Abstract
Non-alcoholic steatohepatitis (NASH) is the potentially progressive form of non-alcoholic fatty liver disease (NAFLD). NAFLD and NASH are very common in most regions of the world and are on trajectory to become the most common liver disease at a global scale. Risk for high prevalence and progressiveness include visceral obesity and Type 2 diabetes. The conundrum of NAFLD is related to the rapid increase in its global burden with very low awareness among most general providers, as well as a lack of widespread availability of fully validated non-invasive diagnostic and prognostic tests and limited treatment options. Currently, lifestyle modification with diet and exercise are the best options. A large number of clinical trials are being developed to provide drug therapeutic options with patients with NASH and moderate to advanced fibrosis.
Key Points
1. There is limited knowledge about non-alcoholic fatty liver disease (NAFLD) in general healthcare settings, despite a rapid increase in global diagnoses.
2. There exist limited treatment options for NAFLD, and a lack of widespread availability of diagnostic testing.
3. Researchers are developing clinical trials in order to provide therapeutic drug options for patients with non-alcoholic steatohepatitis (NASH), the potentially progressive form of the disease.
EPIDEMIOLOGY
Changing socioeconomic conditions around the world have led to environmental changes promoting major chronic diseases.1 In this context, there has been a rapid increase in the prevalence of obesity and Type 2 diabetes (T2D). While these disease states are associated with many chronic diseases, they are also the drivers for one of the leading causes of chronic liver disease, specifically non-alcoholic fatty liver disease (NAFLD).2-8 NAFLD, a biologically and clinically heterogeneous disease, is an umbrella term used to describe a broad spectrum of histological conditions that are characterised by hepatic fat accumulation. A subtype of NAFLD or non-alcoholic steatohepatitis (NASH) is histologically diagnosed with hepatic fat in conjunction with liver cell injury. NASH is associated with an increase in both hepatic and non-hepatic morbidity and mortality, as well as impairment of health-related quality of life and substantial economic burden.9-19
Currently, 25–30% of the adult population are estimated to have NAFLD, while 8–10% of children and young adolescents are reported to have NAFLD. These prevalence rates are higher in populations with obesity and diabetes.20 In contrast, it is important to recognise that NAFLD can be found in those who are not obese (sometimes referred to as lean NAFLD).21-24 In fact, up to 40% within the NAFLD adult population can be considered to be non-obese.24 Despite the non-lean terminology, most of these patients have insulin resistance and may have visceral obesity.22-24
It is estimated that about 15–20% of patients with NASH can progress, leading to the development of cirrhosis, hepatocellular carcinoma (HCC), end-stage liver disease, and death.2,3 In fact, NASH is the second most common indication for liver transplantation in the USA.25 From all cancers globally, HCC is now the second leading cause of years of life lost, and NASH is a growing cause of HCC.2
Within the younger population, NAFLD can be diagnosed around the age of puberty (11–13 years old) and about a quarter of these children may already have NASH.26-28 In addition, it has been reported that for each 1‐unit gain in BMI Z-score among children aged 7–13 years, the risk for cirrhosis is increased by 16% in adulthood.28 These data are worrisome as it indicates the potential growing wave of NASH-related liver disease in the decades to come.
Finally, the consensus is that the prevalence of NAFLD increases with age. The peak prevalence of NAFLD for males is between the ages of 50–60 years (29.3%),29 while for females the peak time is noted for those over the age of 65 (25.4%).30,31 Based on NHANES III data, the prevalence rates for males by age have been cited as 16.1% in those aged 30–40 years old; 22.3% in those aged 41–50 years old; and 27.6% in those over 60 years old.30 For females, the prevalence of NAFLD was 12.5% in those aged 30–40 years old; 16.1% in those aged 41–50 years old; and 21.6% for those 51–60 years.30 During assessment of disease burden according to gender, researchers found that females aged 50 years and older were 17% more likely to develop NASH, and 56% more likely to develop advanced fibrosis compared with males of similar ages.31
PATHOPHYSIOLOGY OF NON-ALCOHOL FATTY LIVER DISEASE NON-ALCOHOLIC STEATOHEPATITIS
As noted, NASH is part of the systemic disease that is multifactorial with complex metabolic associations. Insulin resistance, T2D, and visceral obesity appear to be key pathogenic drivers for the development of NASH.1,21 They contribute to increased levels of free fatty acids and carbohydrates, which then places excess lipotoxic and metabolic loads on the liver leading to hepatic lipid accumulation, liver cell injury, inflammation, activation of Stellate cells, and fibrosis (Figure 1).32-34
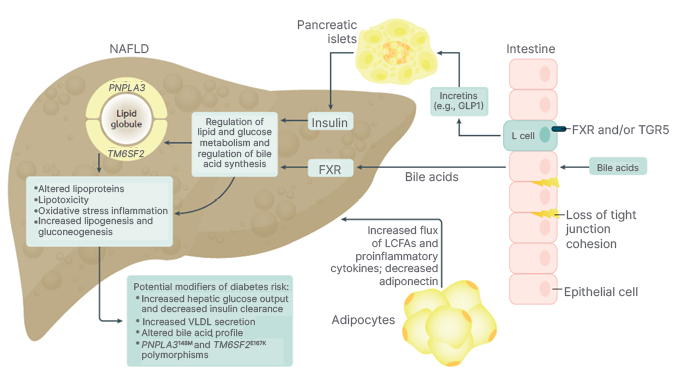
Figure 1: Pathophysiology of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis.
