Competition between Silicifiers and Non-silicifiers in the Past and Present Ocean and Its Evolutionary Impacts
- 1Department of Earth Sciences, Bristol University, Bristol, United Kingdom
- 2Department of Applied Mathematics and Theoretical Physics, Centre for Mathematical Sciences, University of Cambridge, Cambridge, United Kingdom
- 3Institut de Biologie de l'Ecole Normale Supérieure, Ecole Normale Supérieure, Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, PSL Research University, Paris, France
- 4Department of Geology, Lund University, Lund, Sweden
- 5Stellenbosch Institute for Advanced Study, Stellenbosch, South Africa
- 6Institut Pierre-Simon-Laplace, Laboratoire des Sciences du Climat et de l'Environnement, Gif-sur-Yvette, France
- 7Aix Marseille Univ, Université de Toulon, Centre National de la Recherche Scientifique/INSU, IRD, MIO, UM 110, Marseille, France
Competition is a central part of the evolutionary process, and silicification is no exception: between biomineralized and non-biomineralized organisms, between siliceous and non-siliceous biomineralizing organisms, and between different silicifying groups. Here we discuss evolutionary competition at various scales, and how this has affected biogeochemical cycles of silicon, carbon, and other nutrients. Across geological time we examine how fossils, sediments, and isotopic geochemistry can provide evidence for the emergence and expansion of silica biomineralization in the ocean, and competition between silicifying organisms for silicic acid. Metagenomic data from marine environments can be used to illustrate evolutionary competition between groups of silicifying and non-silicifying marine organisms. Modern ecosystems also provide examples of arms races between silicifiers as predators and prey, and how silicification can be used to provide a competitive advantage for obtaining resources. Through studying the molecular biology of silicifying and non-silicifying species we can relate how they have responded to the competitive interactions that are observed, and how solutions have evolved through convergent evolutionary dynamics.
Introduction
Biomineralization refers to the precipitation of minerals by living organisms (Simkiss and Wilbur, 1989). It may occur as a by-product of the normal metabolism of the organism under indirect genetic control—related to the cellular processes that create the conditions for incidental biomineral formation—and with no pre-concentration of specific mineral ions. Alternatively, the composition of the biominerals formed can be entirely dependent on the environmental conditions, for example, the formation of iron oxide by brown algae (Lee and Kugrens, 1989). By contrast, biologically controlled biomineralization requires direct genetic control, generates characteristically patterned structures, and involves selective uptake and pre-concentration of mineral ions.
In nature, we observe a wide array of biominerals (see Figure 1), ranging from iron oxide to strontium sulfate (Raven and Knoll, 2010), with calcareous biominerals being particularly notable (Knoll, 2003; Knoll and Kotrc, 2015). However, the most taxonomically widespread biomineral is silica (SiO2·nH2O), being present in all eukaryotic supergroups (Marron et al., 2016b). Notwithstanding, the degree of silicification can vary even between closely related taxa, from being found in composite structures with other biominerals (e.g., limpet teeth; Sone et al., 2007), to forming minor structures (e.g., ciliate granules; Foissner et al., 2009) or being a major structural constituent of the organism (Preisig, 1994). The most extreme degree of silicification is evident in the diatoms, where almost all species have an obligate requirement for silicon to complete cell wall formation and cell division (Darley and Volcani, 1969; Martin-Jézéquel et al., 2000). Biogeochemically and ecologically, diatoms are believed to be the most important silicifiers in modern marine ecosystems, with radiolarians (polycystine and phaeodarian rhizarians), silicoflagellates (dictyochophyte and chrysophyte stramenopiles), and sponges with prominent roles as well. In contrast, the major silicifiers in terrestrial ecosystems are the land plants (embryophytes), with other silicifying groups (e.g., testate amoebae) having a minor role.
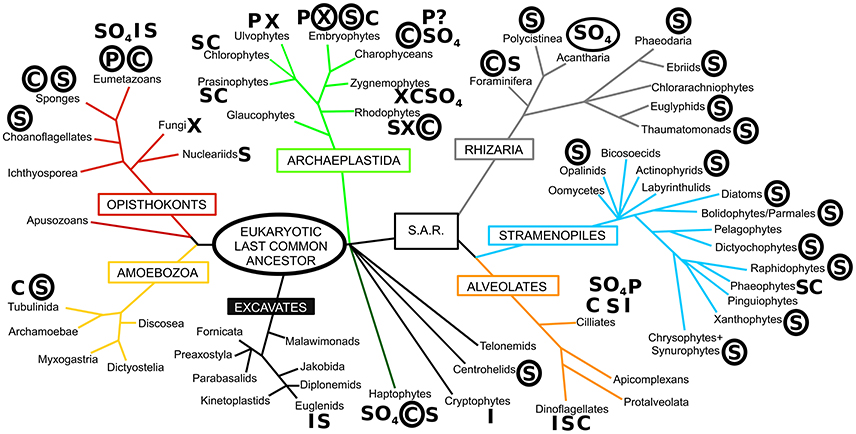
Figure 1. The diversity of biomineralization across the eukaryotes. The phylogeny is based on that of Adl et al. (2012) with major eukaryotic supergroups named in boxes. Letters next to taxon names denote the presence of biomineralization, with circled letters indicating prominent and widespread use of that biomineral. S, silica; C, calcium carbonate; P, calcium phosphate; I, iron (magnetite/goethite); X, calcium oxalate; SO4, sulfates (calcium/barium/strontium), ? denotes uncertainty in report. Based on Ensikat et al. (2016), Gal et al. (2012), Knoll (2003), Knoll and Kotrc (2015), Marron et al. (2016b), Raven and Knoll (2010), Weich et al. (1989), and references therein.
Broadly, biomineralized structures are believed to have evolved and diversified where the energetic cost of biomineral production is less than the expense of producing an equivalent organic structure (Mann, 2001; Raven and Waite, 2004; Finkel and Kotrc, 2010). Raven (1983) calculated that the energetic costs of silicic acid uptake and silica structure formation is substantially more efficient than forming the same volume of an organic structure (~20x more for lignin and 10x for polysaccharides like cellulose). Based on the structural model of biogenic silica of Hecky et al. (1973), Lobel et al. (1996) identified by biochemical modeling a low-energy reaction pathway for nucleation and growth of silica. The combination of organic and inorganic components within biomineralized structures often results in enhanced properties compared to exclusively organic or inorganic materials. With respect to biogenic silica, this can result in the production of much stronger structures, such as siliceous diatom frustules having the highest strength per unit density of any known biological material (Hamm et al., 2003; Aitken et al., 2016), or sponge spicules being many times more flexible than an equivalent structure made of pure silica (Ehrlich et al., 2008; Shimizu et al., 2015). As a result, biogenic silica structures are utilized for support (Weaver et al., 2007), feeding (Nesbit and Roer, 2016), predation defense (Pondaven et al., 2007; Friedrichs et al., 2013; Hartley et al., 2016) and environmental protection as a component of cyst walls (Preisig, 1994). Biogenic silica also has useful optical properties for light transmission and modulation in organisms as diverse as plants (Schaller et al., 2013), diatoms (Fuhrmann et al., 2004; Yamanaka et al., 2008; Romann et al., 2015), sponges (Sundar et al., 2003), and molluscs (Dougherty et al., 2014). There is also evidence that silicification is used as a detoxification response in snails (Desouky et al., 2002) and plants (Neumann and zur Nieden, 2001), sequestering harmful substances such as aluminum and zinc within the biogenic silica to ensure the correct functioning of cellular metabolism. Diatom biosilica has even been suggested to play a role as a pH buffer for the enzymatic activity of carbonic anhydrase, aiding the acquisition of inorganic carbon for photosynthesis (Milligan and Morel, 2002).
The myriad of functions and benefits of biomineralization raises an important question: why do some organisms biomineralize while others do not? Furthermore, why is there such a diversity of biominerals besides silicon, when silicon is so abundant, comprising 28% of the Earth's crust? The answer to these questions lies in the evolutionary interplay between biomineralization and geochemistry, and in the competitive interactions that have arisen from these dynamics. Fundamentally whether an organism produces silica or not involves evolutionary trade-offs and competition between silicifiers themselves, and with non-silicifying organisms (both those which utilize other biominerals, and non-mineralizing groups). Mathematical models and controlled experiments of resource competition in phytoplankton have demonstrated the rise to dominance of different algal species based on nutrient backgrounds in defined media. These have been part of fundamental studies in ecology (Tilman, 1977; Sommer, 1994). However, the vast diversity of organisms that thrive in a complex array of biotic and abiotic interactions in oceanic ecosystems are a challenge to such minimal models and experimental designs, whose parameterization and possible combinations, respectively, limit the interpretations that can be built on them. Here we broadly extend our attention into other types of data from which competition can be inferred: the geological record, the distribution of species in modern marine ecosystems, and phenomena at the cellular and molecular levels (summarized in Table 1).
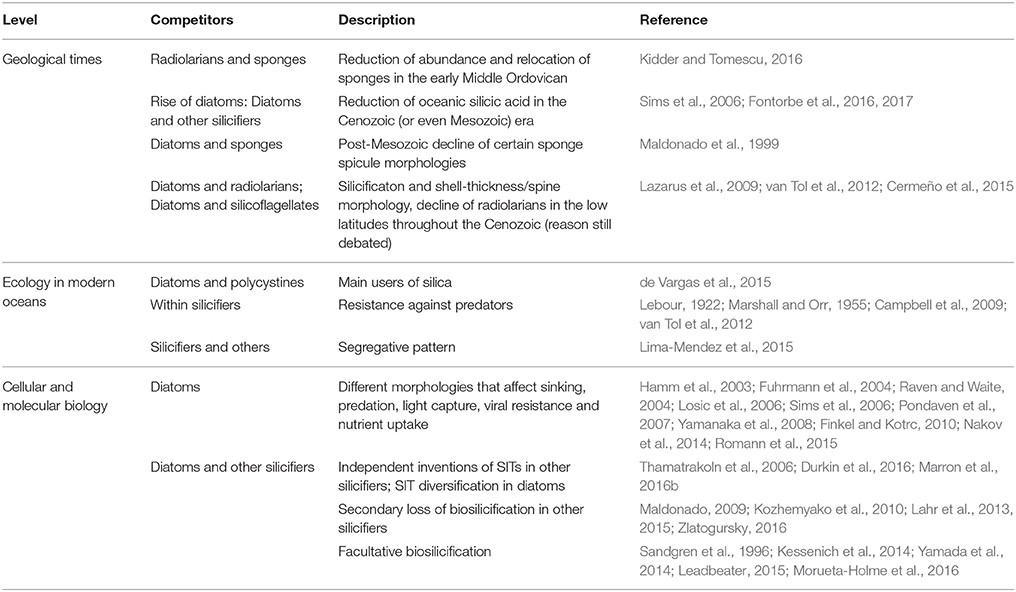
Table 1. Examples of competitive interactions involving the use of silica, as seen from geology, ecology, and cellular/molecular biology.
Evolutionary Competition Across Geological Time
Competition between organisms is usually driven by a limited supply of a commonly used resource. For organisms that are biomineralizers of silica, orthosilicic acid—which we will refer to as silicic acid throughout, to distinguish from cellular silicon—is one such important limiting resource (Tilman et al., 1982). Silicic acid is released by the dissolution of biogenic silica and silicate minerals by biological and chemical weathering processes, and by hydrothermal activity, up to a solubility limit of 1.2–2.2 mM in water depending on the ambient physicochemical properties (Gunnarsson and Arnórsson, 2000). The drawdown of oceanic silicic acid through geological time has been attributed to biological utilization (Siever, 1991) and may have resulted in skeletal changes in marine silicifiers (Maldonado et al., 1999; Racki and Cordey, 2000; Lazarus et al., 2009; Finkel and Kotrc, 2010; Finkel et al., 2010; van Tol et al., 2012). To what extent these changes are a result of competition between silicifiers is still matter of debate.
The evolution of organisms that transport silicon into their cells has a long history (Marron et al., 2016b), likely going back to the evolution of cyanobacteria in the Archean (Dvorák et al., 2014). As silicic acid is a small molecule, and is mostly present in an undissociated form (Si(OH)4) under seawater pH conditions (Del Amo and Brzezinski, 1999), it is possible for silicic acid to diffuse across the cell membrane (Raven, 1983; Thamatrakoln and Hildebrand, 2008). In the high silicic acid Precambrian ocean, diffusion from the surrounding Si-saturated seawater could result in silica precipitating freely within the cytoplasm, interfering with cellular processes and disabling the functioning of the cell. Consequently, silicon transporters are proposed to have arisen initially to prevent intracellular toxicity by removing it from the cytoplasm (Marron et al., 2016b). Recently, cyanobacteria microfossils that contain nanocrystals of greenalite with the approximate composition of Si2O5(OH)4Fe2+ (Lepot et al., 2017) have been found in the 1.88 Ga Gunflint Iron Formation, suggesting Fe biomineralization, which may also have protected oxygenic photosynthesisers against Fe2+ toxicity during the Paleoproterozoic.
It is inferred from the proliferation of biomineralized fossils in the geological record at the end of the Proterozoic and into the early Paleozoic that the ocean witnessed large reductions in silicic acid (Conley et al., 2017). The appearance of biomineralization (Kouchinsky et al., 2011; Knoll and Kotrc, 2015) likely led to evolutionary “arms races” with organisms using biomineralized structures to reduce predation rates, resulting in competition for an important shared resource (Smith and Szathmáry, 1995). This arms race produced a proliferation of non-silicic acid users and some highly efficient silicic acid users, the latter outcompeting inefficient users leading to their extinction.
There is widespread evidence in the geological record for such a decline in silicic acid and coincidental macroevolutionary changes in silicifying groups. For example, changes in chert precipitation occurred due to the increased abundance of radiolarians in the lower to early Middle Ordovician, which may have reduced the abundance of sponges overall and forced the relocation of remaining sponge species from shallow to deep-water environments as surface waters became depleted in silicic acid (Kidder and Tomescu, 2016). In addition to the geological record, the timing of independent losses of silicon transporters (Marron et al., 2016b) further support the hypothesis that significant declines in silicic acid concentrations during the Paleozoic (Conley et al., 2017) led to evolutionary changes in the biochemical pathways of silicification and efficiency of silicon uptake.
However, it was the evolution of diatoms with their superior ability to utilize Si, due to their unique complement of Silicon Transporter (SIT) genes (Hildebrand et al., 1997, 1998), that led to the reduction of oceanic silicic acid concentrations to the low levels observed in the global ocean today (Tréguer and De La Rocha, 2013). Diatoms, with their obligate requirement for Si to complete their cell cycle, are strong competitors for silicic acid. This, combined with diatom features not related to Si (e.g., nutrient acquisition and storage, light harvesting, bloom formation), must have produced new competitive interactions that were previously unseen in the Paleozoic oceans (Knoll and Follows, 2016). Recent studies have robustly demonstrated the presence of very low oceanic silicic acid concentrations since at least 60 Ma, most likely as a result of the drawdown of silicic acid by diatom biomineralization (Fontorbe et al., 2016, 2017), which is tens of millions of years before the time period envisioned by Siever (1991) and others. Conley et al. (2017) have hypothesized that if such a global decrease in oceanic silicic acid concentrations occurred, it must predate the Cenozoic and perhaps began with the appearance of silicifying diatoms in the Mesozoic (Sims et al., 2006).
Both the geological record and molecular phylogenetics concur that, whilst the majority of the main morphological groups of diatoms had arisen by the end of the Cretaceous (Kotrc and Knoll, 2015), there was a rapid expansion and diversification of diatoms in the Cenozoic (Siever, 1991; Figure 2). The most recent compilations have shown two short-lived major abundance peaks near the Eocene–Oligocene boundary and in the late Oligocene, with a shift in diatom abundance in sediments during the middle Miocene to globally higher values which have largely persisted to the modern day (Lazarus et al., 2014; Renaudie, 2016). There remains a lively debate in the scientific literature surrounding the drivers of the diatom expansion, mostly relating to shifts in the supply of silicic acid to the ocean due to changes in climate and weathering regimes. A correlation between a recent diatom diversity compilation and paleoclimate archives (oxygen and carbon isotopes from carbonates) indicates that there could be a direct link between temperature and diatom evolution throughout the Neogene (Lazarus et al., 2014). Climatically induced changes in oceanic circulation and mixing due to the opening of marine gateways may have altered nutrient availability in the euphotic zone and driven macroevolutionary shifts in the size of marine pelagic diatoms through the Cenozoic (Finkel et al., 2005). For example, geochemical archives point toward an increase in silicic acid supply to the surface Southern Ocean at the Eocene-Oligocene boundary, likely due to the opening of the Drake Passage and Tasman Seaway and the formation of a “proto circum-Antarctic current,” and coinciding with the large peak in diatom diversification (Egan et al., 2013). The rapid rise of diatoms in the Cenozoic has also been attributed to the impact of orogeny on weathering (Misra and Froelich, 2012) with periods of enhanced continental weathering fluxes and increased silicic acid input to the oceans (Cermeño et al., 2015). Correlation between diatom abundance peaks and shifts in seawater strontium and osmium isotopic composition also hint at a strong control by silicate weathering on diatom deposition (Finkel et al., 2005). However, it is a major challenge to tease apart the impacts of oceanic circulation and weathering on diatom diversification and abundance due to the inherent coincidence in timing of the major orogenic episodes and shifts in oceanic circulation throughout the Cenozoic (Benoiston et al., 2017).
