Comparative Evaluation of Safety and Efficacy of Alternate Schedule (AS) of Sunitinib in Asian and Non-Asian Patient Population for the Treatment of Metastatic Renal Cell Cancer (mRCC): A Meta-Analysis
Abstract
BACKGROUND:
Treatment of metastatic renal cell carcinoma (mRCC) using traditional schedule (TS, 4/2) of Sunitinib is associated with higher adverse effects compared to the alternate schedule (AS, 2/1 upfront or when switched from TS).
OBJECTIVE:
This meta-analysis aims to compare the safety, efficacy, and percentage of patients requiring dose reduction or dose interruption between Asian (AP) and non-Asian population (NAP) receiving AS of sunitinib.
METHODS:
Electronic databases (PubMed, EMBASE, Cochrane Library) were searched to identify studies published in the English language between May 2009- May 2019, which included patients (>18 years) with mRCC receiving AS of sunitinib. Data were analyzed using the random effect model and t-test. P < 0.05 was considered statistically significant.
RESULTS:
Of 1922, 16 studies were included (8 AP, 8 NAP). Among all grade AEs, mucositis (RR:0.22; 95% CI:0.12–0.40), cardiotoxicity (RR: 0.52; 95% CI: 0.31–0.88), nausea (RR:0.21; 95% CI: 0.10–0.44), hand-foot syndrome (RR:0.33; 95% CI:0.13–0.83), rash (RR: 0.52; 95% CI: 0.34–0.79), and aspartate transaminase (RR:0.57; 95% CI:0.33–0.98) were more common in AP. Leukopenia (RR:2.57; 95% CI:1.47–4.49), proteinemia (RR:4.45; 95% CI:2.12–9.33), and stomatitis (RR:4.33; 95% CI:2.6–7.23) occurred more commonly in NAP. Further, PFS was significantly longer in NAP, while longer OS was observed in AP (p < 0.001). Dose reduction was significantly higher in AP than NAP (52.08% vs. 40.6%, p = 0.0088).
CONCLUSION:
Safety profile of AS of sunitinib was similar with variations in the efficacy, dose reduction between AP and NAP. Sunitinib dose or schedule modification may mitigate AEs and enhance efficacy outcomes in mRCC by extending the treatment duration.
KEYMESSAGES
The alternate schedule of sunitinib showed similar safety but varying efficacy profile in the Asian and non-Asian population with metastatic renal cell carcinoma.
Sunitinib dose or schedule modification in Asian patients with symptomatic management of AEs should be considered in therapy management to help patients remain longer on therapy.
INTRODUCTION
Kidney/renal cancer is one of the most common malignancies in the world, ranked ninth among the most common cancers occurring in men and 14th in women [1]. The incidence of renal cancer per 100,000 population is 4.4, with a cumulative risk of 0.51% [2]. About 2.4% of cancers are renal cancer [3], among which approximately 90% are renal cell carcinoma (RCC) [4]. Metastatic RCC (mRCC) accounts for 1/3rd of all RCC [5]. In the United States, the incidence of kidney and renal pelvis cancer during 2013-18 was reported to be 16,90,000 and mortality during 2014-18 was 3,60,000 [6]. According to GLOBOCAN 2020, renal cancer accounted for 431,288 new cases and 179,368 deaths across the globe [7]. The Asian sub-continent reported the highest prevalence of renal cancer in 2020, with 156,470 new cases and 80,251 deaths. Further, in the Indian scenario, the incidence rate of kidney cancer was reported in 16,861 patients and mortality in 9897 cases [8].
Renal cell carcinoma (RCC) is characterized by its high vascularity due to alteration in the von Hippel-Lindau (VHL) tumor suppressor gene, leading to the accumulation of transcription factors such as hypoxia-inducible factor 1 alpha and 2 alpha (HIF-1α, HIF-2α). These factors mediate the overexpression of vascular endothelial growth factor (VEGF) [11]. Metastatic RCC (mRCC) was initially treated with cytokine therapy along with interleukin-2 (IL-2) or interferon-alpha (IFN-α). Further, the landscape of RCC treatment has changed because of a greater understanding of cytokines and their involvement in tumor immunology, which includes discovering immune checkpoint inhibitors for RCC treatment [12]. Later, VEGF receptor (VEGFR) inhibitors, including targeted therapy with tyrosine kinase inhibitors (TKIs), angiogenesis inhibitors, and mammalian target of rapamycin (mTOR), became the standard of care for mRCC [13].
Further, TKIs are a part of the immuno-oncology combinations that have significantly increased the efficacy outcomes (PFS and OS) in RCC [14]. Therefore, understanding the frequency and management of TKI toxicities is critical in RCC treatment. Vascular endothelial growth factor inhibitors revolutionized the treatment of mRCC. Amongst the VEGF inhibitors, sunitinib is an oral multi-targeted TKI of VEGFR1, VEGFR2, and VEGFR3, which received United States Food and Drug Administration (FDA)-approval in 2006. As per Schmid et al., 2016, Sunitinib was considered the gold standard. It became the first-line treatment for treating mRCC after it showed better efficacy than interferon-alpha (IFN-α) in a phase III trial. The standard dosage of sunitinib for mRCC treatment is 50 mg taken orally every day for four consecutive weeks, followed by a duration of 2 weeks off (also called schedule 4/2) [15].
The emergence of new immuno-oncology therapies has revamped the therapeutic strategy for patients with RCC and modified the position of previous approaches, including VEGF inhibitors [16]. However, Sunitinib still holds its place in the clinical practice guidelines for mRCC. According to NCCN 2020 guidelines, sunitinib is the preferred first-line treatment for favorable risk clear cell mRCC patients [17]. Even ESMO 2019 guidelines consider sunitinib (category I, A evidence) a preferred treatment option for good risk mRCC patients [18]. European Association of Urology, 2018 guidelines strongly recommend sunitinib in favorable risk mRCC patients [19]. Despite its efficacy in mRCC, sunitinib schedule 4/2 is associated with many toxicities, which requires therapy management like symptomatic management of AEs, and dose interruptions, dose reduction or schedule modification (2 weeks on, 1 week off) [15, 20]. Interestingly, standard schedule of sunitinib demonstrated much higher toxicities in the Asian population (AP) than in non-Asian population (NAP) [21, 22]. Various dosing regimens of sunitinib were therefore tried to overcome the toxicities. Several studies reported that starting an alternate schedule (AS), i.e., 2-week on treatment, 1-week off (schedule 2/1) upfront or switching from schedule 4/2 to AS reduced the toxicity of sunitinib [20, 23–26].
Rationale
Dose/Schedule modification is a part of therapy management with sunitinib, which helps patients remain on therapy because of better tolerability, ultimately resulting in better efficacy outcomes. However, none of the studies conducted to date have analyzed and compared the role of schedule modification, in terms of efficacy and safety, in AP and NAP. The main objective was to compare the safety (overall and Grade 3-4 adverse events [AEs]) and efficacy (progression-free survival [PFS] and overall survival [OS]) between the AP and NAP on treatment with sunitinib schedule 2/1 (AS) and to observe any significant difference in safety profile with AS between these two populations and find out the reason for any differences.
MATERIALS AND METHODS
Electronic databases
Electronic databases such as PubMed, EMBASE, and Cochrane Library were searched to identify articles published between May 2009 and May 2019. The inclusion of studies was based on patient/ population/ problem-intervention-comparator-outcome (PICO) strategy that was employed to identify concepts required for constructing search strategies.
Search strategy
Articles published in the English language were considered for inclusion. Reference lists of the articles that were selected were searched to identify relevant articles. Duplicates were removed after manual curation. Articles were then scanned by title and abstract. From the identified publications, meeting abstracts, conference abstracts with insufficient data, case reports, pharmacoeconomic studies, reviews, and meta-analyses were excluded. Unique full-text articles meeting the inclusion criteria were considered for inclusion in the meta-analysis.
Selection criteria
All clinical trials and retrospective studies which included patients with mRCC who were receiving AS of sunitinib in first line setting in patients with mRCC and were more than 18 years of age, were considered for inclusion (Table 1). Further, studies evaluating safety outcomes such as incidence of all-grade AEs, grade 3-4 AEs and incidence of different types of AEs; efficacy outcomes such as progression-free survival (PFS) and overall survival (OS) were considered for inclusion in the meta-analysis. The percentage of patients requiring dose reduction or treatment interruption was also determined in the included studies.
Table 1
List of Inclusion Criteria
| Population | Intervention | Outcomes | Study design |
| mRCC patients receiving alternate schedule (AS) of sunitinib, 18 years or older in age | Sunitinib (AS) | Safety: Incidence of all grade adverse event, incidence of different types of adverse events | All randomized controlled trials and retrospective studies |
| Efficacy: Progression-free survival (PFS), Overall survival (OS) |
Search terms
Search terms used to identify relevant articles in PubMed were Carcinoma, Renal Cell/drug therapy OR Carcinoma, Renal Cell/therapy AND sunitinib. Filters applied to identify articles of interest in PubMed included clinical trial, clinical trial, phase I, clinical trial, phase II, clinical trial, phase III, clinical trial, phase IV, comparative study, controlled clinical trial, journal article, randomized controlled trial, humans.
The search terms used to identify articles from EMBASE included kidney metastasis/exp AND sunitinib/exp AND ([article]/lim OR [article in press]/lim OR [conference abstract]/lim OR [conference paper]/lim) AND [english]/lim AND [humans]/lim AND ([embase]/lim OR [medline]/lim). The medical subject heading (MeSH) search descriptor used to identify articles from Cochrane database was [carcinoma, renal cell] explode all trees and sunitinib.
Endpoints evaluated
The primary endpoint of this meta-analysis was as follows:
1. To compare the safety of AS of sunitinib in AP with that in NAP. Safety assessments included the incidence of different types of AEs in AP vs. NAP (all grades and Grade 3/4).
The secondary endpoints were as follows:
1. To compare the efficacy of AS of sunitinib in AP vs. NAP. The efficacy assessments included median PFS and OS in AP vs. NAP.
