Bifidobacterium breve MCC1274 Supplementation Increased the Plasma Levels of Metabolites with Potential Anti-Oxidative Activity in APP Knock-In Mice
Abstract
Background:
We previously reported the effects of a probiotic strain, Bifidobacterium breve MCC1274, in improving cognitive function in preclinical and clinical studies. Recently, we demonstrated that supplementation of this strain led to decreased amyloid-β production, attenuated microglial activation, and suppressed inflammation reaction in the brain of APP knock-in (AppNL - G - F) mice.
Objective:
In this study, we investigated the plasma metabolites to reveal the mechanism of action of this probiotic strain in this Alzheimer’s disease (AD)-like model.
Methods:
Three-month-old mice were orally supplemented with B. breve MCC1274 or saline for four months and their plasma metabolites were comprehensively analyzed using CE-FTMS and LC-TOFMS.
Results:
Principal component analysis showed a significant difference in the plasma metabolites between the probiotic and control groups (PERMANOVA, p = 0.03). The levels of soy isoflavones (e.g., genistein) and indole derivatives of tryptophan (e.g., 5-methoxyindoleacetic acid), metabolites with potent anti-oxidative activities were significantly increased in the probiotic group. Moreover, there were increased levels of glutathione-related metabolites (e.g., glutathione (GSSG)_divalent, ophthalmic acid) and TCA cycle-related metabolites (e.g., 2-Oxoglutaric acid, succinic acid levels) in the probiotic group. Similar alternations were observed in the wild-type mice by the probiotic supplementation.
Conclusion:
These results suggest that the supplementation of B. breve MCC1274 enhanced the bioavailability of potential anti-oxidative metabolites from the gut and addressed critical gaps in our understanding of the gut-brain axis underlying the mechanisms of the probiotic action of this strain in the improvement of cognitive function.
INTRODUCTION
Alzheimer’s disease (AD) is the primary cause of dementia and is fast becoming one of the most burdensome and expensive diseases this century [1]. More than 50 million people will be diagnosed with dementia, and the number of these patients could go as high as 152 million in 2050 [2], as many predict. AD is linked to amyloid-β (Aβ) deposition causing senile plaques. It is also often accompanied by neurofibrillary tangles due to the tau protein’s hyperphosphorylation [3]. However, various factors such as oxidative stress [4], chronic inflammation [5], and abnormal glucose metabolism [6], are also associated with the disease. Since these neurological pathologies progress slowly over more than 20 years, much emphasis has been placed on early AD diagnosis and how to prevent its onset [7]. Significant research has led to the identification of blood biomarkers and studies of preventive interventions of change in daily life activities such as diet and exercise habits.
Recently, many studies have focused on the microbiome-gut-brain axis, a growing concept in which the brain interacts with the gut mediated by gut microbiota (GM). In humans, several studies have reported the difference of GM in both American [8], Chinese, [9], and Japanese patients with AD [10]. In addition, studies using germ-free AD-like model mice show a noticeable reduction of cerebral Aβ, indicating a role of GM in the early pathological change of AD [11]. The communication between the gut and the brain is achieved mainly via direct neural pathways such as the vagus nerve, pathways through the immune system and endocrine system, and pathways by which metabolites reach the brain via blood vessel [12]. Therefore, examining perturbation in microbial metabolites could help reveal the microbial signatures that help improve the prognosis or ameliorate the progression of AD. Metabolomics studies in AD were well conducted in blood [13], urine [14], brain [15], or cerebrospinal fluid [16]. Recently, a reduction of five short-chain fatty acids (SCFAs) and altered profile of tryptophan derivatives were identified as signatures for pre-onset and progression of AD [17].
Our group focuses on the benefits of a probiotic strain Bifidobacterium breve MCC1274 (B. breve MCC1274, synonym: B. breve A1) in improving cognitive function. In a human randomized controlled trial study of people with suspected mild cognitive impairment, immediate memory and delayed memory related to memory ability, and visuospatial/construction related to spatial recognition were significantly improved by supplementing B. breve MCC1274 for 16 weeks compared to the placebo group [18]. In addition, this strain consumption improves memory and reduces the hippocampus’s inflammatory response in AD-like model mice in which Aβ is directly injected into the brain [19].
Recently, the effect of this strain in improving cognitive function has been evaluated using APPNL - G - F mice, a representative AD-like model of overproducing Aβ without overexpressing amyloid precursor protein (APP) [20]. Data indicated that the oral supplementation of this strain prevents memory impairment and decreases hippocampal Aβ levels. Furthermore, increased expression of α-disintegrin and metalloproteinase 10 (ADAM10), a typical α-secretase in the brain, specifically in the hippocampus, was associated with these effects. In addition, the probiotic treatment also enhanced the PKC-ERK-HIF-1α pathway, which is a transcriptional activator of ADAM10. Also, it attenuated microglial activation, which led to reduced mRNA expression levels of pro-inflammatory cytokines in the brain. These studies gave insight into the benefits of the probiotic treatment, such as reducing Aβ deposition and suppressing inflammatory reaction in the brain, which is closely related to the pathologies of cognitive impairment. However, the ligands that activated these signaling pathways or how they interacted from the gut to the brain remain undefined. Therefore, to understand the mechanism of action and further explore the pharmaceutical use potential of this strain, we conducted a metabolomics analysis of plasma of AD-like model (APPNL - G - F) mice and wild-type mice with or without B. breve MCC1274 treatment.
MATERIALS AND METHODS
Sample preparation
The plasma samples were obtained from studies performed at Nagoya City University which were approved by Nagoya City University Institutional Care and Use of Laboratory Animals committee to understand the mechanisms of action and explore this probiotic pharmaceutical potential. Plasma samples of AD-like model mice were obtained from our previous study, which were orally supplemented with B. breve MCC1274 (AT group) or saline (AC group) for four months [20]. In addition, two-month-old C57BL/6J wild-type mice (6 males and 6 females per group) were also administrated in the same way with B. breve MCC1274 (WT group) or saline (WC group) for comparison with the AD-like models as described in the previous study [21]. Plasma was promptly frozen in liquid nitrogen after separation from blood and then stored at –80°C. Twelve plasma samples were obtained per group. In order to ensure sufficient volume to perform metabolomic analysis, each sample was thawed and three samples were randomly pooled, and finally four samples for each group were analyzed.
Metabolomic analysis with CE-FTMS
40μL of plasma was added to 160μL of methanol containing the internal standard (H3304-1002, Human Metabolome Technologies, Inc. (HMT)) at 0°C to inhibit enzyme activity. After this solution was mixed thoroughly with 120μL of Milli-Q water, 240μL of this mixture was centrifuged with a Millipore 5 kDa cutoff filter (ULTRAFREE MC PLHCC, HMT) at 9,100× g for 120 min at 4°C to remove macromolecules. The filtrate was evaporated to dryness under vacuum and then dissolved in 40μL of Milli-Q water, followed by donation to HMT’s ω Scan package using capillary electrophoresis Fourier transform mass spectrometry (CE-FTMS) based on the methods described previously [22, 23]. The spectrometer was scanned from m/z 60 to 900 in positive mode and from m/z 70 to 1,050 in negative mode, respectively.
Metabolomic analysis with LC-TOFMS
80μL of plasma was added to 240μL of 1% formic acid/acetonitrile containing the internal standard (same as CE-FTMS) at 0°C. The mixture was centrifuged at 2,300× g, 4°C for 5 min and then filtered using a hybrid SPE phospholipid cartridge (Hybrid SPE - Phospholipid 30 mg/mL, SUPELCO) to remove phospholipids. The filtrate was then evaporated to dryness under nitrogen, dissolved in 80μL of 50% isopropanol (v/v), and conducted a metabolomic analysis using liquid chromatography time-of-flight mass spectrometry (LC-TOFMS) based on the methods previously described (HMT’s LC package) [24, 25]. Briefly, LC-TOFMS analysis was performed using an Agilent 1200 HPLC pump, an ODS column (2 mm×50 mm, two μm i.d.), and an Agilent 6210 time-of-flight mass spectrometer (Agilent Technologies, USA). The system was controlled by MassHunter (Agilent Technologies, USA), and the spectrometer was scanned from m/z 50 to 1,000.