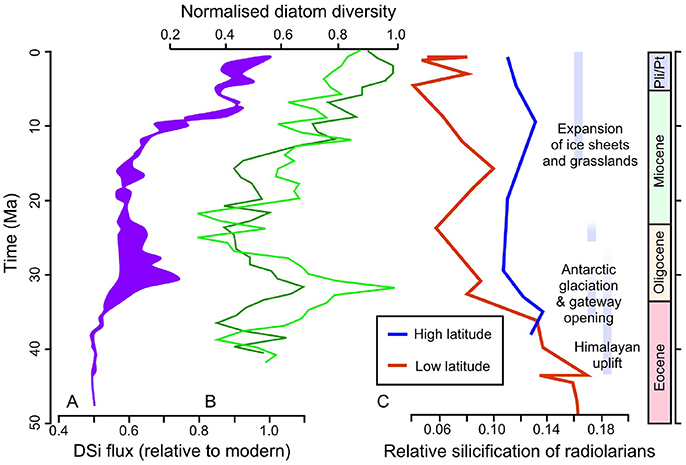
Figure 2. Cenozoic records of silicifying organisms and environmental drivers. (A) An approximate range of silicic acid fluxes relative to modern fluxes, encompassing global flux estimates modeled using the Cenozoic lithium isotope record of Misra and Froelich (2012) and Southern Ocean fluxes modeled using marine clay mineralogy. Note that a number of key assumptions are made to model these fluxes: the fluxes were modeled assuming a change in the ratio of suspended clays to silicic acid through time in order to account for a potential increase in incongruent silicate weathering, and a two-fold increase in silicic acid content of rivers over the last 50 My. (B) Diatom diversity curves calculated using the unweighted lists subsampling method of Cermeño et al. (2015) (lime green line) and the consensus diversity curve of Lazarus et al. (2014) (dark green line line). (C) Calculated silicification of radiolarians through the Cenozoic, from Lazarus et al. (2009).
The expansion of diatoms, with their strong affinity for silicic acid, is likely to have led to competition with other silificiers, especially in intermediate and shallow depths where silicic acid is present only in low—and potentially limiting—concentrations. Here the fossil record provides an archive for examining possible signs of competition in the geological past. The post-Mesozoic decline of certain sponge spicule morphologies, indicative of high silicic acid conditions in specimens from shallower waters, has been interpreted as showing competitive exclusion of sponges by diatoms (Maldonado et al., 1999). However, the exact timing of the decline in these spicules from shallower waters relative to diatom-driven changes in silicic acid is not well-constrained. If oceanic silicic acid declined earlier than the Cretaceous-Paleogene boundary (Conley et al., 2017), then the exclusion of sponges from shallower waters either occurred due to competition at an earlier stage, or was driven by an alternative factor.
Changes in the size of radiolarians in the fossil record also provide evidence of macroevolution likely driven by competition. The silicification and shell-thickness of radiolarians in the low latitudes decline throughout the Cenozoic, whilst the higher latitude specimens remain invariant (Lazarus et al., 2009). This latitudinal pattern of change suggests that radiolarians increasingly struggled to obtain sufficient silicic acid in the lower latitude, oligotrophic waters, which were increasingly depleted of silicic acid due to a combination of diatom drawdown and shifts in oceanic circulation. However, despite the coincidence in timing, the decline in low-latitude radiolarian size does not necessarily point to competition as a driver of macroevolution. Mathematical models of competition between radiolarians and diatoms have been used to investigate this problem further. These experiments show that the reduction in radiolarian shell size is not sufficient to explain diatom diversification changes, which were more likely driven by an increase in resources and were linked inherently to their geographical distribution (Cermeño et al., 2015; Figure 2). However, these models are limited by the availability of reliable data on silicic acid inputs and fossil abundance records.
A combination of competitive interactions for silicic acid with other silicifiers and the effect of predator-prey arms races may govern macroevolutionary trends in silicoflagellate morphology (van Tol et al., 2012). Across the Cenozoic, silicoflagellate skeletons show two diverging trends: spineless species become smaller, whereas spiny species display decreased levels of silicification but increased numbers and length of spines. It has been interpreted that grazing pressure necessitated a siliceous skeleton for protection, as other phytoplankton (e.g., coccolithophores) also possessed biomineralized defenses. However, competition from diatoms reduced silicic acid availability and therefore increased the cost of producing such a skeleton. In response silicoflagellates may have evolved either to maintain the degree of silicification but became smaller overall, or to utilize spines as a method of retaining a large size and defensive structure but at a lower silicon requirement. These evolutionary trade-offs saw the extinction of large, spineless silicoflagellates as ecosystems changed through geological time to feature both high grazing pressure and low silicon availability.
One additional route for examining competition as a driver of silicifier macroevolution is the timing and extent of biochemical changes in silicon uptake pathways. The evolution of biochemical pathways for silicification have been investigated by resolving the different gene families that express, for example, silicon transporters in different silicifiers (Marron et al., 2016b). The affinity for silicic acid and the uptake efficiency of the different transporter families could then be assessed to determine the potential for competitive interaction between different silicifying groups, between and within major taxonomic groupings. Isotope geochemistry could also be used as an additional tool for investigating this problem. Silicon transport into and within the cell is likely the basis for the fractionation of stable Si isotopes. All silicifiers investigated to date fractionate Si isotopes relative to the seawater in which they grow, but sponges have a more variable and potentially greater isotopic fractionation than either diatoms or radiolarians (de la Rocha et al., 1997; Hendry and Robinson, 2012; Hendry et al., 2014; Abelmann et al., 2015). Such isotopic fractionation may be related to the biochemical pathways involved in Si metabolism, and may reflect the organism's affinity for silicic acid and efficiency of uptake and utilization. As such, Si isotopes may be a useful tool for examining the evolution of silicification.
Evolutionary Competition in Modern Ecosystems
There are numerous examples of silicified structures being used by organisms to gain a competitive advantage in contemporary ecosystems. The siliceous lorica of choanoflagellates show a range of morphologies (Leadbeater et al., 2009), differing in size and density of siliceous components. This has been connected to niche partitioning, from large open structures that are required to maintain a planktonic lifestyle through the water column, to densely packed lorica of biofilm-inhabiting species (Leadbeater, 2015). The diatom Phaeodactylum tricornutum also displays evidence for optimizing its degree of silicification for deeper and colder waters (Zhao et al., 2014). Some wetland plants, notably rice, employ silica as a supplement to cellulose or lignin for structural strengthening, which allows taller growth and aids in competition for light (Cooke and Leishman, 2011).
The optical properties of silica can also be employed to aid in the absorption of light by photosynthetic organisms. Diatom frustules have been proposed to modulate solar wavelengths and direction for optimum absorption of light for photosynthesis (Yamanaka et al., 2008; Romann et al., 2015), while amorphous silica (in combination with microsporine-like amino acids) can help protect against harmful ultraviolet radiation (Ingalls et al., 2010). These adaptations can establish competitive interactions with other photosynthetic organisms that use different biominerals (e.g., calcium carbonate in coccolithophores Taylor et al., 2017, calcium oxalate in plants He et al., 2014), or organic components (e.g., flavonoids, Schaller et al., 2013) for similar photoprotective purposes.
Analysis of the distribution of silicifiers in the contemporary ocean at large spatial scale can bring additional insights about the evolution of competition between different groups. During the course of the Tara Oceans expedition (Karsenti et al., 2011; Bork et al., 2015), a worldwide characterization of pelagic plankton ecosystems was performed using DNA metabarcoding and microscopy correlated with key environmental parameters in a way that would, beyond acquisition of data, create a well-structured dataset to address broad ecological and evolutionary questions. Using a non-destructive in situ imaging system to visualize organisms directly in the water column (Underwater Vision Profiler, UVP), Biard et al. (2016) focused on the abundance of giant Rhizaria in a variety of pelagic ecosystems in the upper 500 meters of the water column. Rhizaria is a supergroup of unicellular eukaryotes composed of three subphyla: Radiolaria, Cercozoa, and Foraminifera, some of which are silicified (Moreira et al., 2007; Figure 1). Radiolaria are divided into two major lineages: the siliceous skeleton producing Polycystinea (including the two orders Nassellaria and Spumellaria) and non-silicified Spasmaria (including Acantharia and Taxopodida; Krabberød et al., 2011). Phaeodaria, an asymbiotic rhizarian taxon with widespread biosilicification and extensively silicified lineages, was initially classified in Radiolaria and is now placed among the Cercozoa as revealed by molecular phylogeny (Polet et al., 2004). Phaeodaria was the most important contributor to rhizarian biomass at all latitudes in the 100–500 m depth layer, displaying an even distribution worldwide. On the contrary, photosymbiotic non-silicified Collodaria dominated the top 100 m of the water column at low latitudes, showing that these orders of Rhizaria display different ecological preferences and vertical stratification.
Additionally, the use of the 18S ribosomal DNA molecular marker to chart microbial diversity by metabarcoding (Taberlet et al., 2012) enabled a high-resolution taxonomic description of planktonic communities across several depths and various size fractions with reasonable accuracy (de Vargas et al., 2015), and was found to be a good proxy for cell number, at least within the diatoms (Malviya et al., 2016). Analysis of the Tara Oceans metabarcoding data revealed that diatom diversity was high in the open ocean, contrary to what was generally considered, and also confirmed diatom prevalence in regions of high productivity and at high latitudes. Ocean circulation choke points such as Cape Agulhas and the Drake Passage were found to be important in constraining diatom distribution and diversity (Malviya et al., 2016).
Beyond individual studies of major silicifying groups, comparative analysis can be performed using the Tara Oceans data set (de Vargas et al., 2015). Functional annotation of the silicifying organisms (based on Marron et al., 2016b), followed by mapping of their distribution across the global ocean, reveals major patterns (Figure 3). The silicifier community is largely size-delineated: the smallest size fraction (0.8–5 micron) contains a large diversity of silicifying organisms in nearly constant proportions. Dictyochophyceae, Polycystinea, and Chrysophyceae are the major taxonomic groups present in this size fraction, together with Bacillariophyta (diatoms) at some locations. Although less abundant, the constant presence of Centrohelida and Choanoflagellates suggests that ecological niche enables the coexistence of several taxonomic groups.
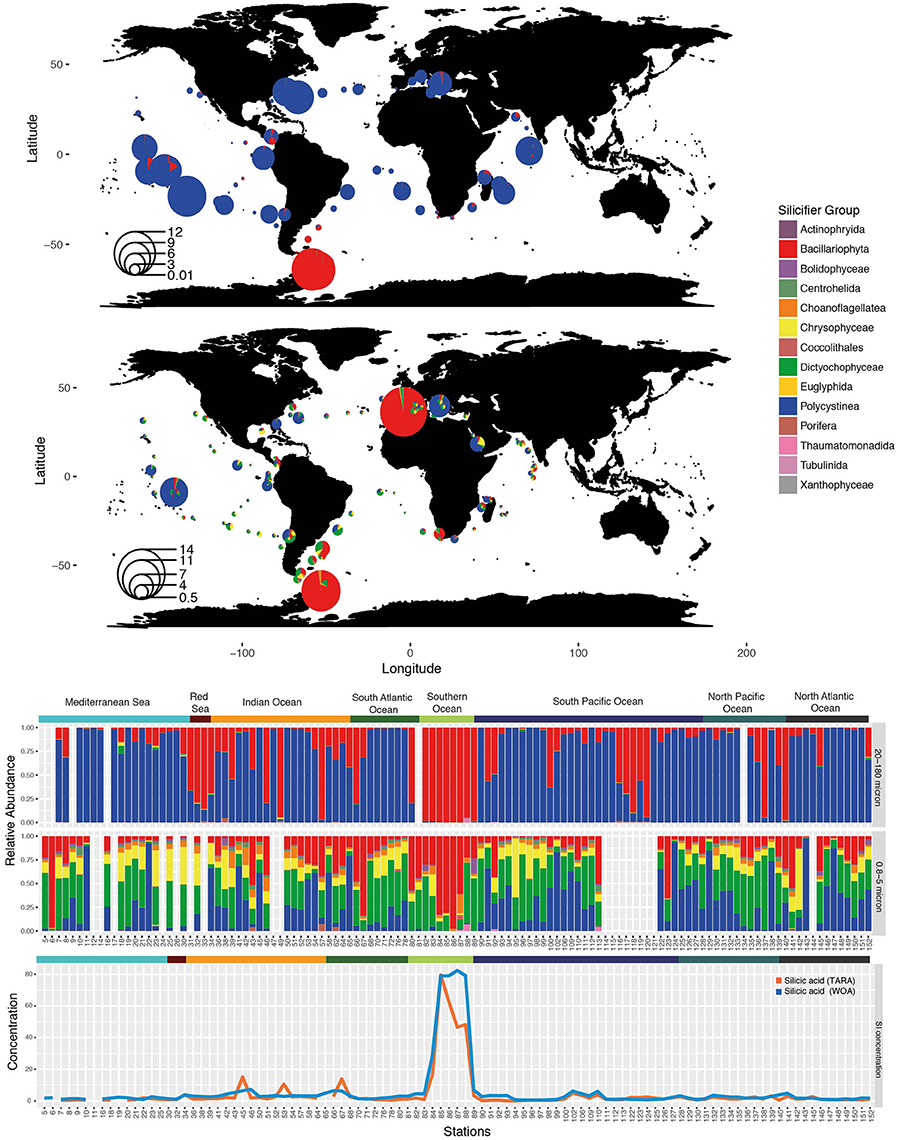
Figure 3. Distribution of silicifiers in the sunlit ocean based on metabarcoding abundance data from the Tara Oceans expedition. (Top) Silicifiers in surface waters of the 20–180 micron size fraction—divide radius by 20 for log transformed relative abundance. (Middle) Silicifiers in surface waters of the 0.8–5 micron size fraction—divide radius by 30 for log transformed relative abundance. The size of the bubble corresponds to the importance of silicifiers with respect to the whole planktonic community. (Bottom) Composition of the silicifiers' community in surface waters at each sampling station and in situ silicic acid concentrations (in μM) obtained either from discrete Tara Oceans samples or from the World Ocean Atlas (woa13). Ocean provinces are indicated.
A larger size fraction of micro-plankton (20–180 microns) displays a very different trend. Diversity within the silicifier community drops, and is composed essentially of only Bacillariophyta and Polycystinea, so much so that both taxonomic groups represent over 99% percent of the micro-planktonic silicifier community across the vast majority of the global ocean. Diatoms and polycystines occur in highly variable proportions, where diatoms dominate the cold high-latitude regions. Co-existence between both groups is rare, whereby the presence of one of the organisms appears to exclude the other, which may also reflect special adaptations to nutritional environments as opposed as eutrophic, oligotrophic, or HNLC areas.
Diatom expansion 65 Ma ago has been attributed to their superior competitive ability for silicic acid uptake relative to radiolarians, the latter experiencing a reduction in weight of their tests (Harper and Knoll, 1975). It is therefore expected that at high silicic acid concentrations, Radiolaria, with their inferior silicic acid uptake ability should have a better chance to thrive alongside diatoms. Yet, in the modern ocean, regions with high silicic acid concentrations (Southern Ocean, Tara stations TARA_084 to TARA_089) are still strongly dominated by large populations of diatoms (Platt et al., 2009) in which they can represent over three quarters of the whole planktonic community (Figure 3), according to the metabarcoding survey. This hints to the possibility that biotic or abiotic factors other than silicic uptake are responsible for diatom dominance.