2. To determine the percentage of patients requiring dose reduction or treatment interruption in AP and NAP.
Data extraction
From the included articles, the study number, study name, authors’ information, study design, study objective, study methods, study population, sample size, interventions, dose and route of administration, outcomes, safety information (AEs/serious adverse events, SAEs), efficacy information (PFS and OS), dose reduction and dose interruption related information were collected. Based on the existing literature, major focus was on the following clinically relevant AEs, i.e., mucositis, rash, hemorrhage, nausea, hand-foot syndrome (HFS), hypertension, lipase increase, liver dysfunction, anemia, neutropenia, thrombocytopenia, leukopenia, hypertension, fatigue, dysgeusia, appetite loss, anorexia, vomiting, stomatitis, diarrhea, abdominal pain, hypothyroidism, cardiotoxicity, and aspartate transaminase (AST). Further, AEs/SAEs of all grades and Grade 3,4 were obtained.
Statistical analysis
Meta-analysis was performed based on the available safety and efficacy data from the included studies. A zero value for an AE could mean either that incidence was zero or the study had not reported that AE. Statistically, AE incidence between AP and NAP (overall for each AE and for Grade 3 & 4 for each AE) were compared using a random effect model. The pooled relative risk for dichotomous outcomes with a 95% confidence interval (CI) was calculated, and meta-analysis was performed using MedCalc for Windows, version 15.0 (MedCalc Software, Ostend, Belgium). The efficacy endpoints (PFS and OS) were statistically analyzed by using a t-test. The percentage of patients requiring dose reduction and/or treatment interruption was presented as n (%) and were statistically analyzed using a z-test. Mean and SD for conducting meta-analysis was calculated using the guidelines in the article by Hozo SP et al. [27]. A p-value of less than 0.05 was considered statistically significant. All analysis was conducted using SPSS software version 18 (Chicago, Released 2008).
RESULTS
Search results
Our systematic search retrieved 1922 publications. From these, 363 duplicates were removed. The remaining 1,559 records were screened by title and abstracts. Then a detailed analysis was done to exclude records not satisfying the requirements for the meta-analysis. A total of 1,475 records were excluded. The remaining 84 records were then screened for full text, of which 65 not meeting the required criteria were removed (reviews and commentaries [n = 26], conference abstracts [n = 9], non-human studies [n = 8], safety and efficacy outcomes missing [n = 12], and unavailability of relevant data [n = 10]). Of the remaining 19 full-text articles assessed for eligibility, 16 studies were finally included in the meta-analysis. The search results are presented in a PRISMA flow chart in Fig. 1.
Fig. 1
PRISMA flow diagram of included studies in the meta-analysis.
Study characteristics
All the 16 studies included in the meta-analysis were published between May 2009 and May 2019 and included eight AP [23, 28–34] and eight NAP [20, 24–26, 35–38] studies with a total of 1140 patients. Of these, 329 were AP (28.9 %) and 811 were NAP (71.1 %). All studies included in the analysis allowed either a switch from traditional to alternate, upfront AS, or both. The studies included were mostly retrospective cohorts (n = 12), followed by randomized controlled trials (RCT; n = 1) and non-RCTs (n = 3). (Table 2). Most of the studies used the response evaluation criteria in solid tumors (RECIST) criteria for assessing and grading the tumor response, while the AEs were graded using different versions of common terminology criteria for adverse events (CTCAE) [39, 40].
Table 2
Characteristics of studies included in the meta-analysis
| Design | References | |
| Asian Population Studies | ||
| Lee 2015 | Randomized control trial | [29] |
| Miyake 2018 | Retrospective | [32] |
| Miyake 2015 | Retrospective | [31] |
| Kondo 2014 | Retrospective | [23] |
| Iwamoto 2018 | Retrospective | [28] |
| Ohzeki 2014 | Retrospective | [33] |
| Pan 2015 | Retrospective | [34] |
| Makino 2014 | Non-randomized | [30] |
| Non-Asian Population studies | ||
| Neri 2012 | Non-randomized phase II | [38] |
| Atkinson 2014 | Retrospective | [20] |
| Bracarda 2015 | Retrospective | [25] |
| Bjarnason 2014 | Retrospective | [35] |
| Boegemann 2018 | Retrospective | [36] |
| Ezz El Din 2017 | Retrospective | [37] |
| Najjar 2014 | Retrospective | [24] |
| Jonasch 2018 | Non-randomized phase II | [26] |
SAFETY ASSESSMENT OF SUNITINIB: COMPARATION BETWEEN AP AND NAP
All grade adverse events
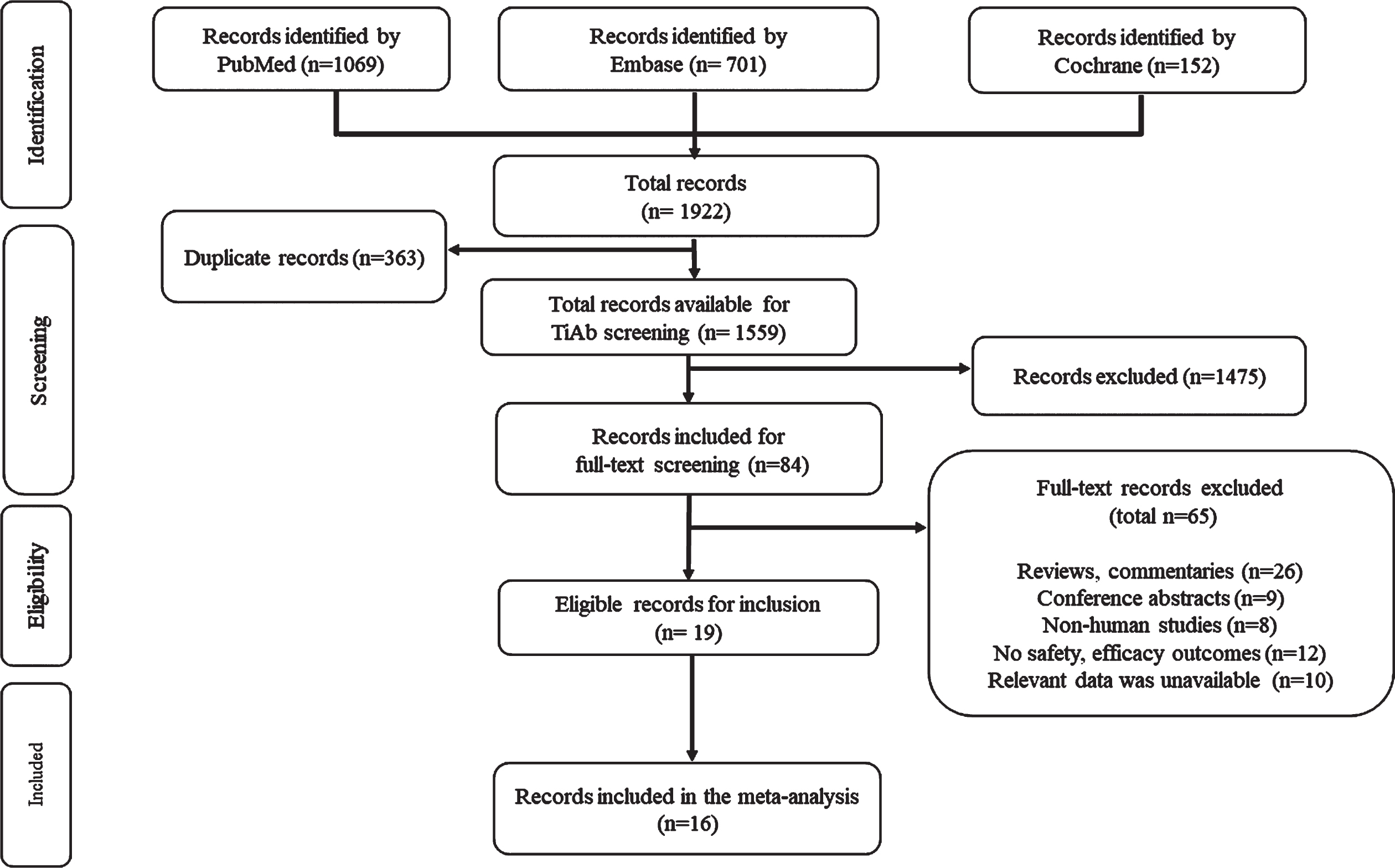
Leukopenia (RR: 2.57; 95% CI: 1.47–4.49), proteinemia (RR:4.45; 95% CI: 2.12–9.33), nausea (RR:0.21; 95% CI: 0.10–0.44), (stomatitis (RR: 4.33; 95% CI: 2.6–7.23), HFS (RR: 0.33; 95% CI: 0.13–0.83), rash (RR: 0.52; 95% CI: 0.34–0.79), cardiotoxicity (RR: 0.52; 95% CI: 0.31–0.88), mucositis (RR: 0.22; 95% CI: 0.12–0.40), and AST (RR: 0.57; 95% CI: 0.33–0.98) were found to be significantly different between NAP and AP (Table 3). Mucositis, cardiotoxicity, nausea, HFS, rash and AST were more prevalent in AP, whereas leukopenia, proteinemia and stomatitis occurred more commonly in NAP. However, pooled estimate of all grade AEs (RR: 0.77; 95% CI: 0.52–1.16; p = 0.216) was not significantly different between the populations with high heterogeneity (I2 = 86.50%, 95% CI: 80.51–90.64, p < 0.0001). The forest plot representing comparison of all grade AEs in AP vs. NAP population is depicted in Fig. 2.
