A Computer-Assisted Systematic Search for Melatonin Derivatives with High Potential as Antioxidants
New melatonin-derivatives with antioxidant potential
Abstract
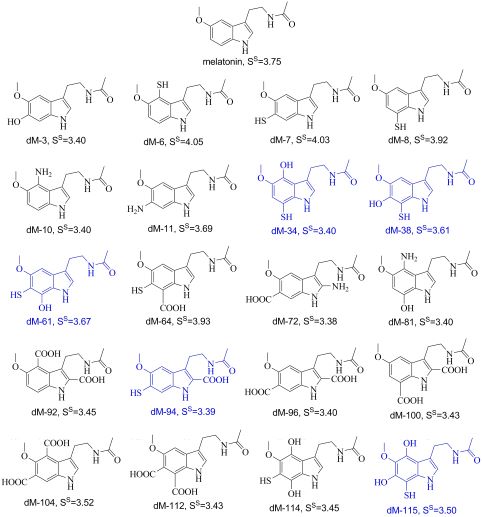
A systematic rational search for newly designed melatonin derivatives, was performed using a computer-assisted protocol. A total of 116 derivatives were generated by adding functional groups (i.e., -OH, -NH2, -SH and -COOH) to the melatonin structure. A selection score (SS) was built to sample the search space, simultaneously considering ADME (absorption, distribution, metabolism, excretion) properties, toxicity and manufacturability (i.e., synthetic accessibility). The search characterized the whole set of designed melatonin derivatives and allowed the selection of a reduced subset of 20 melatonin derivatives that are expected to be the most promising, regarding drug-like behavior. For this subset, several reactivity indices were estimated, as well as their pKa values. According to the gathered data, 5 melatonin derivatives have been identified as the most likely candidates to act as chemical antioxidant (directly scavenging free radicals, by electron transfer and/or H transfer). All of them are predicted to be better for that purpose than melatonin itself or trolox (water soluble vitamin E analog). The findings from this work are expected to motivate further investigations on these molecules, using both theoretical and experimental approaches.
References
synthetic compounds with dual effects upon free radicals and cancer. Curr. Med. Chem.
20 (36): 4451-4459.
2. Matsuda M & Shimomura I (2014) Roles of adiponectin and oxidative stress in obesity-
associated metabolic and cardiovascular diseases. Rev. Endocr. Metab. Disord. 15 (1): 1-10.
3. Eren E, et al. (2014) Heart valve disease: The role of calcidiol deficiency, elevated parathyroid
hormone levels and oxidative stress in mitral and aortic valve insufficiency. Redox Rep. 19 (1):
34-39.
4. Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: Therapeutic
implications for antioxidant treatment. Drugs Aging 18 (9): 685-716.
5. Pohanka M (2014) Alzheimer's disease and oxidative stress: A review. Curr. Med. Chem. 21
(3): 356-364.
6. Pimentel C, Batista-Nascimento L, Rodrigues-Pousada C, & Menezes RA (2012) Oxidative
stress in Alzheimer's and Parkinson's diseases: Insights from the yeast Saccharomyces cerevisiae.
Oxid. Med. Cell. Longev. 2012: 132146.
7.Ramis MR, Esteban S, Miralles A, Tan DX, & Reiter RJ (2015) Protective effects of melatonin
and mitochondria-targeted antioxidants against oxidative stress: A review. Curr. Med. Chem. 22
(22): 2690-2711.
8. Galano A, Tan DX & Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: A
physicochemical examination. J. Pineal Res. 51 (1): 1-16.
9. Hardeland R (2005) Antioxidative protection by melatonin: Multiplicity of mechanisms from
radical detoxification to radical avoidance. Endocrine 27 (2): 119-130.
10. Tan DX, Manchester LC, Terron MP, Flores LJ, & Reiter RJ (2007) One molecule, many
derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species?
J. Pineal Res. 42 (1): 28-42.
11. Tan DX, et al. (2002) Chemical and physical properties and potential mechanisms: melatonin
as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2 (2): 181-197.
12. Miller E, Walczak A, Majsterek I, & Kedziora J (2013) Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J. Neuroimmunol. 257 (1-2): 97-101.
13. Manchester LC, et al. (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59 (4): 403-419.
14. Kaya Y, Savas K, Sarikcioglu L, Yaras N, & Angelov DN (2015) Melatonin leads to axonal regeneration, reduction in oxidative stress, and improved functional recovery following sciatic nerve injury. Curr. Neurovasc. Res. 12 (1): 53-62.
15. Colín-González AL, et al. (2015) On the relationship between the light/dark cycle, melatonin and oxidative stress. Curr. Pharm. Des. 21 (24): 3477-3488.
16. Joshi N, Biswas J, Nath C, & Singh S (2015) Promising Role of Melatonin as Neuroprotectant in Neurodegenerative Pathology. Mol. Neurobiol. 52 (1): 330-340.