The relative abundance of the silica biomineralizing groups with respect to other micro-plankton in the Tara Oceans data (Figure 4) is largely dependent on nutrient availability (silicic acid, nitrate, phosphate), in particular in the Southern Ocean, the Indian Ocean and around the Marquesas Islands in the Pacific Ocean. However, silicic acid concentrations do not appear to be concomitant in specific stations in the Mediterranean Sea or in the North Atlantic Ocean where nutrient concentrations are low and yet silicifiers are highly abundant, perhaps reflecting a time lag between silicic acid availability and diatom uptake and bloom. Moreover, focused studies in the Mediterranean ecosystems do not report diatom dominance, with the exception of the spring bloom in the North West Mediterranean and Adriatic Sea. Most of the Mediterranean Sea is oligotrophic with diatoms restricted to more or less discrete deep layers at the boundary with the Levantine Intermediate Water (the saltiest water mass that forms in the eastern Mediterranean Sea), suggesting a strong bottom-up control by nutrients, including silicic acid (Leblanc et al., 2003; Crombet et al., 2011). Additionally, the North Atlantic planktonic community changes in the course of the productive season, and silicic acid can also limit diatom uptake and growth (Leblanc et al., 2005).
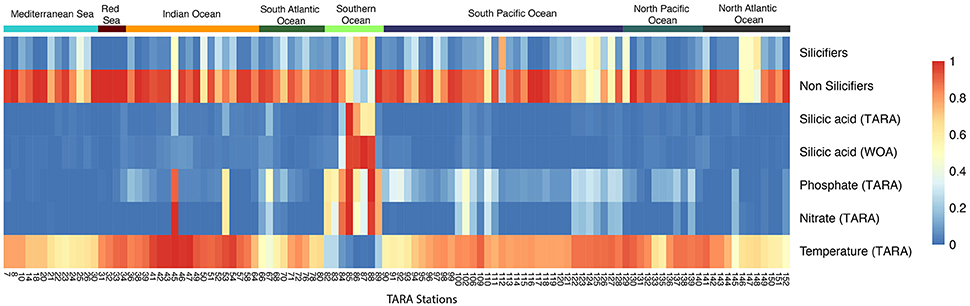
Figure 4. Heatmap of the relative abundance between silicifiers and non-silicifiers and major environmental parameters across Tara Oceans stations. The relative abundance of silicifiers outcompetes that of non-silicifiers in specific locations but not always concomitant with high nutrient availability. Colored rectangles on top of the heatmap correspond to ocean provinces.
Because some diatoms thrive in modestly nutrient-rich regions, other factors must therefore explain their success (Green et al., 2008). The continual reshaping of communities by mortality, allelopathy, symbiosis, and other processes show that community interactions exert strong selective pressure on marine microbes (Strom, 2008). This reflects the “Eltonian shortfall,” introduced by Hortal et al. (2015) in a review on current major flaws in biodiversity research and refers to our lack of knowledge about “biotic interactions.” It is likely that studying these top down pressures on biomineralizing organisms will complement our understanding of their evolution.
A prominent role of biomineralization in modern ecosystems is in defensive and feeding interactions between predators (feeding structures such as teeth) and prey (defensive structures such as spines, shells and tests). In embryophytes, silicification has a wide-ranging defensive role (He et al., 2014; Ensikat et al., 2016; Hartley et al., 2016), from abrasive phytoliths to complex structures such as trichomes, and even by inducing anti-herbivore and anti-pathogen metabolic responses (Ye et al., 2013). These defenses lead to multiple competitive interactions, with differential effects on different types of insect feeding (Massey et al., 2006), between insect and mammalian herbivores, or between large and small mammalian herbivores (Hartley et al., 2016). There are also complex competitive interactions between plants regarding their silicified defenses, which depend on various biotic (plant species, herbivore population cycles) and abiotic factors (soil conditions, climate; Garbuzov et al., 2011; Hartley et al., 2016). This can lead to herbivore-plant specialization and alter plant community and ecosystem structure.
Metazoan herbivores such as copepods (crustaceans) presumably exercise strong pressure on diatoms, silicoflagellates, and polycystines by feeding on them (Lebour, 1922; Marshall and Orr, 1955; Campbell et al., 2009; van Tol et al., 2012). Several feeding experiments have investigated the coevolution between copepods and diatoms. Some adaptations are mechanical: copepods modify their feeding tools by increased silicification of the mouthparts (Itoh, 1970; Miller et al., 1980, 1990; Michels et al., 2012) in response to which diatoms adjust their protective frustules, leading to an arms race that fuels evolutionary processes (Hamm and Smetacek, 2007). Some diatoms that dominate blooms experience less grazing mortality than do co-occurring species (Assmy et al., 2007; Strom et al., 2007): it was shown that in the presence of preconditioned media that contained herbivores, diatoms develop grazing-resistant morphologies such as increased cell wall silicification (Pondaven et al., 2007; Ratti et al., 2013; Zhang et al., 2017). Silicification of diatom genera also depends on their ecological niche, wherein diatoms that thrive at depth under low light and in the nutrient gradient display low growth rates, and thus must be silicified to protect against grazing (Quéguiner, 2013). The silica cell wall therefore provides not only a “constitutive mechanical protection” for the cell but also a plastic trait that responds to grazing pressure promoting the diversification of ecological niches for a single taxonomic group. Differential biosilicification within a specific group may also have major effects on global nutrient recycling such as the decoupling of silicon and carbon cycles through complex biotic interactions influencing sedimentation pathways in the iron-limited Southern Ocean (e.g., Assmy et al., 2013; Quéguiner, 2013).
Experimental evidence suggests that biotic interactions not only shape the arms race between species, but also affect species range (Bateman et al., 2012; Araujo and Rozenfeld, 2014), inducing non-random co-distribution of species at large spatial scales of hundreds of kilometers for macro-organisms (Gotelli et al., 2010), both at regional and continental scales. Therefore, understanding the degree to which occurrences of species are constrained by the distributions of other species at broad scales of resolution and extent likely links back toward ecological and evolutionary mechanisms shaping the success of functional groups. This can be investigated through the use of co-occurrence networks (Gravel et al., 2011). Empirical studies have historically focused on competition (Gause, 1934; Hardin, 1960), revealing that in its extreme form competition leads to co-exclusion of the interacting species (MacArthur, 1972). As part of the recent Tara Oceans expedition, determinants of community structure in global ocean plankton communities were assessed using microbial association networks to create the Tara Oceans Interactome (Lima-Mendez et al., 2015). Pairwise links between species were computed based on how frequently they were found to co-occur in similar samples (positive correlations) or, on the contrary, if the presence of one organism negatively correlated with the presence of another (negative correlations). At a global scale (Figure 5), major taxonomic groups have shown higher positive correlations than negative ones, except diatoms and polycystines (Morueta-Holme et al., 2016). Instead diatoms and polycystines showed an unusually high proportion of negative correlations, which was statistically significant within silicifiers as a whole. This defines diatoms and polycystines as segregators. Conversely, plankton functional traits approaches show that silicifiers display a segregative pattern compared to other major interacting organisms in the plankton, in particular toward meso- and proto-zooplankton as well as parasites (Figure 5). It is therefore the interplay between abiotic and biotic factors that shape the distribution of biomineralizing organisms in the modern ocean, in which competition for silicic acid coupled with differential grazing pressures seem to be important drivers of the spatio-temporal structure of silicifier communities across the ocean. The data in Figures 4, 5 indicate that temperature is a particularly important abiotic factor.
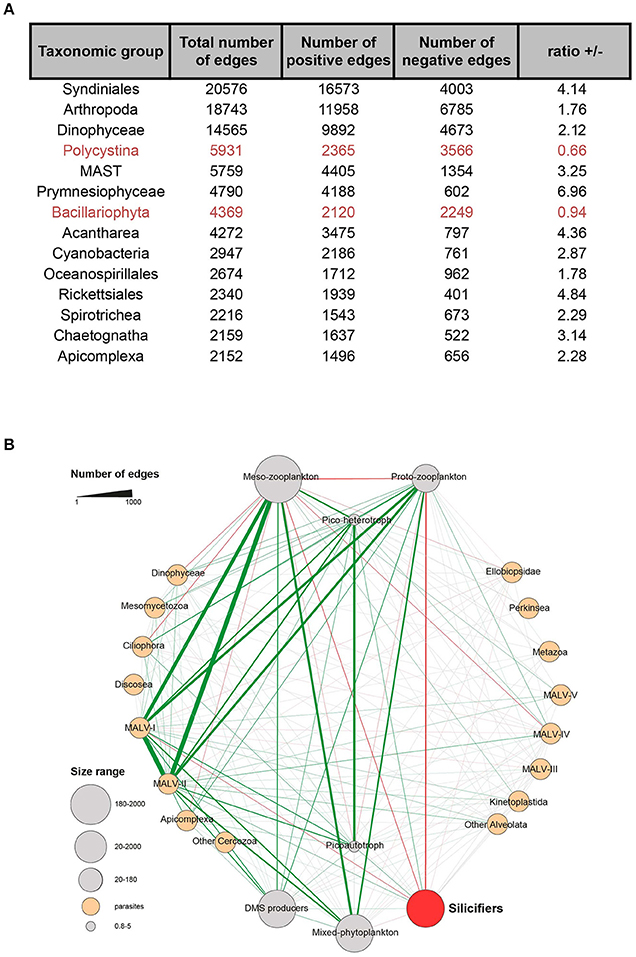
Figure 5. Silicifiers in the Tara Oceans global ocean co-occurrence network. (A) Proportion of positive and negative correlations for the major taxonomic groups. Silicifiers are highlighted in red. (B) Plankton functional types (PFTs) subnetwork. PFTs encapsulate individual barcodes based on their trophic strategy and role in global biogeochemical cycles. Edges width reflects the number of correlations between the corresponding metanodes. Over-represented links (multiple-test corrected P < 0.05) are colored in green if they represent co-presences and in red if they represent exclusions; gray means non-overrepresented combinations. When both co-presences and exclusions were significant, the edge is shown as co-presence. Silicifiers emcompass Bacillariophyta and Polycystina. Figure adapted from Lima-Mendez et al. (2015) and reproduced with permission from AAAS.
Cellular and Molecular Aspects of Evolutionary Competition
Evidence for evolutionary competition can also be found in the molecular biology of biomineralization. The gain, loss and convergence of the molecular mechanisms responsible for nutrient uptake and biomineral patterning illustrates how silicifying and non-silicifying organisms compete for common resources and produce similar solutions to solve these evolutionary problems.
Competition between diatom species has driven their diversification into various ecological niches (Sims et al., 2006; Nakov et al., 2014). This is mirrored in the diversification of diatom morphologies, from the macrostructural level of frustule shapes, spines and chains right down to the different micro- and nanopatterns of the frustule (Gordon et al., 2009; Finkel and Kotrc, 2010). Isolation of macromolecules found in diatom frustules, and in vitro studies of their silica polymerization activity, has led to the development of a model whereby micropatterns are produced by interactions between several components. Silica polymerizes on a structural scaffold composed of glycoproteins and other organic macromolecules, the ammonium fluoride insoluble matrix (Brunner et al., 2009; Tesson and Hildebrand, 2013; Kotzsch et al., 2016). The morphology and mesoscale patterning of the forming diatom silica is also controlled by interactions with the cytoskeleton as the silicon deposition vesicle (SDV) expands, and by components of the membrane surrounding the SDV (the silicalemma; Tesson and Hildebrand, 2010a,b; Tesson et al., 2017). The polymerization rate in these in vitro experiments, and therefore the nanoscale patterning (including formation of plates and pores), has been shown to be influenced by proteins including silaffins (Kröger et al., 2002), silacidins (Wenzl et al., 2008), and long-chain polyamines (LCPAs; Kröger et al., 2000; Sumper and Kröger, 2004). Some unique frustule structures are marked out by them containing specific biosilica-related proteins, such as cingulins that form the girdle bands linking the two valves of the frustule (Scheffel et al., 2011). The number, size and shape of girdle bands vary between diatom species, and this may be connected to variations in cingulin repertoires. Furthermore, it is not only the content and protein sequence of these molecules that has been demonstrated through in vitro experiments to control their silica polymerization and patterning activity, but also their ratios and post-translational processing like cleavage, glycosylation, phosphorylation, or the addition of quaternary ammonium groups (Kröger et al., 2002; Poulsen and Kröger, 2004; Lopez et al., 2005; Sumper et al., 2007; Wenzl et al., 2008).
Diatom morphology can control features such as sinking rates (Smayda, 1970; Raven and Waite, 2004; Nakov et al., 2014), predation (Hamm et al., 2003; Pondaven et al., 2007), light perception (Fuhrmann et al., 2004; Yamanaka et al., 2008; Romann et al., 2015), viral resistance (Losic et al., 2006), and nutrient uptake (Finkel and Kotrc, 2010). Given that it has been demonstrated that the content of the polymerization-influencing components differs between species (Kröger et al., 2000; Sumper and Lehmann, 2006; Bowler et al., 2008), it is conceivable that evolutionary modifications of the silica patterning mechanisms can help individuals to out-compete other diatoms in certain ecological niches. This would eventually lead to the emergence of new species with characteristic morphologies of the siliceous frustule. In this way, the study of diatom silica formation and patterning mechanisms, combined with a phylogenetic framework, can reveal the evolutionary interactions between competition and frustule silicification.
In addition to diatoms, many other siliceous groups feature species-specific morphologies and micropatterning, with only limited variation depending on environmental conditions. Examples include loricate choanoflagellates (Leadbeater et al., 2009), thaumatomonads (Scoble and Cavalier-Smith, 2014), chrysophytes (van Tol et al., 2012), and ascidians (Monniot et al., 1992). Repeatedly we can observe the evolution of similar morphologies in distantly related taxa, such as micropores in the siliceous components of choanoflagellates (Leadbeater, 2015), chrysophytes (Sandgren et al., 1996), diatoms (Finkel and Kotrc, 2010), and haptophytes (Yoshida et al., 2006); spines and spicules in radiolarians (Kunitomo et al., 2006), dictyochophytes (Preisig, 1994), centrohelids (Zlatogursky, 2016), and sponges (Weaver et al., 2007); or tablets and scales in haptophytes (Yoshida et al., 2006), rhizarians (Nomura and Ishida, 2016), synurophytes (Sandgren et al., 1996), amoebozoans (Lahr et al., 2013), and brachiopods (Williams et al., 2001). Though the genes governing the production of these silica patterns are not fully understood, many parallels with the molecular biology of diatom silicification are emerging, such as a role for glycoproteins in choanoflagellates (Gong et al., 2010) and synurophytes (Ludwig et al., 1996), cytoskeleton-mediated shaping of the growing silica structure in multiple taxa (Leadbeater, 2015; Nomura and Ishida, 2016) and the presence of post-translationally modified LCPAs in haptophyte (Durak et al., 2016) and sponge (Matsunaga et al., 2007) silica. These polymerization mechanisms have apparently evolved independently from those in diatoms, suggesting repeated recruitment of similar molecules for silica formation and patterning, and therefore a similar role for silicification-related evolutionary competition and speciation as diatoms.
A critical step in silica biomineralization (Martin-Jézéquel et al., 2000), and therefore a major aspect of evolutionary competition between silicifiers, is the uptake and concentration of silicic acid from the external environment. The nature of this competition has changed over geological time in conjunction with changes in the biogeochemical cycling of silicon (Racki and Cordey, 2000; Maliva et al., 2005; Finkel and Kotrc, 2010; Knoll and Kotrc, 2015; Conley et al., 2017). In turn, this is reflected in the evolutionary molecular biology of silicon transport mechanisms.
The first proteins capable of transporting silicon across a plasma membrane to be identified were the SIT (Silicon Transporter) family (Hildebrand et al., 1997). The SIT protein (see Figure 6A) has a characteristic 10-transmembrane domain structure, with two conserved GXQ motifs arranged in a roughly symmetrical pattern at the cytoplasmic sides of transmembrane helices 2 and 3, and the extracellular sides of transmembrane helices 7 and 8 (Thamatrakoln et al., 2006). These motifs are proposed to be involved in forming an aqueous vestibule to allow an alternating access mechanism for silicon transport (in conjunction with Na+) across the membrane (Knight et al., 2016). This four-GXQ domain characteristic of the SIT gene family is highly distinctive, being found only in the 10-transmembrane domain SITs and in the closely related, 5-transmembrane domain SIT-Ls (Silicon Transporter-Like; Durak et al., 2016). SIT-Ls resemble “halved-SITs” (see Figure 6B), and are believed to be the ancestral genes that underwent duplication to give rise to SITs (Durak et al., 2016; Marron et al., 2016b). This situation is analogous to the gene duplication and fusion events that are believed to have produced pseudo-symmetrical transporter proteins (Keller et al., 2014), exemplified best in the SWEET and semi-SWEET glucose transporters of eukaryotes and bacteria (Feng and Frommer, 2015; Tao et al., 2015).
