Ultrasound features of abdominal thrombosis in COVID 19 patients
Abstract
Purpose:
Abdominal thromboses are a poorly characterized thrombotic complication of COVID-19. The aim of this paper is to report multimodality ultrasound imaging findings of the abdomen in evaluation of thrombotic lesions in hospitalized patients with COVID-19.
Patients & Methods:
In this retrospective observational study, patients admitted to a single University Hospital from April 1, 2020 to April 30, 2022, who tested positive for COVID-19 and developed acute abdominal pain over the course of hospitalization were included. Abdominal ultrasound imaging studies performed in these patients were reviewed, including B mode ultrasound (BMUS), color-coded Doppler ultrasound (CCDS) and contrast enhanced ultrasound (CEUS). Thromboembolic findings on contrast enhanced computed tomography (CTA) were also recorded.
Results:
Finally, 13 cases of abdominal thrombosis in 226 with COVID 19 infections were included (mean age, 56.69±8.97 years; 10 men, 3 women). Thromboembolic events included: iliac thrombosis (n = 4), portal venous (PV) thrombosis (n = 3), superior mesenteric vein (VMS) thrombosis (n = 2), inferior vena cava (IVC) thrombosis (n = 5) and inferior mesenteric vein (VMI) thrombosis (n = 1). In all cases of abdominal thrombosis, during high resolution BMUS scan, intra-luminary hypoechogenic appositional thrombi could be detected. Meanwhile blood flow with reduced speed less than 20 cm/s could be observed by CCDS. High arterial flow speed was a sign of collateral flow changes with diffuse venous dilatation. On CEUS, changes of the microcirculation of the liver, spleen, kidneys or small bowel by infarctions or micro-emboli could be detected. In 3 cases of PV thrombosis and in 2 cases of IVC thrombosis, catheter interventions were successful performed for recanalization without relevant lumen reduction afterwards. In other cases, without interventional procedure, partial recanalization happened with venous flow speed over 15 cm/s and lumen reduction more than 50%.
Conclusions:
Our study highlights those thromboembolic complications can be seen in hospitalized patients with COVID-19. Multimodality ultrasound examinations is helpful for early and accurate diagnosis of these complications.
1Introduction
The World Health Organization (WHO) recognized the coronavirus disease-19 (COVID-19) as a worldwide pandemic on March 11, 2020 [1]. The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), presents an ongoing global threat [2]. Although patients with COVID-19 commonly present with fever, constitutional symptoms, and respiratory symptoms. Thromboembolic complications have been shown to be an important cause of morbidity and mortality in COVID-19 patients both in the general ward and in the ICU, even in patients receiving therapeutic anticoagulation. Gastrointestinal symptoms including diarrhea, vomiting, and abdominal pain were seen in 12–50% of patients with COVID-19 [3].
Thrombotic events are driven by a severe proinflammatory response to COVID-19, generating fibrin deposition and potentially disseminated intravascular coagulation, which confers a much poorer prognosis for patients. In the intensive care setting with critically ill patients, thrombotic complications related to COVID-19 are reported in 25–31% of patients [4–8]. Pulmonary embolism, myocardial infarction and deep vein thrombosis remain as the most common thrombotic events associated with COVID-19. However, development of abdominal thromboses related to COVID-19 infection is sparsely reported in the literature.
With the increasing recognition of gastrointestinal manifestation of COVID-19, various abdominal imaging findings are increasingly being noted. Many studies have evaluated abdominal imaging findings in COVID-19 patients that helped to understand the disease course and potential complications. However, to our knowledge, limited data are available about the ultrasound features of abdominal thrombosis in COVID-19 patients.
Therefore, our purpose was to describe multimodality ultrasound imaging findings of the abdomen in evaluation of thrombotic lesions in hospitalized patients with COVID-19.
2Patients and Methods
2.1Patients and study design
This was a retrospective observational study performed at University Hospital Regensburg. Institutional review board approval was obtained and informed consent was waived. All aspects of the study were performed in accordance with the Declaration of Helsinki.