FXR: farnesoid-X receptor; GLP1: glucagon-like peptide-1; LCFA: long-chain fatty acid; NAFLD: non-alcoholic fatty liver disease; TGR: G protein-coupled receptor; VLDL: very-low-density lipoprotein.
Importantly, a significant amount of focus has been given to the pathophysiology of NAFLD and T2D.35 NAFLD is thought to be associated with hepatic and peripheral insulin resistance, which causes the systemic release of pro-inflammatory cytokines and hepatokines, which promote the development of T2D.35 Another recent study demonstrated that the presence of a fatty liver drives the liver–pancreatic α-cell axis increases glucagon production, which then contributes to the diabetes pathophysiology.36 In this context, the risk of T2D incidence has also been reported to increase as the severity of NAFLD increases. In fact, the presence of NAFLD has been associated with a 2.2 times greater chance of developing T2D, and patients with more advanced stages of liver fibrosis are at even a higher risk of T2D; however, if NAFLD improves or resolves, the risk for diabetes is reduced.37 In fact, another study found that the presence of NAFLD increased the risk of metabolic syndrome to almost the same degree.38
There also appears to be a genetic predisposition involved in the development of NASH. Specifically, the polymorphisms of PNPLA3 and TM6SF2 genes predispose these patients to NASH, and potentially adverse outcomes.34 Environmental factors such as a poor food environment (easy availability of calorie dense processed food), lack of easy access to safe areas for physical activity, poor sleep, and stress may all influence the onset and severity of NAFLD and NASH.39-43
NASH DIAGNOSIS
Hepatic steatosis is defined as an accumulation of triglycerides in >5% of hepatocytes is the first step required for diagnosing NAFLD and NASH. In this light, ultrasound is recommended for those at high-risk for NAFLD (e.g., those with components of metabolic syndrome but especially obesity and T2D).44 In fact, a recent meta-analysis determined that the use of conventional ultrasound has greater diagnostic accuracy than originally thought, especially for those with mild as well as moderate-severe hepatic steatosis (≥30% steatotic hepatocytes).45 In contrast, the diagnosis of NASH and stage of hepatic fibrosis are established through a liver biopsy sample that shows hepatic steatosis, lobular inflammation, and hepatocellular ballooning.46-49 It is important to note that the histology of NASH may differ between young patients and adults. Young patients with NASH are noted to have periportal zone (acinar zone 1) or azonal distribution of steatosis (Type 1) compared with the perivenular zone (acinar zone 3) of steatosis among adults (Type 2).50-52 In regard to inflammation, portal inflammation is more common during youth, whereas lobular inflammation is more common in adults. Ballooning Mallory’s hyaline bodies are infrequent, and hepatocyte ballooning is also rare in young patients, while ballooning degeneration can be present in adults. Finally, fibrosis in the youth is seen as portal fibrosis while, in adults, fibrosis is seen as perisinusoidal fibrosis.50-52
There are major limitations of liver biopsy due to its invasiveness, risks, and costs.53 These limitations have led to significant efforts for establishing validated non-invasive tests (NIT) that can determine the presence and stage of fibrosis.54-59 The NITs can be simple biomarker blood tests such as Fibrosis Score 4 (FIB-4), AST-Platelet Ratio Index (APRI), and NAFLD fibrosis score (NFS). These NITs incorporate ‘indirect’ markers of liver fibrosis, such as aminotransaminases accompanied with clinical parameters (age, sex, presence of insulin resistance/T2D, and andromorphic assessments). There are also ‘complex’ serum biomarker blood tests (e.g., the Enhanced Liver Fibrosis Score [ELF], which incorporates some of the direct markers of fibrogenesis and fibrinolysis such as serum tissue metalloproteinases and hyaluronic acid). Simple NITs and serum biomarkers may be best used in combination as a part of clinical algorithms.57-59