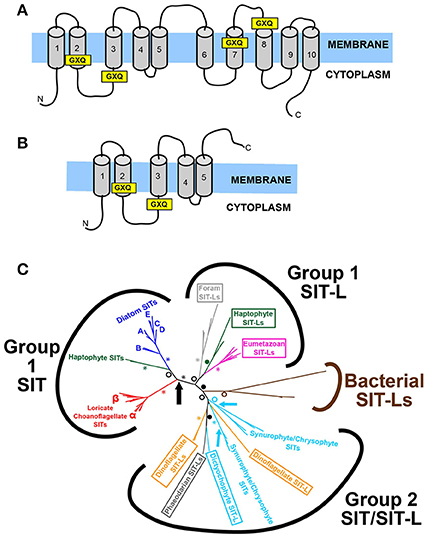
Figure 6. The structure and evolution of SITs and SIT-Ls. (A) Structure of the SIT (Silicon Transporter) protein, demonstrating the characteristic 10 transmembrane domains (TMD) and four GXQ motifs, located at the cytoplasmic side of TMD 2 and 3, and at the extracellular side of TMD 7 and 8. (B) Structure of the SIT-L (Silicon Transporter Like) protein, with 5 TMDs and two GXQ motifs. The SIT structure resembles a “double” SIT-L, with the C-terminal half having been inverted relative to the N-terminal half and fused together. This suggests that SITs evolved by duplication, inversion and fusion of SIT-L subunits. (C) Unrooted radial tree based on a maximum likelihood phylogenetic analysis of SITs and SIT-Ls. The eukaryotic genes form two main subgroups distinct from the bacterial SIT-Ls, and largely follow the species phylogeny except for the paraphyletic stramenopile, rhizarian, and dinoflagellate sequences in Group 2. The diatom and loricate choanoflagellate SIT sub-groups are also shown. Arrows indicate inferred duplication-inversion-fusion events that gave rise to 10 TMD SITs. Brown, Bacteria; Dark Green, Haptophyte; Gray, Rhizarian (Light Gray, Foraminiferan); Bright Red, Choanoflagellate; Magenta, Metazoan; Orange, Dinoflagellate; Dark Blue, Diatoms; Light Blue, Other Stramenopiles; Black, multi-taxon. SIT-L sequences are in boxes. Statistical support values for selected nodes are indicated by asterisks (100% support), closed circles (90–99% support) or open circles (>70% support). Figures adapted from Marron et al. (2016b).
Members of the Silicon Transporter gene family have been identified across a broad taxonomic range, in all eukaryotic supergroups save for archaeplastids, excavates and amoebozoans (with the caveat that no sequence data are currently available from silicifying excavates or amoebozoans) and are also found in some bacterial species. SITs are present in many highly-silicified species, most obviously siliceous stramenopiles (diatoms, chrysophyte/synurophytes, and dictyochophytes) but also siliceous choanoflagellates and haptophytes. In contrast SIT-L genes are often found in primarily calcareous groups (foraminifera, metazoans, calcareous haptophytes), although SIT-Ls were identified in silicifying groups such as phaeodarians and dinoflagellates. What is notable is that though some species contain multiple SITs or SIT-Ls, taxa expressing both SIT and SIT-L genes are rare: of the species examined only the haptophyte Scyphosphaera apsteinii possessed both, with the apparently non-silicifying dictyochophyte genus Florenciella having some evidence for both SIT and SIT-L genes (Marron et al., 2016b). This points to there being some sort of functional difference between SIT and SIT-L transporters, possibly with SITs being superior in some way, or with SIT-L genes being connected to calcification (Durak et al., 2016). It is notable that the geological record of heavily silicifying SIT-L-containing species show a decline in silicification and diversity (Lazarus et al., 2009; van Tol et al., 2012) coincident with the rise of the diatoms from the Mesozoic, again hinting at a “functional superiority” of SITs in competition for silicic acid uptake, though the nature of this advantage remains unresolved. Furthermore, siliceous sponges, which apparently lack either SITs or SIT-Ls (Marron et al., 2016b), show a similar decline in their degree of silicification and ecological dominance over this time (Maldonado et al., 1999). Again, this is suggestive of SIT-based mechanisms providing some type of competitive advantage over other uptake systems as silicic acid levels fell over geological time and competition for silicon increased.
When the SIT/SIT-L phylogeny is compared to species phylogenies, an interesting picture emerges (see Figure 6C). Three main eukaryotic clades are resolved: a monophyletic SIT-only clade containing diatom, choanoflagellate, and haptophyte genes; a monophyletic SIT-L only clade containing foraminferan, metazoan, and haptophyte genes; and a polyphyletic clade featuring both SIT and SIT-L genes from radiolarians, dinoflagellates and various non-diatom stramenopiles (Marron et al., 2016b). In this analysis, the stramenopile SITs branch in two distinct groups with high statistical support, with diatoms being separate from chrysophytes/synurophytes, and dictyochophytes. This distinction also emerges when the phylogeny is analyzed with an alignment of SIT-L, SIT N-terminal and SIT C-terminal sequences (i.e., artificially splitting the SITs into two, roughly equal 5-transmembrane sequences each containing two GXQ motifs). In this case the diatom N- and C-termini branch with the relevant N-and C-termini of the choanoflagellate and haptophyte SITs. The 5-transmembrane sequences of other stramenopile SITs however branch paraphyletically, again together with radiolarian and dinoflagellate SIT-Ls. These phylogenies provide a strong molecular signal for the stramenopile SITs having evolved via multiple, independent duplications. In this scenario, the diatom SITs arose from a single gene duplication and fusion event that also gave rise to the haptophyte and loricate choanoflagellate SITs, and must have occurred early in eukaryotic evolution, before these groups diverged deep in the Precambrian (Parfrey et al., 2011). The phylogenetic signal supports the scenario that the other stramenopile SITs arose from at least two other duplication-fusion events within the ochrophyte stramenopile clade itself, but after diatoms diverged, potentially as recently as the Mesozoic (Brown and Sorhannus, 2010; Derelle et al., 2016). It is hypothesized that this is a remarkable case of convergent molecular evolution, and that the independent invention of SITs from SIT-Ls was in response to competition for silicic acid from the rise of the diatoms after the Jurassic period (Marron et al., 2016b). As diatoms came to dominate the global silicon cycle, other heavily silicifying groups required more complex or more efficient uptake mechanisms and transporters to compete. What also emerges from these molecular analyses is that the last common ancestor of all ochrophytes must have possessed an SIT-L gene, and that diatoms have lost these SIT-Ls, again hinting at a superiority of SITs over SIT-L-based uptake systems in the competition for silicon.
Diatom domination of marine phytoplankton can be attributed to several ecological advantages (Armbrust et al., 2004), but of particular relevance to silicification is that they possess multiple modes of silicic acid uptake (Martin-Jézéquel et al., 2000; Thamatrakoln and Hildebrand, 2008). At lower silicic acid levels (≤30 μM), the majority of uptake is by SIT-mediated active transport, while at higher concentrations silicic acid enters the cell by diffusion (Thamatrakoln and Hildebrand, 2008; Shrestha and Hildebrand, 2015). The concentration gradient is created by binding of silicic acid to intracellular binding components in the cytoplasm, the character of which remains unknown (Thamatrakoln and Hildebrand, 2008; Spinde et al., 2011). This means that at higher silicic acid levels, diatoms can internally control uptake depending on the rate of silica polymerization in the SDV. The cytoplasmic binding compounds also allow diatoms to maintain a soluble intracellular silicon pool, with spare capacity permitting “surge uptake” of silicic acid following short-term silicon starvation (Thamatrakoln and Hildebrand, 2008). This is highly advantageous, allowing diatoms to take advantage of transient silicon sources in patchy environments like ocean gyres. The different modes of diatom silicic acid uptake are in contrast to other silicifiers such as sponges, which appear to only employ active transport and display maximum uptake efficiency at much higher silicic acid concentrations (Maldonado et al., 2011).
At ecologically-relevant silicon concentrations experienced by diatoms in modern oceans, active SIT-mediated uptake dominates. Diatom species generally possess multiple SIT genes (Hildebrand et al., 1998). These paralogs display differences in expression levels, the timing of their expression in the cell cycle, protein abundances and their sub-cellular localization (Thamatrakoln and Hildebrand, 2007; Sapriel et al., 2009; Shrestha et al., 2012; Shrestha and Hildebrand, 2015). These differences are believed to allow neofunctionalization of each SIT, evolving roles as silicon sensors, silicon transporters, targeting of different SIT proteins to specific cellular locations (Shrestha and Hildebrand, 2015), specialization for the uptake of certain silicon species (Sapriel et al., 2009) or for differential characteristics of substrate affinity versus transport capacity. This allows for maximal uptake at various silicic acid concentrations in the external environment (Thamatrakoln et al., 2006; Thamatrakoln and Hildebrand, 2007, 2008). This suite of SITs is hypothesized to allow adaptation to varying conditions and allows the diatom cell to sense silicon availability, ensuring that it can meet the silicon requirements of frustule synthesis and so complete cell division.
This highlights the central role that silicon and silicification play in diatom biology (Martin-Jézéquel et al., 2000). Indeed, silicic acid limitation influences wider diatom cellular biology and results in transcriptional changes of genes related to multiple metabolic pathways (Mock et al., 2008; Shrestha et al., 2012), just as is seen for other essential nutrients like iron or nitrate. It is therefore unsurprising that diatoms have evolved such complex systems to accumulate and compete for silicic acid. This even extends to chemosensory responses of the motile raphid pennate diatom Seminavis robusta toward sources of silicic acid, and remarkably away from sources of germanium (Bondoc et al., 2016), since germanium exposure disrupts biosilicification and is therefore toxic to siliceous organisms (Marron et al., 2016a).
Phylogenetic evidence (see Figure 6C) demonstrates that SIT diversification occurred within the diatom lineage itself (Marron et al., 2016b). More detailed phylogenetic analyses of diatom SITs can be divided into five main clades (A–E), though the deep branching order between these clades is poorly resolved due to low statistical support (Durkin et al., 2016). The paraphyletic Clade B was found to be the most basal, and Clade B-type SITs are found throughout the various diatom groups. The other clades, however, have limited taxonomic distributions, for example Clade A only being present in pennate diatoms, and Clade E being unique to the Thalassiosirales. Therefore, diatom SITs underwent multiple independent duplications and diversifications within different lineages, with the more recently evolving diatom lineages (e.g., raphid pennates) having more complex SIT repertoires. This complexity is reflected in transcriptional analyses of diatoms under nutrient starvation, and in natural diatom assemblages. The expression levels and ratios of the various SIT clades differ according to silicic acid levels, with the more derived SIT types being more prominent over the basal Clade B SITs in low silicic acid environments compared to waters with higher silicic acid levels. This is hypothesized to allow diatom species to co-occur by employing different approaches to utilize limited silicic acid, and to out-compete other silicifying groups (Durkin et al., 2016). It also suggests a certain “directionality” of diatom SIT evolution, with new SIT clades evolving in new lineages to adapt to the drawdown of ocean silicic acid concentrations that occurred as the diatoms themselves diversified and came to dominate the oceans (Sims et al., 2006; Finkel and Kotrc, 2010).
Silicon Transporter diversification has also occurred in other taxa (see Figure 6C), with some stramenopiles expressing multiple SITs, and evidence for independent duplications of SIT-Ls in both foraminifera (Ammonia sp.) and Phaeodarian radiolarians (Marron et al., 2016b). The most prevalent case of this is in the siliceous loricate choanoflagellates, which display evidence for a SIT gene duplication event early in their evolution (Leadbeater, 2015). All loricate choanoflagellates examined were found to possess two SIT gene types, termed SITα and SITβ (Marron et al., 2016b). Transcriptional analysis of these SIT types under varying silicic acid concentrations demonstrated that the SITα genes were highly expressed, and their expression levels responded to silicic acid availability, while the SITβ genes were always expressed at a low level, irrespective of the environmental silicic acid concentration. This closely resembles the situation in diatoms (see above), where some SIT genes are highly expressed and silicon-responsive, while other types have low expression unresponsive to silicon, suggesting that SITα and SITβ have evolved different roles, possibly as specialized transporters or silicon sensors (Thamatrakoln and Hildebrand, 2007; Shrestha and Hildebrand, 2015). The similar diversification and sub-functionalization of SITs in parallel in both choanoflagellates and diatoms is proposed to be an example of convergent evolution in response to increased competition for silicic acid. This mirrors the hypothesis for the convergent evolution of 10-transmembrane domain SITs in the stramenopiles (see above). It is likely that future research will identify other cases of transporter diversification due to increased competition for silicic acid, potentially in the multiple rhizarian SIT-Ls, horsetail silicon transporters or the proposed silicon-related Lsi2-like gene family found in the siliceous sponges (Grégoire et al., 2012; Marron et al., 2016b; Vivancos et al., 2016).
The phylogenetic distribution of silicon-related active transporters reveals evidence for multiple and widespread losses of SIT, SIT-L, and Lsi2-like genes throughout the eukaryotes (Marron et al., 2016b). There is strong evidence for the loss of SIT-Ls across most bilaterian lineages, of losses of SITs and SIT-Ls across the coccolithophorid haptophytes and for losses of SITs and SIT-Ls multiple times throughout the stramenopiles. Indeed, barring rampant eukaryote-to-eukaryote horizontal gene transfer events (Ku et al., 2015), it is likely that SITs and/or SIT-Ls were present in the last common ancestor of all eukaryotes, and that multiple independent gene loss events only occurred after the main eukaryotic lineages and supergroups had diverged. Similarly, the distribution of Lsi2-like genes across the eukaryotes strongly supports that they were present in the eukaryotic last common ancestor (Marron et al., 2016b), but that they were independently lost in multiple lineages, for example deuterostome eumetazoans, fungi or apicomplexans.
It has been hypothesized that these active silicon transporters were originally required as part of a detoxification mechanism for life in the high-silicon Precambrian ocean (Marron et al., 2016b). Geological evidence for abiotic silica precipitation in sediments from the Neoproterozoic demonstrates that at the time that the eukaryote supergroups were originating and diversifying (Parfrey et al., 2011) oceanic silicic acid concentrations were high enough to allow autopolymerization into silica (Iler, 1979; Maliva et al., 1989, 2005; Siever, 1992; Grenne and Slack, 2003). Uncontrolled formation of silica free in the cytoplasm, potentially mediated by localized pH environments or accelerated by polyamines, would cause massive disruption to cellular metabolism. Unprotected by a membrane the freely polymerizing silica could occlude and adsorb proteins, nucleic acids, and other macromolecules within its structure or on its surface (e.g., Betancor and Luckarift, 2008; Vandeventer et al., 2013). Therefore organisms must have required a system for silicon homeostasis, to bind or sequester silicic acid within the cytoplasm and then remove it from the cell. This is reflected in the bidirectional transportation capacity of SITs, where they could be employed to transport silicon out of the cell against a concentration gradient (Thamatrakoln and Hildebrand, 2008; Knight et al., 2016). In ancient oceans there would have been a strong selective pressure to maintain a molecular machinery for silicon detoxification, as has also been suggested for the employment of mucins as anti-calcification mechanisms in early animals (Marin et al., 1996; Wood et al., 2017).
Major geochemical and biological upheavals around the Precambrian/Cambrian boundary changed the competitive interactions and evolutionary pressures acting on these detoxification and homeostasis mechanisms. Most notable was the appearance and diversification of biomineralized hard parts (Knoll, 2003; Knoll and Kotrc, 2015), believed to have originated by modification and co-opting of the detoxification mechanisms (Wood et al., 2017). The innovation of biomineralized structures for feeding, movement and protection established an evolutionary arms race (Knoll, 2003; Cohen, 2005). This gave rise to biomineralization becoming more significant, with organisms producing larger and more prominent calcified or silicified structures. The biosilicified structures would have acted as silicon sinks, with the burial of biogenic silica skeletons sequestering silicon in the sediments. The outcome of this was that by the early Phanerozoic oceanic silicic acid concentrations were substantially reduced from Neoproterozoic levels (Maliva et al., 1989; Racki and Cordey, 2000), below the threshold for autopolymerization and removing the threat of harmful silica precipitation free in the cytoplasm. This would have removed the selective pressure to possess silicon detoxification mechanisms, and organisms could gain a competitive advantage by not diverting resources to such an unnecessary metabolic pathway, hence resulting in gene losses. The exception were those organisms which had re-deployed the silicon homeostasis machinery to produce siliceous structures. In these cases, silicon became a scarce resource, requiring the evolution of more sophisticated transport systems in the acquiring of silicic acid uptake to meet the metabolic requirements of biosilicification. This reflects the evolutionary biology of other elements, where they are initially toxic, before becoming metabolically useful and finally ending up as limiting nutrients and the subject of evolutionary competition (Rickaby, 2015).