The inclusion criteria were: 1) adult patients (>18 years) who were admitted to our hospital between April 1, 2020 to April 30, 2022; 2) Patients who tested positive for SARS-CoV-2; 3) Patients who developed acute abdominal pain or decreasing hemoglobin levels (fall in hemoglobin level of 2 g/dL or more) during hospitalization; 4) Patients who underwent multimodality ultrasound and CT examinations of the abdomen in our department.
The exclusion criteria were: 1) Penetration for ultrasound more than 25 cm; 2) Patients with critical cardiac outcome, acute infarctions or stroke; 3) Patients with colon interposition by the liver lobe; 4) Patients with arterial hypertension and cardiac failure, who could not accept CEUS examinations.
Demographics, clinical data (presence of acute abdominal pain, etc.), hospital admission, ultrasound and CT imaging findings and outcome of the patients during hospitalization were collected from electronic medical records.
Laboratory data within 3 days before ultrasound imaging were considered. Laboratory data collected included D-dimer level (ng/mL, reference range less than 500 ng/mL) prothrombin time (PT, s, reference range 13.2–16.4 s), and platelets (×103/μL, reference range 150–400×103/μL).
2.2Image acquisition
All examinations were carried out by an investigator with many years of experience in ultrasound with more than 3000 examinations over more than 20 years. The examinations were always carried out under maximum protective measures with also ICU patients being included. First, focussed assessment with sonography (FAST) was performed and documented for free fluid. Then pleural examinations were done for effusions and the lungs for extent of consolidations.
All abdominal examinations were carried out with high-end ultrasound devices using multi-frequency convex probes from 1 to 6 MHz. Liver vascularization was performed using color-coded Doppler ultrasound (CCDS) for the portal vein, hepatic veins, and hepatic artery (HA). The volume status of the inferior vena cava (IVC) was documented. If thrombosis was suspected, additional examinations of the splenic vein, the superior mesenteric vein, the venous confluency and the renal veins were carried out on both sides. For this purpose, the flow parameters were adapted to low flow, pulse repeated frequency (PRF) or scale < 1000 KHz, wall filter < 100 KHz, color gain and Doppler gain high to the limits of artefacts caused by pulsations. The criteria for thrombosis were a lack of full compressibility of an early distended vein with missing breathing modulations in BMUS. In the CCDS, the best possible sound angles at 30 to 60 degrees were evaluated as evidence of thrombosis in various sound positions.
CEUS was performed in bolus technique as intravenous administration of 1–2.4 ml sulfur hexafluoride microbubbles contrast agent SonoVue® (Bracco SpA, Milan, Italy) with 10 ml NaCL (saline solution) via cubital vein or by catheters of the central venous system. Possible history of contrast intolerance was considered a contraindication. Impaired renal function or renal insufficiency or changes in thyroid function were not considered contraindications according to the EFSUMB guidelines. The examinations were performed dynamically with the contrast harmonic low MI technique, mechanical index less than 0.16, depth-adapted with the modalities general (GEN), resolution (RES) or penetration (PEN) and digitally documented.
The CEUS examinations of the entire liver performed in sweep technique included continuous acquisition of the arterial phase 10 to 15 s after bolus administration to the portal venous phase over 1 minute, in case of vascular changes with optimal acquisition of the hepatic hilus to be able to detect possible a portal venous thrombosis. Then the venous confluence and splenic vein were examined. During the late venous phase after 1 up to 2 minutes, spleen, kidney and liver parenchymal structures were examined for reduced contrast enhancement like infarctions or peripheral embolic lesions.
Short cine loops of the portal venous (PV), mesenteric or renal venous system were stored over 5 to 10 s up to a late phase of 5 to 6 minutes, supplemented by single images that could be stored during the cine sequences specifically related to findings. The images were saved in DICOM format and automatically sent to PACS for independent analysis.