Annotation of metabolites detected by CE-FTMS and LC-TOFMS
Peaks were extracted with the automated integration software MasterHands (Keio University, Japan), and peak information such as m/z, peak area, retention time (RT), and migration time (MT) was obtained [26]. Signal peaks that correspond to known metabolite isotopes, additional ions, and other product ions were excluded, and the remaining peaks were annotated according to the HMT metabolite database based on their m/z values and RT or MT. The internal standard and sample volume then normalized the areas of each annotated peak to calculate the relative amounts of the metabolites.
Quantifying the concentration of characteristic metabolites
The CE-FTMS and LC-TOFMS systems support the quantification of a limited numbers of metabolites using a one-point calibration curve based on their respective standard compounds. In addition, the concentrations of the indole-3-propionic acid (IPA) were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS; Vanquish HPLC connected with TSQ-FORTIS, Thermo Fisher Scientific, USA) based on previous research with some modifications [27]. In brief, chromatographic separation was performed using an XBridge® C18 column (Waters Corporation, Milford) (4.6×150 mm, 3.5μm) with mobile phase A (containing 1 g/L ammonium acetate in water) and mobile phase B (containing 1 g/L ammonium formate and 0.1% formic acid in methanol) at a flow rate of 0.2 mL/min and gradient elution at 2% B. Concentration quantification was performed by comparing peak areas of ions with those of the corresponding standards and internal standard (1-methyl-2-oxindole, Sigma-Aldrich).
Statistical analysis
Statistical analysis was performed using R software (version 3.6.0). The comparison between probiotic and saline groups in the principal component analysis was assessed by permutation manova using the adonis package in R. Also, intergroup comparisons of each metabolite were performed by Welch’s t-test.
RESULTS
Metabolomic analysis
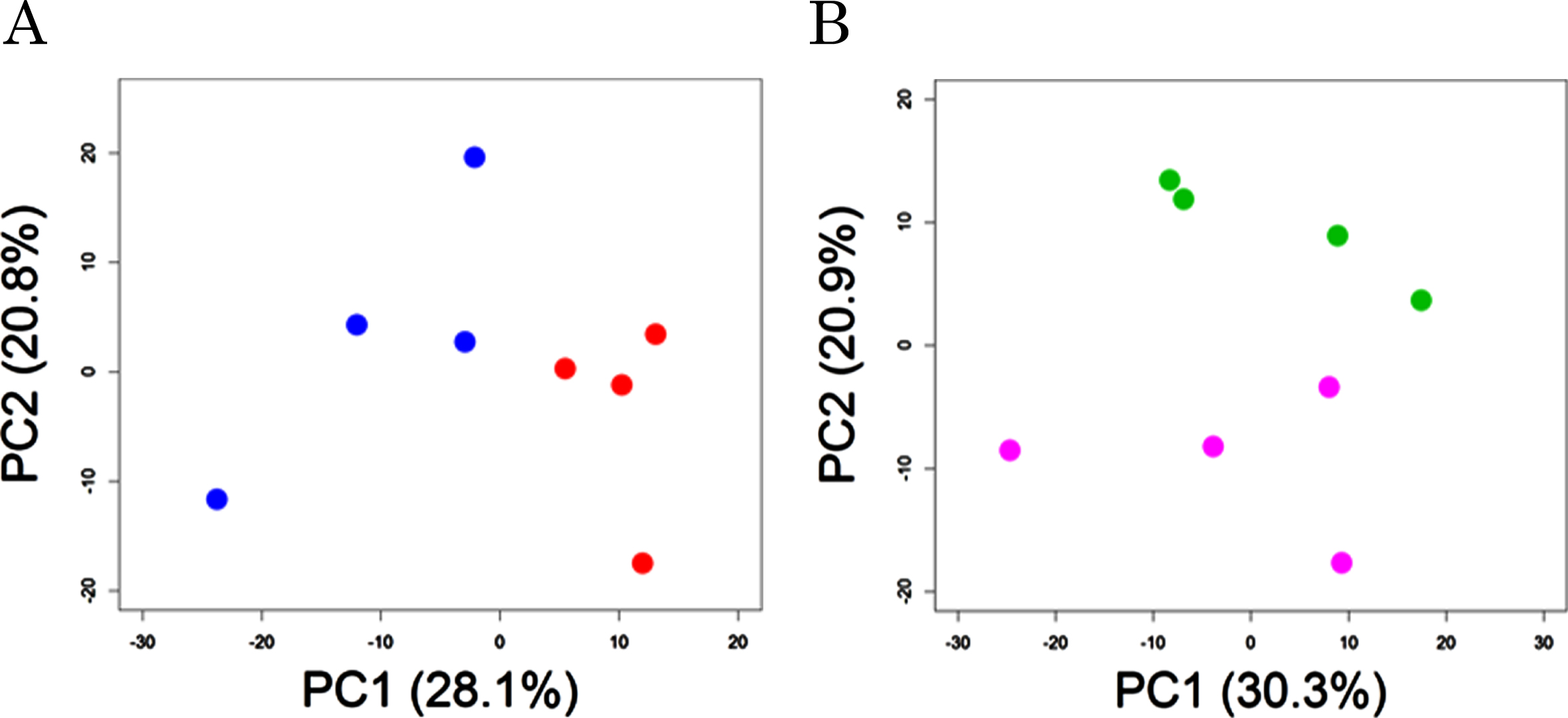
Plasma metabolomic analysis by CE-FTMS and LC-TOFMS detected a total of 400 (hydrophilic) and 212 (lipophilic) peaks, respectively. 63 of the 400 hydrophilic and 22 of the 212 lipophilic were significantly different between probiotic group (supplemented with B. breve MCC1274, AT group) and control (AC groups) in the AD-like model mice (Supplementary Table 1). Principal component analysis showed a significant difference between AT and AC groups (PERMANOVA, p = 0.03, Fig. 1A). In addition, of the compounds in the wild-type groups, 46 of the 400 hydrophilic and 7 of the 212 lipophilic were significantly different between the probiotic (WT) and control (WC) groups (Supplementary Table 2). Principal component analysis showed a tendency of difference between WT and WC groups (PERMANOVA, p = 0.09, Fig. 1B).
Fig. 1
Principal component analysis of plasma metabolites. AD-like model mice (A) and wild-type mice (B) were orally supplemented with B. breve MCC1274 (red or purple circles) or saline (blue or green circles). p values show the comparison of group difference as determined by permutation MANOVA.