In some cases, the solution to the problem of this scarcity was to secondarily lose biosilicification and the associated molecular mechanisms. An excellent example of this occurs within the haptophytes. Some species, such as Prymnesium neolepis (Yoshida et al., 2006) and S. apsteinii (Drescher et al., 2012) produce wholly- or partially siliceous scales, while some calcareous species such as Calcidiscus leptoporus possess SIT-Ls and have a metabolic requirement for silicon to complete normal production of their calcified scales (Durak et al., 2016). Other species however, have lost SIT-Ls (and SITs), and show no effect of germanium toxicity disrupting biomineralization. One such species is Emiliania huxleyi, a calcifying haptophyte known to produce blooms of major ecological importance, and which is believed to have evolved relatively recently in the Cenozoic (Liu et al., 2010; Taylor et al., 2017). What is notable is that E. huxleyi blooms often occur following diatom blooms, raising the possibility that while diatoms enjoy a competitive advantage in silicon-replete waters, non-silicifying species like E. huxleyi have evolved to out-compete and succeed siliceous phytoplankton in silicon-deplete waters where they can continue to grow and produce biomineralized structures (Durak et al., 2016). In this way, the abandonment of any metabolic requirement for silicon opens new ecological niches and provides opportunities to gain new competitive advantages.
Molecular phylogenetics has revealed many other cases where non-silicifying species have evolved from heavily silicified ancestors. This is evident in land plants, with basal groups tending to be highly siliceous (e.g., liverworts) while some derived taxa (e.g., conifers) have low silicon contents and lack phytoliths (Hodson et al., 2005). Although there is some debate as to the evolutionary relationships between the main sponge groups (Sperling et al., 2010), the widespread distribution of non-siliceous species in otherwise siliceous sponge clades, and the similarity of the organic components of siliceous and collagenous skeletons, means that silicification was lost and/or re-evolved multiple times in the sponges (Ehrlich et al., 2007a,b; Maldonado, 2009; Kozhemyako et al., 2010). Similar evidence for independent losses and reinventions of biosilicification can be found in the centrohelids, a taxonomically enigmatic group of protists (Zlatogursky, 2016). Based on current phylogenetic data, centrohelids have evolved silicified structures ranging from scales to spines, but in some species these have been modified into organic-only scales and spines, or even been reduced so that the cell is covered only by a mucous sheath. Parallel evolution is also observed in the Arcellid testate amoebae, with convergent gains and losses of biosiliceous or organic tests (Lahr et al., 2013, 2015), or even the ability to form a test by agglutination of exogenous siliceous particles such as quartz grains, a strategy also developed by some coastal tintinnids.
A possible analog of the scenarios that saw these losses of biosilicification is the situation where silicification is facultative in some organisms (as opposed to the obligate silicification found in groups such as diatoms). This can occur under conditions of extreme silicon limitation, as in the tectiform choanoflagellates or synurophytes which produce naked, but otherwise healthy, cells if starved of silicon (Sandgren et al., 1996; Leadbeater, 2015). It may also be connected to stages in the life cycle, for example in juvenile brachiopods (Williams et al., 2001), or resembling in the haploid/diploid distinction between calcified and uncalcified stages of the E. huxleyi life cycle (Taylor et al., 2017) as has recently been suggested to occur in loricate choanoflagellates (Thomsen and Østergaard, 2017). The capacity for facultative silicification could be a combination of the two, as in the diatom P. tricornutum, unique amongst the diatoms in its ability to survive without a siliceous frustule. A combination of life cycle stage (Kessenich et al., 2014) and silicon availability (Yamada et al., 2014) has been put forward for explaining the evolution of the naked bolidophytes and siliceous parmales. As these are the sister lineages to diatoms the molecular mechanisms regulating their biosilicification are crucial to understanding how the complex silicon-related metabolism of diatoms evolved.
We therefore observe how evolutionary competition and the associated trade-offs are central to the presence of silicification: biomineralized silica structures are only maintained when they confer a competitive advantage. Silicification would be lost when the total metabolic cost of biosilicification exceeds the benefit, for example due to the energetic cost of silicic acid acquisition or silica polymerization, restrictions on mobility due to the presence of a skeleton or the additional weight per unit volume of silica versus organic structures. Alternatively, it could be metabolically cheaper (either in cost of formation or of lifestyle trade-offs) to build a functionally equivalent structure from another biomineral (such as calcium phosphate or calcium carbonate shells), from organic components (e.g., chitin, cellulose, or collagen) or even to utilize the silica structures of other organisms (Lahr et al., 2015). Under this hypothesis these structures would provide similar competitive advantages, such as protection, while allowing survival in new (often silicon-depleted) niches. This could potentially lead to the loss of the molecular mechanisms for silicon transport and silica polymerization, producing a developmental legacy that restricts the potential for future evolutionary innovations, and reinforces the competitive interactions between silicifiers and non-silicifiers.
Future Directions
Recent years have witnessed several new discoveries that have expanded and improved our understanding of silica biomineralization. Fossil sampling and isotopic analyses of new sediment records have modified our view of the geological history of silicon biogeochemistry (Fontorbe et al., 2016). The massive increase in large-scale sequencing data has greatly aided research into the molecular biology of silica biomineralization, both through whole-genome sequencing within groups like diatoms and plants, and via transcriptome sequencing of new and previously poorly researched siliceous taxa (Keeling et al., 2014; Beisser et al., 2017; Caron et al., 2017; Tirichine et al., 2017). Many new genes associated with biosilicification have been identified (Kotzsch et al., 2016), including the recognition that active silicon transporter gene families are much more ancient and widely distributed amongst the eukaryotes than previously thought (Marron et al., 2016b). This has been complemented by the discovery of new silicifying species (Ichinomiya et al., 2011), most notably silicon accumulation in some strains of the hugely ecologically important cyanobacteria Synechococcus (Baines et al., 2012). Combined with metagenomic surveys and geochemical monitoring (Mutsuo et al., 2015; Sunagawa et al., 2015) it will be possible to gain a much deeper knowledge of the role of different marine groups in silicon biogeochemistry and how competitive interactions govern their ecology, distribution and response to changing climatic conditions (Mock et al., 2016).
These advances have contributed to hypotheses for a greater role of silicon in biology, for example in protein folding (Eglin et al., 2006). We are also beginning to recognize a link between phosphate transporters and silicic acid uptake in groups as disparate as cyanobacteria and mammals (Brzezinski et al., 2017; Ratcliffe et al., 2017) that may be underpinned by a more general system for metalloid metabolism (Bienert et al., 2007). This could contribute to satisfying metabolic requirements for silicon in taxa like phaeophyte brown algae, which are known to biosilicify (Mizuta and Yasui, 2012; Tarakhovskaya et al., 2012) but with no currently identified silicon transporter genes (Marron et al., 2016b). These observations blur the boundary between silicifying and non-silicifying species, illustrated by the requirement for silicon in processes such as haptophyte calcium carbonate formation (Durak et al., 2016), amorphous calcium carbonate formation in plant cystoliths (Gal et al., 2012) and in the early developmental stages of the vertebrate calcium phosphate skeleton (Carlisle, 1981); or given the uptake of silicic acid (Fuhrman et al., 1978) and presence of silicon transporters in apparently non-mineralizing species (e.g., Florenciella; Marron et al., 2016b). This has implications for understanding the evolution and competitive interactions between silicifiers and non-silicifiers in the living world.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
KH was funded by the European Research Council (Starter Grant ICY-LAB-678371) and a Royal Society University Research Fellowship.
AM was funded by the European Research Council (Advanced Investigator grant no. 247333) and a Wellcome Trust Senior Investigator Award to Raymond E. Goldstein (University of Cambridge, UK).
CB acknowledges funding from the ERC Advanced Award “Diatomite,” the LouisD Foundation, the Gordon and Betty Moore Foundation, and the French Government “Investissements d'Avenir” programmes MEMO LIFE (ANR-10-LABX-54), PSL* Research University (ANR-1253 11-IDEX-0001-02), and OCEANOMICS (ANR-11-BTBR-0008). CB also thanks the Radcliffe Institute of Advanced Study at Harvard University for a Scholars Fellowship during the 2016-2017 academic year. This article is contribution number 70 of the Tara Oceans project.
BQ acknowledges funding by OSU Pytheas, EUROMARINE consortium, and Labex OT-Med (no. ANR-11-LABEX-0061) from the “Investissements d'Avenir” program of the French National Research Agency through the A*MIDEX project (no. ANR-11-IDEX-0001-02), as sponsors of the SILICAMICS workshop where initiation of this paper took place.
References
Abelmann, A., Gersonde, R., Knorr, G., Zhang, X., Chapligin, B., Maier, E., et al. (2015). The seasonal sea-ice zone in the glacial Southern Ocean as a carbon sink. Nat. Commun. 6, 8136. doi: 10.1038/ncomms9136
Adl, S. M., Simpson, A. G. B., Lane, C. E., Lukeš, J., Bass, D., Bowser, S. S., et al. (2012). The revised classification of eukaryotes. J. Eukaryot. Microbiol. 59, 429–493. doi: 10.1111/j.1550-7408.2012.00644.x
Aitken, Z. H., Luo, S., Reynolds, S. N., Thaulow, C., and Greer, J. R. (2016). Microstructure provides insights into evolutionary design and resilience of Coscinodiscus sp. frustule. Proc. Natl. Acad. Sci. U.S.A. 113, 2017–2022. doi: 10.1073/pnas.1519790113
Araujo, M. B., and Rozenfeld, A. (2014). The geographic scaling of biotic interactions. Ecography 37, 406–415. doi: 10.1111/j.1600-0587.2013.00643.x
Armbrust, E. V., Berges, J. A., Bowler, C., Green, B. R., Martinez, D., Putnam, N. H., et al. (2004). The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306, 79–86. doi: 10.1126/science.1101156
Assmy, P., Henjes, J., Klaas, C., and Smetacek, V. (2007). Mechanisms determining species dominance in a phytoplankton bloom induced by the iron fertilization experiment EisenEx in the Southern Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap 54, 340–362. doi: 10.1016/j.dsr.2006.12.005
Assmy, P., Smetacek, V., Montresor, M., Klaas, C., Henjes, J., et al. (2013). Thick-shelled, grazer-protected diatoms decouple ocean carbon and silicon cycles in the iron-limited Antarctic Circumpolar Current. Proc. Natl. Acad. Sci. U.S.A. 110, 20633–20638 doi: 10.1073/pnas.1309345110
Baines, S. B., Twining, B. S., Brzezinski, M. A., Krause, J. W., Vogt, S., Assael, D., et al. (2012). Significant silicon accumulation by marine picocyanobacteria. Nat. Geosci. 5, 886–891. doi: 10.1038/ngeo1641
Bateman, B. L., Vanderwal, J., Williams, S. E., and Johnson, C. N. (2012). Biotic interactions influence the projected distribution of a specialist mammal under climate change. Divers. Distrib. 18, 861–872. doi: 10.1111/j.1472-4642.2012.00922.x
Beisser, D., Graupner, N., Bock, C., Wodniok, S., Grossmann, L., Vos, M., et al. (2017). Comprehensive transcriptome analysis provides new insights into nutritional strategies and phylogenetic relationships of chrysophytes. PeerJ 5:e2832. doi: 10.7717/peerj.2832
Benoiston, A.-S., Ibarbalz, F. M., Bittner, L., Guidi, L., Jahn, O., Dutkiewicz, S., et al. (2017). The evolution of diatoms and their biogeochemical functions. Proc. R. Soc. B Biol. Sci. 372:20160397. doi: 10.1098/rstb.2016.0397
Betancor, L., and Luckarift, H. R. (2008). Bioinspired enzyme encapsulation for biocatalysis. Trends Biotechnol. 26, 566–572. doi: 10.1016/j.tibtech.2008.06.009
Biard, T., Stemmann, L., Picheral, M., Mayot, N., Vandromme, P., Hauss, H., et al. (2016). In situ imaging reveals the biomass of giant protists in the global ocean. Nature 532, 504–507. doi: 10.1038/nature17652
Bienert, G. P., Schüssler, M. D., and Jahn, T. P. (2007). Metalloids: essential, beneficial or toxic? Major intrinsic proteins sort it out. Trends Biochem. Sci. 33, 20–26. doi: 10.1016/j.tibs.2007.10.004
Bondoc, K. G. V., Heuschele, J., Gillard, J., Vyverman, W., and Pohnert, G. (2016). Selective silicate-directed motility in diatoms. Nat. Commun. 7:10540. doi: 10.1038/ncomms10540
Bork, P., Bowler, C., de Vargas, C., Gorsky, G., Karsenti, E., and Wincker, P. (2015). Tara Oceans studies plankton at planetary scale. Science 348, 873–873. doi: 10.1126/science.aac5605
Bowler, C., Allen, A. E., Badger, J. H., Grimwood, J., Jabbari, K., Kuo, A., et al. (2008). The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456, 239–244. doi: 10.1038/nature07410
Brown, J. W., and Sorhannus, U. (2010). A molecular genetic timescale for the diversification of autotrophic stramenopiles (Ochrophyta): substantive underestimation of putative fossil ages. PLoS ONE 5:e12759. doi: 10.1371/journal.pone.0012759
Brunner, E., Richthammer, P., Ehrlich, H., Paasch, S., Simon, P., Ueberlein, S., et al. (2009). Chitin in biosilica chitin-based organic networks: an integral part of cell wall biosilica in the diatom Thalassiosira pseudonana. Angew. Chem. 48, 9724–9727. doi: 10.1002/anie.200905028
Brzezinski, M. A., Krause, J. W., Baines, S. B., Collier, J. L., Ohnemus, D. C., and Twining, B. S. (2017). Patterns and regulation of silicon accumulation in Synechococcus spp. J. Phycol. 53, 764–761. doi: 10.1111/jpy.12545
Campbell, R. G., Sherrb, E. N., Ashjian, C. J., Plourde, S., Sherrb, B. F., Hille, V., et al. (2009). Mesozooplanktonprey preference and grazing impact in the Western Arctic Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 56, 1274–1289. doi: 10.1016/j.dsr2.2008.10.027
Carlisle, E. M. (1981). “Silicon in bone formation,” in Silicon and Siliceous Structures in Biological Systems, eds T. L. Simpson and B. Volcani (New York, NY: Springer-Verlag), 69–94.