For contrast enhanced computed tomography (CTA), multislice CT examinations in arterial (30–40 s), late portal-venous contrast phase (60–90 s) and if necessary late venous phase after 2 up to 3 minutes would be the reference with 80 to 120 ml iodine-containing contrast medium (Accupaque 350, GE Healthcare Buchler GmbH & Co.KG, Germany) in 5 mm reconstructions axial and coronary, considering possible contraindications.
2.3Image analysis
All ultrasound studies of the abdomen were independently reviewed, in a clinical setting by one abdominal radiologists (10 and 15 years of experience) who was blinded to clinical data.
If thrombosis was present, the location of thromboembolism whether arterial or venous and solid organ or bowel infarction were reviewed.
2.4Statistical analysis
Descriptive statistical analysis is presented as counts and percentages for categorical variables and as mean and standard deviation, or median and range for continuous variables. Patients were divided into two groups, group 1 included patients with thromboembolic complications, and group 2 included patients with no acute findings on ultrasound. Proportions for categorial variables were compared using Chi-Square test. P values for categorical variables were calculated with the Mann Whitney U test. P value less than 0.05 represented a significant difference.
3Results
3.1Demographic and clinical data of the study cohort
A total of 226 adult patients who tested positive for COVID 19 were admitted to University Hospital during the study period. Finally, 13 cases of abdominal thrombosis in 226. Patients with COVID 19 infections were included (mean age, 56.69±8.97 years; 10 men, 3 women). Patients’ data were summarized in Table 1.
Table 1
Demographic data of 13 patients included
| Case | Age | Gender | ilical vein | portal vein (PV) | superior mesenteric vein (VMS) | inferior vena cava (IVC) | inferior mesenteric vein (VMI) | ECMO | ARDS |
| 1 | 63 | m | 0 | 1 | 0 | 1 | 0 | Yes | Yes |
| 2 | 50 | m | 1 | 0 | 0 | 0 | 0 | Yes | Yes |
| 3 | 63 | m | 0 | 1 | 0 | 0 | 0 | No | No |
| 4 | 61 | m | 0 | 1 | 0 | 0 | 1 | No | No |
| 5 | 56 | m | 0 | 0 | 0 | 1 | 0 | Yes | Yes |
| 6 | 73 | m | 0 | 0 | 0 | 0 | 0 | No | No |
| 7 | 44 | w | 0 | 1 | 1 | 0 | 0 | No | No |
| 8 | 50 | m | 0 | 0 | 1 | 0 | 0 | No | No |
| 9 | 60 | w | 1 | 0 | 0 | 1 | 0 | No | Yes |
| 10 | 56 | m | 1 | 0 | 0 | 0 | 0 | No | No |
| 11 | 58 | m | 1 | 0 | 0 | 0 | 0 | No | No |
| 12 | 38 | m | 0 | 0 | 0 | 1 | 0 | Yes | Yes |
| 13 | 65 | w | 0 | 0 | 0 | 1 | 0 | Yes | Yes |
| Mean | 56.69 | ||||||||
| SD | 8.97 |
Localization of the abdominal venous thrombosis; ECMO, extracorporeal membrane oxygenation; ARDS, adult respiratory distress syndrome; IVC, inferior vena cava; VMS, superior mesenteric vein.
Thromboembolic events including: iliac thrombosis (n = 4), PV thrombosis (n = 3), superior mesenteric vein (VMS) thrombosis (n = 2), IVC thrombosis (n = 5) and inferior mesenteric vein (VMI) thrombosis (n = 1).
Adult respiratory distress syndrome (ARDS) was observed in 6 patients (46.2 %, 6/13). Extracorporeal membrane oxygenation (ECMO) was required in 5 patients (38.5 %, 5/13).
In the 3 cases of PV thrombosis and 2 cases of IVC thrombosis, catheter interventions were successful performed for recanalization without relevant lumen reduction afterwards. For those cases without interventional procedure, partial recanalization happened with venous flow speed over 15 cm/s and lumen reduction more than 50%.