17. Rosales-Corral SA, Reiter RJ, Tan DX, Manchester LC, & Liu X (2014) Antioxidant and anti-inflammatory role of melatonin in Alzheimer's neurodegeneration. Aging: Oxidative stress and dietary antioxidants), pp 177-193.
18. Rosales-Corral SA, et al. (2012) Alzheimer's disease: Pathological mechanisms and the beneficial role of melatonin. J. Pineal Res. 52 (2): 167-202.
19. Paterniti I, Cordaro M, Esposito E, & Cuzzocrea S (2016) The antioxidative property of
melatonin against brain ischemia. Expert Rev. Neurother. 16 (7): 841-848.
20. García JJ, et al. (2001) N-acetylserotonin suppresses hepatic microsomal membrane rigidity associated with lipid peroxidation. Eur. J. Pharmacol. 428 (2): 169-175.
21. Oxenkrug G, Requintina P, & Bachurin S (2001) Antioxidant and antiaging activity of N-acetylserotonin and melatonin in the in vivo models. Ann. NY Acad. Sci. 939: 190-199.
22. Bachurin S, et al. (1999) N-acetylserotonin, melatonin and their derivatives improve cognition and protect against β-amyloid-induced neurotoxicity. Ann. NY Acad. Sci. 890: 155-166.
23. Jiang J, et al. (2014) N-acetyl-serotonin protects HepG2 cells from oxidative stress injury induced by hydrogen peroxide. Oxid. Med. Cell. Longev. 2014: 310504.
24. Qi W, et al. (2000) Increased levels of oxidatively damaged DNA induced by chromium(III) and H2O2: Protection by melatonin and related molecules. J. Pineal Res. 29 (1): 54-61.
25. Oxenkrug G (2005) Antioxidant effects of N-acetylserotonin: Possible mechanisms and clinical implications. in Ann. N. Y. Acad. Sci., pp 334-347.
26. López-Burillo S, et al. (2003) Melatonin and its derivatives cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine and 6-methoxymelatonin reduce oxidative DNA damage induced by Fenton reagents. J. Pineal Res. 34 (3): 178-184.
27. Tan DX, Hardeland R, Manchester LC, Galano A, & Reiter RJ (2014) Cyclic-3-hydroxymelatonin (C3HOM), a potent antioxidant, scavenges free radicals and suppresses oxidative reactions. Curr. Med. Chem. 21 (13): 1557-1565.
28. Galano A, Tan DX, & Reiter RJ (2014) Cyclic 3-hydroxymelatonin, a key metabolite enhancing the peroxyl radical scavenging activity of melatonin. RSC Adv. 4 (10): 5220-5227.
29. Tan DX, et al. (2001) N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 15 (12): 2294-2296.
30. Burkhardt S, et al. (2001) DNA oxidatively damaged by chromium(III) and H2O2 is protected by the antioxidants melatonin, N1-acetyl-N2-formyl-5-methoxykynuramine, resveratrol and uric acid. Int. J. Biochem. Cell Biol. 33 (8): 775-783.
31. Manda K, Ueno M, & Anzai K (2007) AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. J. Pineal Res. 42 (4): 386-393.
32. Galano A, Tan DX, & Reiter RJ (2013) On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J. Pineal Res. 54 (3): 245-257.
33. Maharaj DS, et al. (2002) The identification of the UV degradants of melatonin and their ability to scavenge free radicals. J. Pineal Res. 32 (4):257-261.
34. Ressmeyer AR, et al. (2003) Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): Scavenging of free radicals and prevention of protein destruction. Redox Rep. 8 (4): 205-213.
35. Schaefer M & Hardeland R (2009) The melatonin metabolite N1-acetyl-5-methoxykynuramine is a potent singlet oxygen scavenger. J. Pineal Res. 46 (1): 49-52.
36. Guenther AL, et al. (2005) Reactions of the melatonin metabolite AMK (N1-acetyl-5- methoxykynuramine) with reactive nitrogen species: Formation of novel compounds, 3-acetamidomethyl-6-methoxycinnolinone and 3-nitro-AMK. J. Pineal Res. 39 (3): 251-260.
37. Hardeland R, Backhaus C, & Fadavi A (2007) Reactions of the NO redox forms NO+, •NO and HNO (protonated NO-) with the melatonin metabolite N1-acetyl- 5-methoxykynuramine. J. Pineal Res. 43 (4): 382-388.
38. Hardeland R, Backhaus C, Fadavi A, & Hess M (2007) N1-acetyl-5-methoxykynuramine contrasts with other tryptophan metabolites by a peculiar type of NO scavenging: Cyclization to a cinnolinone prevents formation of unstable nitrosamines. J. Pineal Res. 43 (1): 104-105.