Table 3
Comparison of all-grade adverse events in Asian vs. non-Asian population
| Adverse event | NAP* | AP* | Relative risk | 95% CI | p-value |
| ANEMIA | 12/59 | 23/142 | 1.256 | 0.670–2.354 | NS |
| LEUKOPENIA | 17/51 | 21/162 | 2.571 | 1.474–4.487 | S |
| NEUTROPENIA | 6/38 | 15/50 | 0.526 | 0.225–1.228 | NS |
| THROMBOCYTOPENIA | 15/103 | 24/220 | 1.335 | 0.732–2.435 | NS |
| LIPASE INCREASE | 4/4 | 17/17 | – | – | NE |
| HYPERTENSION | 8/118 | 19/136 | 0.485 | 0.221–1.068 | NS |
| PROTEINEMIA | 23/23 | 5/24 | 4.451 | 2.123–9.332 | S |
| FATIGUE | 18/288 | 16/146 | 0.570 | 0.300–1.085 | NS |
| DYSGEUSIA | 41/82 | 6/17 | 1.417 | 0.718–2.794 | NS |
| APPETITE LOSS | 7/7 | 11/11 | – | – | NE |
| ANOREXIA | 2/54 | 5/32 | 0.237 | 0.049–1.151 | NS |
| NAUSEA | 11/99 | 7/13 | 0.206 | 0.097–0.437 | S |
| VOMITING | 8/37 | 3/6 | 0.432 | 0.158–1.185 | NS |
| STOMATITIS | 20/20 | 11/50 | 4.329 | 2.592–7.231 | S |
| DIARRHEA | 16/117 | 12/126 | 1.436 | 0.710–2.906 | NS |
| HAND-FOOT SYNDROME | 6/175 | 15/144 | 0.329 | 0.131–0.827 | S |
| ABDOMINAL PAIN | 9/9 | 15/15 | – | – | NE |
| RASH | 11/22 | 13/13 | 0.519 | 0.340–0.790 | S |
| HEMORRHAGE | 2/4 | 20/20 | 0.512 | 0.213–1.234 | NS |
| HYPOTHYROIDISM | 13/101 | 19/135 | 0.915 | 0.474–1.764 | NS |
| CARDIOTOXICITY | 7/14 | 11/11 | 0.522 | 0.310–0.877 | S |
| MUCOSITIS | 17/150 | 10/19 | 0.215 | 0.116–0.400 | S |
| AST | 12/24 | 3/3 | 0.571 | 0.333–0.980 | S |
| Total (fixed effects) | 285/1599 | 301/1512 | 0.941 | 0.813–1.088 | 0.409 |
| Total (random effects) | 285/1599 | 301/1512 | 0.774 | 0.516–1.161 | 0.216 |
AST: Aspartate aminotransferase; NS: Not significant; S: Significant; NE: Not estimable; NAP: Non-Asian population; AP: Asian population; CI: Confidence interval; *: Median/total events.
Fig. 2
Forest plot representing a comparison of all-grade adverse events in Asian vs. non-Asian population.
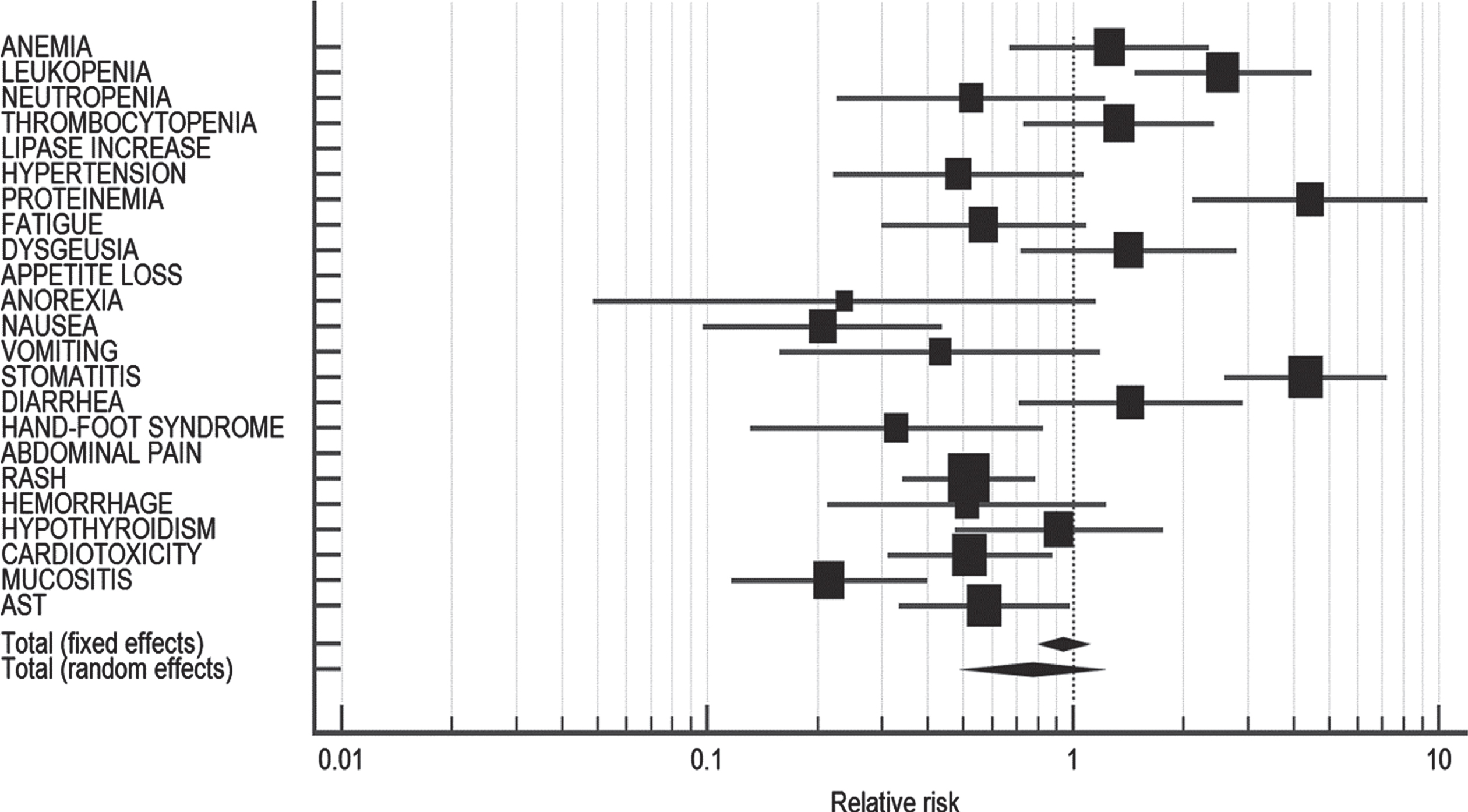
Grade 3-4 adverse events
None of the adverse events individually were found to be statistically different in NAP and AP. Also, pooled estimate of grade 3-4 AEs (RR: 0.74; 95% CI: 0.40–1.37; p = 0.339) was not significantly different in both the populations (I2 = 0.00%, 95% CI: 0.00–10.00, p = 0.9547) (Table 4). The forest plot representing the comparison of grade 3-4 AEs in AP vs. NAP is presented in Fig. 3.
Table 4
Comparison of grade 3-4 adverse events in Asian vs. non-Asian population
| Adverse event | NAP* | AP* | Relative risk | 95% CI | p-value |
| ANEMIA | 2/11 | 3/23 | 1.394 | 0.271–7.176 | NS |
| LEUKOPENIA | 1/6 | 4/23 | 0.958 | 0.130–7.072 | NS |
| NEUTROPENIA | 1/10 | 6/20 | 0.333 | 0.046–2.405 | NS |
| THROMBOCYTOPENIA | 1/8 | 8/59 | 0.922 | 0.132–6.436 | NS |
| HYPERTENSION | 2/30 | 1/19 | 1.267 | 0.123–13.028 | NS |
| FATIGUE | 2/18 | 1/23 | 2.556 | 0.251–26.002 | NS |
| ANOREXIA | 0/3 | 1/3 | 0.333 | 0.0186–5.968 | NS |
| DIARRHEA | 2/91 | 0/8 | 0.489 | 0.0254–9.423 | NS |
| HAND-FOOT SYNDROME | 0/17 | 1/22 | 0.426 | 0.0184–9.847 | NS |
| HYPOTHYROIDISM | 0/2 | 0/4 | – | – | NE |
| CARDIOTOXICITY | 1/2 | 1/1 | 0.667 | 0.167–2.666 | NS |
| MUCOSITIS | 2/12 | 1/2 | 0.333 | 0.0510–2.177 | NS |
| Total (fixed effects) | 14/210 | 27/207 | 0.767 | 0.414–1.421 | 0.399 |
| Total (random effects) | 14/210 | 27/207 | 0.741 | 0.401–1.369 | 0.339 |
NS: Not significant; NE: Not estimable; NAP: Non-Asian population; AP: Asian population; CI: Confidence interval; *: Median/total events.
Fig. 3
Forest plot representing a comparison of grade 3-4 adverse events in Asian vs. non-Asian population.
EFFICACY ASSESSMENT OF SUNITINIB: COMPARATION BETWEEN AP AND NAP
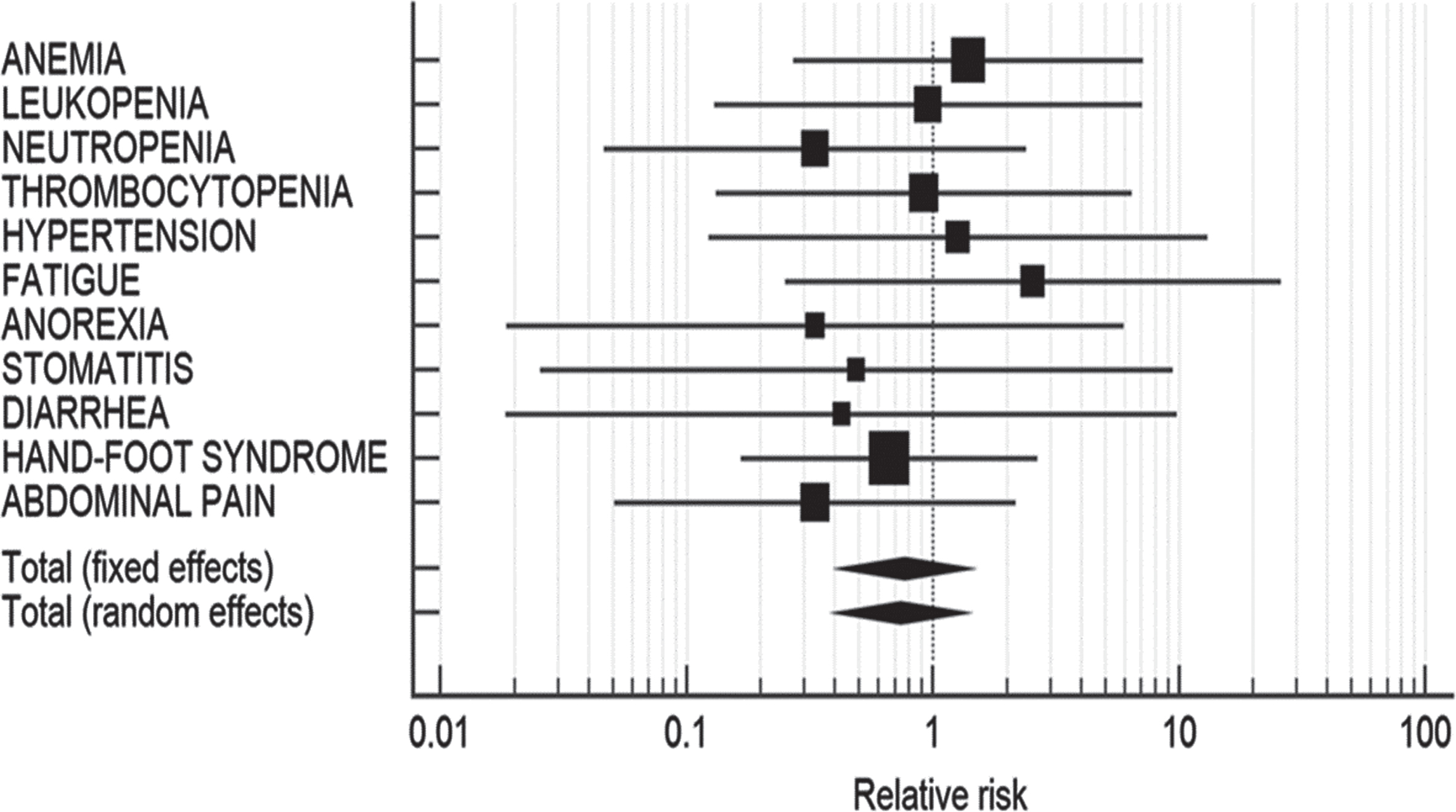
The efficacy of AS of sunitinib was assessed by comparing PFS and OS between AP and NAP. Of the 16 studies included in the present meta-analysis, PFS was evaluated in four AP studies [23, 32–34] and six NAP studies [20, 25, 26, 36–38]. Pooled estimates of PFS of all AP studies were found to be 14.18 (95% CI: 9.38–18.99; p < 0.001) with high heterogeneity (I2 = 73.68%, 95% CI: 26.22–90.61; p = 0.0098) and 15.75 (95% CI: 12.65 –18.85; p < 0.001) for all NAP studies (I2 = 0.00%, 95% CI: 0.00–58.31; p = 0.7068). The PFS for both the populations was found to be significantly different (mean deviation [MD]: 1.57; p < 0.001) in NAP and AP (Table 5), representing longer PFS in NAP (Fig. 4).
