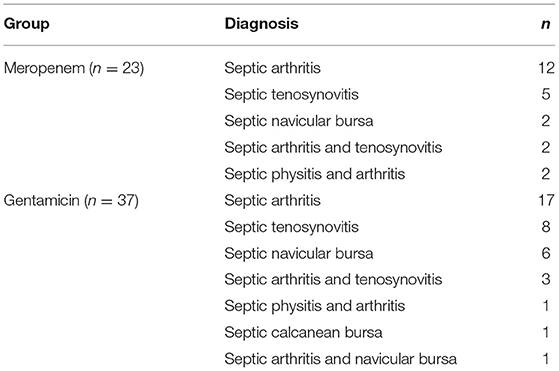
Table 2. Diagnoses for horses administered either meropenem or gentamicin via intravenous regional limb perfusion (IVRLP).
Gentamicin
The diagnoses of the 37 equine patients receiving gentamicin IVRLP are displayed in Table 2. These diagnoses were due to trauma (n = 21), unknown reasons (n = 9), iatrogenic causes (n = 4), or presumptive hematogenous bacterial spread (n = 3). These conditions affected the horses' left hind (n = 14), right hind (n = 9), left front (n = 7), right front (n = 5) limbs, or more than one limb (n = 2). Of the 22 horses that had infected joints, the distal interphalangeal joint (n = 9), the metatarsal/metacarpal-phalangeal joint (n = 4), multiple joints (n = 4), the proximal interphalangeal joint (n = 3), or the tarsocrural joint (n = 2) were affected. Thirty-five of the horses were hospitalized for their diagnosis once, but others were admitted for a second (n = 2) hospitalization.
Diagnostics
Meropenem
Diagnostic procedures performed in the meropenem group include: radiographs (n = 20), culture and susceptibility (n = 15), ultrasound (n = 14), cytology (n = 10), computed tomography (CT, n = 1), magnetic resonance imaging (MRI, n = 1), and nuclear scintigraphy (n = 1). Radiographic reports revealed soft tissues swelling (n = 9), normal anatomy (n = 5), changes to osseous structures (n = 4), and penetrating nails (n = 2). Ultrasound reports were not provided in the records. CT was used to diagnose inflammatory/septic tenosynovitis of the left tarsal sheath in one equine patient. MRI was used to diagnose moderate collateral sesamoidean ligament desmopathy and moderate distal interphalangeal joint effusion in another equine patient in the study.
Ten of the 23 horses in the meropenem group had synovial fluid evaluated by cytology, with fourteen samples total. Structures sampled included joint fluid (n = 11) and tendon sheath fluid (n = 3). Cytology data for the meropenem group is displayed in Table 3. Fifteen horses had joint or synovial fluid submitted for culture, with a total of 16 samples. Of the 15 horses with cultures, eight cases received culture results before starting meropenem whereas seven received the results after already starting meropenem. The susceptibility data showed that out of 16 bacterial isolates with sensitivities reported, 15 were reported as sensitive to imipenem, 13 to ceftiofur, 11 to ampicillin, eight to amikacin, and five to gentamicin. All 15 of the isolates sensitive to imipenem were also sensitive to other antibiotics. Culture and susceptibility results for horses treated with meropenem IVRLP are included by case in Table 4.
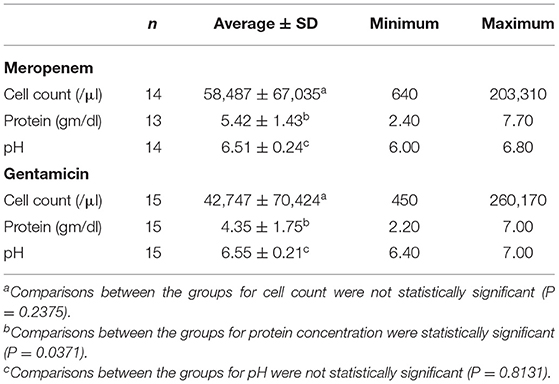
Table 3. Synovial fluid characteristics for groups of horses receiving meropenem (upper) and gentamicin (lower) via intravenous regional limb perfusion (IVRLP).
