Abstract
Azoospermia is a common cause of male infertility; however, surgical sperm retrieval (SSR) and subsequent intracytoplasmic sperm injection offers couples the chance to have a biological child. SSR success is highly variable and dependent on a number of factors. One such factor is male follicle-stimulating hormone (FSH), which has been researched extensively. The aim of this literature review is to ascertain if there is a ‘cut off’ FSH value that correlates with successful SSR, whether this value differs depending on method of SSR, and if there is a correlation between male FSH level and obstetric outcomes. Thirty-five articles were identified and reviewed, with 10 papers suggesting FSH cut off values. These ranged from <8.5 to <25.0 IU/L, with a mean value of 14.0 IU/L. Generally the results suggested that lower FSH values were associated with increased SSR success. Few papers considered pregnancy and birth outcomes following intracytoplasmic sperm injection with surgically retrieved sperm, and there was no clear correlation with male FSH levels. Clinical implications include considering FSH results when counselling patients about both SSR and intracytoplasmic sperm injection. Suggested future research implications are to further investigate the predictive role of FSH in combination with other clinical and endocrinological markers.
Key Points
1. Surgical sperm retrieval is one method to provide males with azoospermia the chance to father a biological child but success depends on numerous variables, including follicle-stimulating hormone.2. Lower follicle-stimulating hormone values were associated with increased surgical sperm retrieval success, especially in obstructive azoospermia.
3. Infertility is associated with feelings of disappointment and a loss of control worldwide, and clinicians have an ethical obligation to provide evidence-based management and individualised care.
INTRODUCTION
Male factor aetiologies account for approximately 50% of infertility in couples,1 with azoospermia diagnosed in up to 15% of infertile males.2 The World Health Organization (WHO) semen analysis parameters are universally used and define azoospermia as an absence of spermatozoa identified in wet or centrifuged ejaculate samples.3 Azoospermia is classified into either obstructive (OA) or non-obstructive causes (NOA), with impaired spermatogenesis. Treatment for both consist of surgical sperm retrieval (SSR) with a variety of techniques. These include testicular sperm extraction with microscopy with (mTESE) or without (TESE), testicular sperm aspiration, microsurgical epididymal sperm aspiration, percutaneous epididymal sperm aspiration (PESA), and fine needle aspiration (FNA). Surgically retrieved spermatozoa are then utilised in intracytoplasmic sperm injection (ICSI). This allows males with azoospermia to father genetically-related children, and remove the necessity for sperm donors or adoption.
Despite several methods, SSR outcomes can be of variable success, particularly in cases of NOA.4 Spermatogenesis is controlled by a complex neuroendocrine axis including follicle-stimulating hormone (FSH). Although the relationship between FSH and SSR has been explored previously, the results have not always been consistent. Therefore, the current evidence was assessed through a literature review to ascertain if FSH levels can correlate with SSR outcomes, in order to update clinicians and help patients make a more informed choice.
METHOD
Objectives
To evaluate if pre-operative serum FSH levels are predictive of subsequent successful SSR (TESE, mTESE, testicular sperm aspiration, microsurgical epididymal sperm aspiration, PESA, FNA) in cases of azoospermia prior to ICSI.
Outcomes
The primary outcome is to ascertain if FSH is predictive of successful SSR, and to determine a ‘cut off’ value. Secondary outcomes include whether different SSR methods have different cut off values, and if there is a correlation between FSH and clinical pregnancy or live birth outcomes following ICSI.
A literature review was conducted by performing a Medline search via Ovid from January 2002–2021. The search terms ‘FSH’, ‘follicle-stimulating hormone’, ‘surgical sperm retrieval’, ‘testicular sperm extraction’, ‘microdissection testicular sperm extraction’, ‘testicular sperm aspiration’, ‘percutaneous epididymal sperm aspiration’, ‘azoospermia*’, and ‘predict*’ were used. Eighty-three articles were identified and screened by the title and abstracts. Inclusion criteria applied to the studies were English language papers, randomised controlled trials (RCT), cohort studies, case-control studies, retrospective and prospective studies, and human-only studies. Exclusion criteria included papers with sample sizes of n=<100, letters to the editor, rapid response articles, case reports, review articles, and abstract proceedings. Two of the authors screened the results and reviewed the 35 full text papers included in the final analysis. Figure 1 depicts the screening processing.
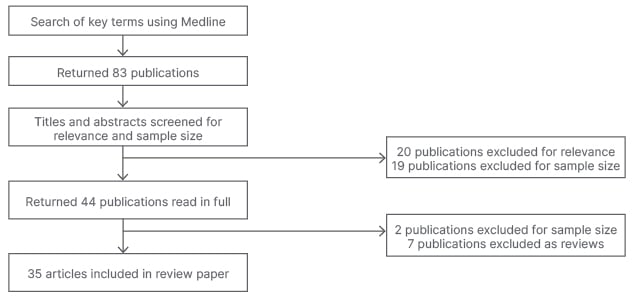
Figure 1: Flowchart summarising the screening process of article selection.