In order to understand the substantial changes due to B. breve MCC1274 supplementation, we focused on the metabolites with a p-value <0.05 and |log2 (fold change)|>1 in the group comparison. Based on these criteria, 23 metabolites were extracted with 21 increased and 2 decreased in the AT mice compared with the AC mice (Fig. 2A, B, Table 1). Whereas 18 compounds were extracted in the wild-type mice, with 17 increased and one decreased in the probiotic group (Fig. 2C, D, Table 2). Similar changes were observed for many metabolites in both the AD and wild-type mice by the oral supplementation of B. breve 1274. Among them, in comparison with the control groups, glutathione (GSSG)_divalent, a disulfide derived from two glutathione molecules, increased significantly for 7.3-fold (p = 0.0014) in AT mice and, although not significantly, it increased for 2.3-fold (p = 0.063) in WT ones. Ophthalmic acid, a tripeptide analog of glutathione, increased significantly for 5.8- (p < 0.0001) and 3.9-fold (p = 0.0117) in AT and WT mice, respectively. Significant increases of glutathione derivatives such as cysteine glutathione disulfide (2.3-fold, p = 0.0025 in AT mice) and S-methylglutathione (3.1-fold, p = 0.0043 in AT and 2.4-fold, p = 0.0014 in WT mice) were also observed. Genistein, an isoflavone derivative, increased significantly for 2.3-fold (p = 0.0004) in AT mice, and there was a tendency of increase (1.9-fold, p = 0.097) in the wild-type ones. Similarly, glycitein, another isoflavone derivative, increased significantly for 2.8-fold (p = 0.0013) in WT mice but not significantly in the AT ones (1.3-fold, p = 0.391). 2-oxoglutaric acid, an intermediate in the TCA cycle, increased significantly for 2.5- (p = 0.0072) and 2.1-fold (p = 0.0215) in AT and WT mice, respectively. Succinic acid, another intermediate in the TCA cycle, increased significantly for 2.3-fold (p = 0.0222) in AT mice, and although not significantly, it increased by 2.9-fold (p = 0.060) in WT ones. 5-methoxyindoleacetic acid (5-MIAA), a tryptophan derivative, increased 2.5- (p = 0.0062) and 2.0-fold (p = 0.0089) in AT and WT mice, respectively. The simultaneous increase in both models by probiotic supplementation was also found for hypotaurine, butyrylcarnithine, N-Acetylphenylalanine, and 4-Guanidinobutyric acid (Tables 1 and 2).
Fig. 2
Metabolites with differences in each group comparison. Volcano plot and heat map of changing metabolites in AD-like model mice (A, B) and wild type mice (C, D) orally supplemented with B. breve MCC1274 (AT or WT) or saline (AC or WC). Venn diagram of variable metabolites in group comparison (E).
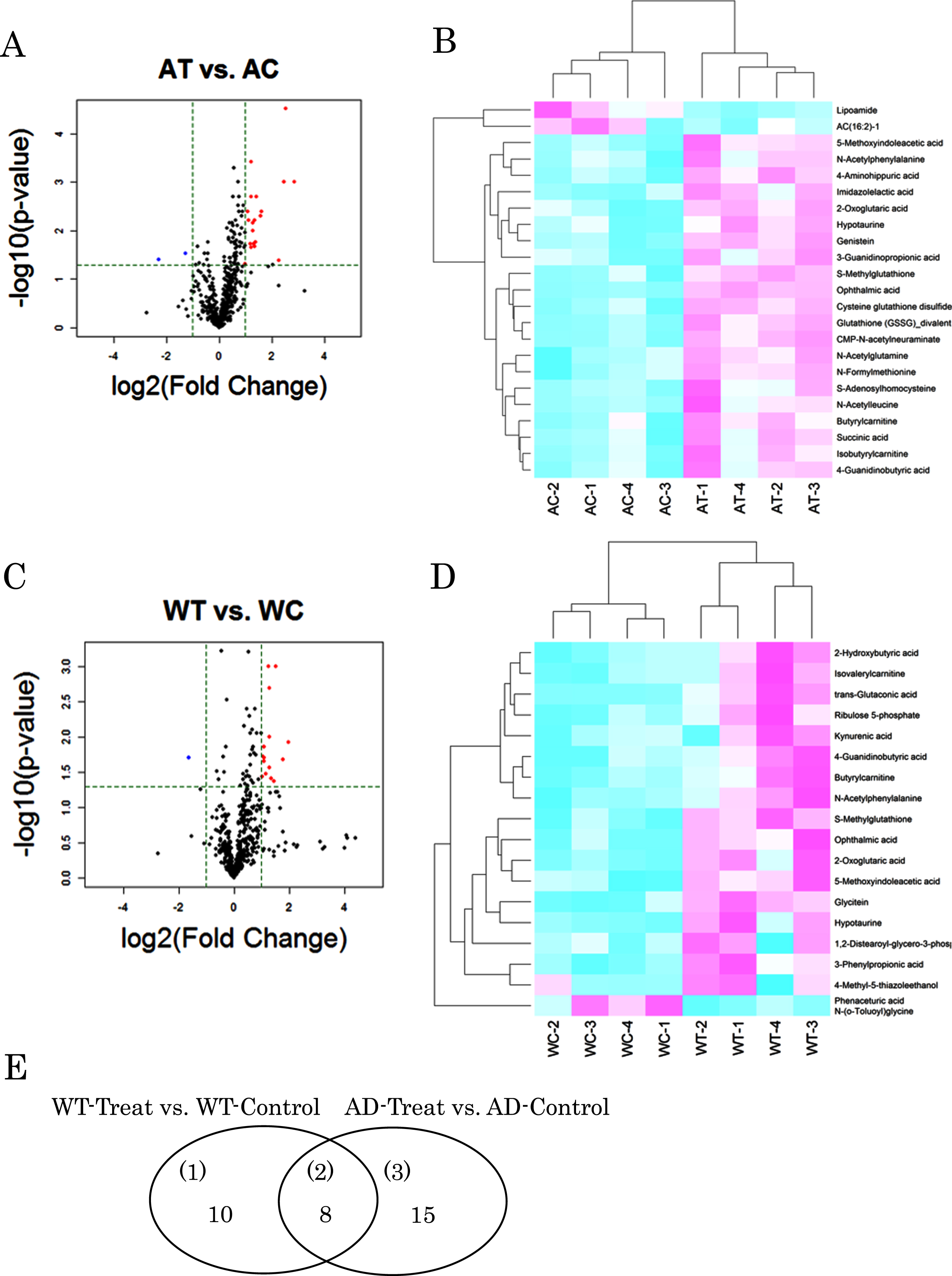
Table 1
The list of metabolites detected in Volcano plot for probiotic (AT) group versus control (AC) group of AD-like model mice
| Method | Compound name | Fold Change | p |
| CE-FTMS | Glutathione (GSSG)_divalent | 7.320 | 0.0014 |
| CE-FTMS | Ophthalmic acid | 5.821 | 0.0000 |
| CE-FTMS | CMP-N-acetylneuraminate | 5.590 | 0.0012 |
| CE-FTMS | N-Acetylleucine | 4.836 | 0.0418 |
| CE-FTMS | S-Adenosylhomocysteine | 4.152 | 0.0496 |
| CE-FTMS | S-Methylglutathione | 3.076 | 0.0043 |
| CE-FTMS | Imidazolelactic acid | 3.007 | 0.0054 |
| CE-FTMS | 4-Aminohippuric acid | 2.676 | 0.0018 |
| CE-FTMS | 4-Guanidinobutyric acid | 2.626 | 0.0170 |
| CE-FTMS | Isobutyrylcarnitine | 2.531 | 0.0210 |
| CE-FTMS | Butyrylcarnitine | 2.498 | 0.0191 |
| CE-FTMS | Hypotaurine | 2.457 | 0.0104 |
| CE-FTMS | 2-Oxoglutaric acid | 2.457 | 0.0072 |
| CE-FTMS | Succinic acid | 2.317 | 0.0222 |
| CE-FTMS | Cysteine glutathione disulfide | 2.312 | 0.0025 |
| CE-FTMS | N-Acetylphenylalanine | 2.292 | 0.0195 |
| CE-FTMS | N-Formylmethionine | 2.153 | 0.0063 |
| CE-FTMS | N-Acetylglutamine | 2.132 | 0.0043 |
| CE-FTMS | 3-Guanidinopropionic acid | 2.002 | 0.0475 |
| CE-FTMS | Lipoamide | 0.205 | 0.0391 |
| LC-TOFMS | 5-Methoxyindoleacetic acid | 2.538 | 0.0062 |
| LC-TOFMS | Genistein | 2.320 | 0.0004 |
| LC-TOFMS | AC(16:2)-1 | 0.410 | 0.0291 |
Fold change means the ratio of average value of AT/AC. p values are by Welch’s t test.