Table 5
Comparison of progression-free survival in Asian vs. non-Asian population
| Study | Estimate | SE | 95% CI | z | p-value | Test for Heterogeneity |
| Asian | ||||||
| Ohzeki 2014 [33] | 11.300 | 3.110 | 5.20–17.39 | |||
| Kondo 2014 [23] | 18.400 | 0.240 | 17.93–18.87 | Q = 11.39 | ||
| Pan 2015 [34] | 9.500 | 4.380 | 0.92–18.09 | DF = 3 | ||
| Miyake 2018 [32] | 13.800 | 3.090 | 7.74–19.86 | P = 0.0098 | ||
| I2 (inconsistency) = 73.68% | ||||||
| Total (fixed effects) | 18.305 | 0.238 | 17.84–18.77 | 76.84 | <0.001 | 95% CI for I2 = 26.22–90.61 |
| Total (random effects) | 14.183 | 2.450 | 9.38–18.99 | 5.79 | <0.001 | |
| Non-Asian | ||||||
| Neri 2012 [38] | 13.00 | 2.860 | 7.39–18.61 | |||
| Atkinson 2014 [20] | 14.50 | 2.070 | 10.44–18.56 | |||
| Bracarda 2015 [25] | 10.40 | 6.090 | –1.54–22.34 | Q = 2.96 | ||
| Boegemann 2018 [36] | 15.10 | 2.120 | 10.95–19.26 | DF = 5 | ||
| Ezz El Din 2017 [37] | 17.00 | 1.860 | 13.35–20.65 | P = 0.7068 | ||
| Jonasch 2018 [26] | 13.70 | 1.350 | 11.05–16.35 | |||
| Total (fixed effects) | 14.51 | 0.83 | 12.89 –16.13 | 17.52 | <0.001 | I2 (inconsistency) = 0.00% |
| Total (random effects) | 15.75 | 1.58 | 12.65 –18.85 | 17.52 | <0.001 | 95% CI for I2 = 0.00 –58.31 |
| t-test for PFS | <0.001 | MD = 1.57 |
PFS: Progression-free survival; CI: Confidence interval; MD: Mean deviation.
Fig. 4
Forest plot representing a comparison of progression-free survival in Asian (A) vs. non-Asian population (B).
Among the studies evaluating AS of sunitinib in AP, Ohzeki et al. (2014) reported the median PFS to be 11.3 months, while Pan et al. (2015) reported the median PFS to be 9.5 months in the transition group (shifted from schedule 4/2 to 2/1) and 11.2 months in patients with upfront 2/1 group [33, 34]. On the other hand, NAP demonstrated a better PFS with sunitinib therapy. The median PFS reported by Neri et al. (2013) was 13 months, while it was 17 months by Ezz El Din et al. (2017) [37, 38]
Overall, of the 16 studies included, three AP studies [29, 32, 33] and five NAP studies [20, 25, 36–38] evaluated the OS with sunitinib among mRCC patients. Pooled estimates of OS of all NAP studies were found to be 25.65 (95% CI: 19.77–31.53; p < 0.001) with high heterogeneity (I2 = 66.39%, 95% CI: 12.5–87.09; p = 0.0181) and 33.07 (95% CI: 25.67–40.44; p < 0.001) with I2 = 0.00%, 95% CI: 0.00–91.77; p = 0.665 for all AP studies. The OS was found to be significantly different (MD: –7.42; p < 0.001) between the studies of NAP and AP (Table 6), representing longer OS in AP (Fig. 5).
Table 6
Comparison of overall survival in Asian vs. non-Asian population
| Study | Estimate | SE | 95% CI | Z | P-value | Test for Heterogeneity |
| Asian | ||||||
| Lee 2015 [29] | 30.50 | 5.89 | 18.96–42.04 | |||
| Ohzeki 2014 [33] | 32.10 | 6.25 | 19.85–44.35 | Q = 0.815 | ||
| Miyake 2018 [32] | 39.20 | 7.91 | 23.69–54.70 | DF = 2 | ||
| P = 0.665 | ||||||
| Total (fixed effects) | 33.067 | 3.77 | 25.67–40.44 | 8.77 | <0.001 | I2 (inconsistency) = 0.00% |
| Total (random effects) | 33.067 | 3.76 | 25.67–40.44 | 8.77 | <0.001 | 95% CI for I2 = 0.00–91.77 |
| Non-Asian | ||||||
| Neri 2012 [38] | 20.00 | 1.84 | 16.39–23.61 | |||
| Atkinson 2014 [20] | 33.00 | 7.47 | 18.36–47.64 | Q = 11.90 | ||
| Boegemann 2018 [36] | 38.10 | 5.51 | 27.30–48.90 | DF = 4 | ||
| Bracarda 2015 [25] | 23.20 | 2.65 | 18.01–28.39 | |||
| Ezz El Din 2017 [37] | 23.00 | 5.88 | 11.48–34.52 | P = 0.0181 | ||
| Total (fixed effects) | 22.65 | 1.39 | 19.92–25.38 | 16.29 | <0.001 | I2 (inconsistency) = 66.39% |
| Total (random effects) | 25.65 | 2.99 | 19.77–31.53 | 8.55 | <0.001 | 95% CI for I2 = 12.50–87.09 |
| t -test for OS | <0.001 | MD = –7.42 |
OS: Overall survival; CI: Confidence interval; MD: Mean deviation.
Fig. 5
Forest plot representing a comparison of overall survival in Asian (A) vs. non-Asian population (B).
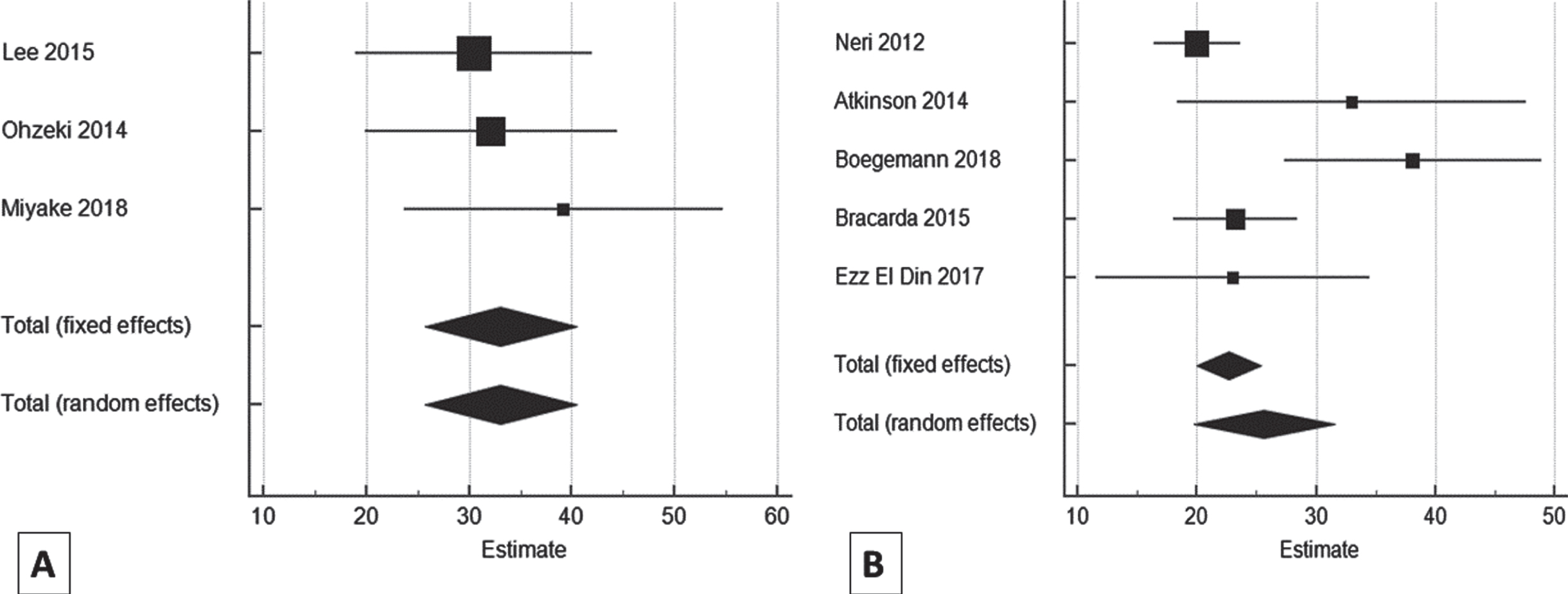
Of the AP studies included in this meta-analysis, Lee et al. (2015) reported median OS to be 30.5 months, while among the NAP studies, Ezz El Din et al. (2017) reported a lower median OS (23 months) in mRCC patients receiving sunitinib [29, 37].
