Abstract
Rheumatoid arthritis (RA) is a common systemic rheumatic disease. While the most visible manifestation of RA is articular involvement, it is a true systemic disease with the potential to affect multiple organs. Methotrexate (MTX) is the most commonly used medication to treat RA. MTX pneumonitis (MTX-pneu) is a rare disease entity reported in MTX users. It usually develops acutely or subacutely in the first year of treatment. MTX-pneu presents with cough, dyspnoea, and often fever. Pre-existing lung disease is a major risk factor and the clinical diagnosis is based on MTX exposure, symptoms, and laboratory and imaging findings. Treatment involves MTX cessation and high-dose glucocorticoids. Interstitial lung disease (ILD) is a common manifestation of RA with clinical RA-ILD affecting up to 10% of patients. RA-ILD tends to be a more indolent process than MTX-pneu and frequently develops over years but can also be acute. Similar to MTX-pneu, RA-ILD presents with cough, dyspnoea, and often fever. Risk factors include age, male sex, disease activity, seropositivity, and smoking. Treatment is aimed at optimal control of RA disease and within this strategy there may be particular roles for rituximab, tocilizumab, and abatacept. Antifibrotics may also have a role. Given the distinct pathologies, the differentiation of these two entities is crucial. The treatment approach differs significantly and what is beneficial for one may be harmful for the other. In this paper, the authors discuss and contrast contemporary knowledge of MTX-pneu and RA-ILD.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disorder affecting 0.5–1.0% of the global population. While primarily seen as a condition affecting joints, it is more accurately a systemic inflammatory disease which can affect multiple organ systems including the lungs. Among the extra-articular manifestations, respiratory disease is the second most common cause of death after cardiovascular disease.1 A large autopsy study of 1,246 RA cases from Japan corroborates the lung involvement, including interstitial lung disease (ILD), second to infection as the most common cause of death.2
Given the significant role of ILD as part of the natural history of RA, there has been much controversy over the role of methotrexate (MTX) as the anchor disease-modifying antirheumatic drug (DMARD). On one side of the debate is the idea that MTX may cause ILD in RA. On the other side, including the view of the authors, is that ILD or fibrosis in the expected usual interstitial pneumonia (UIP) pattern is a result of poorly controlled RA and the resultant active systemic inflammation. As to how uncontrolled chronic inflammation predisposes to lymphoproliferative disorders and amyloidosis, it is the authors’ view that in the context of RA, poor disease control predisposes to RA-related interstitial lung disease (RA-ILD). As such, there is an association or correlation between MTX use and ILD, but there is a confounding variable, namely that most cases have underlying RA; a classic case of how correlation does not imply causation. There is also a historic channelling bias as patients with more severe RA were traditionally both more likely to develop RA-ILD and to be treated with MTX.3,4
A supported association between MTX and ILD first appeared in the literature over 30 years ago.5 An important distinction to be made is what is meant by ILD and if it is present at baseline before treatment with MTX. In terms of MTX and lung injury, the most commonly reported manifestation is MTX-related pneumonitis (M-pneu) which can be difficult to distinguish clinically from underlying RA-ILD.6 In short, this is where much confusion arises and why MTX and its putative role in the lung is misunderstood. MTX may very rarely cause drug-related pneumonitis but it also may have a protective effect against progressive RA-ILD, these being two distinct pathological entities. Elucidating the precise cause of respiratory symptoms in an RA patient may be difficult but it is crucial to guide treatment. A comparison of the features of MTX-pneu, RA-ILD, idiopathic pulmonary fibrosis (IPF), and pneumocystis jiroveci pneumonia (as an example of an atypical infection) is shown in Table 1. The aim of this manuscript is to review the current understanding of MTX-pneu and RA-ILD.