Table 2
The list of metabolites detected in Volcano plot for probiotic group (WT) versus control (WC) in wild type mice
| Method | Compound name | Fold Change | p |
| CE-FTMS | Ophthalmic acid | 3.908 | 0.0117 |
| CE-FTMS | Hypotaurine | 3.447 | 0.0210 |
| CE-FTMS | Kynurenic acid | 2.705 | 0.0416 |
| CE-FTMS | Butyrylcarnitine | 2.555 | 0.0389 |
| CE-FTMS | Ribulose 5-phosphate | 2.461 | 0.0274 |
| CE-FTMS | 4-Guanidinobutyric acid | 2.459 | 0.0274 |
| CE-FTMS | 3-Phenylpropionic acid | 2.427 | 0.0100 |
| CE-FTMS | S-Methylglutathione | 2.363 | 0.0014 |
| CE-FTMS | Isovalerylcarnitine | 2.237 | 0.0337 |
| CE-FTMS | N-Acetylphenylalanine | 2.133 | 0.0215 |
| CE-FTMS | 2-Oxoglutaric acid | 2.130 | 0.0143 |
| CE-FTMS | 4-Methyl-5-thiazoleethanol | 2.109 | 0.0202 |
| CE-FTMS | trans-Glutaconic acid | 2.093 | 0.0187 |
| CE-FTMS | 2-Hydroxybutyric acid | 2.028 | 0.0371 |
| CE-FTMS | Phenaceturic acid N-(o-Toluoyl)glycine | 0.320 | 0.0200 |
| LC-TOFMS | Glycitein | 2.825 | 0.0013 |
| LC-TOFMS | 1,2-Distearoyl-glycero-3-phosphocholine | 2.453 | 0.0020 |
| LC-TOFMS | 5-Methoxyindoleacetic acid | 1.977 | 0.0089 |
Fold change means the ratio of average value of WT/WC. p values are by Welch’s t test.
Quantitative analysis of markedly altered metabolites
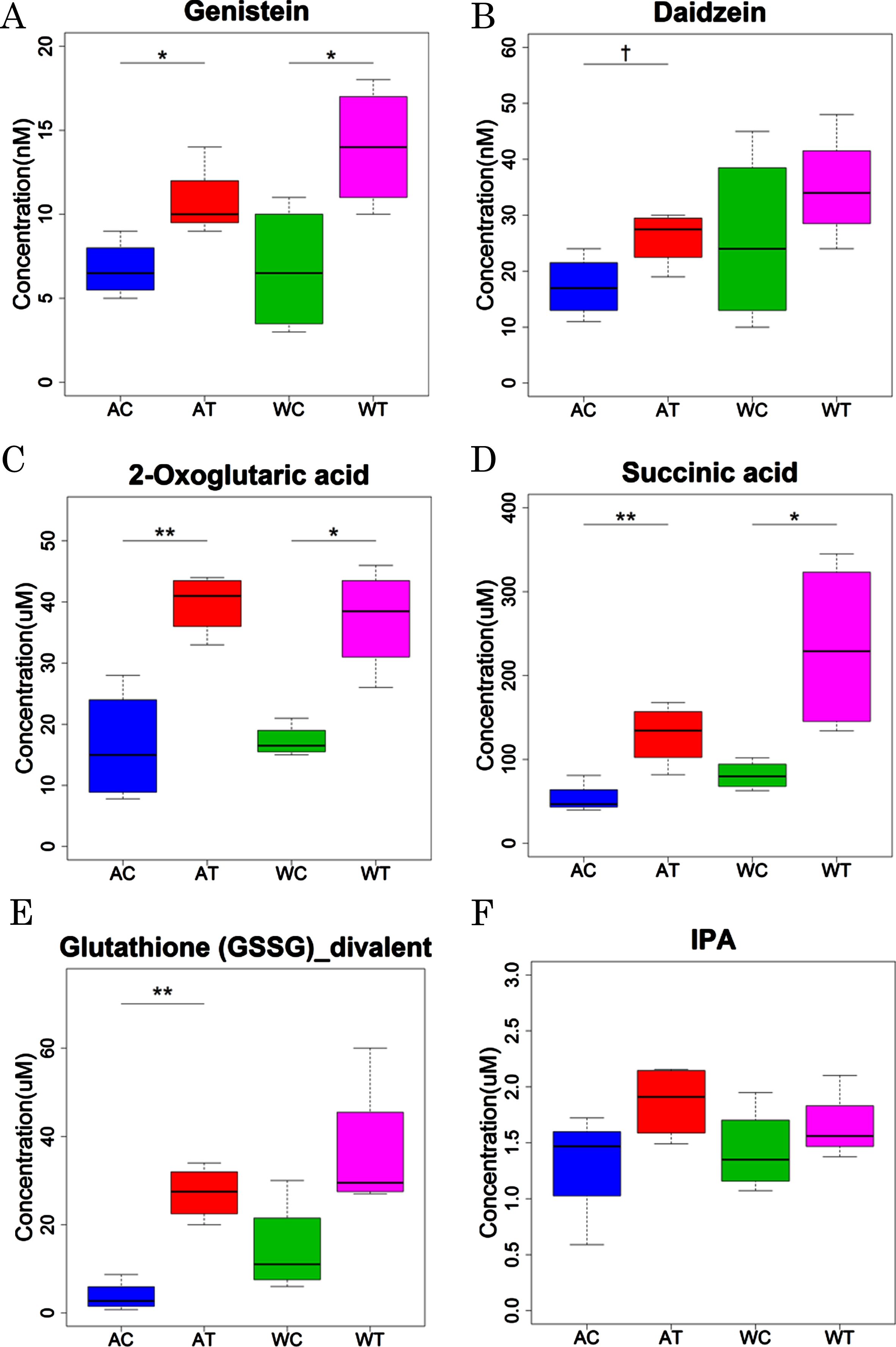
CE-FTMS and LC-TOFMS analysis showed some alterations in the metabolisms related to glutathione, isoflavone, TCA cycle, and tryptophan by B. breve MCC1274 supplementation. Since the CE-FTMS and LC-TOFMS analysis showed only the relative contents of each metabolite in the samples, we conducted a quantitative analysis of some metabolites related to these altered metabolisms (Fig. 3). For the isoflavone-related metabolites, the levels of genistein, an aglycones form of genistin, were significantly increased by the probiotic supplementation in both the AD-like model and wild-type mice (Fig. 3A). Daidzein, an aglycones form of daidzin, tended to be increased by the probiotic supplementation, although the difference was not significant between probiotic and saline groups (Fig. 3B). For the TCA cycle-related metabolites, both the 2-oxoglutaric acid and succinic acid levels were significantly increased by the probiotic supplementation in both the AD-like model and wild-type mice (Fig. 3C, D). For the glutathione-related metabolites, the levels of glutathione (GSSG)_divalent were increased significantly in AD-like model mice (p < 0.01) and tended to be increased in wild-type mice (p = 0.06) (Fig. 3E). For the tryptophan-related metabolites, the concentration of IPA tended to be elevated by the probiotic supplementation, although without statistical significance in both the AD-like model and wild-type mice (Fig. 3F). The concentrations of other compounds are summarized in Supplementary Tables 4 and 5. Quantitative analysis confirmed the increased levels of other metabolites related to the TCA cycle, including pyruvic acid, lactic acid, citric acid, isocitric acid, fumaric acid, and malic acid, by the probiotic supplementation in both the AD-like model and wild-type mice.