DOSE REDUCTION ASSESSMENT OF SUNITINIB: COMPARATION BETWEEN AP AND NAP
Dose reduction data were available for five studies [23, 28, 29, 32, 34] in AP and four studies in NAP (Fig. 6)). Out of 288 patients in AP (the total number of patients from the studies in which dose reduction-related data was available), 150 (52.08%) patients underwent dose reduction. For AP, the pooled estimates of the percentage of patients requiring dose reduction in all Asian studies were found to be 51.26% (95% CI: 19.07–82.87; p < 0.0001) with high heterogeneity (I2 = 97.13 %; 95% CI: 95.29–98.25; p < 0.0001). Among the NAP studies, out of 133 patients, 54 (40.6%) patients underwent dose reduction. The pooled estimates of the percentage of patients requiring dose reduction in non-Asian studies were found to be 35.9% (95% CI: 18.54–55.42; p < 0.0001) with high heterogeneity (I2 = 80.48 %; 95% CI: 48.61–92.58; p < 0.0015) (Table 7). Dose reduction between the AP and NAP was significantly different (z = 2.6182; p = 0.0088), with a higher dose reduction in AP.
Fig. 6
Forest plot representing a comparison of dose reduction in Asian (A) vs. non-Asian (B) population.
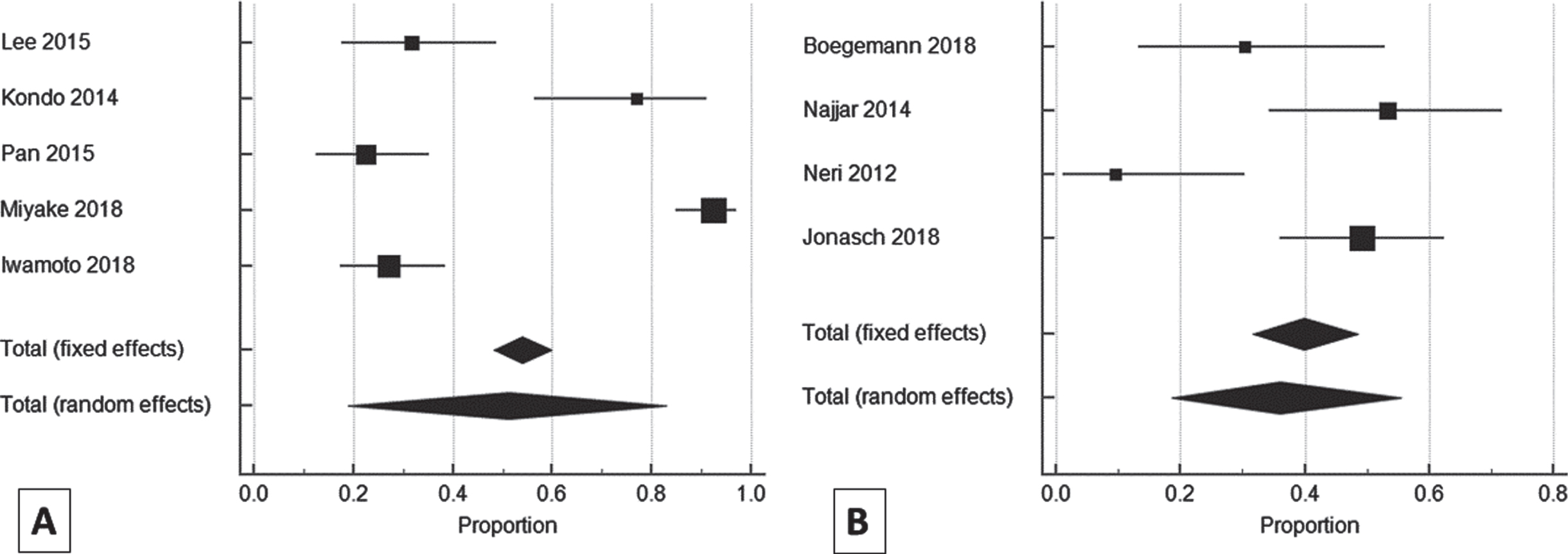
Table 7
Pooled estimates of the percentage of patients requiring dose reduction in Asian vs. non-Asian population
| Study | Total number of patients | Proportion (%) | 95% CI |
| Asian | |||
| Lee 2015 [29] | 38 | 31.579 | 17.50–48.65 |
| Kondo 2014 [23] | 26 | 76.923 | 56.35–91.03 |
| Pan 2015 [34] | 58 | 22.414 | 12.51–35.27 |
| Miyake 2018 [32] | 92 | 92.391 | 84.95–96.89 |
| Iwamoto 2018 [28] | 74 | 27.027 | 17.36–38.61 |
| Total (fixed effects) | 288 | 54.064 | 48.17–59.87 |
| Total (random effects) | 288 | 51.256 | 19.07–82.86 |
| Non-Asian | |||
| Boegemann 2018 [36] | 23 | 30.43 | 13.21–52.92 |
| Najjar 2014 [24] | 30 | 53.33 | 34.33–71.66 |
| Neri 2012 [38] | 21 | 9.52 | 1.17–30.38 |
| Jonasch 2018 [26] | 59 | 49.15 | 35.89–62.50 |
| Total (fixed effects) | 133 | 39.95 | 31.68–48.66 |
| Total (random effects) | 133 | 35.90 | 18.54–55.42 |
CI: confidence interval.
TREATMENT INTERRUPTION ASSESSMENT OF SUNITINIB: COMPARISON BETWEEN AP AND NAP
Among eight AP studies, only 50% (n = 4) studies reported data related to treatment interruption (Fig. 7) [23, 28, 32, 34]. Out of 250 patients in AP, treatment was interrupted in only 94 (37.6%) patients. For AP, the pooled estimates of dose reduction of all Asian studies were found to be 34.44% (95% CI: 19.75–50.85; p < 0.0001) with high heterogeneity (I2 = 85.43% (95% CI: 64.05–94.09; p = 0.0001) (Table 8). Treatment interruption data was not available for NAP.
Fig. 7
Forest plot representing dose interruption in Asian population.
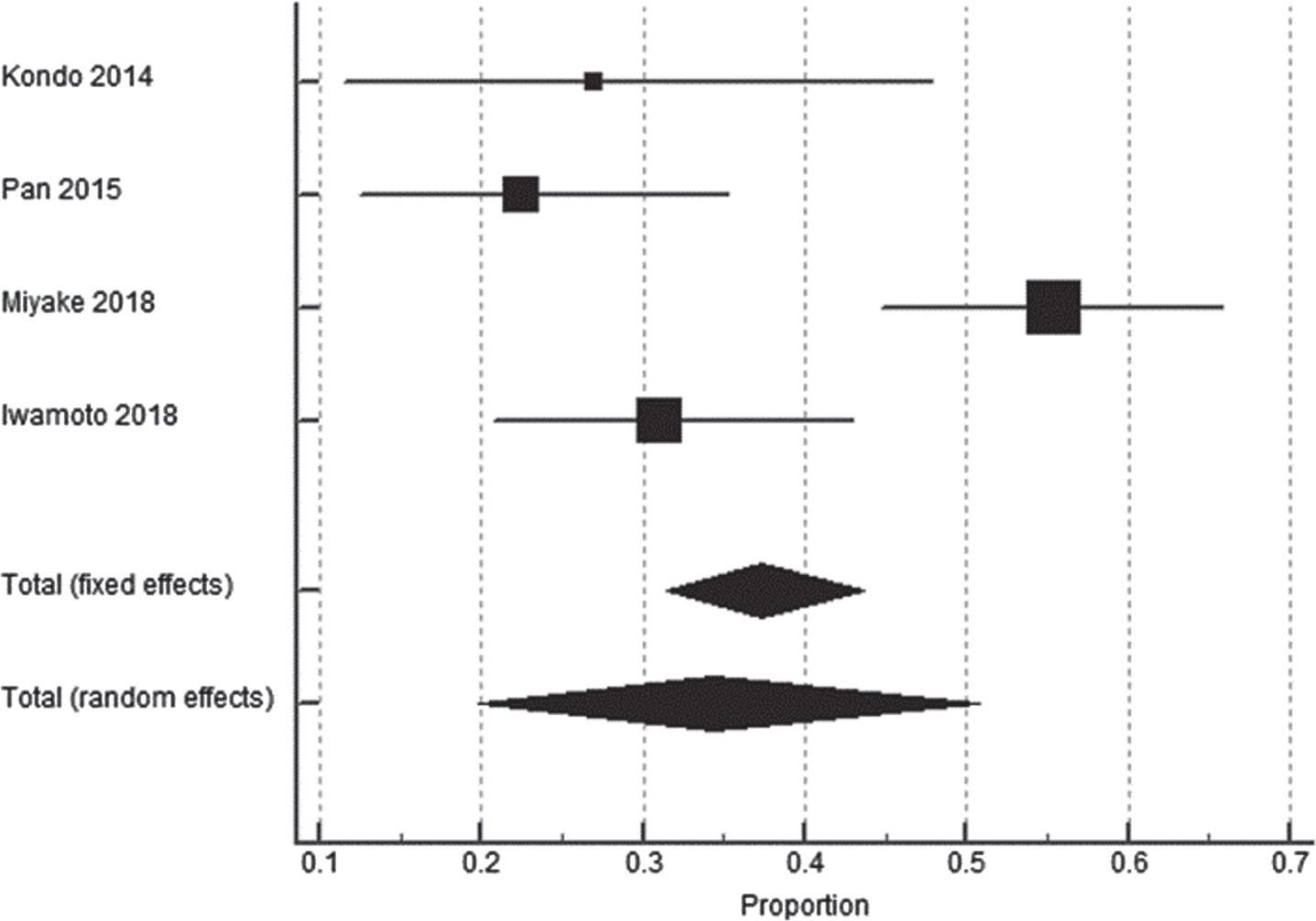
Table 8
Pooled estimates of the percentage of patients requiring dose interruption in Asian population
| Study | Total number of patients | Proportion (%) | 95% CI |
| A sian | |||
| Kondo 2014 [23] | 26 | 26.92 | 11.57–47.79 |
| Pan 2015 [34] | 58 | 22.41 | 12.51–35.27 |
| Miyake 2018 [32] | 92 | 55.43 | 44.70–65.81 |
| Iwamoto 2018 [28] | 74 | 31.08 | 20.83–42.91 |
| Total (fixed effects) | 250 | 37.35 | 31.39–43.62 |
| Total (random effects) | 250 | 34.44 | 19.75–50.85 |
CI: confidence interval.