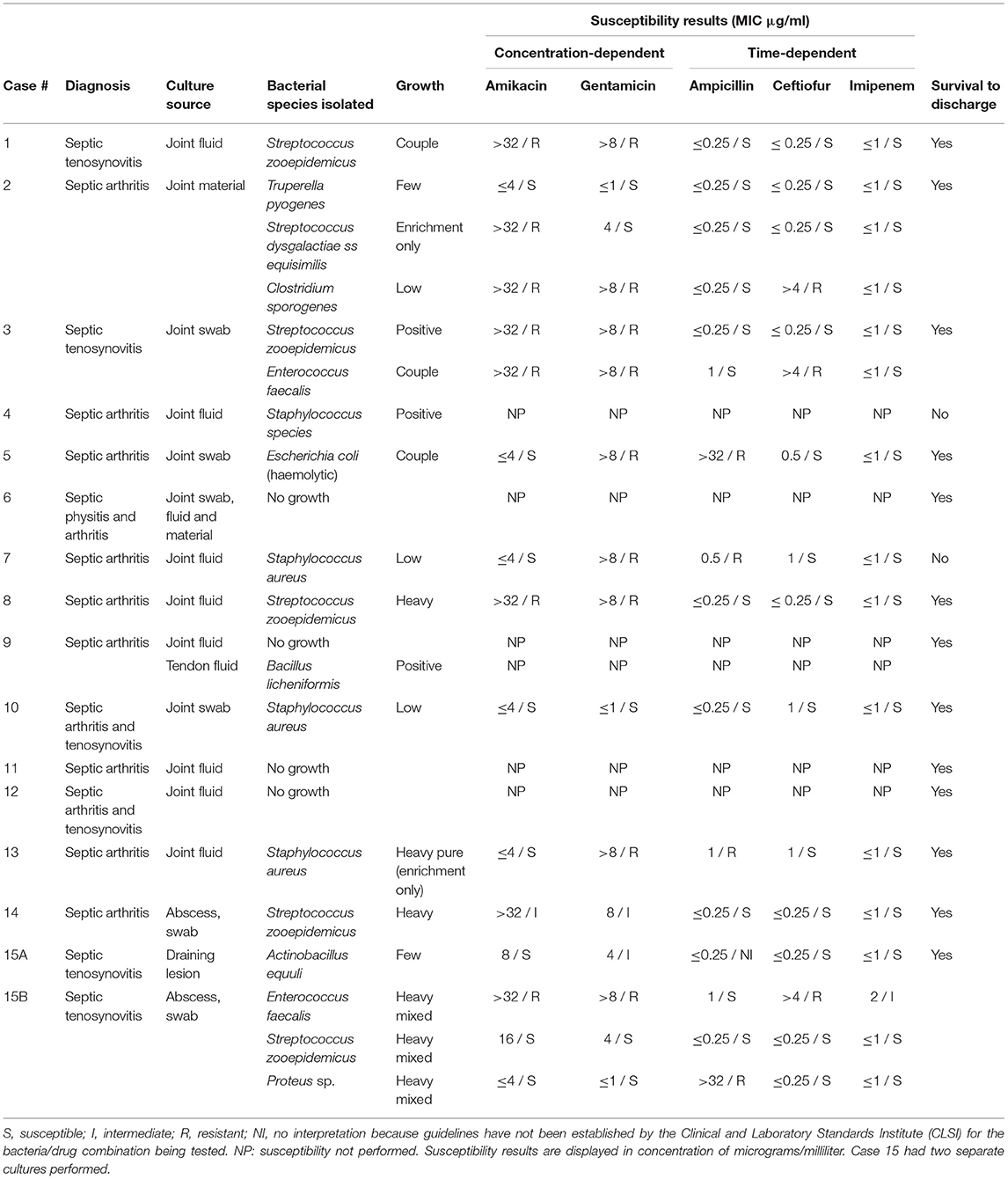
Table 4. Bacterial culture and susceptibility results of horses treated with Meropenem via intravenous regional limb perfusion with growth characteristics as reported by the laboratory.
Gentamicin
Diagnostic procedures performed in the gentamicin group include: radiographs (n = 31), culture and susceptibility (n = 13), ultrasound (n = 13), cytology (n = 12), and MRI (n = 1). Radiographic reports revealed soft tissues swelling (n = 17), penetrating wounds (n = 6), changes to boney structures (n = 6), and normal anatomy (n = 2). Ultrasound reports were not provided in the records for the gentamicin group. MRI was used to diagnose a laceration of the soft tissues proximolateral to the left metatarsophalangeal joint in one equine patient in the study.
Twelve of the 37 gentamicin group horses had synovial fluid evaluated by cytology, with seventeen samples total. Structures sampled included joint fluid (n = 10) and tendon sheath fluid (n = 7). Cytology data for the gentamicin group is displayed in Table 3. The susceptibility data showed that, out of ten bacterial isolates with sensitivities reported, nine were reported by the laboratory as sensitive to imipenem, nine to ceftiofur, seven to amikacin, seven to gentamicin, and seven to ampicillin. All nine of the isolates sensitive to imipenem were also sensitive to other antibiotics. Culture and susceptibility results for horses treated with gentamicin IVRLP are included by case in Table 5.