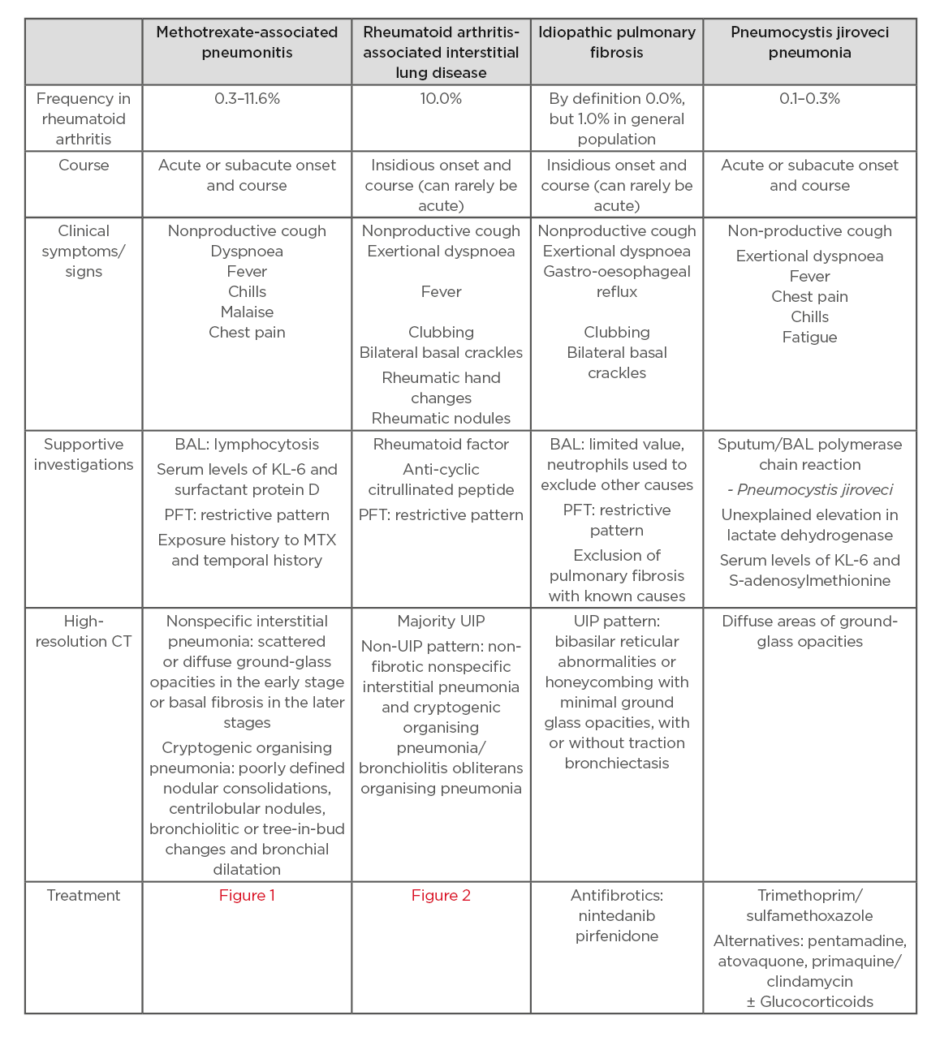
Table 1: Comparison of features of methotrexate-associated pneumonitis, RA-ILD, IPF, and PJP.
BAL: bronchoalveolar lavage; PFT: pulmonary function tests; UIP: usual interstitial pneumonia.
METHODS
A systematic literature search for relevant articles using PubMed, the Cochrane central register of controlled trials, and Embase was done. The search was performed with no date limits and last updated on 4th March 2020. The keywords ‘Methotrexate’ OR ‘rheumatoid arthritis’ AND (‘lung’ OR ‘respiratory’) were used. Reference lists of relevant articles were also reviewed.