DISCUSSION
This meta-analysis compared AS of sunitinib outcomes between AP and NAP by pooling data from eight AP and eight NAP studies. Our analysis observed no significant difference in the overall incidence of all grade and grade 3-4 AEs across both the populations in pooled estimate analysis. Among all grade AEs: mucositis, cardiotoxicity, nausea, HFS, rash and AST were more prevalent in AP whereas, leukopenia, proteinemia and stomatitis occurred more commonly in NAP.
Owing to the high frequency of the dose-limiting toxicity (DLTs) caused by schedule 4/2, the AS of sunitinib is favored. Numerous studies have been conducted which revealed that AS is associated with a lower risk of DLTs and help in maintaining a high relative dose intensity (RDI) which facilitates an equivalent therapeutic effect as schedule 4/2 [23–25, 28, 31]. Hence, the schedule 2/1 regime is commonly implemented in clinical practice, especially when patients are unable to tolerate the 4/2 schedule.
This meta-analysis includes safety and efficacy data of 16 studies with 1140 patients with mRCC receiving sunitinib in AS (2/1). Despite the limited availability of studies (comparing the period of literature search of 10 years with the number of studies included) and heterogeneity of studies, there is evidence that sunitinib in AS is associated with 22 AEs, including anemia, leukopenia, neutropenia, thrombocytopenia, lipase increase, hypertension, fatigue, dysgeusia, appetite loss, anorexia, nausea, vomiting, stomatitis, diarrhea, HFS, abdominal pain, rash, hemorrhage, hypothyroidism, cardiotoxicity, mucositis and AST. Available literature also shows that patients receiving sunitinib treatment experience these AEs through the prevalence/incidence varied due to ethnic differences [21, 24, 41–44].
A study conducted on the Asian (non-western) population reported fatigue (70%), hand-foot syndrome (62%), skin rash (58%), mucositis (58%), anorexia (42%), skin discoloration (42%), as the most common AEs in AP taking sunitinib [45]. In concordance, our analysis also reported mucositis, skin rash and HFS to be more common in AP. Hand-foot syndrome is a sunitinib treatment-induced AE which affects the QoL of patients [23]. Its clinical presentation, as well as histopathology, is different in patients receiving sunitinib from the ones receiving chemotherapy. The risk of incidence of HFS seems to be highest within the first two to four weeks of treatment [46]. It has been found to be more prevalent in AP [21, 47]. A relationship between polymorphism of CYP3A5*3 and low response and tolerability of sunitinib has been established in 101 Spanish mRCC patients [48]. The CYP3A5*3 is commonly observed in AP than Caucasians (34% vs. 4%), due to which there is a difference in the incidence of HFS in both the ethnic groups, representing higher incidence in AP [49, 50]. Pan et al. (a study conducted in AP) has also reported HFS as one of the most common AEs (73/108, 67.6%) [34]. Also, the previously conducted studies report a higher frequency of HFS in AP than NAP (Western population) [51]. As per the findings of Liu et al., 2017, HFS was more prevalent in AP than the Caucasian population, with an almost similar prevalence pattern for other AEs between both populations [21]. Even in our study, HFS was more prevalent in AP.
Guo et al., 2018 reported hematologic AEs more common in AP than NAP. There were few non-hematologic AEs also (stomatitis [30 vs. 13%], HFS [64 vs. 50%], hypothyroidism [28 vs. 16%], increased blood creatinine [27 vs. 17%], increased blood lactate dehydrogenase [24 vs. 14%], constipation [18 vs. 8%], increased blood thyroid-stimulating hormone [18 vs. 9%], and yellow skin [24 vs. 2%]) which commonly occurred among AP vs. NAP [52]. This meta-analysis also showed a similar trend for HFS and mucositis except for a few AEs that were more prevalent in NAP, including hematological AEs e.g., leukopenia. The I2 value was high for all grade AEs due to their heterogenous nature among the studies.
Previous literature provides an extensive and possible explanation for the higher risk of toxicity in AP due to high drug exposure in AP. A study assessing the population pharmacokinetics of sunitinib and SU12662 (its primary metabolite) in patients with mRCC, gastrointestinal stromal tumor and other solid tumors reported 15% higher drug exposure (indicated as area under curve and maximal plasma drug concentration) in AP in comparison to other ethnical groups [53]. Moreover, lean body mass also influences toxicity and clinical activity of sunitinib as lower lean body mass have a higher drug exposure [54]. Mir et al., 2016 also reported that AP (non-western patients) exhibit different behaviour with sunitinib therapy compared to NAP/Western patients. As per the study, AP experience more mucocutaneous AEs [45]. According to the study conducted by Lear et al., 2009, which compared total body fat to lean mass ratio in Aboriginal, Chinese, European, and South Asian individuals with differences in insulin resistance, South Asians have a phenotype of high-fat mass and low lean mass [55]. Also, AP has a relatively lower body surface area than the other ethnic groups, which may attribute to higher drug exposure in AP. However, no such association was observed in our analysis which was in concordance with the findings of the previous studies conducted on AP [56] as well as NAP [53]. The difference in AE profiles between AP and NAP with sunitinib treatment may be attributed to variations in ethnic tolerances for certain AEs. Disparities in toxicity reported may also be related to racial differences in drug absorption and metabolization capabilities [57–60]. We need more translation efforts to provide insight into the role of these factors and to decide if other factors could be involved [52].
Our pooled analysis showed that the difference in PFS was statistically significant between AP and NAP, with PFS higher in NAP. Lee et al., 2014 evaluated and compared the safety and efficacy of sunitinib in Asian and non-Asian patients with mRCC and reported median PFS higher in NAP (AP vs NAP: 8.7 versus 10.9 months) than AP, which was in concordance to our analysis [61]. However, Liu et al. did not find any statistical difference in PFS and OS between AP and NAP (Caucasian) [21]. As per our analysis, OS was significantly higher in AP than NAP. This finding was also supported by Lee et al., 2014 where AP showed longer median OS than NAP (18.9 versus 18.4 months) [61].
Several studies have compared the efficacy of AS of sunitinib with the traditional schedule in AP and NAP and reported longer PFS and OS in AS. Ezz El Din et al. reported PFS and OS similar in both the groups (median PFS: 15 months in 4/2; 17 months in 2/1, median OS: 24 months in 4/2; 23 months in 2/1) [37]. A meta-analysis of 13 studies comparing sunitinib’s 4/2 schedule with 2/1 reported significantly shorter PFS in the 4/2 group than the transition group (4/2-to-2/1-schedule: HR: 2.30 [95% CI: 1.07, 4.99]) [62]. A recent meta-analysis conducted in 2019 also supports that 2/1 schedule is more beneficial than 4/2 and reported that treatment with 2/1 decreased the risk of disease progression or death by 25% [63].
Studies reported that some sunitinib-related AEs such as hypertension, neutropenia, thrombocytopenia, and HFS are predictive clinical biomarkers of efficacy in patients with mRCC [64–66]. A higher number of sunitinib-related AEs has also been reported to be an independent marker of longer median PFS and median OS [67]. A higher incidence of hypothyroidism has also been associated with improved clinical outcomes [66, 68]. An alternative schedule or switching from a traditional schedule to an alternative schedule makes sunitinib more tolerable to patients, so they remain on therapy for a more extended period leading to better efficacy outcomes [36].
The AS of sunitinib has been found as a more tolerable schedule compared to the traditional schedule [29]. However, in the present meta-analysis, the AS of sunitinib was also associated with dose reduction and treatment interruption, which was more prevalent in AP than NAP. Generally, the patients initiated with 50.0 mg of sunitinib dose, which was reduced to 37.5 mg and further to 25.0 mg [29, 32, 36]. The factors which led to dose reduction majorly included treatment-induced toxicity [46]. According to Kondo et al., three factors, i.e., age above 65 years, high serum creatinine level (>2 mg/dl) and body weight below 50 kg, led to dose reduction. In the presence of one of the factors, the dose was reduced to 37.5 mg, while in the presence of ≥2 factors, the dose was reduced to 25.0 mg [23]. As per Miyake et al., 2018, the dose was modified based on the age, body weight, and physiological functions of the patient [32]. Sunitinib, at an initial dose, ≤25.0 mg/day, is not effective. Hence, further dose reduction is not advisable/ recommended, and administration of another targeted agent other than sunitinib can be an optimal treatment choice [28]. Therefore, to maintain the therapeutic efficacy of sunitinib, attention should be paid for patient tolerability to avoid early DLT development.
Among AP studies, Lee et al., 2015 reported at least one dose reduction (50 mg/day to 37.5 mg/day) in 32% of patients, while the proportion of patients who underwent dose reduction within six months after initiation was 21% in schedule 2/1. The findings of Kondo et al., 2014 showed a dose reduction in 76.92% of patients [23, 29]. A dose reduction in 22.41%, 92.39% and 27.03% of patients were observed in Pan et al., 2015; Miyake et al., 2018; and Iwamoto et al., 2018, respectively [28, 32, 34]. Among NAP studies, a dose reduction in 9.52%, 30.43%, 53.3 %, and 49.15 % patients were observed in Neri et al., 2012;Boegemann et al., 2018, Najjar et al., 2014; and Jonasch et al., 2018 respectively [26, 36, 38, 41].
Studies also report that reducing the drug dose may lead to decreased incidence or risk of AEs. Nagata et al., 2015 conducted a study in AP (six Japanese patients with RCC) which reported that patients taking 50 mg of sunitinib as initial dose developed grade 2 or 3 thrombocytopenia. Therefore, a minimum trough dosage of<100 ng/mL of sunitinib could eliminate severe thrombocytopenia, and to attain the same, the initial dose of sunitinib in most Japanese patients should be lowered to 37.5 mg or 25 mg [69].