3.2Ultrasound Imaging findings
Various ultrasound findings could be detected in our patients’ cohort. In all cases of abdominal thrombosis, during high resolution BMUS scan, intra-luminary hypoechogenic appositional thrombi could be detected [Fig. 1] [Fig. 2]. Meanwhile blood flow with reduced speed less than 20 cm/s could be observed by CCDS [Fig. 3]. High arterial flow speed was a sign of collateral flow changes with diffuse venous dilatation [Fig. 4]. On CEUS, changes of the microcirculation of the liver, spleen, kidneys or small bowel by infarctions or micro-emboli could be detected [Fig. 5].
Fig. 1
A case of abdominal venous thrombosis happened during severe COVID-19 infection. B mode ultrasound (BMUS) scan shows hypoechoic thrombosis of the inferior vena cava (a, arrow). Color-coded Doppler ultrasound (CCDS) shows flow separation into the lumen of the portal vein (b, arrow).
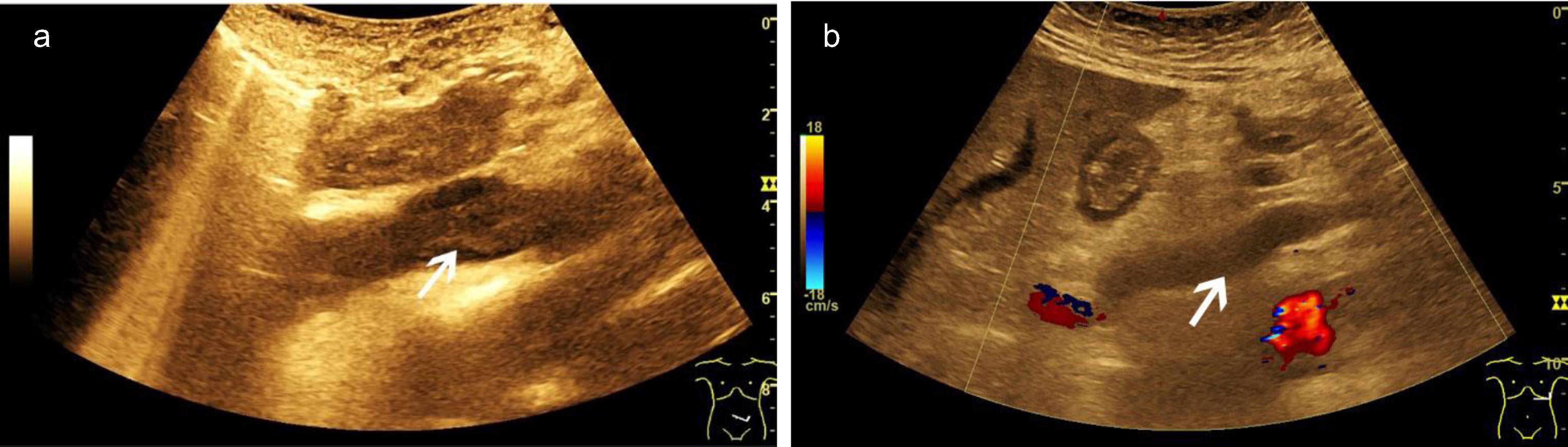
Fig. 2
Optimizing ultrasound examinations of the portal vein. B mode ultrasound (a, left) and B flow scan shows blood flow of the portal vein (a, right). Color-coded Doppler ultrasound (CCDS) (b, left) and Power Doppler show flow in the lumen of the portal vein (b, right). Hemodynamic assessment of the hepatic artery with CCDS including high resolution (HR) and Glazing Flow (c).