RESULTS
Thirty-five articles met the inclusion criteria and were analysed. Table 1 outlines the study design, SSR technique, results, and authors’ comments about the selected articles.
Twenty-seven studies considered NOA only; one study considered OA only; and seven studies included mixed aetiologies for azoospermia. Ten out of the 35 studies gave cut off FSH values predicting SSR success. These ranged from <8.5 to <25.0 IU/L, with a mean value of 14.0 IU/L. The results suggest lower FSH levels are associated with increased SSR success, although five papers did not demonstrate any significant relationship between FSH levels and SSR outcomes. Nine publications described pregnancy or birth related outcomes in addition to SSR results.7,13,16,24,31,32,35,37,38 Five of these papers described ICSI outcomes following SSR procedures and consideration to FSH levels.
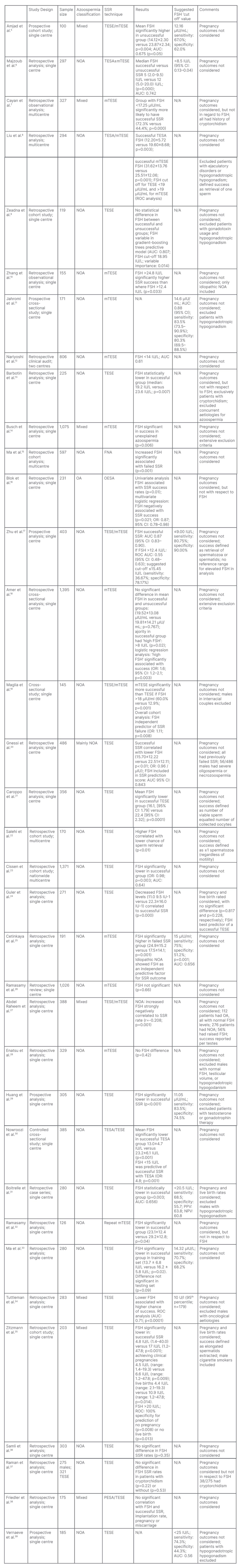
Table 1: Study characteristics and association between follicle-stimulating hormone and surgical sperm retrieval.
AUC: area under receiver operating characteristic curve; CI: confidence interval; FSH: follicle-stimulating hormone; FNA: fine needle aspiration; mTESE: testicular sperm extraction with microscopy; N/A: not applicable; NOA: azoospermia with a non-obstructive cause; NPV: negative predictive value; OA: azoospermia with an obstructive cause; OESA: open epididymal spermatozoa aspiration; OR: odds ratio; PESA: percutaneous epididymal sperm aspiration; PPV: positive predictive value; ROC: receiver operating characteristic curve; SSR: surgical sperm retrieval; TESE: testicular sperm extraction without microscopy.
DISCUSSION
Indications for Surgical Sperm Retrieval
NOA is more common than OA, which is reflected in the respective proportion of articles covering each diagnosis. Additionally, some cases of OA can be treated with surgical correction to restore normal anatomy and avoid the necessity for SSR. However, males may show a continuum of disorder, with features of both NOA and OA.7,35 Therefore, classification of azoospermia may be of debatable value when counselling couples regarding treatment options, and an individualised approach to each case is preferred.
NOA testicular histopathological classifications include normal spermatogenesis, hypospermatogenesis, maturation arrest, and Sertoli cell-only syndrome. Genetic anomalies such as Klinefelter syndrome and Y chromosome microdeletions were in the exclusion criteria of 10 of the papers,5,15,17-19,21,29,30,34,39 despite being well-established causes of azoospermia. Huang et al.29 did identify FSH was not predictive for males with Klinefelter syndrome. Papers had variable exclusion criteria to decrease the risk of confounders; however, this made comparison by the authors more challenging.
Surgical Sperm Retrieval Techniques
Some publications compared mTESE with traditional techniques. Majzoub et al.6 found that although FSH levels <8.5m IU/mL gave similar success rates between the two techniques, when all FSH ranges were considered, mTESE was more successful.
The authors’ results have previously stated that SSR is less successful when males have higher pre-operative FSH levels, yet these articles suggest that males with higher FSH levels may benefit from microdissection techniques.8,19,34 However, the number of publications comparing the two methodologies were very limited. Microdissection SSR requires advanced surgical training and is a longer procedure, therefore conferring marked resource implications and may reduce cost effectiveness.
FNA and PESA are simple, low cost, well-tolerated procedures, but as they are performed blind, they can yield less sperm compared to other SSR methods. Males may then have to undergo an additional TESE. Only one article about FNA15 and one article including PESA38 was identified, which is insufficient to conclude what FSH values would confer benefit from these procedures.