Dose interruption data was limited to AP. Kondo et al., 2014; Pan et al., 2015; Miyake et al., 2018; and Iwamoto et al., 2018 reported 26.92%, 22.41%, 55.43%, and 31.08 % incidence of dose interruption, respectively. Further, Kondo et al. reported a significantly lower dose interruption in the 2/1 schedule than the 4/2 schedule (27 versus 53% p = 0.04) [23].Our meta-analysis revealedsome promising results, showing that AS of sunitinib has a comparable toxicity profile in AP and NAP Further, higher PFS in NAP and higher OS, dose reduction in AP was observed. However, there were certain strengths and limitations to our meta-analysis, and we have done our best to address them.
Based on the findings of the current review, PFS was found to be shorter in Asian population. Mucositis, cardiotoxicity, nausea, rash, AST and HFS were also common in Asian population. Association of these toxicities with poor survival would reveal the drivers of dose reduction, treatment interruption that caused shorter PFS in Asian population. Further, emphasis on monitoring and managing certain toxicities would prevent affecting the efficacy. However, there are only two studies in the AP with regression data. Since the number is not sufficient, we could not carry out the required analysis, which is a limitation of the study. Though there are some studies and reviews comparing AS with traditional sunitinib schedule, none of them compared the AS of sunitinib in AP versus NAP. This is the first meta-analysis comparing AS of sunitinib in AP versus NAP. Most studies were retrospective, a few were clinical trials, so the comparison of results may not have been possible. This situation was overcome by adopting appropriate statistical tests for analysis of safety and efficacy, respectively. Also, until data from extensive prospective studies or large multi-center trials are not available, our data from retrospective studies adds real value. The studies included in our meta-analysis were primarily conducted in a single center and had few patients. Their ethnicity was not mentioned, but since they enrolled in a country, the patients were considered to be AP or NAP based on the region. Hence, the current meta-analysis identifies the need for large multi-center research in AP and NAP with properly defined ethnicity to compare the safety and efficacy profile of AS of sunitinib.
CONCLUSION
The study demonstrated that the safety of AS of sunitinib in AP were similar to NAP. However, AP experienced all-grade mucositis, cardiotoxicity, nausea, rash, AST and HFS more frequently, while leukopenia, proteinemia and stomatitis were more prevalent in NAP. Therefore, schedule 2/1 of sunitinib can be considered a suitable treatment option for Asian patients with mRCC. Longer PFS in NAP and longer OS and higher dose reduction in AP was observed. Moreover, dose adjustment/ reduction in Asian patients may also be considered to help patients remain on therapy for a longer time. However, large multi-center trials are required to confirm or refute the findings in an extensive population set of Asian and non-Asian origin.
ACKNOWLEDGMENTS
Medical writing and editorial support was provided by Dr. Mudili Sivaprasad and Esha Bahi at Turacoz Healthcare Solutions (www.Turacoz.com) and was funded by Pfizer.
FUNDING
Pfizer.
AUTHOR CONTRIBUTIONS
Dr. Amit Joshi was involved in providing clinical data analysis, manuscript review & editing and had access to data. Dr. Ishan Patel was involved in literature search, planning of statistical analysis, data analysis, data interpretation, manuscript preparation/editing and review and had access to data. Dr. Manmohan Singh was involved in concept and designing, data analysis, manuscript editing and review and had access to data. Dr. Pratiksha Kapse was involved in concept and designing, data analysis, manuscript editing and review and had access to data.
CONFLICT OF INTEREST
Amit Joshi has no conflict of interest to report.
Ishan Patel and Manmohan Singh are employed by Pfizer whereas Pratiksha Kapse was employed by Pfizer at the time of manuscript preparation. They have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/KCA-210122.
REFERENCES
[1] | Fund WCR . Kidney cancer statistics 2018 [Available from: https://www.wcrf.org/dietandcancer/cancer-trends/kidney-cancer-statistics. |
[2] | Capitanio U , Bensalah K , Bex A , Boorjian SA , Bray F , Coleman J , et al. Epidemiology of Renal Cell Carcinoma. Eur Urol. (2019) ;75: (1):74–84. |
[3] | Gangadaran SGD . Current Management Options in Metastatic Renal Cell Cancer. Oncol Rev. (2017) ;11: (2):339. |
[4] | Ljungberg B , Campbell SC , Cho HY , Jacqmin D , Lee JE , Weikert S , et al. The epidemiology of renal cell carcinoma. J Eur Urol. (2011) ;60: (4):615–21. |
[5] | Gong J , Maia MC , Dizman N , Govindarajan A , Pal SK . Metastasis in renal cell carcinoma: Biology and implications for therapy. Asian J Urol. (2016) ;3: (4):286–92. |
[6] | Siegel RL , Miller KD , Fuchs HE , Jemal A . Cancer Statistics, 2021. CA Cancer J Clin. (2021) ;71: (1):7–33. |
[7] | Sung H , Ferlay J , Siegel RL , Laversanne M , Soerjomataram I , Jemal A , et al. Global Cancer Statistics GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. (2021) ;71: (3):209–49. |
[8] | GLOBOCAN 2020: estimated cancer incidence, mortality and prevalence worldwide in 2020. [Internet].World health Organization. 2020 [cited 20 October 2021]. |
[9] | Globocan. Asia 2018 [Available from: https://gco.iarc.fr/today/data/factsheets/populations/935-asia-fact-sheets.pdf. |
[10] | ICMR-NCRP. Trends over time for all sites and on selected leading sites of cancer. Chapter 10 Time trends in cancer incidence rate 2012-2015. Three Year Report of Population Based Cancer Registries 2006-2008. Available from: http://ncdirindia.org/NCRP/ALL_NCRP_REPORTS/PBCR_REPORT_2012_2014/ALL_CONTENT/PDF_Printed_Version/Chapter10_Printed.pdf 2010 |
[11] | Minardi D , Lucarini G , Santoni M , Mazzucchelli R , Burattini L , Conti A , et al. Survival in patients with clear cell renal cell carcinoma is predicted by HIF-1α expression. Anticancer Res. (2015) ;35: (1):433–8. |
[12] | Hasanov E , Gao J , Tannir NM . The Immunotherapy Revolution in Kidney Cancer Treatment: Scientific Rationale and First-Generation Results. Cancer J. (2020) ;26: (5):419–31. |
[13] | Rodriguez-Vida A , Hutson TE , Bellmunt J , Strijbos MH . New treatment options for metastatic renal cell carcinoma. ESMO open. (2017) ;2: (2):e000185. |
[14] | IO/TKI as first line therapy. International Kidney Cancer Symposium; November 6-72020. |
[15] | Schmid TA , Gore ME . Sunitinib in the treatment of metastatic renal cell carcinoma. Therapeutic Advances in Urology. (2016) ;8: (6):348–71. |
[16] | Rini BI , McDermott DF , Hammers H , Bro W , Bukowski RM , Faba B , et al. Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of renal cell carcinoma. J Immunother Cancer. (2016) ;4: (1):81. |
[17] | NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Kidney Cancer. 2020. |
[18] | Escudier B , Porta C , Schmidinger M , Rioux-Leclercq N , Bex A , Khoo V , et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019: :1–15. |
[19] | Powles T , Albiges L , Staehler M , Bensalah K , Dabestani S , Giles RH , et al. Updated European Association of Urology Guidelines: Recommendations for the Treatment of First-line Metastatic Clear Cell Renal Cancer. Eur Urol. (2018) ;73: (3):311–5. |
[20] | Atkinson BJ , Kalra S , Wang X , Bathala T , Corn P , Tannir NM , et al. Clinical outcomes for patients with metastatic renal cell carcinoma treated with alternative sunitinib schedules. J Urol. (2014) ;191: (3):611–8. |
[21] | Liu X , Fiocco M , Swen JJ , Guchelaar H-J . Assessment of ethnic differences in sunitinib outcome between Caucasian and Asian patients with metastatic renal cell carcinoma: a meta-analysis. J Acta Oncologica. (2017) ;56: (4):582–9. |
[22] | Hong MH , Kim HS , Kim C , Ahn JR , Chon HJ , Shin SJ , et al. Treatment outcomes of sunitinib treatment in advanced renal cell carcinoma patients: a single cancer center experience in Korea. Cancer Res Treat. (2009) ;41: (2):67–72. |
[23] | Kondo T , Takagi T , Kobayashi H , Iizuka J , Nozaki T , Hashimoto Y , et al. Superior tolerability of altered dosing schedule of sunitinib with 2-weeks-on and 1-week-off in patients with metastatic renal cell carcinoma—comparison to standard dosing schedule of 4-weeks-on and 2-weeks-off. Jpn J Clin Oncol. (2014) ;44: (3):270–7. |
[24] | Najjar Y , Mittal K , Elson P , Wood L , Garcia J , Dreicer R , et al. A 2weeks on and 1week off schedule of sunitinib is associated with decreased toxicity in metastatic renal cell carcinoma. Eur J Cancer. (2014) ;50: (6):1084–9. |
[25] | Bracarda S , Iacovelli R , Boni L , Rizzo M , Derosa L , Rossi M , et al. Sunitinib administered on 2/1 schedule in patients with metastatic renal cell carcinoma: the RAINBOW analysis. Ann Oncol. (2015) ;26: (10):2107–13. |
[26] | Jonasch E , Slack RS , Geynisman DM , Hasanov E , Milowsky MI , Rathmell WK , et al. Phase II Study of Two Weeks on, One Week off Sunitinib Scheduling in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. (2018) ;36: (16):1588–93. |
[27] | Hozo SP , Djulbegovic B , Hozo I . Estimating the mean and variance from the median, range, and the size of a sample. J BMC Medical Research Methodology. (2005) ;5: (1):13. |
[28] | Iwamoto K , Ishihara H , Takagi T , Kondo T , Yoshida K , Iizuka J , et al. Evaluation of relative dose intensity during the early phase of first-line sunitinib treatment using a 2-week-on/1-week-off regimen for metastatic renal cell carcinoma. Med Oncol. (2018) ;35: (6):78. |