39. Maharaj DS, Walker RB, Glass BD, & Daya S (2003) 6-Hydroxymelatonin protects against cyanide induced oxidative stress in rat brain homogenates. J. Chem. Neuroanat. 26 (2): 103-107.
40. Maharaj DS, et al. (2005) 6-Hydroxymelatonin protects against quinolinic-acid-induced oxidative neurotoxicity in the rat hippocampus. J. Pharm. Pharmacol. 57 (7): 877-881.
41. Pérez-González A, Galano A, Alvarez-Idaboy JR, Tan DX, & Reiter RJ (2017) Radical-trapping and preventive antioxidant effects of 2-hydroxymelatonin and 4-hydroxymelatonin: Contributions to the melatonin protection against oxidative stress. Biochim. Biophys. Acta 1861 (9): 2206-2217.
42. Tan DX, et al. (2010) The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 85 (3): 607-623.
43. Reiter RJ, et al. (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell. Mol. Life Sci. 74 (21): 3863-3881.
44. Acuña-Castroviejo D, et al. (2014) Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 71 (16): 2997-3025.
45. Reiter RJ, Tan DX, & Fuentes-Broto L (2010) Melatonin: A multi-tasking molecule. Prog. Brain Res. 181: 127-151.
46. Reiter RJ, Tan DX, & Galano A (2014) Melatonin: Exceeding expectations. Physiology (Bethesda) 29 (5):325-333.
47. Reiter RJ, et al. (2015) Phytomelatonin: Assisting plants to survive and thrive. Molecules 20 (4): 7396-7437.
48. Wiechmann AF & Sherry DM (2013) Role of melatonin and its receptors in the vertebrate retina. Int. Rev. Cell Mol. Biol. 300: 211-242.
49. Kim TK, et al. (2013) Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 27 (7):2742-2755.
50. Pinato L, et al. (2013) Selective protection of the cerebellum against intracerebroventricular LPS is mediated by local melatonin synthesis. Brain Struct. Funct. 220 (2):1-14.
51. Venegas C, et al. (2012) Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52 (2): 217-227.
52. Cruz MHC, Leal CLV, Cruz JF, Tan DX, & Reiter RJ (2014) Essential actions of melatonin in protecting the ovary from oxidative damage. Theriogenology 82 (7): 925-932.
53. Peschke E (2008) Melatonin, endocrine pancreas and diabetes. J. Pineal Res. 44 (1): 26-40.
54. Suofu Y, et al. (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. U. S. A. 114 (38): E7997-E8006.
55. Wang L, et al. (2017) Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 63 (3). doi: 10.1111/jpi.12429.
56. Reiter RJ, Tan DX, Rosales-Corral S, & Manchester LC (2013) The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini-Rev. Med. Chem. 13 (3): 373-384.
57. Hevia D, et al. (2015) Melatonin uptake through glucose transporters: A new target for melatonin inhibition of cancer. J. Pineal Res. 58 (2): 234-250.
58. Huo X, et al. (2017) Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 62 (4)., doi: 10.1111/jpi.12390.
59. Ceraulo L, et al. (1999) Interactions of melatonin with membrane models: Portioning of melatonin in AOT and lecithin reversed micelles. J. Pineal Res. 26 (2): 108-112.
60. Bonnefont-Rousselot D & Collin F (2010) Melatonin: Action as antioxidant and potential applications in human disease and aging. Toxicology 278 (1): 55-67.
61. Jahnke G, et al. (1999) Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci. 50 (2): 271-279.
62. Andersen LPH, Gögenur I, Rosenberg J, & Reiter RJ (2016) The safety of melatonin in humans. Clin. Drug Investig. 36 (3):169-175.
63. Vijayalaxmi, Meltz ML, Reiter RJ, & Herman TS (1999) Melatonin and protection from genetic damage in blood and bone marrow: Whole-body irradiation studies in mice. J. Pineal Res. 27 (4):221-225.
64. Vijayalaxmi, Meltz ML, Reiter RJ, Herman TS, & Kumar K S (1999) Melatonin and protection from whole-body irradiation: Survival studies in mice. Mutat. Res. 425 (1): 21-27.
65. Kaya H, Delibas N, Serteser M, Ulukaya E, & Özkaya O (1999) The effect of melatonin on lipid peroxidation during radiotherapy in female rats. Strahlenther. Onkol. 175 (6):285-288.
66. Nordlund JJ & Lerner AB (1977) The effects of oral melatonin on skin color and on the release of pituitary hormones. J. Clin. Endocrinol. Metab. 45 (4): 768-774.
67. Tan DX, et al. (2000) Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept. 9 (3-4): 137-159.
68. Gurer-Orhan H & Suzen S (2015) Melatonin, its metabolites and its synthetic analogs as multi-faceted compounds: Antioxidant, prooxidant and inhibitor of bioactivation reactions. Curr. Med. Chem. 22 (4):490-499.