Fig. 3
Quantitative analysis of representative metabolites in AD-like model mice and wild-type mice orally supplemented with B. breve MCC1274 (AT or WT) or saline (AC or WC). p values were calculated by Welch’s t test.
DISCUSSION
In this study, we found that supplementation of B. breve MCC1274, a probiotic that has been reported to improve cognitive function, dramatically altered plasma metabolites in both AD-like model and wild-type mice. In particular, the analysis in the volcano plot identified soy isoflavones such as genistein, intermediary metabolites of the TCA cycle including 2-oxoglutaric acid and succinic acid, and antioxidants such as glutathione (GSSG)_divalent as altered metabolites, and quantitative analysis confirmed the changed levels of some of these substances.
Isoflavone metabolites were the most notably altered by the probiotic supplementation. Soy and soy foods are rich sources of isoflavones, which have been shown to possess several biological activities. Most of the isoflavones, daidzin, genistin, and glycitin, similar to other types of polyphenols, are generally consumed as glycosides, which are poorly absorbed from the small intestine [28]. A significant fraction can persist in the colon, where they encounter the gut microbiota and are hydrolyzed by bacterial β-glucosidases to their corresponding bioactive aglycones, daidzein, genistein, and glycitein [29]. In this study, the concentrations of genistein were significantly increased in both AD-like model mice and wild type mice, those of glycitein were increased in the wild-type group, and the concentrations of daidzein tended to be increased in the AT group. Previously, B. breve MCC1274 has been shown to possess high β-glucosidase activity and a solid capability to convert daidzin and other polyphenols to their aglycons [30]. Indeed, it was shown that oral supplementation of B. breve MCC1274 significantly enhanced the plasma concentration of daidzein in rats. Therefore, we proposed that the supplementation of live B. breve MCC1274 is responsible for the gut release of aglycones from isoflavone glycosides in the dietary sources. Soy isoflavones have been reported to have multiple functions, including anti-cancer, anti-oxidative, and anti-inflammation effects [31, 32]. It has been shown in in vivo studies that genistein can pass through the blood-brain barrier, antagonize the toxicity of Aβ, and have a neuroprotective effect [32]. The mechanism of the antioxidant effect involves a reducing agent that directly removes hydroxyl radicals and enhances glutathione biosynthesis via the nuclear factor-erythroid 2-related factor 2 (Nrf2) pathway [33]. Nrf2 is a master transcription factor that responds to oxidative stress, and it has been reported that hyperactivation of Nrf2 in AD-like model mice enhances glutathione synthase and decreases inflammatory cytokines [34]. Genistein has also been shown to inhibit the production of Aβ by promoting the expression of α-secretase and the degradation of presenilin. It is known that PKC-MAPK-ERK is a transcriptionally active signaling pathway for α-secretase induced by genistein [35]. These isoflavones act on the estrogen receptors (ERs), ER-α and ER-β. In addition, they have an exceptionally high affinity for ER-β [36], which is explicitly expressed in the hippocampus but not in the cerebral cortex [37]. Furthermore, there is an isoform of ER called G-protein coupled receptor ER1 (GPER) that binds to G-protein coupled receptor (GPCR), which is expressed in the hippocampus [38], and is known to activate PKC, MAPK/ERK, and Nrf2 [39]. We have shown that B. breve MCC1274 supplementation led to decreased microglial activation in the hippocampus and the gene expression of cytokines IL-6 and IL-1β in the hippocampus and cortex in the AD-like model mice [20]. In addition, the PKC-MAPK-ERK pathway was explicitly enhanced in the hippocampus by B. breve MCC1274 supplementation. The specific effect in the hippocampus is unclear, but the interaction of isoflavones with regional-located ERs may be at play.
We found that IPA, an indole derivative of tryptophan generated by gut microbes, increased by probiotic supplementation in the AT group. Microbial tryptophan catabolites affect various physiological processes and may contribute to intestinal and systemic homeostasis in health and disease [40]. Previous studies have shown the production of indole-3-lactic acid (ILA) by B. breve MCC1274 [27]. These indole derivatives may potentially improve intestinal barrier function [41], regulate the gut mucosal immune system [42], and modulate inflammatory responses in an AhR-dependent way [43]. It has been reported that IPA acts on pregnane X receptors (PXR) in the gut to inhibit TNF-α production and simultaneously improve intestinal barrier function [44], and that IPA has antioxidant and inhibitory neuronal cell death effects on hippocampal neurons [45]. In this study, it is possible that the ILA produced by B. breve MCC1274 was transformed to IPA by other microbes in the gut, although the details await future study. The transformation of ILA to IPA has been previously reported by Clostridium sporogenes. We also observed a significant increase of 5-MIAA, an analog of IPA. Although the physiological function of 5-MIAA remains to be clarified, it has been shown that it is produced by a probiotic strain Lactobacillus rhamnosus LGG and acts as an activator of Nrf2 in the modulation of hepatic susceptibility to oxidative injury [47].
We observed an increased level of lactic acid in the AD-like model mice by the probiotic supplementation. Acetic acid and lactic acid are the primary metabolites of Bifidobacterium. We could not analyze the concentration of acetic acid since it is an internal standard in the measurements in this study. However, in a previous study, we observed that the probiotic supplementation significantly increased the plasma level of acetic acid, and supplementation of acetate partially improved the cognitive function activity in mice where Aβ is injected into mouse brains [19]. Notably, acetate also possesses anti-oxidative and anti-inflammation activities [48], and has been shown to contribute to microglial metabolic modulation and inhibition of phagocytosis [49].
On the other hand, lactic acid, together with other metabolic intermediates, fuels the TCA cycle. Alterations in metabolites responsible for the TCA cycle, including 2-oxoglutaric acid, succinic acid, and many other metabolites, were associated with the probiotic supplementation. It is interesting since dysregulation of glucose metabolism, glycolysis, TCA cycle, oxidative phosphorylation (OXPHOS), and pentose phosphate pathway are seen in aged and AD brains [50]. The TCA cycle is one of the most important metabolic pathways for producing the energy sources required to perform vital functions and is generally driven by the metabolism of carbohydrates, lipids, and amino acids. It has been reported that the expression of genes related to lipid metabolism and organic acid metabolism was increased in the liver when mice are treated with this probiotic [51]. In addition, the promotion of β-oxidation via estrogen-related receptors [52], for which soy isoflavones are ligands, and effective oxidative phosphorylation by the Nrf2 pathway are also considered an essential function of the TCA cycle [53]. In addition, as shown in Supplementary Table 5, lactic acid was significantly decreased in the AC group compared to the WC group. A similar phenomenon has been observed in a human study [54], and the underlying mechanism could be owing to the impairment of glucose metabolism in the pathophysiology of AD.
In this study, the probiotic supplementation increased glutathione (GSSG)_divalent and relative metabolites. These changes are thought to be associated with the oxidative stress caused by the accumulation of Aβ in the brains of AD-like model mice [55]. Neuronal inflammation is associated with a broad spectrum of neurodegenerative diseases, including AD. Several studies have shown that Aβ can induce cerebral oxidative stress and activate microglia and astrocytes, leading to neuroinflammation, neuronal damage injury, and cognitive impairment [56, 57]. The probiotic supplementation led to an enhanced glutathione biosynthesis in response to activating the Nrf2 pathway triggered by isoflavones and tryptophan derivatives in the probiotic group [34]. We observed a superficial level for the reduced glutathione in this study. The reason may be that the reduced glutathione in plasma is rapidly metabolized with a half-life of a few minutes by γ-glutamyltranspeptidase [58], and that the plasma concentration are much lower than intracellular level [59]. Nevertheless, these results suggest that B. breve MCC1274 supplementation led to significant suppression of oxidative reaction in the brain.