Finally, assessment of liver stiffness through elastography (transient elastography, magnetic resonance elastography, etc.) is also being established as important radiologic NITs.55 Again, the use of these tests is optimised in the context of algorithms that use risk stratification and simple NITs.57-59 As more work continues in the field of NITs, it is important to establish validated algorithms to accurately risk stratify patients at risk, who are seen in primary care and endocrinology practices.57
NASH FIBROSIS, FIBROSIS PROGRESSION, AND MORTALITY
Stage of hepatic fibrosis, presence of T2D, and increasing number of components of metabolic syndrome as well as PNPLA3 can play an important role for determining prognosis of patients with NASH.1,21,39,60 In this context, a recent prospective study of 1,773 persons with NAFLD, where 1,330 persons had NASH, conducted over a median of 4 years, found that all-cause mortality increased with increasing fibrosis stages, which increased from 0.32 deaths per 100 person-years for stage F0–F2 to 0.89 deaths per 100 persons-years for stage F3, and 1.76 deaths per 100 person-years for stage F4.60 Such findings validated prior results that came from retrospective data. The investigators also noted that the incidence of liver-related complications such as variceal haemorrhage, ascites, encephalopathy, and hepatocellular cancer increased with fibrosis stage. Other notable findings included that compared with patients with stage F0–F2 fibrosis, patients with stage F4 fibrosis had a higher incidence of T2DM (7.53 versus 4.45 events per 100 person-years), and experienced a decrease in their estimated glomerular filtration rate of more than 40%.60 On the other hand, investigators reported that the incidence of cardiac events and non-hepatic cancers were similar across all the fibrosis stages. Finally, they reported that in their multivariable analysis controlling for age, sex, race, diabetes status, and baseline histologic severity, all-cause mortality was increased almost seven times (average hazard ratio: 6.8; 95% confidence interval [CI]: 2.2–21.3) following an incidence of any hepatic decompensation event and the overall all cause death rate was higher in this group than the expected death rate (0.57 deaths per 100 person-years versus 0.40 deaths per 100 person-years, respectively).
Another study conducted in the USA had similar findings. In this study, investigators estimated that in the USA there are 9.8 million people living with NASH, where 6.5 million were living with fibrosis stages F0–F2; 2 million were living with NASH and fibrosis stage F3; and 1.3 million were living with NASH and cirrhosis (F4). These investigators also reported the incidence rate and the numbers of annual deaths attributable to NASH and NASH fibrosis such that the mortality rate for F3 and F4 fibrosis was 0.89 and 1.76 deaths per 100 person-years, respectively, with 17,800 annual deaths for F3 and 22,800 annual deaths for F4.4 These same investigators also provided forecasts for other countries reporting the relatively similar results.61-63
Another study attempted to discern time to the development of severe liver disease.64 These investigators found that regardless of having NAFL or NASH, it was the presence and the stage of fibrosis that dictated the time to severe liver disease and mortality. In this study, with a mean follow up of 20 years (range 0–40 years), the researchers reported that the median time until 10% of the patients developed liver decompensation was 33.4 years for F0 (95% CI: 24.2–42.6); 34.1 years for F1 (95% CI: 25.1–43.2); 22.7 years for F2 (95% CI: 13.7–31.7); 11.8 years for those with F3 (95% CI: 4.3–19.4), and 5.6 years for those with F4 (95% CI: 0.9–10.3 [hl]Figure 2[/hl]).64
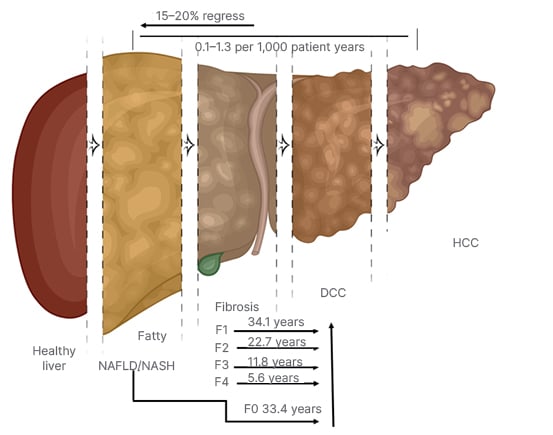
Figure 2: Non-alcoholic steatohepatitis fibrosis progression and regression.