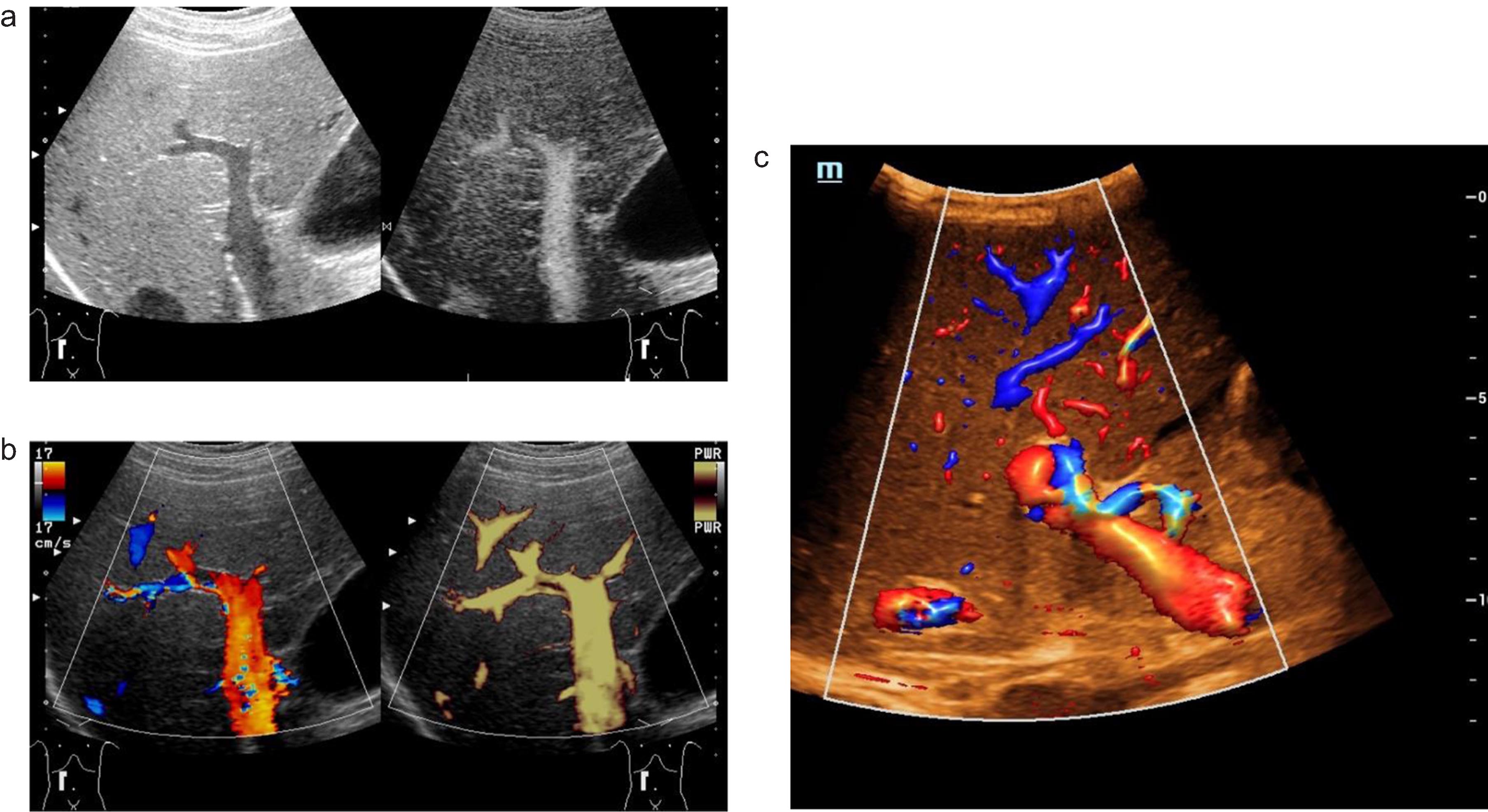
Fig. 3
A case of abdominal venous thrombosis happened after severe COVID-19 infection. Color-coded Doppler shows hypoechoic thrombosis of the superior mesenteric vein (a, arrow). Color-coded Doppler ultrasound (CCDS) shows flow separation into the lumen of the portal vein after thrombosis (b, arrow). Color-coded Doppler (CCDS) flow changes after thrombosis of the portal vein (c).