Caron, D. A., Alexander, H., Allen, A. E., Archibald, J. M., Armbrust, E. V., Bachy, C., et al. (2017). Probing the evolution, ecology and physiology of marine protists using transcriptomics. Nat. Rev. Microbiol. 15, 6–20. doi: 10.1038/nrmicro.2016.160
Cermeño, P., Falkowski, P. G., Romero, O. E., Schaller, M. F., and Vallina, S. M. (2015). Continental erosion and the Cenozoic rise of marine diatoms. Proc. Natl. Acad. Sci. U.S.A. 112, 4239–4244. doi: 10.1073/pnas.1412883112
Cohen, B. L. (2005). Not armour, but biomechanics, ecological opportunity and increased fecundity as keys to the origin and expansion of the mineralized benthic metazoan fauna. Biol. J. Linn. Soc. 85, 483–490. doi: 10.1111/j.1095-8312.2005.00507.x
Conley, D. J., Frings, P. J., Fontorbe, G., Clymans, W., Stadmark, J., Hendry, K. R., et al. (2017). Biosilicification drives a decline of dissolved Si in the oceans through geologic time. Front. Mar. Sci. 4:397. doi: 10.3389/fmars.2017.00397
Cooke, J., and Leishman, M. R. (2011). Is plant ecology more siliceous than we realise? Trends Plant Sci. 16, 61–68. doi: 10.1016/j.tplants.2010.10.003
Crombet, Y., Leblanc, K., Quéguiner, B., Moutin, T., Rimmelin, P., Ras, J., et al. (2011). Deep silicon maxima in the stratified oligotrophic Mediterranean Sea. Biogeosciences 8, 459–475. doi: 10.5194/bg-8-459-2011
Darley, W. M., and Volcani, B. E. (1969). Role of silicon in diatom metabolism: a silicon requirement for deoxyribonucleic acid synthesis in the diatom silicon requirement for DNA synthesis in the diatom Cylindrotheca fusiformis Reimann and Lewin. Exp. Cell Res. 58, 334–342. doi: 10.1016/0014-4827(69)90514-X
de la Rocha, C., Brzezinski, M. A., and DeNiro, M. J. (1997). Fractionation of silicon isotopes by marine diatoms during biogenic silica formation, Geochim. Cosmochim. Acta 61, 5051–5056. doi: 10.1016/S0016-7037(97)00300-1
de Vargas, C., Audic, S., Henry, N., Decelle, J., Mahé, F., Logares, R., et al. (2015). Eukaryotic plankton diversity in the sunlit ocean. Science 348:1261605. doi: 10.1126/science.1261605
Del Amo, Y., and Brzezinski, M. A. (1999). The chemical form of dissolved Si taken up by marine diatoms. J. Phycol. 35, 1162–1170. doi: 10.1046/j.1529-8817.1999.3561162.x
Derelle, R., López-García, P., Timpano, H., and Moreira, D. (2016). A phylogenomic framework to study the diversity and evolution of stramenopiles (=heterokonts). Mol. Biol. Evol. 33, 2890–2898. doi: 10.1093/molbev/msw168
Desouky, M., Jugdaohsingh, R., McCrohan, C. R., White, K. N., and Powell, J. J. (2002). Aluminum-dependent regulation of intracellular silicon in the aquatic invertebrate Lymnaea stagnalis. Proc. Natl. Acad. Sci. U.S.A. 99, 3394–3399. doi: 10.1073/pnas.062478699
Dougherty, L. F., Johnsen, S., Caldwell, R. L., and Marshall, N. J. (2014). A dynamic broadband reflector built from microscopic silica spheres in the “disco” clam Ctenoides ales. J. R. Soc. Interface 11:20140407. doi: 10.1098/rsif.2014.0407
Drescher, B., Dillaman, R. M., and Taylor, A. R. (2012). Coccolithogenesis in Scyphosphaera apsteinii (Prymnesiophyceae). J. Phycol. 48, 1343–1361. doi: 10.1111/j.1529-8817.2012.01227.x
Durak, G. M., Taylor, A. R., Walker, C. E., Probert, I., de Vargas, C., Audic, S., et al. (2016). A role for diatom-like silicon transporters in calcifying coccolithophores. Nat. Commun. 7:10543. doi: 10.1038/ncomms10543
Durkin, C. A., Koester, J. A., Bender, S. J., and Armbrust, E. V. (2016). The evolution of silicon transporters in diatoms. J. Phycol. 52, 716–734. doi: 10.1111/jpy.12441
Dvorák, P., Casamatta, D. A., Poulíčkov,á, A., Hašler, P., Ondrej, V., and Sanges, R. (2014). Synechococcus: 3 billion years of global dominance. Mol. Ecol. 23, 5538–5551, doi: 10.1111/mec.12948
Egan, K., Rickaby, R. E. M., Hendry, K. R., and Halliday, A. N. (2013). Opening the gateways for diatoms primes Earth for Antarctic glaciation. Earth Planet. Sci. Lett. 375, 34–43. doi: 10.1016/j.epsl.2013.04.030
Eglin, D., Shafran, K. L., Livage, J., Coradin, T., and Perry, C. C. (2006). Comparative study of the influence of several silica precursors on collagen self-assembly and of collagen on Si speciation and condensation. J. Mater. Chem. 16, 4220–4230. doi: 10.1039/B606270A
Ehrlich, H., Heinemann, S., Heinemann, C., Simon, P., Tabachnick, K. R., Bazhenov, V. V., et al. (2008). Nanostructural organization of naturally occurring composites - Part I: silica-collagen-based biocomposites. J. Nanomater. 2008:623838. doi: 10.1155/2008/623838
Ehrlich, H., Krautter, M., Hanke, T., Simon, P., Knieb, C., Heinemann, S., et al. (2007a). First evidence of the presence of chitin in skeletons of marine sponges - Part II: glass sponges (Hexactinellida: Porifera). Biophys. J. 308, 473–483. doi: 10.1002/jez.b.21174
Ehrlich, H., Maldonado, M., Spindler, K., Eckert, C., Hanke, T., and Born, R. (2007b). First evidence of chitin as a component of the skeletal fibers of marine sponges. Part, I. Verongidae (Demospongia : Porifera). J. Exp. Zool. B. Mol. Dev. Evol. 308B, 347–356. doi: 10.1002/jez.b.21156
Ensikat, H.-J., Geisler, T., and Weigend, M. (2016). A first report of hydroxylated apatite as structural biomineral in Loasaceae-plants' teeth against herbivores. Sci. Rep. 6:26073. doi: 10.1038/srep26073
Feng, L., and Frommer, W. B. (2015). Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem. Sci. 40, 480–486. doi: 10.1016/j.tibs.2015.05.005
Finkel, Z. V., and Kotrc, B. (2010). Silica use through time: macroevolutionary change in the morphology of the diatom fustule. Geomicrobiol. J. 27, 596–608. doi: 10.1080/01490451003702941
Finkel, Z. V., Katz, M. E., Wright, J. D., Schofieid, O. M., and Falkowski, P. G. (2005). Climatically driven macroevolutionary patterns in the size of marine diatoms over the Cenozoic. Proc. Natl. Acad. Sci. U.S.A. 102, 8927–8932. doi: 10.1073/pnas.0409907102
Finkel, Z. V., Matheson, K. A., Regan, K. S., and Irwin, A. J. (2010). Genotypic and phenotypic variation in diatom silicification under paleo-oceanographic conditions. Geobiology 8, 433–445. doi: 10.1111/j.1472-4669.2010.00250.x
Foissner, W., Weissenbacher, B., Krautgartner, W.-D., and Lütz-Meindl, U. (2009). A cover of glass: first report of biomineralized silicon in a ciliate, Maryna umbrellata (Ciliophora: Colpodea). J. Eukaryot. Microbiol. 56, 519–530. doi: 10.1111/j.1550-7408.2009.00431.x
Fontorbe, G., Frings, P. J., De La Rocha, C. L., Hendry, K. R., and Conley, D. J. (2016). A silicon depleted North Atlantic since the Palaeogene: evidence from sponge and radiolarian silicon isotopes. Earth Planet. Sci. Lett. 453, 67–77. doi: 10.1016/j.epsl.2016.08.006
Fontorbe, G., Frings, P. J., De La Rocha, C. L., Hendry, K. R., and Conley, D. J. (2017). Enrichment of dissolved silica in the deep Equatorial Pacific during the Eocene-Oligocene. Paleoceanography, 32, 848–863. doi: 10.1002/2017PA003090
Friedrichs, L., Hörnig, M., Schulze, L., Bertram, A., Jansen, S., and Hamm, C. (2013). Size and biomechanic properties of diatom frustules influence food uptake by copepods. Mar. Ecol. Prog. Ser. 481, 41–51. doi: 10.3354/meps10227
Fuhrman, J., Chisholm, S., and Guillard, R. (1978). Marine alga Platymonas sp. accumulates silicon without apparent requirement. Nature 272, 244–246. doi: 10.1038/272244a0
Fuhrmann, T., Landwehr, S., El Rharbl-Kucki, M., and Sumper, M. (2004). Diatoms as living photonic crystals. Appl. Phys. B Lasers Opt. 78, 257–260. doi: 10.1007/s00340-004-1419-4
Gal, A., Hirsch, A., Siegel, S., Li, C., Aichmayer, B., Politi, Y., et al. (2012). Plant cystoliths : a complex functional biocomposite of four distinct silica and amorphous calcium carbonate phases. Chem. A Eur. J. 18, 10262–10270. doi: 10.1002/chem.201201111
Garbuzov, M., Reidinger, S., and Hartley, S. E. (2011). Interactive effects of plant-available soil silicon and herbivory on competition between two grass species. Ann. Bot. 108, 1355–1363. doi: 10.1093/aob/mcr230
Gause, G. F. (1934). Experimental analysis of Vito Volterra's mathematical theory of the struggle for existence. Science 79, 16–17.
Gong, N., Wiens, M., Schröder, H. C., Mugnaioli, E., Kolb, U., and Müller, W. E. G. (2010). Biosilicification of loricate choanoflagellate: organic composition of the nanotubular siliceous costal strips of Stephanoeca diplocostata. J. Exp. Biol. 213, 3575–3585. doi: 10.1242/jeb.048496
Gordon, R., Losic, D., Tiffany, M. A., Nagy, S. S., and Sterrenburg, F. A. (2009). The Glass Menagerie: diatoms for novel applications in nanotechnology. Trends Biotechnol. 27, 116–127. doi: 10.1016/j.tibtech.2008.11.003
Gotelli, N. J., Graves, G. R., and Rahbek, C. (2010). Macroecological signals of species interactions in the Danish avifauna. Proc. Natl. Acad. Sci. U.S.A. 107, 5030–5035. doi: 10.1073/pnas.0914089107
Gravel, D., Massol, F., Canard, E., Mouillot, D., and Mouquet, N. (2011). Trophictheory of island biogeography. Ecol. Lett. 14, 1010–1016. doi: 10.1111/j.1461-0248.2011.01667.x
Green, J. L., Bohannan, B. J. M., and Whitaker, R. J. (2008). Microbial biogeography: from taxonomy to traits. Science 320, 1039–1043. doi: 10.1126/science.1153475
Grégoire, C., Rémus-Borel, W., Vivancos, J., Labbé, C., Belzile, F., and Bélanger, R. R. (2012). Discovery of a multigene family of aquaporin silicon transporters in the primitive plant Equisetum arvense. Plant J. 72, 320–330. doi: 10.1111/j.1365-313X.2012.05082.x
Grenne, T., and Slack, J. F. (2003). Paleozoic and Mesozoic silica-rich seawater: evidence from hematitic chert (jasper) deposits. Geology 31, 319–322. doi: 10.1130/0091-7613(2003)031<0319:PAMSRS>2.0.CO;2
Gunnarsson, I., and Arnórsson, S. (2000). Amorphous silica solubility and the thermodynamic properties of H4SiO4 in the range of 0° to 350°C at Psat. Geochim. Cosmochim. Acta 64, 2295–2307. doi: 10.1016/S0016-7037(99)00426-3
Hamm, C. E., Merkel, R., Springer, O., and Jurkojc, P. (2003). Architecture and material properties of diatom shells provide effective mechanical protection. Nature 421, 841–843. doi: 10.1038/nature01416
Hamm, C., and Smetacek, V. (2007). “Armor: why, when, and how,” in Evolution of Primary Producers in the Sea, eds P. Falkowski and A. H. Knoll (Amsterdam: Elsevier), 311–332.
Hardin, G. (1960). The competitive exclusion principle. Science 131, 1292–1297. doi: 10.1126/science.131.3409.1292
Harper, H. E., and Knoll, A. H. (1975). Silica, diatoms, and cenozoic radiolarian evolution. Geology 3, 175–177. doi: 10.1130/0091-7613(1975)3<175:SDACRE>2.0.CO;2
Hartley, S. E., DeGabriel, J. L., and Cooke, J. (2016). The ecology of herbivore-induced silicon defences in grasses. Funct. Ecol. 30, 1311–1322. doi: 10.1111/1365-2435.12706
He, H., Veneklaas, E. J., Kuo, J., and Lambers, H. (2014). Physiological and ecological significance of biomineralization in plants. Trends Plant Sci. 19, 166–174. doi: 10.1016/j.tplants.2013.11.002
Hecky, R. E., Mopper, K., Kilham, P., and Degens, E. T. (1973). The amino acids and sugar composition of diatom cell-walls. Mar. Biol. 19, 323–331. doi: 10.1007/BF00348902
Hendry, K. R., and Robinson, L. F. (2012). The relationship between silicon isotope fractionation in sponges and silicic acid concentration: modern and core-top studies of biogenic opal. Geochim. Cosmochim. Acta 81, 1–12. doi: 10.1016/j.gca.2011.12.010
Hendry, K. R., Robinson, L. F., McManus, J. F., and Hays, J. D. (2014). Silicon isotopes indicate enhanced carbon export efficiency in the North Atlantic during deglaciation. Nat. Commun. 5, 3107. doi: 10.1038/ncomms4107
Hildebrand, M., Dahlin, K., and Volcani, B. E. (1998). Characterization of a silicon transporter gene family in Cylindrotheca fusiformis: sequences, expression analysis, and identification of homologs in other diatoms. Mol. Gen. Genet. 260, 480–486. doi: 10.1007/s004380050920
Hildebrand, M., Volcani, B. E., Gassman, W., and Schroeder, J. (1997). A gene family of silicon transporters. Nature 385, 688–689. doi: 10.1038/385688b0
Hodson, M. J., White, P. J., Mead, A., and Broadley, M. R. (2005). Phylogenetic variation in the silicon composition of plants. Ann. Bot. 96, 1027–1046. doi: 10.1093/aob/mci255
Hortal, J., de Bello, F., Diniz-Filho, J. A. F., Lewinsohn, T. M., Lobo, J. M., and Ladle, R. J. (2015). Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 46, 523–549. doi: 10.1146/annurev-ecolsys-112414-054400
Ichinomiya, M., Yoshikawa, S., Kamiya, M., Ohki, K., Takaichi, S., and Kuwata, A. (2011). Isolation and characterization of parmales (Heterokonta/Heterokontophyta/Stramenopiles) from the Oyashio Region, Western North Pacific. J. Phycol. 47, 144–151. doi: 10.1111/j.1529-8817.2010.00926.x
Ingalls, A. E., Whitehead, K., and Bridoux, M. C. (2010). Tinted windows: the presence of the UV absorbing compounds called mycosporine-like amino acids embedded in the frustules of marine diatoms. Geochim. Cosmochim. Acta 74, 104–115. doi: 10.1016/j.gca.2009.09.012
Itoh, K. (1970). A consideration on feeding habits of planktonic copepods in relation to the structure of their oral parts. Bull. Plankton Soc. Japan 17:1–10.
Karsenti, E., Acinas, S. G., Bork, P., Bowler, C., De Vargas, C., Raes, J., et al. (2011). A holistic approach to marine eco-systems biology. PLoS Biol. 9:e1001177. doi: 10.1371/journal.pbio.1001177
Keeling, P. J., Burki, F., Wilcox, H. M., Allam, B., Allen, E. E., Amaral-Zettler, L. A., et al. (2014). The marine microbial eukaryote transcriptome sequencing project (MMETSP): illuminating the functional diversity of eukaryotic life in the oceans through transcriptome sequencing. PLoS Biol. 12:e1001889. doi: 10.1371/journal.pbio.1001889
Keller, R., Ziegler, C., and Schneider, D. (2014). When two turn into one: evolution of membrane transporters from half modules. Biol. Chem. 395, 1379–1388. doi: 10.1515/hsz-2014-0224
Kessenich, C. R., Ruck, E. C., Schurko, A. M., Wickett, N. J., and Alverson, A. J. (2014). Transcriptomic insights into the life history of bolidophytes, the sister lineage to diatoms. J. Phycol. 50, 977–983. doi: 10.1111/jpy.12222
Kidder, D. L., and Tomescu, I. (2016). Biogenic chert and the Ordovician silica cycle. Palaeogeogr. Palaeoclimatol. Palaeoecol. 458, 29–38. doi: 10.1016/j.palaeo.2015.10.013
Knight, M. J., Senior, L., Nancolas, B., Ratcliffe, S., and Curnow, P. (2016). Direct evidence of the molecular basis for biological silicon transport. Nat. Commun. 7:11926. doi: 10.1038/ncomms11926
Knoll, A. H. (2003). “Biomineralization and evolutionary history,” in Reviews in Mineralogy and Geochemistry: Biomineralization, eds P. M. Dove, J. J. Deyoreo, and S. Weiner (Washington, DC: Mineralogical Society of America), 329–356.
Knoll, A. H., and Follows, M. J. (2016). A bottom-up perspective on ecosystem change in Mesozoic oceans. Proc. R. Soc. B 283:20161755. doi: 10.1098/rspb.2016.1755
Knoll, A. H., and Kotrc, B. (2015). “Protistan skeletons: a geologic history of evolution and constraint,” in Evolution of Lightweight Structures, ed C. Hamm (Dordrecht: Springer), 1–16.