[29] | Lee JL , Kim MK , Park I , Ahn JH , Lee DH , Ryoo HM , et al. RandomizEd phase II trial of Sunitinib four weeks on and two weeks off versus Two weeks on and One week off in metastatic clear-cell type REnal cell carcinoma: RESTORE trial. Ann Oncol. (2015) ;26: (11):2300–5. |
[30] | Makino K , Yoda K , Tomoishi J , Kume H . Efficacy and tolerability of a low-dose, 2-week administration of sunitinib followed by a week rest (2/1 schedule) for metastatic renal cell carcinoma: a single center experience of six cases. BMC Res Notes. (2014) ;7: :872. |
[31] | Miyake H , Harada K , Miyazaki A , Fujisawa M . Improved health-related quality of life of patients with metastatic renal cell carcinoma treated with a 2 weeks on and 1 week off schedule of sunitinib. Med Oncol. (2015) ;32: (3):78. |
[32] | Miyake H , Matsushita Y , Watanabe H , Tamura K , Suzuki T , Motoyama D , et al. Significance of introduction of alternative dosing schedule for sunitinib during first-line treatment of patients with metastatic renal cell carcinoma. Med Oncol. (2018) ;35: (10):133. |
[33] | Ohzeki T , Fukasawa S , Komaru A , Namekawa T , Sato Y , Takagi K , et al. Efficacy of traditional and alternative sunitinib treatment schedules in Japanese patients with metastatic renal cell carcinoma. Int J Urol. (2014) ;21: (10):1065–8. |
[34] | Pan X , Huang H , Huang Y , Liu B , Cui X , Gan S , et al. Sunitinib dosing schedule 2/1 improves tolerability, efficacy, and health-related quality of life in Chinese patients with metastatic renal cell carcinoma. Urol Oncol. (2015) ;33: (6):268 e9–15. |
[35] | Bjarnason GA . Can individualized sunitinib dose and schedule changes optimize outcomes for kidney cancer patients? Can Urol Assoc J. (2016) ;10: (11-12Suppl7):S252-S5. |
[36] | Boegemann M , Hubbe M , Thomaidou D , Blackburn S , Bent-Ennakhil N , Wood R , et al. Sunitinib Treatment Modification in First-Line Metastatic Renal Cell Carcinoma: Analysis of the STAR-TOR Registry. Anticancer Res. (2018) ;38: (11):6413–22. |
[37] | Ezz El Din M . Sunitinib 4/2 Versus 2/1 Schedule for Patients With Metastatic Renal Cell Carcinoma: Tertiary Care Hospital Experience. Clin Genitourin Cancer. (2017) ;15: (3):e455–e62. |
[38] | Neri B , Vannini A , Brugia M , Muto A , Rangan S , Rediti M , et al. Biweekly sunitinib regimen reduces toxicity and retains efficacy in metastatic renal cell carcinoma: a single-center experience with 31 patients. Int J Urol. (2013) ;20: (5):478–83. |
[39] | CTCAE. Common Terminology Criteria for Adverse Events (CTCAE) 2017 [Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf |
[40] | Coy HJ , Douek ML , Ruchalski K , Kim HJ , Gutierrez A , Patel M , et al. Components of Radiologic Progressive Disease Defined by RECIST 1.1 in Patients with Metastatic Clear Cell Renal Cell Carcinoma. Radiology. (2019) ;292: (1):103–9. |
[41] | Najjar YG , Mittal K , Elson P , Wood L , Garcia JA , Dreicer R , et al. A 2 weeks on and 1 week off schedule of sunitinib is associated with decreased toxicity in metastatic renal cell carcinoma. Eur J Cancer. (2014) ;50: (6):1084–9. |
[42] | Kollmannsberger C , Soulieres D , Wong R , Scalera A , Gaspo R , Bjarnason G . Sunitinib therapy for metastatic renal cell carcinoma: recommendations for management of side effects. Can Urol Assoc J. (2007) ;1: (2 Suppl):S41–54. |
[43] | Motzer RJ , Hutson TE , Tomczak P , Michaelson MD , Bukowski RM , Rixe O , et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. (2007) ;356: (2):115–24. |
[44] | Kim HS , Hong MH , Kim K , Shin SJ , Ahn JB , Jeung HC , et al. Sunitinib for Asian patients with advanced renal cell carcinoma: a comparable efficacy with different toxicity profiles. Oncology. (2011) ;80: (5-6):395–405. |
[45] | Mir MH , Changal KH , Aziz SA , Bhat GM , Lone AR . Sunitinib in metastatic renal cell carcinoma (mRCC): a developing country experience. Do our patients behave differently than the Western patients? Int Urol Nephrol. (2016) ;48: (11):1811–6. |
[46] | Sehdev S . Sunitinib toxicity management - a practical approach. Can Urol Assoc J. (2016) ;10: (11-12Suppl7):S248–S51. |
[47] | Akaza H , Naito S , Ueno N , Aoki K , Houzawa H , Pitman Lowenthal S , et al. Real-world use of sunitinib in Japanese patients with advanced renal cell carcinoma: efficacy, safety and biomarker analyses in consecutive patients. J Clin Oncol. (2015) ;45: (6):576–83. |
[48] | Garcia-Donas J , Esteban E , Leandro-Garcia LJ , Castellano DE , Gonzalez del Alba A , Climent MA , et al. Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: a multi-centre, observational, prospective study. Lancet Oncol. (2011) ;12: (12):1143–50. |
[49] | Teo YL , Wee HL , Chue XP , Chau NM , Tan MH , Kanesvaran R , et al. Effect of the CYP3A5 and ABCB1 genotype on exposure, clinical response and manifestation of toxicities from sunitinib in Asian patients. Pharmacogenomics J. (2016) ;16: (1):47–53. |
[50] | Diekstra MH , Klumpen HJ , Lolkema MP , Yu H , Kloth JS , Gelderblom H , et al. Association analysis of genetic polymorphisms in genes related to sunitinib pharmacokinetics, specifically clearance of sunitinib and SU2. Clin Pharmacol Ther. (2014) ;96: (1):81–9. |
[51] | Zhou A . Management of sunitinib adverse events in renal cell carcinoma patients: the Asian experience. Asia Pac J Clin Oncol. (2012) ;8: :132–44. |
[52] | Guo J , Jin J , Oya M , Uemura H , Takahashi S , Tatsugami K , et al. Safety of pazopanib and sunitinib in treatment-naive patients with metastatic renal cell carcinoma: Asian versus non-Asian subgroup analysis of the COMPARZ trial. J Hematol Oncol. (2018) ;11: (1):69. |
[53] | Houk BE , Bello CL , Kang D , Amantea M . A population pharmacokinetic meta-analysis of sunitinib malate (SU8) and its primary metabolite (SU2) in healthy volunteers and oncology patients. Clin Cancer Res. (2009) ;15: (7):2497–506. |
[54] | Narjoz C , Cessot A , Thomas-Schoemann A , Golmard JL , Huillard O , Boudou-Rouquette P , et al. Role of the lean body mass and of pharmacogenetic variants on the pharmacokinetics and pharmacodynamics of sunitinib in cancer patients. Invest New Drugs. (2015) ;33: (1):257–68. |
[55] | Lear SA , Kohli S , Bondy GP , Tchernof A , Sniderman AD . Ethnic variation in fat and lean body mass and the association with insulin resistance. J Clin Endocrinol Metab. (2009) ;94: (12):4696–702. |
[56] | Uemura H , Shinohara N , Yuasa T , Tomita Y , Fujimoto H , Niwakawa M , et al. A phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into the treatment, efficacy and safety. Jpn J Clin Oncol. (2010) ;40: (3):194–202. |
[57] | Mizuno T , Terada T , Kamba T , Fukudo M , Katsura T , Nakamura E , et al. ABCG2 421C>A polymorphism and high exposure of sunitinib in a patient with renal cell carcinoma. Ann Oncol. (2010) ;21: (6):1382–3. |
[58] | Kitada M . Genetic polymorphism of cytochrome P450 enzymes in Asian populations: focus on CYP2D6. Int J Clin Pharmacol Res. (2003) ;23: (1):31–5. |
[59] | Bernard S , Neville KA , Nguyen AT , Flockhart DA . Interethnic differences in genetic polymorphisms of CYP2D6 in the U.S. population: clinical implications. Oncologist. (2006) ;11: (2):126–35. |
[60] | Phan VH , Moore MM , McLachlan AJ , Piquette-Miller M , Xu H , Clarke SJ . Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opin Drug Metab Toxicol. (2009) ;5: (3):243–57. |
[61] | Lee SH , Bang YJ , Mainwaring P , Ng C , Chang JW , Kwong P , et al. Sunitinib in metastatic renal cell carcinoma: an ethnic Asian subpopulation analysis for safety and efficacy. Asia Pac J Clin Oncol. (2014) ;10: (3):237–45. |
[62] | Abogunrin S , Ashaye A , Fahrbach K , Cappelleri J , Sandin R , Ramaswamy K . Sunitinib Dosing Schedules in The Management of Metastatic Renal Cell Carcinoma: A Meta-Analysis. Value Health. (2016) ;19: (7):A711. |
[63] | Abogunrin S , Ashaye AO , Cappelleri JC , Clair AG , Fahrbach K , Ramaswamy K , et al. Safety and effectiveness of classical and alternative sunitinib dosing schedules for metastatic renal cell carcinoma: a meta-analysis. Future Oncol. (2019) ;15: (18):2175–90. |
[64] | Donskov F , Michaelson MD , Puzanov I , Davis MP , Bjarnason GA , Motzer RJ , et al. Sunitinib-associated hypertensionand neutropenia as efficacy biomarkers in metastatic renal cell carcinoma patients. Br J Cancer. (2015) ;113: (11):1571–80. |
[65] | Rautiola J , Donskov F , Peltola K , Joensuu H , Bono P . Sunitinib-induced hypertension, neutropaenia and thrombocytopaeniaas predictors of good prognosis in patients with metastatic renalcell carcinoma. BJU Int. (2016) ;117: (1):110–7. |
[66] | Maraz A , Cserhati A , Uhercsak G , Szilagyi E , Varga Z , Kahan Z . [Therapeutic significance of sunitinib-induced “off-target” side effects]. Magy Onkol. (2014) ;58: (3):167–72. |
[67] | Nagyivanyi K , Budai B , Biro K , Gyergyay F , Noszek L , Kuronya Z , et al. Synergistic Survival: A New Phenomenon Connected to Adverse Events of First-Line Sunitinib Treatment in Advanced Renal Cell Carcinoma. Clin Genitourin Cancer. (2016) ;14: (4):314–22. |
[68] | Schmidinger M , Vogl UM , Bojic M , Lamm W , Heinzl H , Haitel A , et al. Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer. (2011) ;117: (3):534–44. |
[69] | Nagata M , Ishiwata Y , Takahashi Y , Takahashi H , Saito K , Fujii Y , et al. Pharmacokinetic-pharmacodynamic analysisof sunitinib-induced thrombocytopenia in Japanese patients with renal cell carcinoma. Biol Pharm Bull. (2015) ;38: (3):402–10. |