Altered levels were also observed on other metabolic pathways such as butyrylation and N-acetylation. Butyric acid is an SCFA produced by gut bacteria in the colon. However, no change was observed in the butyric acid level in this study; the mechanisms for the enhanced butyrylation are not well understood. Enhanced N-acetylated metabolites were observed for most of the amino acids, including N-acetylleucine, a compound developed for possible treatment for several neurological disorders [60]. The N-acetylation is supposed to be triggered by N-acetyltransferase, an enzyme commonly expressed by Bifidobacterium species. Nevertheless, future studies are needed to understand the mechanisms and physiological functions of these altered metabolic pathways with the effects of the probiotic strain in improving cognitive function.
There are several limitations to this study. First, since only plasma metabolites were evaluated, a more comprehensive metabolomic analysis with other samples such as feces, liver, and brain should be performed for validation. Then, there is a need to investigate transcriptomic analysis to understand how gene expression has changed in the brain and liver. Another limitation of this study is that we could only quantify some of the metabolites due to technical restrictions. In addition, whether the changes in metabolites detected in these mice could be extrapolated to humans, who have more complex dietary habits and gut microbiota, needs to be studied in the future.
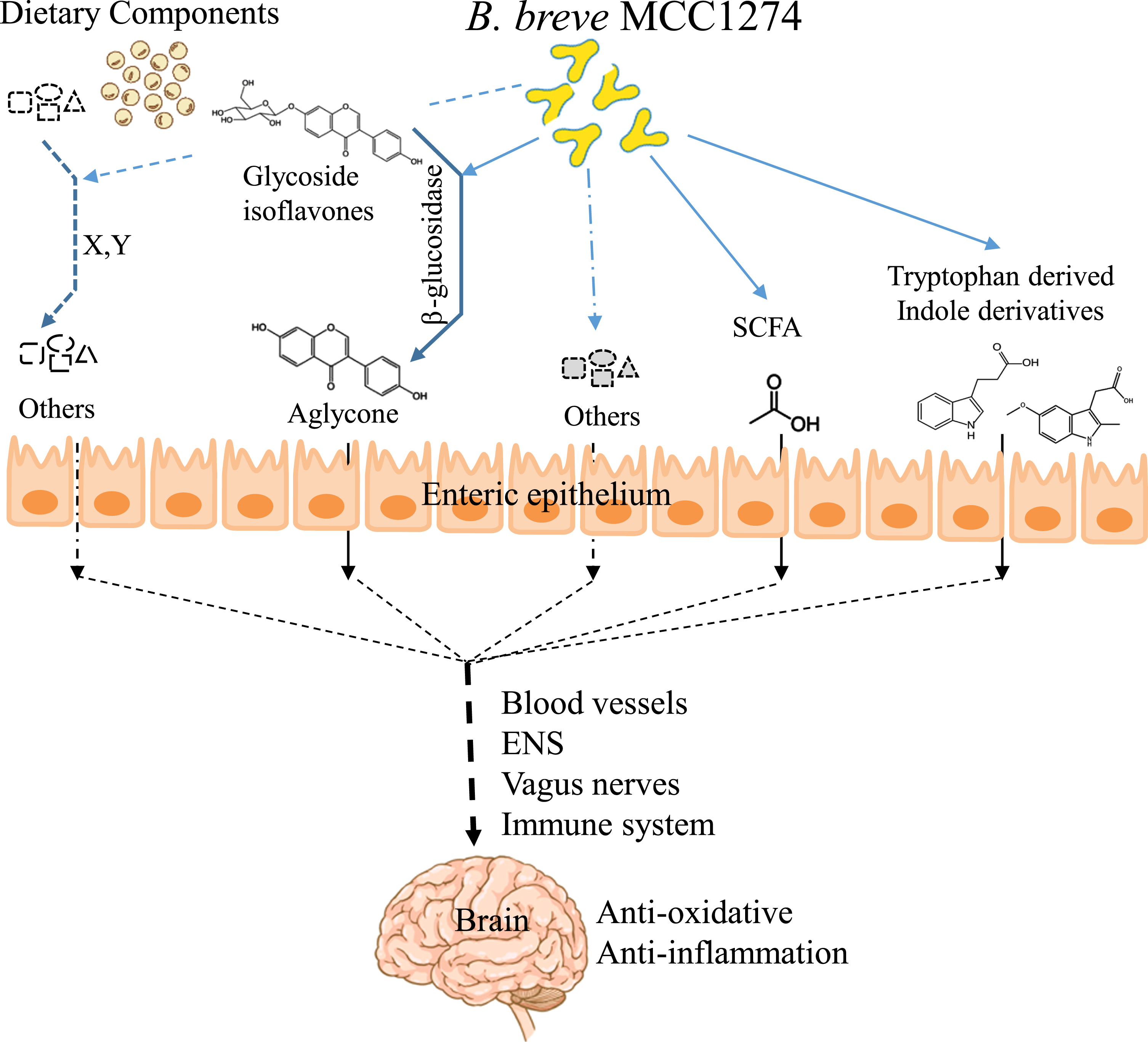
However, this study is valuable because it reveals a part of the pathway by which B. breve MCC1274 signals act from the gut to the brain, which has not been previously elucidated. Taking together with our previous findings, we can propose the following as parts of the possible mechanisms of action of this probiotic: 1) conversion of dietary soy isoflavones in the gut to a bioavailable aglycone form; 2) production of indole derivatives from tryptophan; and 3) generation of SCFAs (Fig. 4). These molecules can cross the blood-brain barrier and contribute to activating the TCA cycle and the Nrf2 pathway, which reduces excessive oxidative stress and Aβ accumulation in the brain. In addition, since various pathways, including the enteric nervous system, vagus nerve, or the immune system, connect the brain and gut, the elucidation of the individual effects of these molecules generated in the gut by B. breve MCC1274 awaits future study.
Fig. 4
Illustration of possible mechanisms by B. breve MCC1274 in improving cognitive function.
ACKNOWLEDGMENTS
We acknowledge Dr. Francois Bernier for his assistance in reviewing the manuscript. This work was supported by a Grant-in-Aid for Scientific Research B 16H05559 (to M.M.), a Grant-in-Aid for Challenging Exploratory Research 15K15712 (to M.M.), and Grant-in-Aid for Scientific Research C 20K07762 (to C-G. J.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0479r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220479.