METHOTREXATE PNEUMONITIS
Epidemiology
The prevalence of M-pneu as documented in the literature ranges anywhere from 0.3–11.6%, with the caveat that diagnostic criteria used for M-pneu are not consistent across studies.7-10 A previous comprehensive literature review of 3,463 RA patients treated with MTX reported a 2% rate of some form of lung toxicity with only 15 cases (0.43%) of MTX-pneu.11
In an even more favourable review of seven trials and 1,630 patients without RA, but with diagnoses ranging from psoriasis; psoriatic arthritis; or inflammatory bowel disease, who were treated with either MTX or placebo, did not show a statistically significant increase in adverse respiratory events within 52 weeks of treatment and only one case of pneumonitis was identified in the MTX group.12 This provides some evidence of the safety of MTX itself and may suggest that there are factors attributable to the inherent RA disease process which increase the risk for acute lung injury when subjected to MTX. Of particular interest, since 2001, there has been no reported cases of M-pneu across all randomised clinical trials of MTX in RA.13 The recent CIRT trial of MTX enrolled 6,158 patients and reported 6 possible cases of pneumonitis in the MTX group compared to 1 case in the placebo group, but there was insufficient evidence to confirm that these cases were definite MTX-pneu.14
Clinical Presentation and Symptoms
While distinguishing between M-pneu and RA-ILD can be difficult given the overlap of clinical and histological features, M-pneu tends to have an acute or subacute course with a propensity for developing within the first year of treatment.15-17 This reiterates the need to study the baseline respiratory function of RA patients in clinical studies prior to commencing DMARD. This would help to distinguish between the subsequent development of M-pneu or RA-ILD.
While the presentation may be nonspecific, the symptomatology of M-pneu typically may include fever, chills, malaise, nonproductive cough, dyspnoea, and chest pain. The presentation tends to be either acute with progressive symptoms over days, or subacute with an insidious onset over weeks.18
Mild blood eosinophilia has been noted in 25–40% of cases of subacute M-pneu by some authors and similarly a small case series demonstrated lymphopenia in the context of M-pneu, with a return to normal once lung function is restored.6,7,19,20 These signs may not be reliable but can serve as a clue to the aetiology of lung involvement.
Pathogenesis
M-pneu is generally considered to be a hypersensitivity reaction. In vitro studies suggest that IL-8 plays a role in the pathogenesis and it is known that MTX can trigger IL-8 secretion within airway epithelial cells, with resultant increased levels found in both peripheral blood samples and bronchoalveolar lavage (BAL) samples.21-23 To the best of the authors’ knowledge, IL-8 inhibition for pneumonitis has not been used in clinical practice with pilot trials terminating early.
Risk Factors
Various risk factors for M-pneu have been identified but have not always been reliably replicated in other studies. These include age >60, diabetes, hypoalbuminaemia, previous DMARD exposure, chronic kidney disease, male sex, increased Health Assessment Questionnaire (HAQ) score, decreased pain Visual Analog Scale (VAS), and crucially, pre-existing lung disease.24 In a case-control study intended to identify and investigate risk factors for M-pneu, pre-existing lung disease was found to confer increased risk with an odds ratio of 7.1 (95% confidence interval: 1.1–45.4).25 Taking this into consideration, the most recent iteration of the British Society of Rheumatology’s guidelines note that while not an absolute contraindication to traditional DMARD initiation, caution should be exercised in commencing treatment in those with poor respiratory reserve.26 The concern being that these patients with poor baseline respiratory function (e.g., diffusing capacity for carbon monoxide <40%) are less able to tolerate any occurrence of drug-induced pneumonitis.