69. Reiter RJ, et al. (2008) Biogenic amines in the reduction of oxidative stress: Melatonin and its metabolites. Neuro Endocrinol. Lett. 29 (4):391-398.
70. Galano A, Medina ME, Tan DX, & Reiter RJ (2015) Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: A physicochemical analysis. J. Pineal Res. 58 (1): 107-116.
71. Galano A & Reiter RJ (2018) Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 65 (1):e12514.
72. Galano A, Tan DX, & Reiter RJ (2018) Melatonin: A versatile protector against oxidative
DNA damage. Molecules 23 (3): 23(3). pii: E530. doi: 10.3390/molecules23030530.
73. Romero A, et al. (2014) A review of metal-catalyzed molecular damage: Protection by
melatonin. J. Pineal Res. 56 (4): 343-370.
74. Majidinia M, et al. (2017) Melatonin: A pleiotropic molecule that modulates DNA damage response and repair pathways. J. Pineal Res. 63 (1): art. e12416, DOI 12410.11111/jpi.12416.
75. Boutin JA (2016) Quinone reductase 2 as a promising target of melatonin therapeutic actions. Expert Opin. Ther. Targets 20 (3): 303-317.
76. Galano A (2015) The role of indoleamines in reducing free radical damage and oxidative stress: A physicochemical perspective. Indoleamines: Sources, Role in Biological Processes and Health Effects), pp 1-41.
77. Suzen S (2013) Melatonin and synthetic analogs as antioxidants. Curr. Drug Del. 10 (1): 71-75.
78. Johns JR & Platts JA (2014) Theoretical insight into the antioxidant properties of melatonin and derivatives. Org. Biomol. Chem. 12 (39): 7820-7827.
79. Tsia PL & Hu MK (2003) Free radical scavenging and antioxidative activity of melatonin derivatives. J. Pharm. Pharmacol. 55 (12): 1655-1660.
80. Ates-Alagoz Z, Coban T, & Buyukbingol E (2006) Synthesis and antioxidant activity of new tetrahydro-naphthalene-indole derivatives as retinoid and melatonin analogs. Arch. Pharm. 339 (4): 193-200.
81. Ateş-Alagöz Z, Coban T, & Suzen S (2005) A comparative study: Evaluation of antioxidant activity of melatonin and some indole derivatives. Med. Chem. Res. 14 (3): 169-179.
82. Suzen S, Bozkaya P, Coban T, & Nebioǧlu D (2006) Investigation of the in vitro antioxidant behaviour of some 2-phenylindole derivatives: Discussion on possible antioxidant mechanisms and comparison with melatonin. J. Enzyme Inhib. Med. Chem. 21 (4):405-411.
83. Shirinzadeh H, Eren B, Gurer-Orhan H, Suzen S, & Özden S (2010) Novel indole-based analogs of melatonin: Synthesis and in vitro antioxidant activity studies. Molecules 15 (4): 2187-2202.
84. Yilmaz AD, Coban T, & Suzen S (2012) Synthesis and antioxidant activity evaluations of melatonin-based analogue indole-hydrazide/hydrazone derivatives. J. Enzyme Inhib. Med. Chem. 27 (3):428-436.
85. Gürkök G, Coban T, & Suzen S (2009) Melatonin analogue new indole hydrazide/hydrazone derivatives with antioxidant behavior: Synthesis and structureactivity relationships. J. Enzyme Inhib. Med. Chem. 24 (2): 506-515.
86. Suzen S, Cihaner SS, & Coban T (2012) Synthesis and comparison of antioxidant properties of indole-based melatonin analogue indole amino acid derivatives. Chem. Biol. Drug Des. 79(1): 76-83.
87. Galano A (2016) Computational-aided design of melatonin analogues with outstanding multifunctional antioxidant capacity. RSC Advances 6 (27): 22951-22963.
88. Lipinski CA, Lombardo F, Dominy BW, & Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 46 (1-3): 3-26.
89. Ghose AK, Viswanadhan VN, & Wendoloski JJ (1999) A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1 (1): 55-68.
90. Veber DF, et al. (2002) Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45 (12): 2615-2623.
91. Leeson PD & Davis AM (2004) Time-related differences in the physical property profiles of oral drugs. J. Med. Chem. 47 (25): 6338-6348.
92. Zhu H, et al. (2008) Combinatorial QSAR modeling of chemical toxicants tested against Tetrahymena pyriformis. J. Chem. Inf. Model. 48 (4): 766-784.
93. Boda K, Seidel T, & Gasteiger J (2007) Structure and reaction based evaluation of synthetic accessibility. J. Comput. Aided Mol. Des. 21 (6): 311-325.
94. Bonnet P (2012) Is chemical synthetic accessibility computationally predictable for drug and lead-like molecules? A comparative assessment between medicinal and computational chemists. Eur. J. Med. Chem. 54: 679-689.