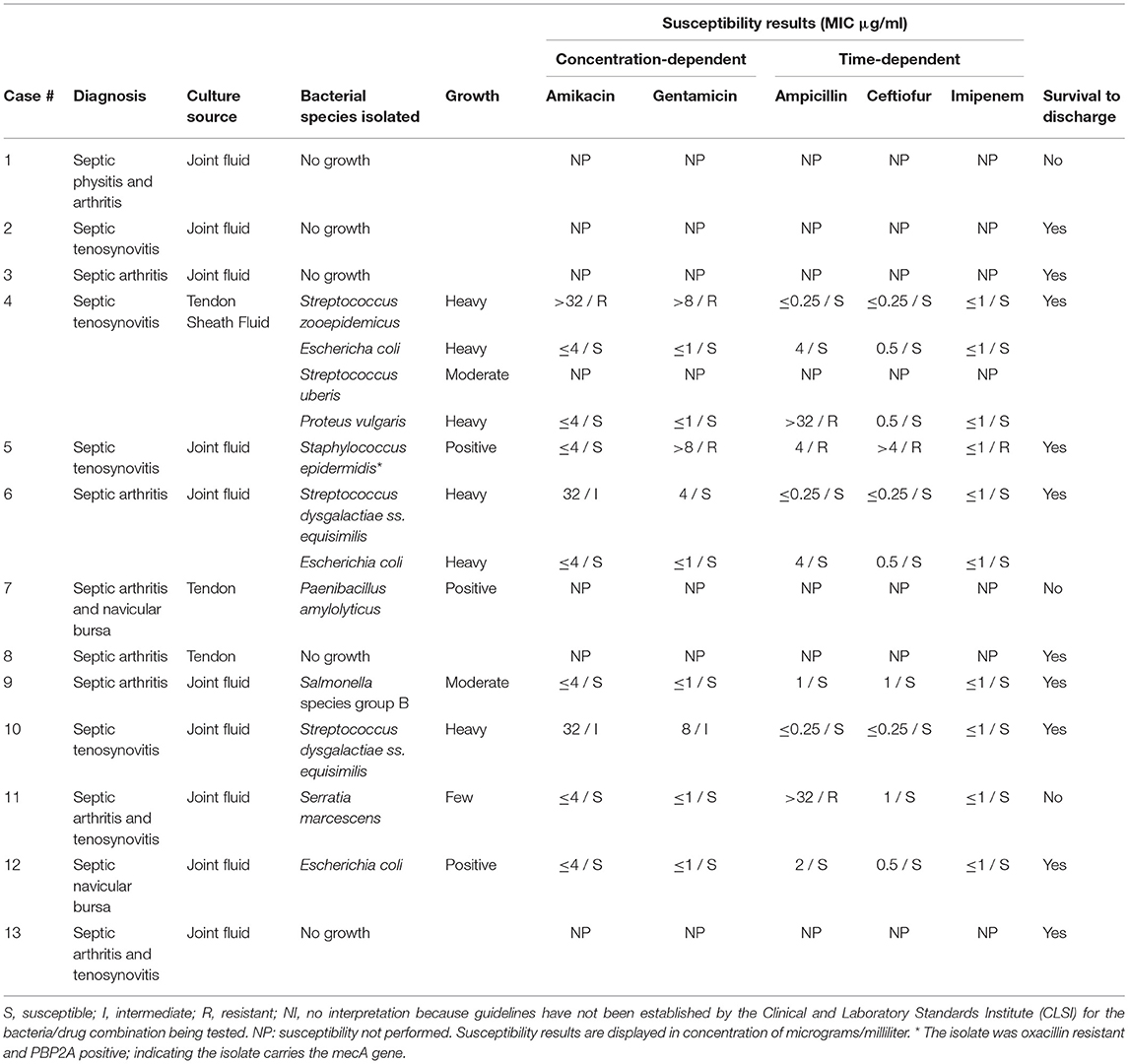
Table 5. Bacterial culture and susceptibility results of horses treated with Gentamicin via intravenous regional limb perfusion with growth characteristics as reported by the laboratory.
Comparisons
Comparisons between groups for cell count of fluid submitted for cytology revealed no significant differences (meropenem: 58,487 ± 67,035 cells/μl; gentamicin: 42,747 ± 70,424; p = 0.2375). Comparisons of protein concentration revealed a significant difference (meropenem: 5.42 ± 1.43 gm/dL; gentamicin: 4.35 ± 1.75; p = 0.0371). Comparisons of joint fluid pH revealed no significant differences (meropenem: 6.51 ± 0.24; gentamicin: 6.55 ± 0.21; p = 0.8131).
Treatment Regimen
Meropenem
Each of the 23 horses in the meropenem group was treated with at least one IVRLP of the affected limb, with the median number of IVRLP with meropenem being 2 (range 1–7). Horses received IVRLP with other medications, such as gentamicin (n = 12), amikacin (n = 1), or both gentamicin and amikacin (n = 2) before switching to meropenem. There were also nine horses that received meropenem as the first drug given via IVRLP. Of the 15 horses that switched to meropenem, the reasons for the change in therapy included: showing no improvement with other treatments (n = 7), not specifically stated (n = 5), or culture and susceptibility results (n = 3).
All of the meropenem group IVRLP were performed using 1 gram (one vial) of meropenem, combined with saline and/or mepivacaine. Dilution and application information is present in Supplementary File 2.
Of the 23 horses in the meropenem group, all of them received systemic antibiotics as well as anti-inflammatories. Concurrently administered antibiotics for the meropenem IVRLP group is displayed in Table 6. The concurrently administered anti-inflammatories for the meropenem IVRLP group are displayed in Table 7. Surgery reports were also evaluated for the 16 cases that underwent a surgical procedure while hospitalized. These procedures included through-and-through needle lavage only (n = 5), arthroscopy/tenoscopy and lavage (n = 6), lavage and debridement (n = 4), and arthrodesis (n = 1). All 16 of these procedures were done under general anesthesia.