REFERENCES
[1] | Scheltens P , De Strooper B , Kivipelto M , Holstege H , Chételat G , Teunissen CE , Cummings J , van der Flier WM ((2021) ) Alzheimer’s disease. Lancet 397: , 1577–1590. |
[2] | Alzheimer’s Disease International (2019) The costs of dementia: Advocacy, media and stigma. InWorld Alzheimer Report 2019. Attitudes to Dementia, Alzheimer’s Disease International, London, pp. 100-101. |
[3] | Tiwari S , Venkata A , Kaushik A , Adriana Y , Nair M ((2019) ) Alzheimer’s disease diagnostics and therapeutics market. Int J Nanomedicine 14: , 5541–5554. |
[4] | Tönnies E , Trushina E ((2017) ) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57: , 1105–1121. |
[5] | Newcombe EA , Camats-Perna J , Silva ML , Valmas N , Huat TJ , Medeiros R ((2018) ) Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J Neuroinflammation 15: , 276. |
[6] | Butterfield DA , Halliwell B ((2019) ) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20: , 148–160. |
[7] | Lewandowska M , Bednarski W ((2003) ) The characteristics of fermentation abilities of K. fragilis immobilized on Siran. Commun Agric Appl Biol Sci 68: , 497–501. |
[8] | Vogt NM , Kerby RL , Dill-McFarland KA , Harding SJ , Merluzzi AP , Johnson SC , Carlsson CM , Asthana S , Zetterberg H , Blennow K , Bendlin BB , Rey FE ((2017) ) Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7: , 13537. |
[9] | Liu P , Wu L , Peng G , Han Y , Tang R , Ge J , Zhang L , Jia L , Yue S , Zhou K , Li L , Luo B , Wang B ((2019) ) Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun 80: , 633–643. |
[10] | Saji N , Tsuduki T , Murotani K , Hisada T , Sugimoto T , Kimura A , Niida S , Toba K , Sakurai T ((2022) ) Relationship between the Japanese-style diet, gut microbiota, and dementia: A cross-sectional study. Nutrition 94: , 111524. |
[11] | Harach T , Marungruang N , Duthilleul N , Cheatham V , Mc Coy KD , Frisoni G , Neher JJ , Fåk F , Jucker M , Lasser T , Bolmont T ((2017) ) Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota.. Sci Rep 7: , 46856.41802. Erratum: |
[12] | Sampson TR , Mazmanian SK ((2015) ) Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17: , 565–576. |
[13] | MahmoudianDehkordi S , Arnold M , Nho K , Ahmad S , Jia W , Xie G , Louie G , Kueider-Paisley A , Moseley MA , Thompson JW , St John Williams L , Tenenbaum JD , Blach C , Baillie R , Han X , Bhattacharyya S , Toledo JB , Schafferer S , Klein S , Koal T , Risacher SL , Kling MA , Motsinger-Reif A , Rotroff DM , Jack J , Hankemeier T , Bennett DA , De Jager PL , Trojanowski JQ , Shaw LM , Weiner MW , Doraiswamy PM , van Duijn CM , Saykin AJ , Kastenmüller G , Kaddurah-Daouk R ; Alzheimer’s Disease Neuroimaging Initiative and the Alzheimer Disease Metabolomics Consortium ((2019) ) Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-An emerging role for gut microbiome. Alzheimers Dement 15: , 604.76-92. Erratum: |
[14] | Cui Y , Liu X , Wang M , Liu L , Sun X , Ma L , Xie W , Wang C , Tang S , Wang D , Wu Q ((2014) ) Lysophosphatidylcholine and amide as metabolites for detecting Alzheimer disease using ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry-based metabonomics. J Neuropathol Exp Neurol 73: , 954–963. |
[15] | Varma VR , Oommen AM , Varma S , Casanova R , An Y , Andrews RM , O’Brien R , Pletnikova O , Troncoso JC , Toledo J , Baillie R , Arnold M , Kastenmueller G , Nho K , Doraiswamy PM , Saykin AJ , Kaddurah-Daouk R , Legido-Quigley C , Thambisetty M ((2018) ) Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med 15: , e1002482. |
[16] | Kaddurah-Daouk R , Rozen S , Matson W , Han X , Hulette CM , Burke JR , Doraiswamy PM , Welsh-Bohmer KA ((2011) ) Metabolomic changes in autopsy-confirmed Alzheimer’s disease. Alzheimers Dement 7: , 309–317. |
[17] | Wu L , Han Y , Zheng Z , Peng G , Liu P , Yue S , Zhu S , Chen J , Lv H , Shao L , Sheng Y , Wang Y , Li L , Li L , Wang B ((2021) ) Altered gut microbial metabolites in amnestic mild cognitive impairment and Alzheimer’s disease: Signals in host–microbe interplay. Nutrients 13: , 228. |
[18] | Xiao J , Katsumata N , Bernier F , Ohno K , Yamauchi Y , Odamaki T , Yoshikawa K , Ito K , Kaneko T ((2020) ) Probiotic Bifidobacterium breve in improving cognitive functions of older adults with suspected mild cognitive impairment: A randomized, double-blind, placebo-controlled trial. J Alzheimers Dis 77: , 139–147. |
[19] | Kobayashi Y , Sugahara H , Shimada K , Mitsuyama E , Kuhara T , Yasuoka A , Kondo T , Abe K , Xiao J ((2017) ) Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci Rep 7: , 13510. |
[20] | Abdelhamid M , Zhou C , Ohno K , Kuhara T , Taslima F , Abdullah M , Jung C-G , Michikawa M ((2021) ) Probiotic Bifidobacterium breve prevents memory impairment through the reduction of both amyloid-β production and microglia activation in APP knock-in mouse. J Alzheimers Dis 85: , 1555–1571. |
[21] | Abdelhamid M , Zhou C , Jung C-G , Michikawa M ((2022) ) Probiotic Bifidobacterium breve MCC1274 mitigates Alzheimer’s disease-related pathologies in wild-type mice. Nutrients 14: , 2543. |
[22] | Okamoto N , Ikenouchi A , Watanabe K , Igata R , Fujii R , Yoshimura R ((2021) ) A metabolomics study of serum in hospitalized patients with chronic schizophrenia. Front Psychiatry 12: , 763547. |
[23] | Sasaki K , Sagawa H , Suzuki M , Yamamoto H , Tomita M , Soga T , Ohashi Y ((2019) ) Metabolomics platform with capillary electrophoresis coupled with high-resolution mass spectrometry for plasma analysis. Anal Chem 91: , 1295–1301. |
[24] | Ohashi Y , Hirayama A , Ishikawa T , Nakamura S , Shimizu K , Ueno Y , Tomita M , Soga T ((2008) ) Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol Biosyst 4: , 135–147. |
[25] | Ooga T , Sato H , Nagashima A , Sasaki K , Tomita M , Soga T , Ohashi Y ((2011) ) Metabolomic anatomy of an animal model revealing homeostatic imbalances in dyslipidaemia. Mol Biosyst 7: , 1217–1223. |
[26] | Sugimoto M , Wong DT , Hirayama A , Soga T , Tomita M ((2010) ) Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 6: , 78–95. |
[27] | Sakurai T , Odamaki T , Xiao JZ ((2019) ) Production of indole-3-lactic acid by bifidobacterium strains isolated fromhuman infants. Microorganisms 7: , 340. |
[28] | Setchell KDR , Brown NM , Zimmer-Nechemias L , Brashear WT , Wolfe BE , Kirschner AS , Heubi JE ((2002) ) Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr 76: , 447–453. |
[29] | Branca F , Lorenzetti S ((2005) ) Health effects of phytoestrogens. Forum Nutr 57: , 100–111. |
[30] | Yao R , Wong CB , Nakamura K , Mitsuyama E , Tanaka A , Kuhara T , Odamaki T , Xiao JZ ((2019) ) Bifidobacterium breve MCC1274 with glycosidic activity enhances isoflavone bioavailability. }. Benef Microbes 10: , 521–531. |
[31] | Peng Y , Shi Y , Zhang H , Mine Y , Tsao R ((2017) ) Anti-inflammatory and anti-oxidative activities of daidzein and its sulfonic acid ester derivatives. J Funct Foods 35: , 635–640. |
[32] | Duan X , Li Y , Xu F , Ding H ((2021) ) Study on the neuroprotective effects of Genistein on Alzheimer’s disease. Brain Behav 11: , e02100. |