95. Frisch MJ, et al. (2009) Gaussian 09 (Gaussian, Inc., Wallingford, CT, USA).
96. Zhao Y, Schultz NE, & Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. Journal of Chemical Theory and Computation 2 (2):364-382.
97. Marenich AV, Cramer CJ, & Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113 (18):6378-6396.
98. Zhao Y & Truhlar DG (2008) How well can new-generation density functionals describe the energetics of bond-dissociation reactions producing radicals? J. Phys. Chem. A 112 (6): 1095-1099.
99. Galano A & Alvarez-Idaboy JR (2013) A computational methodology for accurate predictions of rate constants in solution: Application to the assessment of primary antioxidant activity. J. Comput. Chem. 34 (28): 2430-2445.
100. Pérez-González A, et al. (2017) Estimation of empirically fitted parameters for calculating pK a values of thiols in a fast and reliable way. Theor. Chem. Acc. 137 (1): art. 5 (10 pages).
101. Galano A, et al. (2016) Empirically Fitted Parameters for Calculating pKa Values with Small Deviations from Experiments Using a Simple Computational Strategy. J. Chem. Inf. Model. 56 (9): 1714-1724.
102. Galano A, Francisco Marquez M, & Pérez-González A (2014) Ellagic acid: An unusually versatile protector against oxidative stress. Chem. Res. Toxicol. 27 (5): 904-918.
103. Marino T, Galano A, & Russo N (2014) Radical scavenging ability of gallic acid toward OH and OOH radicals. Reaction mechanism and rate constants from the density functional theory. J. Phys. Chem. B 118 (35):10380-10389.
104. Galano A, Raul Alvarez-Idaboy J, & Francisco-Márquez M (2010) Mechanism and branching ratios of hydroxy ethers + ·oH gas phase reactions: Relevance of H bond interactions. J. Phys. Chem. A 114 (28):7525-7536.
105. León-Carmona JR, Martínez A, & Galano A (2014) New free radicals to measure antiradical capacity: A theoretical study. J. Phys. Chem. B 118 (34): 10092-10100.
106. Pérez-González A, Galano A, & Alvarez-Idaboy JR (2014) Dihydroxybenzoic acids as free radical scavengers: Mechanisms, kinetics, and trends in activity. New J. Chem. 38 (6): 2639-2652.
107. Pérez-González A & Galano A (2013) On the hydroperoxyl radical scavenging activity of two Edaravone derivatives: Mechanism and kinetics. J. Phys. Org. Chem. 26 (3): 261-268.
108. Pérez-González A & Galano A (2012) On the •oH and •oOH scavenging activity of 3-methyl-1-pyridin-2-yl-5-pyrazolone: Comparisons with its parent compound, edaravone. Int. J. Quantum Chem 112 (21): 3441-3448.
109. Álvarez-Diduk R, Ramírez-Silva MT, Galano A, & Merkoçi A (2013) Deprotonation mechanism and acidity constants in aqueous solution of flavonols: A combined experimental and theoretical study. J. Phys. Chem. B 117 (41): 12347-12359.
110. Medina ME, Galano A, & Alvarez-Idaboy JR (2014) Theoretical study on the peroxyl radicals scavenging activity of esculetin and its regeneration in aqueous solution. Phys. Chem. Chem. Phys. 16 (3): 1197-1207.
111. Rebollar-Zepeda AM & Galano A (2016) Quantum mechanical based approaches for predicting pKa values of carboxylic acids: evaluating the performance of different strategies. RSC Adv. 6 (113):112057-112064.
112. Ortiz JV (1999) Toward an Exact One-Electron Picture of Chemical Bonding. Adv. Quantum Chem. 35: 33-52.
113. Ortiz JV (2013) Electron propagator theory: An approach to prediction and interpretation in quantum chemistry. Wiley Interdisciplinary Reviews: Computational Molecular Science 3 (2): 123-142.
114. Ortiz JV (1996) Partial third-order quasiparticle theory: Comparisons for closed-shell ionization energies and an application to the Borazine photoelectron spectrum. J. Chem. Phys. 104 (19): 7599-7605.
115. Pérez-González A, Galano A, & Ortiz JV (2014) Vertical ionization energies of free radicals and electron detachment energies of their anions: A comparison of direct and indirect methods versus experiment. J. Phys. Chem. A 118 (31):6125-6131.
116. Parr RG, Szentpály Lv, & Liu S (1999) Electrophilicity Index. J. Am. Chem. Soc. 121 (9): 1922-1924.
117. Chattaraj PK, Maiti B, & Sarkar U (2003) Philicity: A Unified Treatment of Chemical Reactivity and Selectivity. J Phys. Chem. A 107 (25):4973-4975.
118. Gázquez JL, Cedillo A, & Vela A (2007) Electrodonating and Electroaccepting Powers. J. Phys. Chem. A 111 (10): 1966-1970.