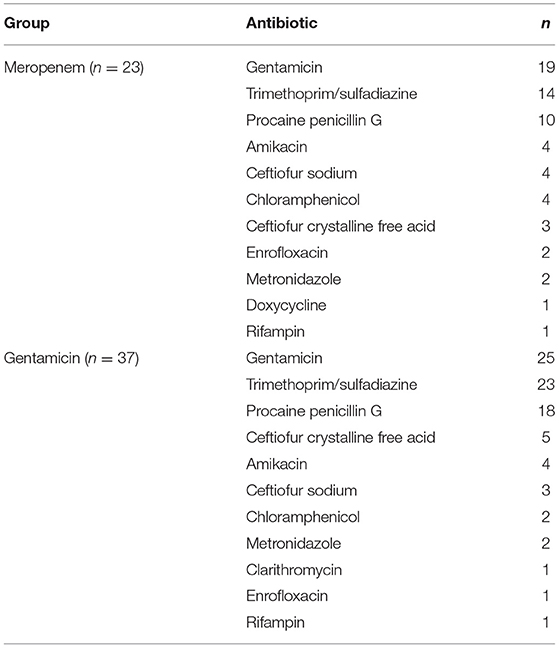
Table 6. Concurrently administered antimicrobials for horses administered either meropenem or gentamicin via intravenous regional limb perfusion (IVRLP).
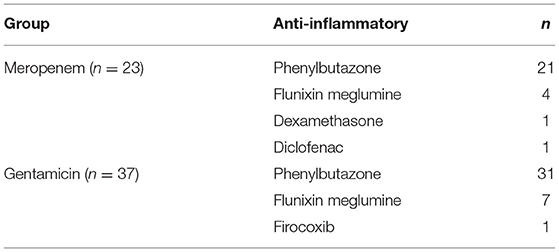
Table 7. Concurrently administered anti-inflammatories for horses administered either meropenem or gentamicin via intravenous regional limb perfusion (IVRLP).
Gentamicin
Of the IVRLP performed using gentamicin, all received at least one IVRLP with gentamicin, with a median number of gentamicin treatments being 3 (range 1 to 11). All were combined with saline and/or mepivacaine. Dilution and application information is present in Supplementary File 2.
Of the 37 horses in the gentamicin group, all 37 of them received systemic antibiotics as well as anti-inflammatories. Concurrently administered antibiotics for the gentamicin IVRLP group is displayed in Table 6. The concurrently administered anti-inflammatories for the gentamicin IVRLP group is displayed in Table 7. Surgery reports were also evaluated for the 19 cases that underwent a surgical procedure while hospitalized. These procedures included through-and-through needle lavage only (n = 10), arthroscopy/tenoscopy only (n = 3), navicular bursotomy (n = 3), sequestrum removal and through-and-through needle lavage (n = 2), and through-and-through needle lavage and wound debridement (n = 1). All but one of these procedures were done under general anesthesia, with one exception, which was performed with an abaxial distal limb block.
Outcomes
Meropenem
Twenty-one of the 23 horses (91%) in the meropenem group survived to discharge, but two were euthanized in hospital due to poor response to intervention. The two horses that were euthanized prior to discharge were diagnosed with septic arthritis of the distal interphalangeal joint and septic arthritis of the proximal interphalangeal joint. These horses were treated for 12 and 29 days respectively. Adverse effects due to some aspect of the treatment regimen or hospitalization was noted in four patients, who experienced colitis (n = 3) and impaction colic (n = 1). The other 19 patients did not have any adverse effects during their stay in the hospital according to the medical records.
Follow-up was obtained from 11 cases in the meropenem group. Of these, seven reported that their horse was sound enough to return to normal activity, three to reduced activity, and one was retired completely. For the 10 horses that were able to return to work, it took an average of 9 +/– 4 months to return to work. Eight of these horses require treatments, such as anti-inflammatory (n = 4), steroid joint injections (n = 3), topical ointments (n = 2), and antibiotics (n = 1), as maintenance. One of these owners reported recurrence of the infection, four reported problems with wound healing and excessive scarring, and two reported arthritis related to the injury. After leaving the hospital two owners noticed the adverse effects of diarrhea (n = 2).
Gentamicin
Thirty-one of the 37 horses (84%) in the gentamicin group survived to discharge, but six were euthanized in hospital due to poor prognosis. The six horses that were euthanized prior to discharge were diagnosed with septic arthritis of the distal interphalangeal joint (n = 2), septic physitis and arthritis (n = 1), septic arthritis of the metatarsal-phalangeal joint (n = 1), septic arthritis and septic navicular bursa (n = 1), and septic arthritis and tenosynovitis (n = 1). The time to euthanasia was on average five days, ranging from one to 11 days. Adverse effects due to some aspect of the treatment regimen was noted in only five patients, who experienced colitis (n = 4) and impaction colic (n = 1). The other 32 patients did not have any adverse effects during their stay in the hospital according to the medical records.