[33] | Froyen EB , Steinberg FM ((2011) ) Soy isoflavones increase quinone reductase in hepa-1c1c7 cells via estrogen receptor beta and nuclear factor erythroid 2-related factor 2 binding to the antioxidant response element. J Nutr Biochem 22: , 843–848. |
[34] | Uruno A , Matsumaru D , Ryoke R , Saito R , Kadoguchi S , Saigusa D , Saito T , Saido TC , Kawashima R , Yamamoto M ((2020) ) Nrf2 suppresses oxidative stress and inflammation in App knock-in Alzheimer’s disease model mice. Mol Cell Biol 40: , e00467–19. |
[35] | Uddin MS , Kabir MT ((2019) ) Emerging signal regulating potential of genistein against Alzheimer’s disease: A promising molecule of interest. Front Cell Dev Biol 7: , 197. |
[36] | Morito K , Hirose T , Kinjo J , Hirakawa T , Okawa M , Nohara T , Ogawa S , Inoue S , Muramatsu M , Masamune Y ((2001) ) Interaction of phytoestrogens with estrogen receptors α andβ. Biol Pharm Bull 24: , 351–356. |
[37] | Warfvinge K , Krause DN , Maddahi A , Edvinsson JCA , Edvinsson L , Haanes KA ((2020) ) Estrogen receptors α, β and GPER in the CNS and trigeminal system - molecular and functional aspects. J Headache Pain 21: , 131. |
[38] | Almey A , Milner TA , Brake WG ((2015) ) Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav 74: , 125–138. |
[39] | Wang C , Huang W , Lin J , Fang F , Wang X , Wang H ((2020) ) Triclosan-induced liver and brain injury in zebrafish (Danio rerio) via abnormal expression of miR-125 regulated by PKCα/Nrf2/p53 signaling pathways. Chemosphere 241: , 125086. |
[40] | Roager HM , Licht TR ((2018) ) Microbial tryptophan catabolites in health and disease. Nat Commun 9: , 3294. |
[41] | Dodd D , Spitzer MH , Van Treuren W , Merrill BD , Hryckowian AJ , Higginbottom SK , Le A , Cowan TM , Nolan GP , Fischbach MA , Sonnenburg JL ((2017) ) A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551: , 648–652. |
[42] | Cervantes-Barragan L , Chai JN , Tianero MD , Di Luccia B , Ahern PP , Merriman J , Cortez VS , Caparon MG , Donia MS , Gilfillan S , Cella M , Gordon JI , Hsieh C , Colonna M ((2017) ) Lactobacillus reuteri induces gut intraepithelial CD4+CD8α α+ T cells. Science 357: , 806–810. |
[43] | Wilck N , Matus MG , Kearney SM , Olesen SW , Forslund K , Bartolomaeus H , Haase S , Mahler A , Balogh A , Marko L , Vvedenskaya O , Kleiner FH , Tsvetkov D , Klug L , Costea PI , Sunagawa S , Maier L , Rakova N , Schatz V , Neubert P , Fratzer C , Krannich A , Gollasch M , Grohme DA , Corte-Real BF , Gerlach RG , Basic M , Typas A , Wu C , Titze JM , Jantsch J , Boschmann M , Dechend R , Kleinewietfeld M , Kempa S , Bork P , Linker RA , Alm EJ , Muller DN ((2017) ) Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551: , 585–589. |
[44] | Venkatesh M , Mukherjee S , Wang H , Li H , Sun K , Benechet AP , Qiu Z , Maher L , Redinbo MR , Phillips RS , Fleet JC , Kortagere S , Mukherjee P , Fasano A , Le Ven J , Nicholson JK , Dumas ME , Khanna KM , Mani S ((2014) ) Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41: , 296–310. |
[45] | Hwang IK , Yoo KY , Li H , Park OK , Lee CH , Choi JH , Jeong YG , Lee YL , Kim YM , Kwon YG , Won MH ((2009) ) Indole-3-propionic acid attenuates neuronal damage and oxidative stress in the ischemic hippocampus. J Neurosci Res 87: , 2126–2137. |
[46] | Wikoff WR , Anfora AT , Liu J , Schultz PG , Lesley SA , Peters EC , Siuzdak G ((2009) ) Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A 106: , 3698–3703. |
[47] | Saeedi BJ , Liu KH , Owens JA , Hunter-Chang S , Camacho MC , Eboka RU , Chandrasekharan B , Baker NF , Darby TM , Robinson BS , Jones RM , Jones DP , Neish AS ((2020) ) Gut-resident Lactobacilli activate hepatic Nrf2 and protect against oxidative liver injury.. Cell Metab 31: , 956–968.e5. |
[48] | Moriyama M , Kurebayashi R , Kawabe K , Takano K , Nakamura Y ((2016) ) Acetate attenuates lipopolysaccharide-induced nitric oxide production through an anti-oxidative mechanism in cultured primary rat astrocytes. Neurochem Res 41: , 3138–3146. |
[49] | Erny D , Dokalis N , Mezö C , Castoldi A , Mossad O , Staszewski O , Frosch M , Villa M , Fuchs V , Mayer A , Neuber J , Sosat J , Tholen S , Schilling O , Vlachos A , Blank T , Gomez de Agüero M , Macpherson AJ , Pearce EJ , Prinz M ((2021) ) Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab 33: , 2260–2276.e7. |
[50] | Yan X , Hu Y , Wang B , Wang S , Zhang X ((2020) ) Metabolic dysregulation contributes to the progression of Alzheimer’s disease. Front Neurosci 14: , 530219. |
[51] | Kondo S , Kamei A , Xiao JZ , Iwatsuki K , Abe K ((2013) ) Bifidobacterium breve B-3 exerts metabolic syndrome-suppressing effects in the liver of diet-induced obese mice: A DNA microarray analysis. Benef Microbes 4: , 247–252. |
[52] | Uchitomi R , Nakai S , Matsuda R , Onishi T , Miura S , Hatazawa Y , Kamei Y ((2019) ) Genistein, daidzein, and resveratrols stimulate PGC-1β-mediated gene expression. Biochem Biophys Reports 17: , 51–55. |
[53] | Holmström KM , Baird L , Zhang Y , Hargreaves I , Chalasani A , Land JM , Stanyer L , Yamamoto M , Dinkova-Kostova AT , Abramov AY ((2013) ) Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open 2: , 761–770. |
[54] | Verri M , Aquilani R , Ricevuti G , Rondanelli M , Ghitti M , Bongiorno AI , Venturini L , Buonocore D , Boschi F , Dossena M ((2018) ) Plasma energy substrates at two stages of Alzheimer’s disease in humans. Int J Immunopathol Pharmacol 32: , 1–7. |
[55] | Mohmmad Abdul H , Wenk GL , Gramling M , Hauss-Wegrzyniak B , Butterfield DA ((2004) ) APP and PS-1 mutations induce brain oxidative stress independent of dietary cholesterol: Implications for Alzheimer’s disease. Neurosci Lett 368: , 148–150. |
[56] | Akiyama H , Barger S , Barnum S , Bradt B , Bauer J , Cole GM , Cooper NR , Eikelenboom P , Emmerling M , Fiebich BL , Finch CE , Frautschy S , Griffin WS , Hampel H , Hull M , Landreth G , Lue L , Mrak R , Mackenzie IR , McGeer PL , O’Banion MK , Pachter J , Pasinetti G , Plata-Salaman C , Rogers J , Rydel R , Shen Y , Streit W , Strohmeyer R , Tooyoma I , Van Muiswinkel FL , Veerhuis R , Walker D , Webster S , Wegrzyniak B , Wenk G , Wyss-Coray T ((2011) ) Inflammation and Alzheimer’s disease. Neurobiol Aging 21: , 383–421. |
[57] | Kempuraj D , Thangavel R , Natteru PA , Selvakumar GP , Saeed D , Zahoor H , Zaheer S , Iyer SS , Zaheer A ((2016) ) Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine 1: , 1003. |
[58] | Magnani M , Novelli G , Palloni R ((1984) ) Human plasma glutathione oxidation in normal and pathological conditions. Clin Physiol Biochem 2: , 287–289. |
[59] | Giustarini D , Colombo G , Garavaglia ML , Astori E , Portinaro NM , Reggiani F , Badalamenti S , Aloisi AM , Santucci A , Rossi R , Milzani A , Dalle-Donne I ((2017) ) Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic Biol Med 112: , 360–375. |
[60] | Strupp M , Bayer O , Feil K , Straube A ((2019) ) Prophylactic treatment of migraine with and without aura with acetyl-dl-leucine: A case series. J Neurol 266: , 525–529. |