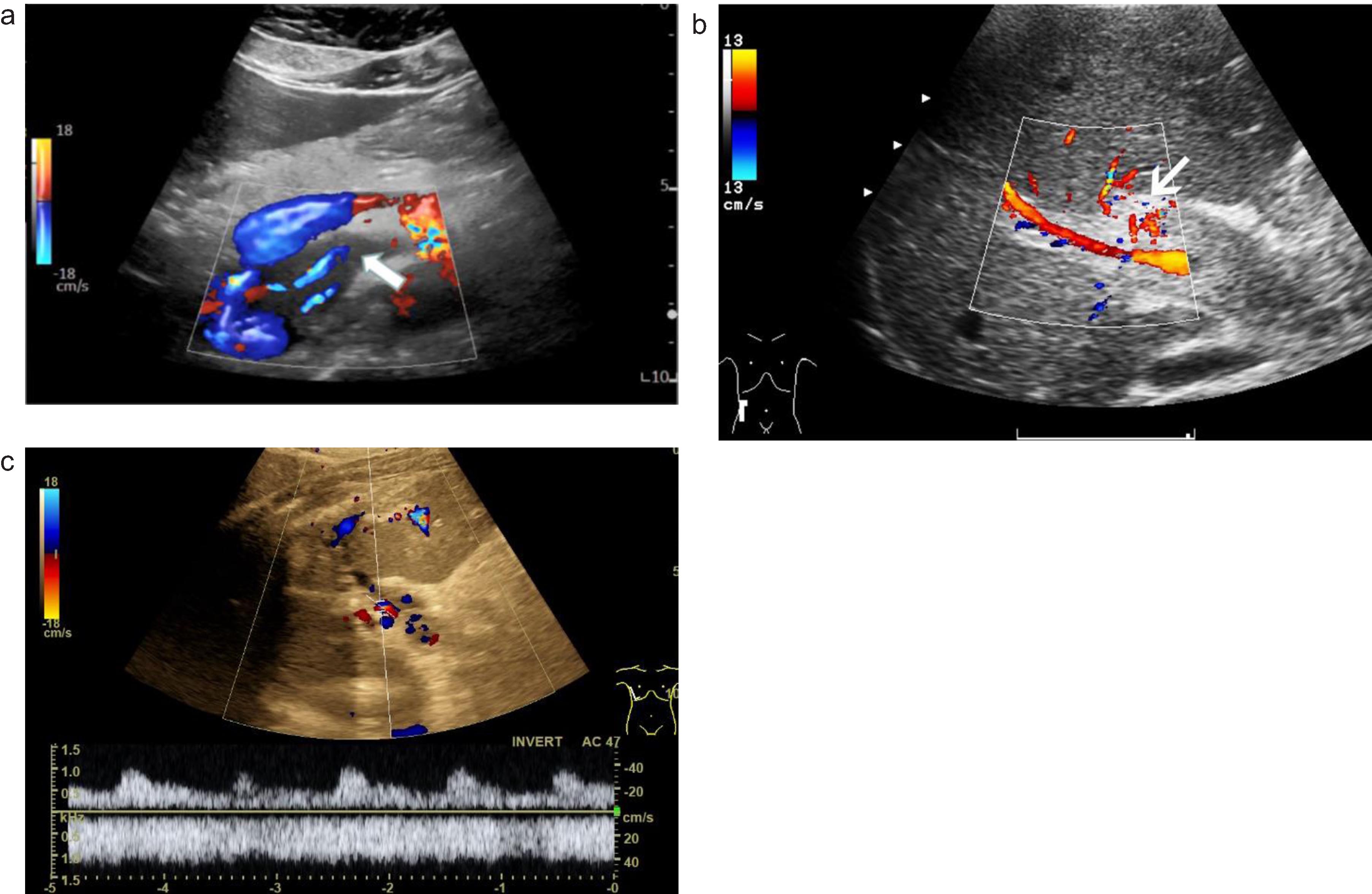
Fig. 4
Aneurysm of the portal vein during severe COVID-19 infection. B mode ultrasound scan (a), B-flow (b), color-coded Doppler ultrasound (CCDS, c) and power Doppler (d) show enlargement of vascular lumen with blood flow.