Follow-up was obtained from 22 cases in the gentamicin group; of these, 12 reported that their horse was sound enough to return to normal activity, six to reduced activity, and four were retired completely. For the 18 horses that were able to return to work, it took an average of 7.69 +/– 4.46 months to return to work. Five of these horses require treatments, such as anti-inflammatories (n = 3), antibiotics (n = 1) and corrective shoeing (n = 1), as maintenance. Two of these owners reported recurrence of the infection, seven reported problems with wound healing and excessive scar tissue, and two reported arthritis related to the injury. After discharge from the hospital no owners noticed adverse effects.
Comparisons
The overall survival to discharge was 86% (52/60), with meropenem being 91% (21/23) and gentamicin being 84% (31/37). The differences in survival to discharge between groups were not statistically significant (p = 0.6983). When the outcomes of the horses that were contacted in each group were compared no statistically significant differences were detected between the meropenem and gentamicin groups with respect to return to work (meropenem: 7/11; gentamicin 12/22; p = 0.7193), reduced work (meropenem: 3/11; gentamicin: 6/22; p = 1) or retired/euthanized (meropenem: 1/11; gentamicin: 4/22; p = 0.6431). When horses with only positive culture results were compared within each group, no statistically significant differences in outcome were noted (Supplementary File 3).
Discussion
Our study presents the clinical data and outcome for two groups of horses that had treatment augmented with IVRLP meropenem or gentamicin for diagnosis of orthopedic sepsis. While each clinical case is unique and workup can vary based on client resources, case presentation and clinician, the diagnoses were supported by either culture information, altered synovial fluid characteristics, diagnostic imaging, or a combination. Cytology data showed that the average cell counts and protein levels were well-above the normal value range reported to be <1,000 cell/μl (29). Infected synovial structures generally have over 30,000 cells/μl (28), which was seen in both groups. At a total protein over 3.0–4.0 g/dl the structure is likely infected (28, 29), which was the case for both groups in our study. While there were significant differences in the synovial fluid concentrations of both groups (meropenem: 5.42 g/dl; gentamicin: 4.35 g/dl; p = 0.0371) it is important to note that both groups had elevated total protein values which were consistent with infection (28).
Cases that had septic synovitis secondary to a penetrating wound was frequently the metacarpo/metatarsophalangeal joint which is consistent with other reports (11). According to previous studies, the most common infected joint due to trauma was the fetlock, which is similar to findings in this study (22). The majority of bacterial species isolated from cultures of meropenem treated horses were common bacterial species, similar to those found in other reports septic synovial structures (20). Positive cultures in this study also contained less commonly reported bacterial species in equine septic synovitis (e.g., Actinobacillus equuli), creating a diverse group. Trauma was the most commonly identified cause of infection for both gentamicin and meropenem groups, many of which were due to lacerations. The limbs that were affected were also similar in both groups, with the left hind limb having the most infections. Nine horses received meropenem as the first antibiotic given via IVRLP, meaning none of the other possible drugs were attempted first. For the 15 cases where other antibiotics were tried first, only three stated they switched due to results of culture. Almost half of the meropenem horses that had samples submitted for culture received the treatment before culture results were reported. At the institution represented in this study, protocols existed restricting the use of carbapenems to the rare use for severe, life threatening conditions in individual animals when culture and susceptibility testing indicates that it is the only suitable option, however, our results clearly indicate that in many of the reported cases, the necessary criteria for carbapenem use was not met. Retrospective analysis of antimicrobial use practices within the institution for adherence to this policy identified the frequent use of meropenem for IVRLP in horses and prompted the retrospective analysis presented here.
Of the 26 bacterial isolates obtained from culture in either group, 24 displayed MIC ≤1 μg/mL for imipenem and were reported by the laboratory as sensitive. In previous studies, when administered by IVRLP to healthy horses, the concentration of imipenem in the fetlock joint peaked at 60–87 μg/ml and was above 1 μg/ml for roughly 6 h at a 0.5 gm dose (16), suggesting that imipenem may be a viable antibiotic choice in most of these cases. While laboratory-reported susceptibilities to meropenem were not available, studies comparing treatment with meropenem to imipenem found no significant difference in the outcome of serious infections in humans (30). These two antibiotics also exhibit similar in vitro activity and pharmacokinetics (30). All carbapenems, including both meropenem and imipenem, typically produce MIC values of ≤ 1 μg/dl against the commonly isolated susceptible Gram-positive and Gram-negative bacteria in this study such as Staphlyococcus sp., Streptococcus sp., and E. coli. Therefore, extrapolation between imipenem testing, which is routinely tested for using commercially available antimicrobial susceptibility testing panels for equine patients in the United States, for clinical use of meropenem is reasonable (30), although future work is necessary to verify this extrapolation. While meropenem has been shown to have a variable synovial fluid concentration in healthy horses after IVRLP that may not remain above 1 μg/ml for longer than 3–4 h (19), that study used a 0.5 gm dose, and the horses in our study received 1 gram per treatment. While the pharmacokinetics of IVRLP are not as well-known as plasma pharmacokinetics of parenterally administered drugs, it is possible that with an increased IVRLP dosage, an increase of the maximum concentration could be expected, which could allow for higher concentrations to persist for an increased period of time compared to 0.5 gm dosing. Thus, 1 gm dosing may offer improved clinical efficacy over the 0.5 gm dosing that has previously been reported (19). However, laboratory-reported susceptibility to other antibiotics like ceftiofur (n = 22/26), ampicillin (n = 18/26), amikacin (n = 15/26), or gentamicin (n = 12/26) was also frequently present as seen in Table 4.