Haplotype and single nucleotide polymorphisms have not proven to be particularly useful on a global scale, with borderline significance for the latter.27 Interestingly, there seems to be a relationship with increasing latitude and risk for M-pneu. Data from the Ministry of Health of New Zealand suggests that the risk or incidence ratio increases by 16% per degree of increasing latitude.28 Whether this is reflective of a genetic predisposition, single nucleotide polymorphisms, environmental factors, or ultraviolet light exposure and vitamin D level much like the relationship between vitamin D and multiple sclerosis remains unclear.29
Investigations
A diagnosis of M-pneu is typically based on clinical and radiologic findings. While pulmonary function tests (PFT), BAL, or lung biopsy are useful, the latter, at least, may not be practical. BAL can be beneficial as it can be used to rule out infections secondary to immunosuppression. The characteristics of BAL in M-pneu have been well defined in the literature with a systematic review highlighting that the majority (89%) of BAL samples in M-pneu demonstrate lymphocytosis.30 Furthermore, serum levels of KL-6 and surfactant protein D, both expressed by Type II pneumocytes in the lung, are increased in M-pneu and may have utility as novel biomarkers to aid diagnosis, with the caveat that both are increased in other forms of lung pathology including RA-ILD. Careful consideration is needed and relative change to pre-MTX baseline may be more useful than raw values.31,32
As elucidated earlier, invasive investigations are not always practical or indicated. However, a study comprising 44 patients with drug-induced lung injury did conclude that transbronchial lung biopsy was diagnostically helpful in 75% and, as such, may aid diagnosis in conjunction with clinical, laboratory, and radiographic findings.33
The predominant radiographic findings in M-pneu are typical of nonspecific interstitial pneumonia (NSIP) most commonly followed by cryptogenic organising pneumonia (COP)/bronchiolitis obliterans organising pneumonia.34 The NSIP pattern is characterised by diffuse heterogeneous opacities on chest X-ray, scattered or diffuse ground-glass opacities in the early stage, or basal fibrosis in the later stages on CT. The less common COP pattern can be described as demonstrating bilateral scattered heterogeneous or homogeneous opacities with a peripheral distribution in the upper and lower lobes on chest X-ray and poorly defined nodular consolidations, centrilobular nodules, bronchiolitic or tree-in-bud changes, and bronchial dilatation on CT. Imaging findings in MTX-pneu have been reviewed in detail elsewhere.35-38
Diagnostic Criteria
Two proposed diagnostic criteria are those laid out by Searles et al. and those by Kremer et al.6,39 The former has tended to be used most often, where six out of nine criteria must be met for a diagnosis of M-pneu. Baseline PFT abnormalities, such as low forced expiratory volume in 1 second, vital capacity, and diffusing capacity for carbon monoxide, may have prognostic roles and aid in identifying those at higher risk of developing M-pneu.17 Previously, many authors felt that MTX should only be commenced in RA patients if they were believed to have sufficient respiratory reserve to survive M-pneu. However, recent literature would suggest that M-pneu is rarer than previously thought and, given the many proven benefits of MTX, a careful risk–benefit analysis should be made in this group.