DCC: decompensated cirrhosis; HCC: hepatocellular carcinoma; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis.
Other studies have also described the natural history of NASH and NASH fibrosis.65-69 One such study found that approximately 14% of patients with stage F0–F2 fibrosis progressed to stage F3, and 2% progressed to stage F4 over a mean duration of 4.5 years.65 When the investigators actualised these rates, they suggest that there will be 15,000 additional deaths annually among persons whose disease transitions to stage F3 or F4. As mentioned, one of the more common risk factors for progressive liver disease among NAFLD was the presence of T2D. In fact, T2D has been shown to be an independent risk factor for the liver-related mortality in patients with NAFLD and NASH.70-72
Using data from the Global Burden of Disease investigators found that, from 1990–2017, the global disability-adjusted life years from HCC due to NASH increased from 0.71 million to 1.46 million. Geographically, Australasia experienced the largest increase in the burden of HCC due to NASH, with the age-standardised disability-adjusted life years rate increasing by 143.54%. The global prevalence of HCC due to NASH peaked at 60–64 years in males and at 65–69 years in females, and a heavier burden in males compared with females.73
Finally, despite the stage of hepatic fibrosis being a major predictor of mortality, cardiovascular disease is the number one cause of death among those with NAFLD. This is most likely due to the presence of components of metabolic syndrome being associated with mortality among those with NAFLD, and the risk of mortality increasing with each component of metabolic syndrome present.74 However, due to a possible bi-directional relationship between NAFLD and various components of metabolic syndrome, particularly T2D and hypertension, work continues on discerning which disease component precedes what, and whether NAFLD is an independent predictor of cardiovascular mortality or an intermediary step along the cardiometabolic disease trajectory.75
NASH PROGRESSION AND REGRESSION
It is important to note that the natural history of NASH is not linear. Increasing evidence suggests that patients with NASH can progress for a period of time, followed by a period of regression or stability. One small study using paired liver biopsies reported on the progression and regression of patients with NAFLD.66 At baseline, 26 (72%) patients had NAFL (steatosis without liver cell injury) and 10 (28%) patients had NASH. At follow-up, 27% of those with NAFL had progressed to NASH, while 50% of patients with NASH appeared to have regressed as they no longer met the criteria of NASH. Fibrosis was found to progress in 15 (42%), regress in 9 (25%), and remain stable in 12 (33%) patients. They also found that the incidence of T2D was significantly higher in those that had progressed.66
In another study, Wong et al.67 showed that among patients with NASH at baseline, 59% continued to have NASH after 3 years of follow-up while 35% had borderline NASH and 6% of patients regressed to simple steatosis. Additionally, 27% of patients showed fibrosis progression, 48% remained stable, and 25% had fibrosis regression. While another study reported that of the patients who had NASH on baseline biopsy, 93% still had NASH at follow-up (median of 6.6 years); however, 7% had regressed to NAFL, while among those with NAFLD 42% progressed, 40% remained stable, and 18% regressed.68 In a meta-analysis of 11 cohort studies that included 150 persons with biopsy proven NAFL and 261 persons with biopsy proven NASH, investigators found that at baseline 35.8% had fibrosis stage F0; 32.5% had F1; 16.7% had F2; 9.3% had F3; and 5.7% had cirrhosis. When they studied what happened over time, they reported that, over 2,145.5 person-years of follow-up, 33.6% had fibrosis progression, 43.1% remained stable, and 22.3% experienced regression, which translated to an annual fibrosis progression rate in patients with NAFL F0 at baseline to 0.07 stages (95% CI: 0.02–0.11 stages), while for those with NASH experienced an annual progression of 0.14 stages (95% CI: 0.07-0.21 stages). These findings corresponded to one stage of progression over 14.3 years for patients with NAFL (95% CI: 9.1–50.0 years) and 7.1 years for patients with NASH (95% CI: 4.8–14.3 years).66 It is important to note that discrepancies among these results could be due to the length of time of the follow-up, more disease activity at baseline noted in those that progressed, underestimation of the presence of advanced fibrosis due to the limitations of liver biopsies, as well as the risk factors present in the patient populations (Figure 2).64,66-69
THERAPEUTIC INTERVENTIONS