It is important to note that no clinical breakpoints exist specifically for IVRLP in horses and diagnostic laboratories report susceptible (S)/intermediate (I)/resistant (R) based on data related to systemic treatment with those antibiotics. Therefore, all of the interpretations (susceptible or resistant) that are reported for these cases are extrapolated from clinical breakpoints either for systemic use of the same drug for the same bacteria (only a few of these exist for horses) or, more likely, from breakpoints established in humans or other animal species for similar bacteria. In addition, there are no clinical breakpoints for meropenem or imipenem currently approved in any veterinary species, therefore, all interpretations for this class are extrapolated from human breakpoints. While some of these extrapolated breakpoints may closely align with the true epidemiologic cut-off value for non-wildtype (i.e., have acquired resistance genes) for the bacteria of interest, others may not and may be heavily influenced by the pharmacokinetics of the drug in a specific species. Therefore, the confidence level in these interpretations is unfortunately and represents a significant limitation to this study and to many other similar studies in veterinary medicine (31). The use of IVRLP, which leads to significantly higher concentrations at the site of infection when compared to systemic therapy, may be able to overcome increased MIC values which would be otherwise be classified as resistant in some cases. The antimicrobial susceptibility interpretations in this study were reported as the clinician would have seen them, based on what the lab reported, which may also vary from laboratory to laboratory based on which extrapolations are used in each case. In addition, in many of the cases IVRLP was performed with both an antibiotic as well as mepivacaine; while previous studies have shown no effect on antimicrobial activity of amikacin (an aminoglycoside similar to gentamicin) when combined with mepivacaine, no similar studies have been performed using meropenem (32). While the commonly isolated bacteria from equine synovial infections are typically susceptible to the mechanism of action of meropenem, as demonstrated in this study, resistance to meropenem is possible with strains of methicillin-resistant Staphylococcus aureus (MRSA) and carbapenemase-resistant Enterobacteriaceae (CRE) such as Escherichia coli, (33), therefore clinicians should also consider the efficacy of other antibiotics while making decisions on a IVRLP treatment plan (30).
Adverse effects were noted in four horses treated with meropenem but it cannot be determined if this was from concurrent clinical disease, this treatment, or from other medication given. The severe side effects seen in humans given meropenem, such as seizures (34), were not seen in this population. Additionally, the adverse effects of IVRLP of antibiotics described in other species and humans, such as venous scaring, transient pyrexia, thrombosis, bruising, and phlebitis were not observed or noted in the medical records examined in this study (25–27, 35). This lack of adverse effects are similar to what was previous found in a prospective study using healthy horses (19). The overall survival to discharge was 86% (52/60), with meropenem being 91% (21/23) and gentamicin being 84% (31/37). A previous study using various treatments in adult horses had an overall survival of discharge of 85%, very similar to both of the groups in this study (21). Due to the lack of owner response, outcomes were not determined for all horses enrolled in the study. Despite this, no significance differences were found between the outcome of the two groups, with both groups showing mostly positive outcomes. With these outcomes in mind, while meropenem may be an effective antibiotic for septic synovial structures, other more judicious antibiotic treatments may produce similar outcomes.
This study had several additional limitations. The number of horses receiving meropenem via IVRLP at the teaching hospital was limited so only a small population of horses were able to be enrolled in the meropenem group. The retrospective nature of this study is an additional limitation. There is also variability in the clinician treatment protocols, including IVRLP procedure, for the horses enrolled in the study. A variation in clinical presentation is also present, as horses with different stages of infection at different anatomical locations were compared. Not all cases had culture or cytology data so some were diagnosed with diagnostic imaging only, leading to an additional weakness. Variation in ancillary therapies is also a limitation. All of the horses enrolled in the study received systemic antibiotic treatment along with the IVRLP treatment, which limits the ability to identify the complete efficacy of the single agent in the specified treatment groups. Future studies could include multiple institutions, which would allow for more horses to be compared. Also, investigations evaluating specific bacterial diagnoses and treatment protocols would also help to reduce some of the variability for future work.
In conclusion, based on bacterial antimicrobial susceptibility testing and case outcomes, antimicrobial therapy augmented with IVRLP using either meropenem or gentamicin both appear to be an effective treatment for septic synovial structures. Although this study found treatment with meropenem may be effective, treatment with other antimicrobials of lesser critical importance in human medicine is the more ideal choice from an antimicrobial stewardship perspective. Bacterial species may be susceptible to other antibiotics, such as gentamicin, which showed similar outcomes in this study. Restricted antimicrobials, like meropenem, should only be used when the described guidelines, such as proven resistance to less restricted antimicrobials and/or a life-threatening case severe enough to outweigh the risks, are met. If another treatment can lead to a similar outcome, it is preferable option in order to help reduce the development of resistance to critically important antibiotics such as the carbapenems.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
AM conceptualized the study, analyzed patient records as well as results, followed up with clients regarding outcomes, and constructed the manuscript. JS, DT, JT, and AK conceptualized the study, analyzed results, and constructed the manuscript. All authors contributed to and approved the final manuscript.