119. Gázquez JL (2008) Perspectives on the density functional theory of chemical reactivity. J. Mex. Chem. Soc. 52 (1): 3-10.
120. Pearson RG (1963) Hard and Soft Acids and Bases. J. Am. Chem. Soc. 85 (22):3533-3539.
121. Pearson RG (1993) The principle of maximum hardness. Acc. Chem. Res. 26 (5): 250-255.
122. Ortiz JV (2003) Quasiparticle Approximations and Electron Propagator Theory. Int. J. Quantum Chem 95 (4-5): 593-599.
123. Singh RK, Ortiz JV, & Mishra MK (2010) Tautomeric forms of adenine: Vertical ionization energies and Dyson orbitals. Int. J. Quantum Chem 110 (10): 1901-1915.
124. Areti A, Yerra VG, Naidu VGM, & Kumar A (2014) Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol 2 (1): 289-295.
125. Miyagawa T, et al. (2013) Effects of Oral L-Carnitine Administration in Narcolepsy Patients: A Randomized, Double-Blind, Cross-Over and Placebo-Controlled Trial. PLoS ONE 8 (1).: e53707. doi: 10.1371/journal.pone.0053707.
126. Vermersch P, et al. (2012) Masitinib treatment in patients with progressive multiple sclerosis: a randomized pilot study. BMC Neurol 12:36.
127. Folch J, et al. (2015) Masitinib for the treatment of mild to moderate Alzheimer's disease. Expert Rev Neurother 15 (6): 587-596.
128. Bareš M, Kaňovský P, Klajblová H, & Rektor I (2003) Intracortical inhibition and facilitation are impaired in patients with early Parkinson's disease: A paired TMS study. Eur. J. Neurol. 10 (4): 385-389.
129. Fox SH, et al. (2018) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson's disease. Mov. Disord. 33 (8):1248-1266.
130. Niemann N & Jankovic J (2018) Treatment of Tardive Dyskinesia: A General Overview with Focus on the Vesicular Monoamine Transporter 2 Inhibitors. Drugs 78 (5):525-541.
131. Koziróg M, et al. (2011) Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J. Pineal Res. 50 (3): 261-266.
132. Chahbouni M, et al. (2010) Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J. Pineal Res. 48 (3):282-289.
133. Hanaki M, Murakami K, Katayama S, Akagi KI, & Irie K (2018) Mechanistic analyses of the suppression of amyloid β42 aggregation by apomorphine. Bioorg. Med. Chem. 26 (8): 1538-1546.
134. Auffret M, Drapier S, & Vérin M (2018) Pharmacological Insights into the Use of Apomorphine in Parkinson’s Disease: Clinical Relevance. Clin. Drug Investig. 38 (4): 287-312.
135. Tavakoli-Ardakani M, Abbaspour H, Farhadi Nasab A, Mazaheri Meibodi A, & Kheradmand A (2018) Study of the effect of memantine on negative sign in patients with schizophrenia and schizoaffective disorders. Iran J. Pharm. Res. 17 (Special Issue): 122-129.
136. Pucks-Faes E, et al. (2018) Eleven years’ experience with Intrathecal Baclofen – Complications, risk factors. Brain Behav. 8 (5): e00965.
137. McLaughlin MJ, et al. (2018) Pharmacogenomic Variability of Oral Baclofen Clearance and Clinical Response in Children With Cerebral Palsy. PM and R 10 (3):235-243.
138. Porsdam Mann S, de Lora Deltoro P, Cochrane T, & Mitchell C (2018) Is the use of modafinil, a pharmacological cognitive enhancer, cheating? Ethics and Education 13 (2): 251-267.
139. Minzenberg MJ, Yoon JH, Soosman SK, & Carter CS (2018) Altered brainstem responses to modafinil in schizophrenia: Implications for adjunctive treatment of cognition. Transl Psychiatry 8(1): 58. doi: 10.1038/s41398-018-0104-z ..
140. Politi C, Ciccacci C, Novelli G, & Borgiani P (2018) Genetics and Treatment Response in Parkinson’s Disease: An Update on Pharmacogenetic Studies. Neuromolecular Med. 20 (1): 1-17.
141. Gasser UE, Fischer A, Timmermans JP, & Arnet I (2013) Pharmaceutical quality of seven generic Levodopa/Benserazide products compared with original Madopar® / Prolopa®. BMC Pharmacol Toxicol 14: 24. doi: 10.1186/2050-6511-14-24.
142. Taddei RN, Spinnato F, & Jenner P (2017) New Symptomatic Treatments for the Management of Motor and Nonmotor Symptoms of Parkinson's Disease. Int. Rev. Neurobiol. 132: 407-452.
143. Ogino S, Miyamoto S, Miyake N, & Yamaguchi N (2014) Benefits and limits of anticholinergic use in schizophrenia: Focusing on its effect on cognitive function. Psychiatry Clin Neurosci. 68 (1): 37-49.