Understanding the natural history of NASH is also important in the development of therapeutic interventions that may ultimately be effective in changing the trajectory of patients’ long-term outcomes. Currently, the main treatment for NASH is lifestyle management, which involves the loss of body weight of at least 10%, which may be required to have resolution of NASH and improvement of fibrosis.62,63 However, accomplishing and maintaining this weight loss is a challenge due to the multitude of factors that are barriers for sustained weight loss.64 In addition, vitamin E for those without T2D and pioglitazone for patients with pre-diabetes and diabetes have also been recommended.76-80
As such, numerous clinical trials have been ongoing to determine which medications can reach the agreed upon endpoints for a successful trial (either a regression of fibrosis of at least one stage without the progression of NASH or NASH resolution without worsening of fibrosis).81-84 In addition, it appears from a compilation of prior clinical trials that improvement in histologic features (hepatocyte ballooning, Mallory–Denk bodies, and portal inflammation) may also be associated with improvement in fibrosis, which may, in the future, be considered as surrogates for the established clinical trial endpoints.84
However, given that NASH can regress and progress, very few clinical trials have met these clinical trial endpoints that some have suggested that a trial duration should last from 5 to 7 years in order to capture the true efficacy of these medications.56-69 Despite this drawback, several medications are showing promising results such as glucagon-like peptide-1 receptor agonists and sodium–glucose co-transporter 2 inhibitors, which decrease hyperglycaemia and improve cardiovascular health; modulators of bile acid and metabolism, including farnesoid-X receptor agonist obeticholic acid and liver X receptor α inhibitor dithiolethione oltipraz; fibroblast growth factor 19 analogue aldafermin; fibroblast growth factor 21 analogue pegbelfermin; modulators of lipid metabolism (e.g., acetyl-CoA carboxylase inhibitors, stearoyl-CoA desaturase-1 inhibitors, diacylglycerol acyltransferase 2 inhibitors, thyroid hormone receptor-β agonists); and antifibrotic drugs (chemokine receptor inhibitors).35
In addition, it is also important to acknowledge basic science work that may inform future clinical trials.85-87 One study investigated the use of dandelion to prevent the progression of hepatic fibrosis among albino male rats.87 Investigators noted that the use of dandelion did have an antifibrotic effect through the carbon tetrachloride (Chemokine [C-C motif] ligand 4) liver fibrosis system through its ability to be a free radical scavenger and attenuate inflammatory cell activation. Another study using Wister rats investigated the use of olive leaf extract in providing cardiac protection while increasing the effectiveness (decreasing inflammation and oxidative stress) of an antineoplastic drug for HCC (doxorubicin), and found that olive leaf extract may be a useful adjuvant treatment.88 Work on non-invasive tests is also being conducted in conjunction with these basic science studies. One study investigating the effects of Moringa oleifera against fibrosis used MRI textured analysis to determine the antifibrotic effects of Moringa oleifera. MRI textured analysis performed excellently in identifying histological changes when compared with conventional histopathological and liver function tests.89
Bariatric surgery can be a viable alternative for those who are morbidly obese, although bariatric surgery should not be considered the first treatment choice for patients with NASH.90 Efforts must also continue on improving the living environment of many to provide healthy food options and the availability of safe places to exercise.91 Finally, low awareness and recognition of this disease plagues the field of NAFLD/NASH.92 Therefore, efforts must continue to raise awareness of this disease through educating providers and the general population.
SUMMARY
The global burden of NASH, the progressive form of NAFLD, is on the rise. Although many patients may not progress, the sheer number of people with NAFLD across the globe creates a potential tsunami of patients that need to be assessed for risk of progressive liver disease and linked to appropriate care. In this context, lifestyle management with diet and exercise should be the first step. In addition, several drugs are entering into Phase III clinical trials and could potentially provide future therapeutic options. The ongoing research in basic science in both therapeutic and diagnostic areas is encouraging, and may help advance the understanding and treatment of NASH. Raising awareness among providers, patients, and policy makers continues to be of utmost importance as the awareness of NASH increases.