Kotrc, B., and Knoll, A. H. (2015). A morphospace of planktonic marine diatoms. I. Two views of disparity through time. Paleobiology 41, 45–67. doi: 10.1017/pab.2014.4
Kotzsch, A., Pawolski, D., Milentyev, A., Shevchenko, A., Scheffel, A., Poulsen, N., et al. (2016). Biochemical composition and assembly of biosilica-associated insoluble organic matrices from the diatom Thalassiosira pseudonana. J. Biol. Chem. 291, 4982–4997. doi: 10.1074/jbc.M115.706440
Kouchinsky, A., Bengtson, S., Runnegar, B., Skovsted, C., Steiner, M., and Vendrasco, M. (2011). Chronology of early Cambrian biomineralization. Geol. Mag. 149, 221–251. doi: 10.1017/S0016756811000720
Kozhemyako, V. B., Veremeichik, G. N., Shkryl, Y. N., Kovalchuk, S. N., Krasokhin, V. B., Rasskazov, V. A., et al. (2010). Silicatein genes in spicule-forming and nonspicule-forming Pacific demosponges. Mar. Biotechnol. 12, 403–409. doi: 10.1007/s10126-009-9225-y
Krabberød, A. K., Bråte, J., Dolven, J. K., Ose, R. F., Klaveness, D., Kristensen, T., et al. (2011). Radiolaria divided into Polycystina and Spasmaria in combined 18S and 28S rDNA phylogeny. PLoS ONE 6:e23526. doi: 10.1371/journal.pone.0023526
Kröger, N., Deutzmann, R., Bergsdorf, C., and Sumper, M. (2000). Species-specific polyamines from diatoms control silica morphology. Proc. Natl. Acad. Sci. U.S.A 97, 14133–14138. doi: 10.1073/pnas.260496497
Kröger, N., Lorenz, S., Brunner, E., and Sumper, M. (2002). Self-assembly of highly phosphorylated silaffins and their function in biosilica morphogenesis. Science 298, 584–586. doi: 10.1126/science.1076221
Ku, C., Nelson-sathi, S., Roettger, M., Sousa, F. L., Lockhart, P. J., Bryant, D., et al. (2015). Endosymbiotic origin and differential loss of eukaryotic genes. Nature 524, 427–437. doi: 10.1038/nature14963
Kunitomo, Y., Sarashina, I., Iijima, M., Endo, K., and Sashida, K. (2006). Molecular phylogeny of acantharian and polycystine radiolarians based on ribosomal DNA sequences, and some comparisons with data from the fossil record. Eur. J. Protistol. 42, 143–153. doi: 10.1016/j.ejop.2006.04.001
Lahr, D. J. G., Bosak, T., Lara, E., and Mitchell, E. A. D. (2015). The Phanerozoic diversification of silica-cycling testate amoebae and its possible links to changes in terrestrial ecosystems. PeerJ 3:e1234. doi: 10.7717/peerj.1234
Lahr, D. J. G., Grant, J. R., and Katz, L. A. (2013). Multigene phylogenetic reconstruction of the tubulinea (Amoebozoa) corroborates four of the six major lineages, while additionally revealing that shell composition does not predict phylogeny in the arcellinida. Protist 164, 323–339. doi: 10.1016/j.protis.2013.02.003
Lazarus, D. B., Kotrc, B., Wulf, G., and Schmidt, D. N. (2009). Radiolarians decreased silicification as an evolutionary response to reduced Cenozoic ocean silica availability. Proc. Natl. Acad. Sci. U.S.A. 106, 9333–9338. doi: 10.1073/pnas.0812979106
Lazarus, D., Barron, J., Renaudie, J., Diver, P., and Türke, A. (2014). Cenozoic planktonic marine diatom diversity and correlation to climate change. PLoS ONE 9:e84857. doi: 10.1371/journal.pone.0084857
Leadbeater, B. S. C. (2015). The Choanoflagellates: Evolution, Biology and Ecology. Cambridge: Cambridge University Press.
Leadbeater, B. S. C., Yu, Q., Kent, J., and Stekel, D. J. (2009). Three-dimensional images of choanoflagellate loricae. Proc. R. Soc. B Biol. Sci. 276, 3–11. doi: 10.1098/rspb.2008.0844
Leblanc, K., Leynaert, A., Fernandez, I. C., Rimmelin, P., Moutin, T., Raimbault, P., et al. (2005). A seasonal study of diatom dynamics in the North Atlantic during the POMME experiment (2001): evidence for Si limitation of the spring bloom. J. Geophys. Res. 110:C07S14. doi: 10.1029/2004JC002621
Leblanc, K., Quéguiner, B., Garcia, N., Rimmelin, P., and Raimbault, P. (2003). Silicon cycle in the NW Mediterranean Sea: seasonal study of a coastal oligotrophic site. Oceanol. Acta 26, 339–355. doi: 10.1016/S0399-1784(03)00035-5
Lebour, M. V. (1922). The food of plankton organisms. J. Mar. Biol. Assoc. U. K. 12, 644–677. doi: 10.1017/S0025315400009681
Lee, R. E., and Kugrens, P. (1989). Biomineralization of the stalks of Anthophysa vegetans. J. Phycol. 25, 591–596. doi: 10.1111/j.1529-8817.1989.tb00265.x
Lepot, K., Addad, A., Knoll, A. H., Wang, J., Troadec, D., Béché, A., et al. (2017). Iron minerals within specific microfossil morphospecies of the 1.88 Ga Gunflint Formation. Nat. Commun. 8:14890. doi: 10.1038/ncomms14890
Lima-Mendez, G., Faust, K., Henry, N., Decelle, J., Colin, S., Carcillo, F., et al. (2015). Determinants of community structure in the global plankton interactome. Science 348:1262073 doi: 10.1126/science.1262073
Liu, H., Aris-Brosou, S., Probert, I., and De Vargas, C. (2010). A time line of the environmental genetics of the haptophytes. Mol. Biol. Evol. 27, 161–176. doi: 10.1093/molbev/msp222
Lobel, K. D., West, J. K., and Hench, L. L. (1996). Computational model for protein-mediated biomineralization of the diatom frustule. Mar. Biol. 126, 353–360. doi: 10.1007/BF00354617
Lopez, P. J., Desclés, J., Allen, A. E., and Bowler, C. (2005). Prospects in diatom research. Curr. Opin. Biotechnol. 16, 180–186. doi: 10.1016/j.copbio.2005.02.002
Losic, D., Rosengarten, G., Mitchell, J. G., and Voelcker, N. H. (2006). Pore architecture of diatom frustules: potential nanostructured membranes for molecular and particle separations. J. Nanosci. Nanotechnol. 6, 982–989. doi: 10.1166/jnn.2006.174
Ludwig, M., Lind, J., Miller, E., and Wetherbee, R. (1996). High molecular mass glycoproteins associated with the siliceous scales and bristles of Mallomonas splendens (Synurophyceae) may be involved in cell surface development and maintenance. Planta 199, 219–228. doi: 10.1007/BF00196562
MacArthur, R. H. (1972). Geographical Ecology: Patterns in the Distribution of Species. Princeton, NJ: Princeton University Press.
Maldonado, M. (2009). Embryonic development of verongid demosponges supports the independent acquisition of spongin skeletons as an alternative to the siliceous skeleton of sponges. Biol. J. Linn. Soc. 97, 427–447. doi: 10.1111/j.1095-8312.2009.01202.x
Maldonado, M., Carmona, M. C., and Cruzado, A. (1999). Decline in Mesozoic reef-building in sponges explained by silicon limitation. Nature 401, 785–788. doi: 10.1038/44560
Maldonado, M., Navarro, L., Grasa, A., Gonzalez, A., and Vaquerizo, I. (2011). Silicon uptake by sponges: a twist to understanding nutrient cycling on continental margins. Sci. Rep. 1:30. doi: 10.1038/srep00030
Maliva, R. G., Knoll, A. H., and Siever, R. (1989). Secular change in chert distribution: a reflection of evolving biological participation in the silica cycle. Palaois 4, 513–532. doi: 10.2307/3514743
Maliva, R. G., Knoll, A. H., and Simonson, B. M. (2005). Secular change in the Precambrian silica cycle: insights from chert petrology. Geol. Soc. Am. Bull. 117, 835–845. doi: 10.1130/B25555.1
Malviya, S., Scalco, E., Audic, S., Vincent, F., Veluchamy, A., Poulain, J., et al. (2016). Insights into global diatom distribution and diversity in the world's ocean. Proc. Natl. Acad. Sci. 113, E1516–E1525. doi: 10.1073/pnas.1509523113
Mann, S. (2001). Biomineralization. Principles and Concepts in Bioinorganic Materials Chemistry. Oxford: Oxford University Press.
Marin, F., Smith, M., Isa, Y., Muyzer, G., and Westbroek, P. (1996). Skeletal matrices, muci, and the origin of invertebrate calcification. Proc. Natl. Acad. Sci. U.S.A. 93, 1554–1559. doi: 10.1073/pnas.93.4.1554
Marron, A. O., Chappell, H., Ratcliffe, S., and Goldstein, R. E. (2016a). A model for the effects of germanium on silica biomineralization in choanoflagellates. J. R. Soc. Interface 13:20160485. doi: 10.1098/rsif.2016.0485
Marron, A. O., Ratcliffe, S., Wheeler, G. L., Goldstein, R. E., King, N., Not, F., et al. (2016b). The evolution of silicon transport in eukaryotes. Mol. Biol. Evol. 33, 3226–3248. doi: 10.1093/molbev/msw209
Marshall, S. M., and Orr, A. P. (1955). The Biology of a Marine Copepod, Calanus finmarchicus (Gunnerus). London: Oliver and Boyd.
Martin-Jézéquel, V., Hildebrand, M., and Brzezinski, M. A. (2000). Silicon metabolism in diatoms: implications for growth. J. Phycol. 36, 821–840. doi: 10.1046/j.1529-8817.2000.00019.x
Massey, F. P., Ennos, A. R., and Hartley, S. U. E. E. (2006). Silica in grasses as a defence against insect herbivores: contrasting effects on folivores and a phloem feeder. J. Anim. Ecol. 75, 595–603. doi: 10.1111/j.1365-2656.2006.01082.x
Matsunaga, S., Sakai, R., Jimbo, M., and Kamiya, H. (2007). Long-chain polyamines (LCPAs) from marine sponge: possible implication in spicule formation. Chembiochem 8, 1729–1735. doi: 10.1002/cbic.200700305
Michels, J., Vogt, J., and Gorb, S. N. (2012). Tools for crushing diatoms–opal teeth in copepods feature a rubber-like bearing composed of resilin. Sci. Rep. 2:465. doi: 10.1038/srep00465
Miller, C. B., Nelson, D. M., Guillard, R. R. L., and Woodward, B. L. (1980). Effects of media with low silicic acid concentrations on tooth formation in Acartia tonsa (Copepoda, Calanoida). Biol. Bull. 159, 349–363. doi: 10.2307/1541099
Miller, C. B., Nelson, D. M., Weiss, C., and Soeldner, A. H. (1990). Morphogenesis of opal teeth in calanoid copepods. Mar. Biol. 106, 91–101. doi: 10.1007/BF02114678
Milligan, A. J., and Morel, F. M. M. (2002). A proton buffering role for silica in diatoms. Science 297, 1848–1850. doi: 10.1126/science.1074958
Misra, S., and Froelich, P. N. (2012). Lithium isotope history of Cenozoic seawater: changes in silicate weathering and reverse weathering. Science 335, 818–823. doi: 10.1126/science.1214697
Mizuta, H., and Yasui, H. (2012). Protective function of silicon deposition in Saccharina japonica sporophytes (Phaeophyceae). J. Appl. Phycol. 24, 1177–1182. doi: 10.1007/s10811-011-9750-8
Mock, T., Daines, S. J., Geider, R., Collins, S., Metodiev, M., Millar, A. J., et al. (2016). Bridging the gap between omics and earth system science to better understand how environmental change impacts marine microbes. Glob. Chang. Biol. 22, 61–75. doi: 10.1111/gcb.12983
Mock, T., Samanta, M. P., Iverson, V., Berthiaume, C., Robison, M., Holtermann, K., et al. (2008). Whole-genome expression profiling of the marine diatom Thalassiosira pseudonana identifies genes involved in silicon bioprocesses. Proc. Natl. Acad. Sci. U.S.A. 105, 1579–1584. doi: 10.1073/pnas.0707946105
Monniot, F. O., Martojai, R., and Monniot, C. (1992). Silica distribution in ascidian ovaries, a tool for systematics. Systematics 20, 541–552. doi: 10.1016/0305-1978(92)90008-2
Moreira, D., von der Heyden, S., Bass, D., López-García, P., Chao, E., and Cavalier-Smith, T. (2007). Global eukaryote phylogeny: combined small- and large-subunit ribosomal DNA trees support monophyly of Rhizaria, Retaria and Excavata. Mol. Phylogenet. Evol. 44, 255–266. doi: 10.1016/j.ympev.2006.11.001
Morueta-Holme, N., Blonder, B., Sandel, B., McGill, B. J., Peet, R. K., Ott, J. E., et al. (2016). A network approach for inferring species associations from co-occurrence data. Ecography 39, 1139–1150. doi: 10.1111/ecog.01892
Mutsuo, I., Lopes dos Santos, A., Gourvil, P., Yoshikawa, S., Kamiya, M., Ohki, K., et al. (2015). Diversity and oceanic distribution of Parmales and Bolidophyceae, a picoplankton group closely related to diatoms. ISMEJ 10, 2419–2434. doi: 10.1038/ismej.2016.38
Nakov, T., Ashworth, M., and Theriot, E. C. (2014). Comparative analysis of the interaction between habitat and growth form in diatoms. ISME J. 9, 246–255. doi: 10.1038/ismej.2014.108
Nesbit, K. T., and Roer, R. D. (2016). Silicification of the medial tooth in the blue crab Callinectes sapidus. J. Morphol. 277, 1648–1660. doi: 10.1002/jmor.20614
Neumann, D., and zur Nieden, U. (2001). Silicon and heavy metal tolerance of higher plants. Phytochemistry 56, 685–692. doi: 10.1016/S0031-9422(00)00472-6
Nomura, M., and Ishida, K. (2016). Fine-structural observations on siliceous scale production and shell assembly in the testate amoeba Paulinella chromatophora. Protist 167, 303–318. doi: 10.1016/j.protis.2016.05.002
Parfrey, L. W., Lahr, D. J. G., Knoll, A. H., and Katz, L. A. (2011). Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc. Natl. Acad. Sci. U.S.A. 108, 13624–13629. doi: 10.1073/pnas.1110633108
Platt, T., White, G. N. III., Zhai, L., Sathyendranath, S., and Roy, S. (2009). The phenology of phytoplankton blooms: ecosystem indicators from remote sensing. Ecol. Modell. 220, 3057–3069. doi: 10.1016/j.ecolmodel.2008.11.022
Polet, S., Berney, C., Fahrni, J., and Pawlowski, J. (2004). Small-subunit ribosomal RNA gene sequences of Phaeodarea challenge the monophyly of Haeckel's Radiolaria. Protist 155, 53–63. doi: 10.1078/1434461000164
Pondaven, P., Gallinari, M., Chollet, S., Bucciarelli, E., Sarthou, G., Schultes, S., et al. (2007). Grazing-induced changes in cell wall silicification in a marine diatom. Protist 158, 21–28. doi: 10.1016/j.protis.2006.09.002
Poulsen, N., and Kröger, N. (2004). Silica morphogenesis by alternative processing of silaffins in the diatom Thalassiosira pseudonana. J. Biol. Chem. 279, 42993–42999. doi: 10.1074/jbc.M407734200
Preisig, H. R. (1994). Siliceous structures and silicification in flagellated protists. Protoplasma 181, 29–42. doi: 10.1007/BF01666387
Quéguiner, B. (2013). Iron fertilization and the structure of planktonic communities in high nutrient regions of the Southern Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 90, 43–54. doi: 10.1016/j.dsr2.2012.07.024
Racki, G., and Cordey, F. (2000). Radiolarian palaeoecology and radiolarites: is the present the key to the past? Earth Sci. Rev. 52, 83–120. doi: 10.1016/S0012-8252(00)00024-6
Ratcliffe, S., Jugdaohsingh, R., Vivancos, J., Marron, A., Deshmukh, R., Ma, J. F., et al. (2017). Identification of a mammalian silicon transporter. Am. J. Physiol. Cell Physiol. 312, C550–C561. doi: 10.1152/ajpcell.00219.2015
Ratti, S., Knoll, A. H., and Giordano, M. (2013). Grazers and phytoplankton growth in the oceans: an experimental and evolutionary perspective. PLoS ONE 8:e77349. doi: 10.1371/journal.pone.0077349
Raven, J. A. (1983). The transport and function of silicon in plants. Biol. Rev. 58, 179–207. doi: 10.1111/j.1469-185X.1983.tb00385.x
Raven, J. A., and Knoll, A. H. (2010). Non-skeletal biomineralization by eukaryotes: matters of moment and gravity. Geomicrobiol. J. 27, 572–584. doi: 10.1080/01490451003702990
Raven, J. A., and Waite, A. M. (2004). The evolution of silicification in diatoms: inescapable sinking and sinking as escape? New Phytol. 162, 45–61. doi: 10.1111/j.1469-8137.2004.01022.x
Renaudie, J. (2016). Quantifying the Cenozoic marine diatom deposition history: links to the C and Si cycles. Biogeosciences 13, 6003–6014. doi: 10.5194/bg-13-6003-2016
Rickaby, R. E. M. (2015). Goldilocks and the three inorganic equilibria: how Earth's chemistry and life coevolve to be nearly in tune. Philos. Trans. R. Soc. A 373:20140188. doi: 10.1098/rsta.2014.0188
Romann, J., Valmalette, J.-C., Chauton, M. S., Tranell, G., Einarsrud, M.-A., and Vadstein, O. (2015). Wavelength and orientation dependent capture of light by diatom frustule nanostructures. Sci. Rep. 5:17403. doi: 10.1038/srep17403
Sandgren, C. D., Hall, S. A., and Barlow, S. B. (1996). Siliceous scale production in chrysophyte and synurophyte algae.1. Effects of silica-limited growth on cell silica content, scale morphology, and the construction of the scale layer of Synura petersenii. J. Phycol. 32, 675–692. doi: 10.1111/j.0022-3646.1996.00675.x
Sapriel, G., Quinet, M., Heijde, M., Jourdren, L., Tanty, V., and Luo, G. (2009). Genome-wide transcriptome analyses of silicon metabolism in Phaeodactylum tricornutum reveal the multilevel regulation of silicic acid transporters. PLoS ONE 4:e7458. doi: 10.1371/journal.pone.0007458
Schaller, J., Brackhage, C., Bäucker, E., and Dudel, E. G. (2013). UV-screening of grasses by plant silica layer? J. Biosci. 38, 413–416. doi: 10.1007/s12038-013-9303-1
Scheffel, A., Poulsen, N., Shian, S., and Kröger, N. (2011). Nanopatterned protein microrings from a diatom that direct silica morphogenesis. Proc. Natl. Acad. Sci. U.S.A. 108, 3175–3180. doi: 10.1073/pnas.1012842108
Scoble, J. M., and Cavalier-Smith, T. (2014). Scale evolution, sequence phylogeny, and taxonomy of thaumatomonad Cercozoa: 11 new species and new genera Scutellomonas, Cowlomonas, Thaumatospina and Ovaloplaca. Eur. J. Protistol. 50, 270–313. doi: 10.1016/j.ejop.2013.12.005
Shimizu, K., Amano, T., Bari, M. R., Weaver, J. C., Arima, J., and Mori, N. (2015). Glassin, a histidine-rich protein from the siliceous skeletal system of the marine sponge Euplectella, directs silica polycondensation. Proc. Natl. Acad. Sci. U.S.A. 112, 11449–11454. doi: 10.1073/pnas.1506968112
Shrestha, R. P., and Hildebrand, M. (2015). Evidence for a regulatory role of diatom silicon transporters in cellular silicon responses. Eukaryot. Cell 14, 29–40. doi: 10.1128/EC.00209-14
Shrestha, R. P., Tesson, B., Norden-Krichmar, T., Federowicz, S., Hildebrand, M., and Allen, A. E. (2012). Whole transcriptome analysis of the silicon response of the diatom Thalassiosira pseudonana. BMC Genomics 13:499. doi: 10.1186/1471-2164-13-499
Siever, R. (1991). “Silica in the oceans: biological-geochemical interplay,” in Scientists on Gaia, eds S. Schneider and P. Boston (Cambridge, MA: MIT Press), 287–295.