Treatment
The treatment approach is summarised in Figure 1. Discontinuation of MTX is the clear first step in suspected M-pneu. Given that M-pneu is seen as a hypersensitivity reaction, steroids (either methylprednisolone or oral prednisolone) in high doses are often required. There are case reports of benefits from cyclophosphamide
and tocilizumab.40,41
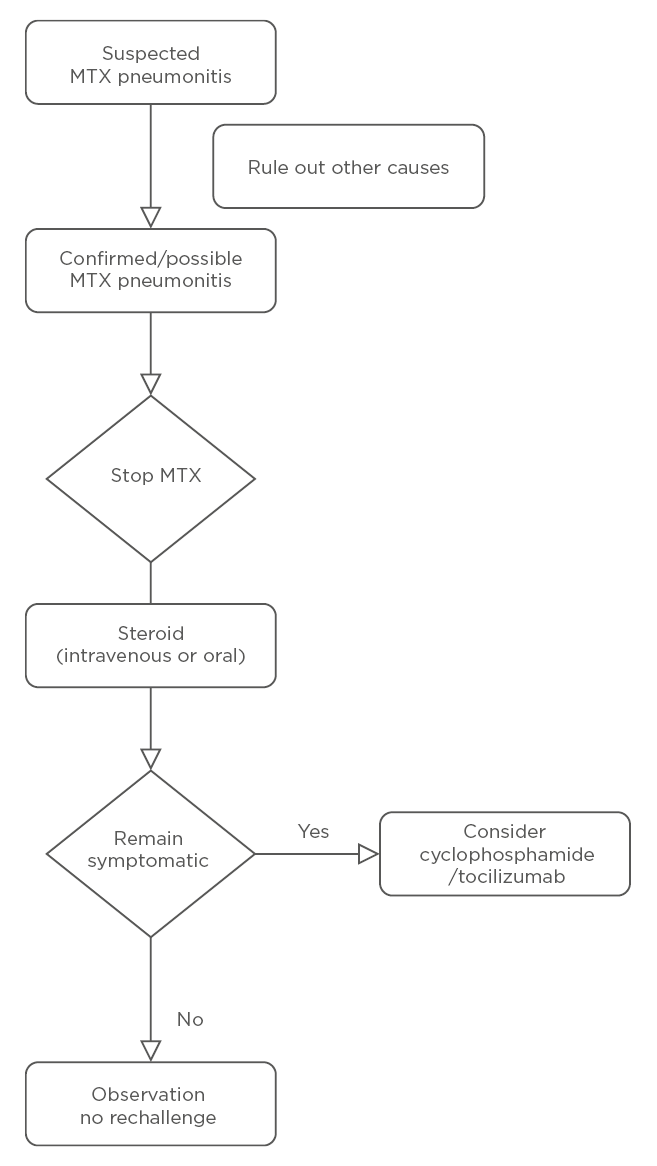
Figure 1: Management of methotrexate-associated pneumonitis.
MTX: methotrexate.
Prognosis
Once MTX is stopped, prognosis in M-pneu tends to be favourable with most recovering fully.18 Three different studies have reported mortality ranging from 13% to as high as 30%.6,9,18,42
Rapid onset of M-pneu following initiation of MTX appears to be associated with poorer prognosis.16
RHEUMATOID ARTHRITIS INTERSTITIAL LUNG DISEASE
Epidemiology
ILD is an under-recognised extra-articular manifestation of RA. The prevalence of RA-ILD varies between studies. Clinical RA-ILD has been estimated to occur in approximately 8–10% of RA patients, with respiratory symptoms preceding articular symptoms in about 10–20% of cases.3,43,44 A study of 140 RA patients by Bharadwaj et al.45 corroborates this, demonstrating the presence of ILD in 9.29% of cases and highlighting that ILD is the most common extra-articular complication of RA. Studies from the UK including the ERAS/ERAN study and the BRILL study have reported a slightly lower prevalence of ILD (3–5%).46,47
Subclinical RA-ILD as evaluated by high resolution CT (HRCT) has been identified in 19–67% of RA patients, while unselected lung biopsy identified evidence of ILD in 80% of patients.48-50
Clinical Presentation and Symptoms
The clinical symptoms of RA-ILD and M-pneu can be difficult to differentiate. RA-ILD, which can be asymptomatic for years, tends to develop insidiously over time in contrast to M-pneu which would more typically present acutely or subacutely with dyspnoea, cough, and fever.35 Traditional articular features of RA can limit mobility and exercise tolerance to the extent that exertional dyspnoea early in the course of RA-ILD is masked by this forced sedentary lifestyle. While subtle radiographic features may be present early in the disease process on HRCT, auscultatory findings may be absent initially but most cases will eventually develop fine bibasal crackles.51 Radiographically, most will develop a UIP pattern often in conjunction with digital clubbing on clinical examination; a pattern very similar to that seen in IPF patients.51 Imaging findings in RA-ILD have been reviewed in detail elsewhere.35,36,52
Pathogenesis and Risk Factors
While there is still much to be understood regarding the pathogenesis of RA-ILD, there is increasing evidence to suggest that the lung may be central in activating the pathological process of RA itself.53-55