Funding
AM stipend for this work was supported by the Iowa State University Summer Scholar program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to acknowledge Deanna Collins, medical record technologist, for her invaluable assistance in obtaining and identifying the records of this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.629627/full#supplementary-material
References
1. Kawano S, Matsumoto K, Hara R, Kuroda Y, Ikawa K, Morikawa N, et al. Pharmacokinetics and dosing estimation of meropenem in Japanese patients receiving continuous venovenous hemodialysis. J Infect Chemother. (2015) 21:476–8. doi: 10.1016/j.jiac.2015.02.011
2. Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother. (2011) 55:4943–60. doi: 10.1128/AAC.00296-11
3. Bidgood T, Papich MG. Plasma pharmacokinetics and tissue fluid concentrations of meropenem after intravenous and subcutaneous administration in dogs. Am J Vet Res. (2002) 63:1622–8. doi: 10.2460/ajvr.2002.63.1622
4. Smith JS, Borts DJ, Slagel CC, Rajewski SM, Bousquet-Melou A, Ferran AA, et al. Pharmacokinetics of Ertapenem in Sheep (Ovis aries) with Experimentally Induced Urinary Tract Infection. Comp Med. (2019) 69:413–8. doi: 10.30802/AALAS-CM-18-000144
6. Scott HM, Acuff G, Bergeron G, Bourassa MW, Gill J, Graham DW, et al. Critically important antibiotics: criteria and approaches for measuring and reducing their use in food animal agriculture. Ann N Y Acad Sci. (2019) 1441:8–16. doi: 10.1111/nyas.14058
7. Gibson KT, McIlwraith CW, Turner AS, Stashak TS, Aanes WA, Trotter GW. Open joint injuries in horses: 58 cases (1980–1986). J Am Vet Med Assoc. (1989) 194:398–404.
8. Gilbertie JM, Schnabel LV, Stefanovski D, Kelly DJ, Jacob ME, Schaer TP. Gram-negative multi-drug resistant bacteria influence survival to discharge for horses with septic synovial structures: 206 Cases (2010–2015). Vet Microbiol. (2018) 226:64–73. doi: 10.1016/j.vetmic.2018.10.009
9. Palmer S, Hogan P Vmd. How to perform regional limb perfusion in the standing horse. Proc Am Assoc Equine Pract. (1999) 45:124–7.
10. Rubio-Martínez LM, Cruz AM. Antimicrobial regional limb perfusion in horses. J Am Vet Med Assoc. (2006) 228:706–12, 655. doi: 10.2460/javma.228.5.706
11. Dahan R, Oreff GL, Tatz AJ, Raz T, Britzi M, Kelmer G. Pharmacokinetics of regional limb perfusion using a combination of amikacin and penicillin in standing horses. Can Vet J. (2019) 60:294–9.
12. Hyde RM, Lynch TM, Clark CK, Slone DE, Hughes FE. The influence of perfusate volume on antimicrobial concentration in synovial fluid following intravenous regional limb perfusion in the standing horse. Can Vet J. (2013) 54:363–7.
13. Rubio-Martínez LM, López-Sanromán J, Cruz AM, Santos M, Andrés MS, Román FS. Evaluation of safety and pharmacokinetics of vancomycin after intravenous regional limb perfusion in horses. Am J Vet Res. (2005) 66:2107–13. doi: 10.2460/ajvr.2005.66.2107
14. Parra-Sanchez A, Lugo J, Boothe DM, Gaughan EM, Hanson RR, Duran S, et al. Pharmacokinetics and pharmacodynamics of enrofloxacin and a low dose of amikacin administered via regional intravenous limb perfusion in standing horses. Am J Vet Res. (2006) 67:1687–95. doi: 10.2460/ajvr.67.10.1687
15. Kelmer G, Tatz AJ, Famini S, Bdolah-Abram T, Soback S, Britzi M. Evaluation of regional limb perfusion with chloramphenicol using the saphenous or cephalic vein in standing horses. J Vet Pharmacol Ther. (2015) 38:35–40. doi: 10.1111/jvp.12140
16. Kelmer G, Tatz AJ, Kdoshim E, Britzi M, Segev G. Evaluation of the pharmacokinetics of imipenem following regional limb perfusion using the saphenous and the cephalic veins in standing horses. Res Vet Sci. (2017) 114:64–8. doi: 10.1016/j.rvsc.2017.02.020
17. Nieto JE, Trela J, Stanley SD, Yamout S, Snyder JR. Pharmacokinetics of a combination of amikacin sulfate and penicillin G sodium for intravenous regional limb perfusion in adult horses. Can J Vet Res. (2016) 80:230–5.