144. Bergman H & Soares-Weiser K (2018) Anticholinergic medication for antipsychotic-induced tardive dyskinesia. Cochrane Database Syst. Rev. 2018 (1): CD000204.
145. Mizuno Y, Shimoda S, & Origasa H (2018) Long-term treatment of Parkinson’s disease with levodopa and other adjunctive drugs. J Neural Transm (Vienna) 125 (1): 35-43.
146. Grimaldi R, et al. (1986) Pharmacokinetic and pharmacodynamic studies following the intravenous and oral administration of the antiparkinsonian drug biperiden to normal subjects. Eur J Clin Pharmacol 29 (6): 735-737.
147. Hauser RA & Holford NHG (2002) Quantitative description of loss of clinical benefit following withdrawal of levodopa-carbidopa and bromocriptine in early Parkinson's disease. Mov Disord 17 (5): 961-968.
148. Zaccara G, et al. (2017) Do antiepileptic drugs increase the risk of infectious diseases? A meta-analysis of placebo-controlled studies. Br J Clin Pharmacol 83 (9): 1873-1879.
149. Steiger MJ, El-Debas T, Anderson T, Findley LJ, & Marsden CD (1996) Double-blind study of the activity and tolerability of cabergoline versus placebo in parkinsonians with motor fluctuations. J Neurol. 243 (1): 68-72.
150. Rinne UK, et al. (1998) Early treatment of Parkinson's disease with cabergoline delays the onset of motor complications. Results of a double-blind levodopa controlled trial. Drugs 55 (SUPPL. 1): 23-30.
151. Oberstadt M, et al. (2018) TDP-43 self-interaction is modulated by redox-active compounds Auranofin, Chelerythrine and Riluzole. Sci. Rep. 8 (1): 2248. doi: 10.1038/s41598-018-20565.
152. Stocchi F, et al. (2010) Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: The STRIDE-PD study. Ann Neurol. 68 (1): 18-27.
153. Hauser RA, et al. (2013) Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson's disease and motor fluctuations: A phase 3 randomised, double-blind trial. Lancet Neurol. 12 (4): 346-356.
154. Takeda A, et al. (2006) A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer's disease. Int J Geriatr Psychiatry 21 (1): 17-28.
155. Epstein J, Sanderson IR, & MacDonald TT (2010) Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br. J. Nutr. 103 (11): 1545-1557.
156. Chen M, et al. (2018) Use of curcumin in diagnosis, prevention, and treatment of Alzheimer's disease. Neural Regen Res 13 (4): 742-752.
157. Schmidt RT, Lee RH, & Spehlmann R (1976) Comparison of dantrolene sodium and diazepam in the treatment of spasticity. J. Neurol. Neurosurg. Psychiatry 39 (4): 350-356.
158. Katrak PH, Cole AMD, Poulos CJ, & McCauley JCK (1992) Objective assessment of spasticity, strength, and function with early exhibition of dantrolene sodium after cerebrovascular accident: A randomized double-blind study. Arch Phys Med Rehabi l 73 (1): 4-9.
159. Glass A & Hannah A (1974) A comparison of dantrolene sodium and diazepam in the treatment of spasticity. Paraplegia 12 (3): 170-174.
160. Ebadi M, Sharma S, Shavali S, & El Refaey H (2002) Neuroprotective actions of selegiline. J. Neurosci. Res. 67 (3): 285-289.
161. Román GC, et al. (2010) Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: Differential effects by hippocampal size. Stroke 41 (6): 1213-1221.
162. Hepnarova V, et al. (2018) The concept of hybrid molecules of tacrine and benzyl quinolone carboxylic acid (BQCA) as multifunctional agents for Alzheimer's disease. Eur. J. Med. Chem. 150: 292-306.
163. Giacobini E (1998) Invited review. Cholinesterase inhibitors for Alzheimer's disease therapy: From tacrine to future applications. Neurochem. Int. 32 (5-6): 413-419.
164. Poewe WH, Deuschl G, Gordin A, Kultalahti ER, & Leinonen M (2002) Efficacy and safety of entacapone in Parkinson's disease patients with suboptimal levodopa response: A 6-month randomized placebo controlled double blind study in Germany and Austria (Celomen study). Acta Neurol. Scand. 105 (4): 245-255.
165. Myllylä VV, et al. (2001) Twelve-month safety of entacapone in patients with Parkinson's disease. Eur. J. Neurol. 8 (1): 53-60.
166. Scorr LM & Factor SA (2018) VMAT2 inhibitors for the treatment of tardive dyskinesia. J. Neuro.l Sci. 389:.43-47.
167. Erkinjuntti T, et al. (2002) Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: A randomised trial. Lancet 359 (9314): 1283-1290.