Multiple risk factors for RA-ILD have been identified including older age, male sex, smoking, disease activity, elevated titres of specific autoimmune antibodies, ethnicity, and certain human leukocyte antigens.3,55-57 In terms of biomarkers, a positive rheumatoid factor or anti-citrullinated peptide antibody are strongly associated with RA-ILD. Other novel biomarkers have emerged, with heat shock protein 90 detected in blood samples of RA-ILD patients as well as BAL samples.58,59 Of particular significance is the gain-of-function MUC5B promoter variant rs35705950, which is strongly associated with ILD involvement, conferring an increased odds ratio of 3.1 in RA patients. The similarities between RA-ILD and IPF are striking. Both typically demonstrate a UIP radiographic pattern with clubbing, and the MUC5B variant is often involved in both, acting as the strongest predictor or known risk factor for IPF.60,61
THE ROLE OF METHOTREXATE IN RHEUMATOID ARTHRITIS-ASSOCIATED INTERSTITIAL LUNG DISEASE
Much has been made of the association between MTX and RA-ILD over the past few decades. While there is an association with MTX and RA-ILD, it is now known to be coincidental and not causative, with the underlying inflammatory process driving ILD. In short, it is the disease and not the drug that causes RA-ILD.62
The seminal studies that explore the role of MTX in RA-ILD are the ERAN and ERAS studies which recruited 2,701 RA patients in the UK and Ireland to the trial with a follow-up period of up to 25 years.46 In this multicentre prospective cohort trial, the diagnosis of ILD was according to standard practice with confirmatory evidence from standard investigations including PFT, chest X-ray, and HRCT. The authors compared the prevalence of RA-ILD in the MTX exposed and the non-MTX exposed groups. They demonstrated that in the MTX exposed group 97.5% (n=1,539) remained ILD free, whereas in the non-MTX exposed group 95.2% (n=1,061) remained ILD free. This is statistically significant and shows that there is no causation between MTX exposure and development of RA-ILD. Patients who developed ILD were, at RA onset, a mean 5.14 years older and mean baseline ESR score of 8.64 mm/hour higher than patients who did not develop ILD. Furthermore, ERAS and ERAN confirmed that higher age of RA onset, male sex, smoking, rheumatoid factor positivity, rheumatoid nodules, higher ESR, and longer time from first RA symptom to first outpatient department visit were independently associated with incident RA-ILD. The authors of the ERAN and ERAS study concluded that the overall prevalence of RA-ILD is 3.7%, in line with the UK BRILL network which reported 2–3% prevalence across its recruiting centres.47 There was no association between MTX exposure and incident RA-ILD. On the contrary, MTX exposure was associated with significantly less RA-ILD and this would suggest a protective effect in delaying the onset of ILD.
Treatment
ILD management in systemic RA is still a challenge due to disease heterogeneity. Asymptomatic patients frequently do not require any specific treatment in comparison to progressive symptomatic patients or those with deteriorating PFT. Histologic subset, if known, can guide treatment approach. Nonfibrotic NSIP and COP/bronchiolitis obliterans organising pneumonia are more likely to have a positive treatment response in comparison to the UIP pattern. Most RA-ILD cases present with a UIP pattern raising the question as to the relative benefits of immunosuppressive versus antifibrotic treatment strategies.
UIP pattern ILD in systemic rheumatic diseases may be more responsive to immunosuppression than IPF.