18. Cox KS, Nelson BB, Wittenburg L, Gold JR. Plasma, subcutaneous tissue and bone concentrations of ceftiofur sodium after regional limb perfusion in horses. Equine Vet J. (2017) 49:341–4. doi: 10.1111/evj.12614
19. Fontenot RL, Langston VC, Zimmerman JA, Wills RW, Sloan PB, Mochal-King CA. Meropenem synovial fluid concentrations after intravenous regional limb perfusion in standing horses. Vet Surg. (2018) 47:852–60. doi: 10.1111/vsu.12940
20. Motta RG, Martins LSA, Motta IG, Guerra ST, Paula CLd, Bolanos CAD, et al. Multidrug resistant bacteria isolated from septic arthritis in horses. Pesquisa Veterinária Brasileira. (2017) 37:325–30. doi: 10.1590/s0100-736x2017000400005
21. Schneider RK, Bramlage LR, Moore RM, Mecklenburg LM, Kohn CW, Gabel AA. A retrospective study of 192 horses affected with septic arthritis/tenosynovitis. Equine Vet J. (1992) 24:436–42. doi: 10.1111/j.2042-3306.1992.tb02873.x
22. Ludwig EK, van Harreveld PD. Equine wounds over synovial structures. Vet Clin North Am Equine Pract. (2018) 34:575–90. doi: 10.1016/j.cveq.2018.07.002
23. Langston VC, Fontenot RL, Byers JA, Andrews CM, Mochal-King CA. Plasma and synovial fluid pharmacokinetics of a single intravenous dose of meropenem in adult horses. J Vet Pharmacol Ther. (2019) 42:525–9. doi: 10.1111/jvp.12770
24. Wiseman LR, Wagstaff AJ, Brogden RN, Bryson HM. Meropenem. Drugs. (1995) 50:73–101. doi: 10.2165/00003495-199550010-00007
25. Knafo SE, Graham JE, Barton BA. Intravenous and intraosseous regional limb perfusion of ceftiofur sodium in an avian model. Am J Vet Res. (2019) 80:539–46. doi: 10.2460/ajvr.80.6.539
26. Spelman L. Regional Digital Intravenous Perfusion In An African Elephant (Loxodonta Africana) IAAAM. 2000.
27. Agarwal P, Agrawal P, Sharma D, Baghel K. Intravenous infusion for the treatment of diabetic and ischaemic non-healing pedal ulcers. J Eur Acad Dermatol Venereol. (2005) 19:158–62. doi: 10.1111/j.1468-3083.2005.01058.x
28. Miagkoff L, Archambault M, Bonilla AG. Antimicrobial susceptibility patterns of bacterial isolates cultured from synovial fluid samples from horses with suspected septic synovitis: 108 cases (2008-2017). J Am Vet Med Assoc. (2020) 256:800–7. doi: 10.2460/javma.256.7.800
29. Getman LM, Sutter WW, Bertone AL. Infections of muscle, joint, and bone. In: Debra C. Sellon MTL, editor. Equine Infectious Diseases. 2nd ed. WB Saunders 2014. p. 60–70.
30. Zhanel GG, Simor AE, Vercaigne L, Mandell L. Canadian Carbapenem Discussion Group. Imipenem and meropenem: Comparison of in vitro activity, pharmacokinetics, clinical trials and adverse effects. Can J Infect Dis. (1998) 9:215–28. doi: 10.1155/1998/831425
31. CLSI. Understanding Susceptibility Test Data as a Component of Antimicrobial Stewardship in Veterinary Settings. Wayne, PA: CLSI 2019.
32. Colbath AC, Wittenburg LA, Gold JR, McIlwraith CW, Moorman VJ. The effects of mepivacaine hydrochloride on antimicrobial activity and mechanical nociceptive threshold during amikacin sulfate regional limb perfusion in the horse. Vet Surg. (2016) 45:798–803. doi: 10.1111/vsu.12515
33. Davis JL, editor. Update on Antimicrobial Drugs in Horses. St. Louis, MO: W.B. Saunders. (2014).
34. Lee YC, Huang YJ, Hung MC, Hung SC, Hsiao CY, Cho HL, et al. Risk factors associated with the development of seizures among adult patients treated with ertapenem: a matched case-control study. PLoS ONE. (2017) 12:e0182046. doi: 10.1371/journal.pone.0182046
Keywords: antibiotics, carbapenem, equine, gentamicin, meropenem, septic synovitis, regional limb perfusion
Citation: Mosichuk AP, Smith JS, Tatarniuk DM, Troy JR and Kreuder AJ (2021) Meropenem Administered via Intravenous Regional Limb Perfusion for Orthopedic Sepsis in Horses: A Clinical Retrospective Study. Front. Vet. Sci. 8:629627. doi: 10.3389/fvets.2021.629627
Received: 15 November 2020; Accepted: 16 February 2021;
Published: 26 March 2021.
Edited by:
Deirdre P. Campion, University College Dublin, IrelandReviewed by:
Christopher Bruce Riley, Massey University, New ZealandNaomi Elisabeth Crabtree, University of Georgia, United States
Copyright © 2021 Mosichuk, Smith, Tatarniuk, Troy and Kreuder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph S. Smith, animal197@gmail.com
 Allison P. Mosichuk
Allison P. Mosichuk Joseph S. Smith
Joseph S. Smith Dane M. Tatarniuk
Dane M. Tatarniuk Jarrod R. Troy
Jarrod R. Troy Amanda J. Kreuder
Amanda J. Kreuder