168. Burns A, et al. (2009) Safety and efficacy of galantamine (Reminyl) in severe Alzheimer's disease (the SERAD study): a randomised, placebo-controlled, double-blind trial. Lancet Neurol.. 8 (1):39-47.
169. Blautzik J, et al. (2016) Functional connectivity increase in the default-mode network of patients with Alzheimer's disease after long-term treatment with Galantamine. Eur. Neuropsychopharmacol. 26 (3): 602-613.
170. Rudolf G, et al. (2018) A novel non-opioid protocol for medically supervised opioid withdrawal and transition to antagonist treatment. Am. J. Drug Alcohol Abuse 44 (3): 302-309.
171. Geldenhuys WJ & Van Der Schyf CJ (2013) Designing drugs with multi-target activity: The next step in the treatment of neurodegenerative disorders. Expert Opin. Drug Discov. 8 (2): 115-129.
172. Weinreb O, Amit T, Bar-Am O, & Youdim MBH (2011) A novel anti-Alzheimer's disease drug, ladostigil. neuroprotective, multimodal brain-selective monoamine oxidase and cholinesterase inhibitor. in Int. Rev. Neurobiol., pp 191-215.
173. Velázquez-Pérez L, et al. (2012) Lisuride reduces involuntary periodic leg movements in spinocerebellar ataxia type 2 patients. Cerebellum 11 (4): 1051-1056.
174. Md S, Haque S, Sahni JK, Baboota S, & Ali J (2011) New non-oral drug delivery systems for Parkinson's disease treatment. Expert Opin. Drug Deliv. 8 (3): 359-374.
175. Zhong HA (2017) ADMET properties: Overview and current topics. Drug Design: Principles and Applications), pp 113-133.
176. Schneider G & Fechner U (2005) Computer-based de novo design of drug-like molecules. Nat. Rev. Drug Discov. 4 (8): 649-663.
177. Chaiyasit W, Elias RJ, McClements DJ, & Decker EA (2007) Role of physical structures in bulk oils on lipid oxidation. Crit. Rev. Food Sci. Nutr. 47 (3): 299-317.
178. Galano A & Alvarez‐Idaboy JR (2018) Computational strategies for predicting free radical scavengers' protection against oxidative stress: Where are we and what might follow? Int. J. Quantum Chem. 118 (13): art. e25665 (25623 pages).
179. Miche H, Brumas V, & Berthon G (1997) Copper(II) interactions with nonsteroidal antiinflammatory agents. II. Anthranilic acid as a potential OH-inactivating ligand. J. Inorg. Biochem. 68 (1): 27-38.
180. Gaubert S, Bouchaut M, Brumas V, & Berthon G (2000) Copper-ligand interactions and physiological free radical processes. Part 3. Influence of histidine, salicylic acid and anthranilic acid on copper-driven Fenton chemistry in vitro. Free Radic. Res. 32 (5): 451-461.
181. Brenk R, et al. (2008) Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. Chem. Med .Chem. 3 (3): 435-444.
182. Alvarez-Idaboy JR & Galano A (2012) On the chemical repair of DNA radicals by glutathione: Hydrogen vs electron transfer. J. Phys. Chem. B 116 (31): 9316-9325.
183. Galano A & Alvarez-Idaboy JR (2011) Glutathione: Mechanism and kinetics of its non-enzymatic defense action against free radicals. RSC Adv. 1 (9): 1763-1771.
184. Gleeson MP, Hersey A, Montanari D, & Overington J (2011) Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discov. 10 (3): 197-208.
185. Zhong HA, Mashinson V, Woolman TA, & Zha M (2013) Understanding the molecular properties and metabolism of top prescribed drugs. Curr Top Med Chem 13(11):1290-1307.
186. Martínez A, Vargas R, & Galano A (2009) What is important to prevent oxidative stress? A theoretical study on electron-transfer reactions between carotenoids and free radicals. J. Phys. Chem. B 113 (35):12113-12120.
187. Martínez A, Rodríguez-Girones MA, Barbosa A, & Costas M (2008) Donator acceptor map for carotenoids, melatonin and vitamins. J. Phys. Chem. A 112 (38):9037-9042.
188. Ulstrup J & Jortner J (1975) The effect of intramolecular quantum modes on free energy relationships for electron transfer reactions. J. Chem. Phys. 63 (10): 4358-4368.
189. Marcus RA & Sutin N (1985) Electron transfers in chemistry and biology. BBA Reviews On Bioenergetics 811 (3): 265-322.
190. Marcus RA (1993) Electron transfer reactions in chemistry: Theory and experiment (Nobel lecture). Angew Chem Int. Ed. Engl. 32 (8): 1111-1121.
191. Galano A (2011) On the direct scavenging activity of melatonin towards hydroxyl and a series
of peroxyl radicals. Phys. Chem. Chem. Phys. 13 (15): 7178-7188.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