Given the overall excellent response of RA to DMARD, it is intuitive that the pulmonary component of the disease is also likely to be responsive to these treatments. The absence of any definitive evidence that RA-ILD is a predominantly immune-mediated inflammatory rather than fibrotic process, however, ensures that this remains controversial. The aim of treatment is to ensure complete overall rheumatoid disease control using whichever available agents to achieve this goal.4,56 The rationale for this strategy is supported by the significant decline in RA-ILD as treatment options have advanced, and the improved articular outcomes achieved by the authors.63 The treatment approach is summarised in Figure 2. The available medication options include glucocorticoids which remain the initial mainstay of therapy but, due to long-term adverse effects, steroid-sparing agents are generally introduced early in the disease course. MTX and leflunomide are important anchor agents in the treatment of RA joint disease. The best available evidence shows no sign of harm in RA-ILD and some evidence of benefit.4,46,64,65 Among the biologic agents rituximab has shown particular promise and has demonstrated improved mortality compared to tumour necrosis factor-inhibitors (TNF-I).66-68 An observational study of 56 patients with RA-ILD treated with rituximab showed that 16% improved and 52% remained stable following treatment.69
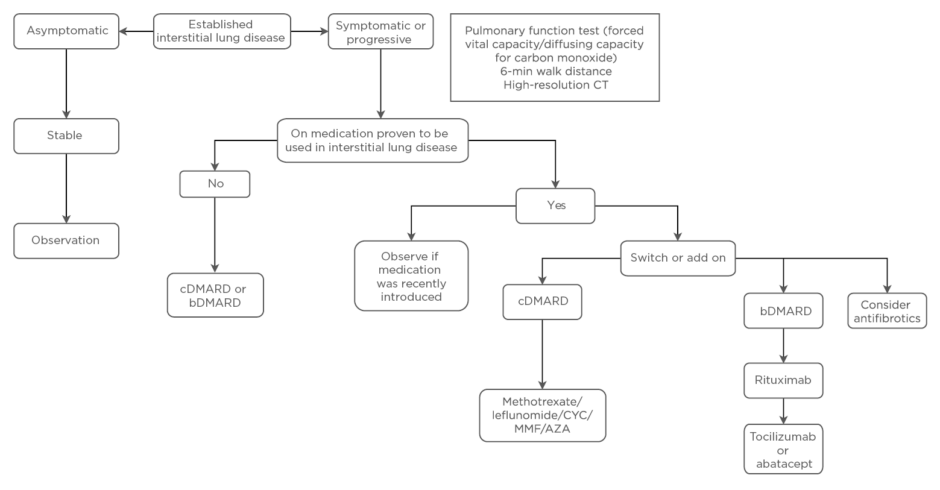
Figure 2: Management of rheumatoid arthritis-associated interstitial lung disease.
AZA: azathioprine; b: biologic; c: classical; CYC: cyclophosphamide; DMARD: disease-modifying antirheumatic drug; MMF: mycophenolate mofetil.
The preference for other biologics in the setting of RA-ILD is less certain. In addition to the study comparing rituximab and TNF-I, another literature review also showed an increased mortality with the use of TNF-I.70 It remains unclear if TNF-I are harmful or if they are merely not as effective as some other biologics in treating RA-ILD. There are some preliminary supportive data for the use of abatacept or tocilizumab for RA-ILD.40,71-74 The role of other agents traditionally used in other forms of connective tissue disease-associated ILD, including cyclophosphamide, mycophenolate mofetil, and azathioprine, is less certain.
The antifibrotic agents pirfenidone and particularly nintedanib have recently sparked interest in the treatment of ILD in the setting of systemic rheumatic disease, including RA, based on initial positive results in the setting of IPF.75 Nintedanib has also demonstrated positive results in systemic sclerosis-related ILD.76 Both antifibrotic agents have been shown to be effective in animal models of RA-ILD.77 The INBUILD study of nintedanib demonstrated efficacy in fibrotic lung disease other than IPF.78 While subgroup analyses were too small to demonstrate statistical significance, patients with RA-ILD appeared to respond similarly to the overall cohort.79 The absolute benefits of these anti-fibrotic agents in terms of lung function appear modest, and must be balanced against the high frequency of adverse events, particularly gastrointestinal issues.78
CONCLUSION
The ultimate choice of therapeutic strategy in RA-ILD relies on the individual patient’s symptoms, comorbidities, and balancing adverse events. A collaborative multidisciplinary team approach between rheumatologists and respiratory physicians is important to ensure optimal care.