Siever, R. (1992). The silica cycle in the Precambrian. Geochim. Cosmochim. Acta 56, 3265–3272. doi: 10.1016/0016-7037(92)90303-Z
Simkiss, K., and Wilbur, K. M. (1989). Biomineralization. Cell Biology and Mineral Deposition. San Diego CA: Academic Press Inc.
Sims, P. A., Mann, D. G., and Medlin, L. K. (2006). Evolution of the diatoms: insights from fossil, biological and molecular data. Phycologia 45, 361–402. doi: 10.2216/05-22.1
Smayda, T. J. (1970). The suspension and sinking of phytoplankton in the sea. Oceanogr. Mar. Biol. Annu. Rev. 8, 353–414.
Smith, J. M., and Szathmáry, E. (1995). The Major Transitions in Evolution. Oxford: Oxford University Press.
Sommer, U. (1994). The impact of light intensity and daylength on silicate and nitrate competition among marine phytoplankton. Limnol. Oceanogr. 39, 1680–1688. doi: 10.4319/lo.1994.39.7.1680
Sone, E. D., Weiner, S., and Addadi, L. (2007). Biomineralization of limpet teeth: a cryo-TEM study of the organic matrix and the onset of mineral deposition. J. Struct. Biol. 158, 428–444. doi: 10.1016/j.jsb.2007.01.001
Sperling, E. A., Robinson, J. M., Pisani, D., and Peterson, K. J. (2010). Where's the glass? Biomarkers, molecular clocks, and microRNAs suggest a 200-Myr missing Precambrian fossil record of siliceous sponge spicules. Geobiology 8, 24–36. doi: 10.1111/j.1472-4669.2009.00225.x
Spinde, K., Pachis, K., Antonakaki, I., Paasch, S., Brunner, E., and Demadis, K. D. (2011). Influence of polyamines and related macromolecules on silicic acid polycondensation: relevance to “Soluble Silicon Pools”? Chem. Mater. 23, 4676–4687. doi: 10.1021/cm201988g
Strom, S. L. (2008). Microbial ecology of ocean biogeochemistry: a community perspective. Science 320, 1043–1045. doi: 10.1126/science.1153527
Strom, S. L., Macri, E. L., and Olson, B. (2007). Microzooplankton grazing in the coastal Gulf of Alaska: variations in top-down control of phytoplankton. Limnol. Oceanogr. 52, 1480–1494. doi: 10.4319/lo.2007.52.4.1480
Sumper, M., and Kröger, N. (2004). Silica formation in diatoms: the function of long-chain polyamines and silaffins. J. Mater. Chem. 14, 2059–2065. doi: 10.1039/B401028K
Sumper, M., and Lehmann, G. (2006). Silica pattern formation in diatoms: species- specific polyamine biosynthesis. ChemBioChem 7, 1419–1427. doi: 10.1002/cbic.200600184
Sumper, M., Hett, R., Lehmann, G., and Wenzl, S. (2007). A code for lysine modifications of a silica biomineralizing silaffin protein. Angew. Chem. 46, 8405–8408. doi: 10.1002/anie.200702413
Sunagawa, S., Coelho, L. P., Chaffron, S., Kultima, J. R., Labadie, K., Salazar, G., et al. (2015). Structure and function of the global ocean microbiome. Science 348, 1–10. doi: 10.1126/science.1261359
Sundar, V. C., Yablon, A. D., Grazul, J. L., Ilan, M., and Aizenberg, J. (2003). Fibre-optical features of a glass sponge. Nature 424, 899–900. doi: 10.1038/424899a
Taberlet, P., Coissac, E., Pompanon, F., Brochmann, C., and Willerslev, E. (2012). Towards next-generation biodiversity assessment using DNA metabarcoding. Mol. Ecol. 21, 2045–2050. doi: 10.1111/j.1365-294X.2012.05470.x
Tao, Y., Cheung, L. S., Li, S., Eom, J.-S., Chen, L.-Q., Xu, Y., et al. (2015). Structure of a eukaryotic SWEET transporter in a homotrimeric complex. Nature 527, 259–263. doi: 10.1038/nature15391
Tarakhovskaya, E. R., Kang, E.-J., Kim, K.-Y., and Garbary, D. J. (2012). Effect of GeO2 on embryo development and photosynthesis in Fucus vesiculosus (Phaeophyceae). Algae 27, 125–134. doi: 10.4490/algae.2012.27.2.125
Taylor, A. R., Brownlee, C., and Wheeler, G. (2017). Coccolithophore cell biology : chalking up progress. Ann. Rev. Mar. Sci. 9, 283–310. doi: 10.1146/annurev-marine-122414-034032
Tesson, B., and Hildebrand, M. (2010a). Dynamics of silica cell wall morphogenesis in the diatom Cyclotella cryptica: substructure formation and the role of microfilaments. J. Struct. Biol. 169, 62–74. doi: 10.1016/j.jsb.2009.08.013
Tesson, B., and Hildebrand, M. (2010b). Extensive and intimate association of the cytoskeleton with forming silica in diatoms: control over patterning on the meso- and micro-scale. PLoS ONE 5:e14300. doi: 10.1371/journal.pone.0014300
Tesson, B., and Hildebrand, M. (2013). Characterization and localization of insoluble organic matrices associated with diatom cell walls: insight into their roles during cell wall formation. PLoS ONE 8:e61675. doi: 10.1371/journal.pone.0061675
Tesson, B., Lerch, S. J. L., and Hildebrand, M. (2017). Characterization of a new protein family associated with the silica deposition vesicle membrane enables genetic manipulation of diatom silica. Sci. Rep. 7, 13457. doi: 10.1038/s41598-017-13613-8
Thamatrakoln, K., Alverson, A. J., and Hildebrand, M. (2006). Comparative sequence analysis of diatom silicon transporters: toward a mechanistic model of silicon transport. J. Phycol. 42, 822–834. doi: 10.1111/j.1529-8817.2006.00233.x
Thamatrakoln, K., and Hildebrand, M. (2007). Analysis of Thalassiosira pseudonana silicon transporters indicates distinct regulatory levels and transport activity through the cell cycle. Eukaryot. Cell 6, 271–279. doi: 10.1128/EC.00235-06
Thamatrakoln, K., and Hildebrand, M. (2008). Silicon uptake in diatoms revisited: a model for saturable and nonsaturable uptake kinetics and the role of silicon transporters. Plant Physiol. 146, 1397–1407. doi: 10.1104/pp.107.107094
Thomsen, H. A., and Østergaard, J. B. (2017). Circumstantial evidence of life history events in loricate choanoflagellates. Eur. J. Protistol. 58, 26–34. doi: 10.1016/j.ejop.2016.12.004
Tilman, D. (1977). Resource competition between plankton algae: an experimental and theoretical approach. Ecology 58, 338–348. doi: 10.2307/1935608
Tilman, D., Kilham, S. S., and Kilham, P. (1982). Phytoplankton community ecology: the role of limiting nutrients. Annu. Rev. Ecol. Syst. 13, 349–372. doi: 10.1146/annurev.es.13.110182.002025
Tirichine, L., Rastogi, A., and Bowler, C. (2017). Recent progress in diatom genomics and epigenomics. Curr. Opin. Plant Biol. 36, 46–55. doi: 10.1016/j.pbi.2017.02.001
Tréguer, P. J., and De La Rocha, C. L. (2013). The world ocean silica cycle. Annu. Rev. Mar. Sci. 5, 477–501. doi: 10.1146/annurev-marine-121211-172346
van Tol, H. M., Irwin, A. J., and Finkel, Z. V. (2012). Macroevolutionary trends in silicoflagellate skeletal morphology: the costs and benefits of silicification. Paleobiology 38, 391–402. doi: 10.1666/11022.1
Vandeventer, P. E., Mejia, J., Nadim, A., Johal, M. S., and Niemz, A. (2013). DNA adsorption to and elution from silica surfaces: influence of amino acid buffers. J. Phys. Chem. B 117, 10742–10749. doi: 10.1021/jp405753m
Vivancos, J., Deshmukh, R., Grégoire, C., Rémus-Borel, W., Belzile, F., and Bélanger, R. R. (2016). Identification and characterization of silicon efflux transporters in horsetail (Equisetum arvense). J. Plant Physiol. 200, 82–89. doi: 10.1016/j.jplph.2016.06.011
Weaver, J. C., Aizenberg, J., Fantner, G. E., Kisailus, D., Woesz, A., Allen, P., et al. (2007). Hierarchical assembly of the siliceous skeletal lattice of the hexactinellid sponge Euplectella aspergillum. J. Struct. Biol. 158, 93–106. doi: 10.1016/j.jsb.2006.10.027
Weich, R. G., Lundberg, P., Vogel, H. J., and Jensén, P. (1989). Phosphorus-31 NMR studies of cell wall-associated calcium-phosphates in Ulva lactuca. Plant Physiol. 90, 230–236. doi: 10.1104/pp.90.1.230
Wenzl, S., Hett, R., Richthammer, P., and Sumper, M. (2008). Silacidins : highly acidic phosphopeptides from diatom shells assist in silica precipitation in vitro. Angew. Chem. 47, 1729–1732. doi: 10.1002/anie.200704994
Williams, A., Lüter, C., and Cusack, M. (2001). The nature of siliceous mosaics forming the first shell of the brachiopod Discinisca. J. Struct. Biol. 134, 25–34. doi: 10.1006/jsbi.2001.4366
Wood, R., Ivantsov, A. Y., and Zhuravlev, A. Y. (2017). First macrobiota biomineralization was environmentally triggered. Proc. R. Soc. B Biol. Sci. 284, 20170059. doi: 10.1098/rspb.2017.0059
Yamada, K., Yoshikawa, S., Ichinomiya, M., Kuwata, A., Kamiya, M., and Ohki, K. (2014). Effects of silicon-limitation on growth and morphology of Triparma laevis NIES-2565 (Parmales, Heterokontophyta). PLoS ONE 9:e103289. doi: 10.1371/journal.pone.0103289
Yamanaka, S., Yano, R., Usami, H., Hayashida, N., Ohguchi, M., Takeda, H., et al. (2008). Optical properties of diatom silica frustule with special reference to blue light. J. Appl. Phys. 103, 74701. doi: 10.1063/1.2903342
Ye, M., Song, Y., Long, J., Wang, R., Baerson, S. R., Pan, Z., et al. (2013). Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc. Natl. Acad. Sci. U.S.A. 110, E3631–E3639. doi: 10.1073/pnas.1305848110
Yoshida, M., Noël, M. H., Nakayama, T., Naganuma, T., and Inouye, I. (2006). A haptophyte bearing siliceous scales: ultrastructure and phylogenetic position of Hyalolithus neolepis gen. et sp. nov. (Prymnesiophyceae, Haptophyta). Protist 157, 213–234. doi: 10.1016/j.protis.2006.02.004
Zhang, S., Liu, H., Ke, Y., and Li, B. (2017). Effect of the silica content of diatoms on protozoan grazing. Front. Mar. Sci. 4:202. doi: 10.3389/fmars.2017.00202
Zhao, P., Gu, W., Wu, S., Huang, A., He, L., Xie, X., et al. (2014). Silicon enhances the growth of Phaeodactylum tricornutum Bohlin under green light and low temperature. Sci. Rep. 4:3958. doi: 10.1038/srep03958
Keywords: diatoms, silicifiers, radiolarians, silicic acid transporters, silicification
Citation: Hendry KR, Marron AO, Vincent F, Conley DJ, Gehlen M, Ibarbalz FM, Quéguiner B and Bowler C (2018) Competition between Silicifiers and Non-silicifiers in the Past and Present Ocean and Its Evolutionary Impacts. Front. Mar. Sci. 5:22. doi: 10.3389/fmars.2018.00022
Received: 22 September 2017; Accepted: 17 January 2018;
Published: 06 February 2018.
Edited by:
Eric 'Pieter Achterberg, GEOMAR Helmholtz Centre for Ocean Research Kiel (HZ), GermanyReviewed by:
Nils Kröger, Technische Universität Dresden, GermanyPascal Jean Lopez, Centre National de la Recherche Scientifique (CNRS), France
Mark Hildebrand, University of California, San Diego, United States
Copyright © 2018 Hendry, Marron, Vincent, Conley, Gehlen, Ibarbalz, Quéguiner and Bowler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chris Bowler, cbowler@biologie.ens.fr
†These authors are joint first authors and have contributed equally to this work.
 Katharine R. Hendry
Katharine R. Hendry Alan O. Marron2†
Alan O. Marron2†  Flora Vincent
Flora Vincent Daniel J. Conley
Daniel J. Conley Marion Gehlen
Marion Gehlen Chris Bowler
Chris Bowler