Strength of Evidence
The main limitations of these results are the heterogenous design of studies, and quality of evidence yielded. No RCTs were identified; however, it may not be feasible to apply RCT methodology to this topic, especially in blinding for surgical procedures.
Only five of the studies were prospective.5,11,17,29,39 All of these papers are single-centre studies only; however, each identifies a FSH cut off for successful SSR, albeit these are still varied: <9 to <25 IU/L. Future prospective studies may help to refine the cut off range. For the remaining papers, it is well established that retrospective studies can carry bias when collecting data, or that crucial data may be missing that can affect reporting.
Many of the studies utilised regression analysis to form a predictive tool to calculate SSR success. Some of these studies included FSH in their algorithms, as they found it to be a significantly predictive factor.23,25
Alternative Variables for Surgical Sperm Retrieval Success
Frequently described clinical markers in the analysed papers included age and BMI. Testicular volume was considered most commonly,6,8,10,15,31-34 but with varying ranges, again making a subgroup analysis difficult. Biomarkers include testosterone,21 fructose,34 α-glucosidase34 and inhibin B,29,39 which was described most frequently, and with good diagnostic accuracy.17,31 The scope of this review intentionally did not consider the role of other variables as FSH is a cheap, established, readily available test. The authors therefore chose to focus on the potential diagnostic benefits of this sole marker.
Limitations
All SSR methods were considered in this review, and there may be different FSH cut offs depending on technique.8 Although many of the studies described surgical techniques and gamete processing, variation is likely to be high. This mirrors global clinical practice, and so may not be a significant limitation to the results.
As previously highlighted, the vast majority of articles regarded NOA, which can bias the inferred application of FSH from this review. When the seven papers which included OA and mixed aetiologies are analysed separately, they are consistent with the existing literature: males with OA have lower FSH and far higher successful SSR compared to males with NOA and higher FSH levels.5,27,38 Little could be concluded from the remaining papers, as they either did not stratify FSH levels or SSR outcomes according to azoospermia classification.
The studies have varying criteria for successful SSR.9,21 For example, one study8 regarded success as retrieval of one sperm, which may be less clinically relevant for ICSI. Different studies used varying normal FSH ranges,34,36 and so descriptions of ‘high’ or ‘low’ levels should be interpreted with caution.
Pregnancy and Birth Outcomes
The ultimate aim for SSR is to produce a healthy baby when combined with ICSI, so it is interesting that so few publications have considered these outcomes. Song et al.’s40 research used FSH as part of a multivariable model, finding FSH significant in prediction of obtaining clinical pregnancy. This study was excluded from this review, as SSR had already occurred and was not the focus of the paper. Zitzmann et al.35 identified 100% specificity for predicting no pregnancy or live births with FSH >20 IU/L (p=0.008). Meanwhile, alternative authors did not find FSH significant in predicting such outcomes.24,31,38 Therefore the correlation between preoperative FSH in SSR, ICSI, and obstetric outcomes seems contested, and warrants further investigation.
Clinical Implication
It is well established that infertility is associated with feelings of disappointment and loss of control, independent of ethnicity and culture.41 The included studies reflect the global scale and impact of azoospermia and SSR treatment. In addition to marked psychological burden, SSR confers similar risks to any surgical procedure: bleeding, infection, scarring, and associated hypogonadism,42 which may further decrease spermatogenesis for repeat procedures.
Clinicians therefore have an ethical obligation when offering fertility treatment to couples, and not adopt a ‘one size fits all’ approach. If ICSI is likely to be futile for specific couples,35 clinicians also have a duty to female partners as oocyte collection carries the previously stated risks in addition to ovarian hyperstimulation syndrome, which can be fatal.
Additional Authors’ Perspective
This work differs from previous reviews, which have either solely focused on microsurgical techniques,43 or the role of FSH in NOA SSR only.44
Additionally, this review summarises important inclusion and exclusion criteria, which may be utilised when providing evidence-based care to specific subsets of patients. Conversely the authors chose to cover all forms of SSR and azoospermia for generalisability of results for patients and clinicians, or where microsurgery may not be available.
CONCLUSION
The authors performed this review with an aim to determine if FSH values can be correlated with success of SSR and, if so, to define a cut off value. This will allow clinicians to counsel patients whether SSR with ICSI is likely to be efficacious, or if pursuing non-biological methods to achieve parenthood may be more appropriate. This review demonstrates that there is an association between lower serum FSH levels and increasingly successful SSR. The authors have not identified a discriminative FSH result to accurately predict SSR outcomes, although a suggested range of values were described. To further elucidate this link, the authors suggest future work should continue to focus on FSH used in combination with other endocrinological and clinical markers.