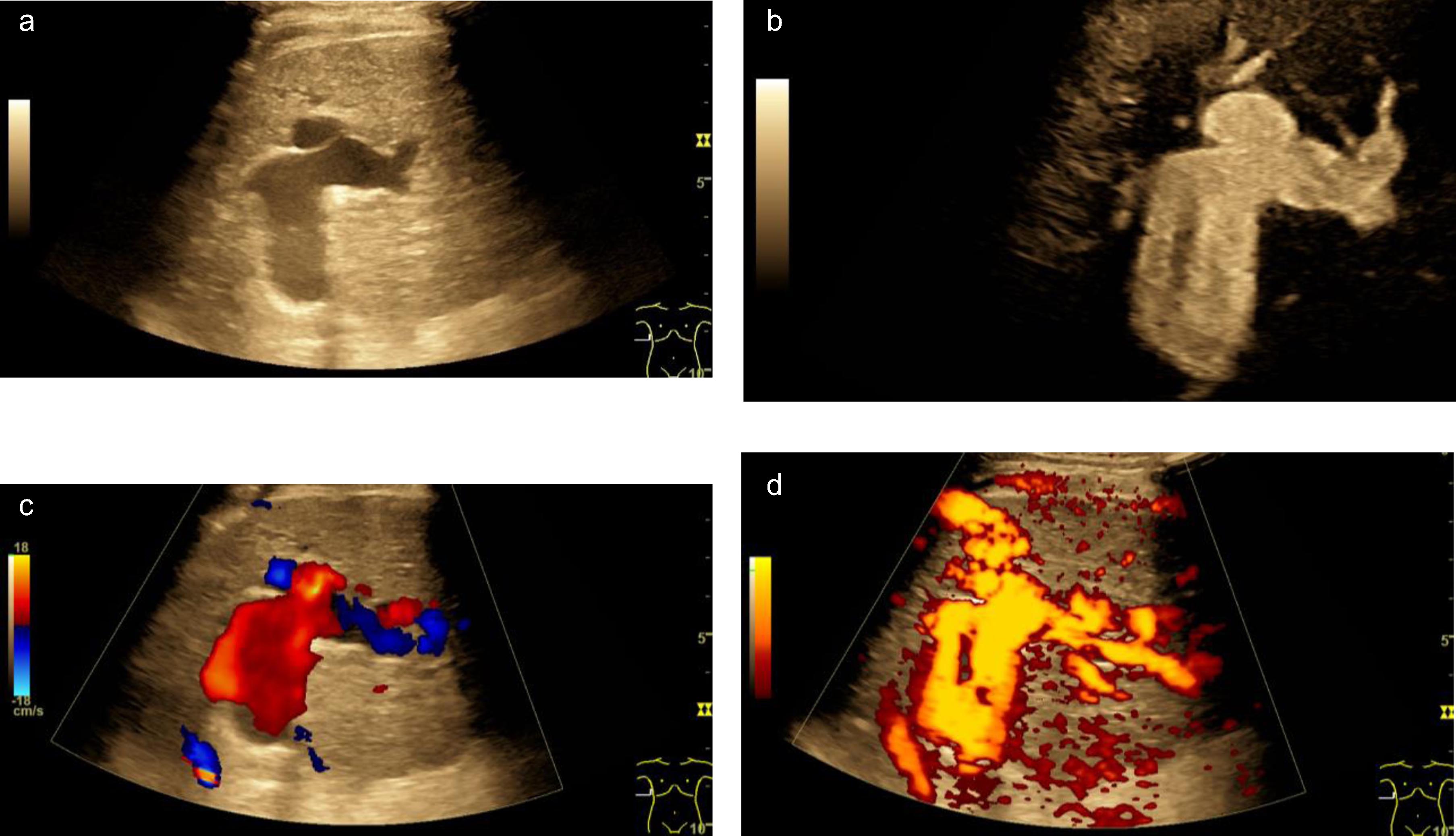
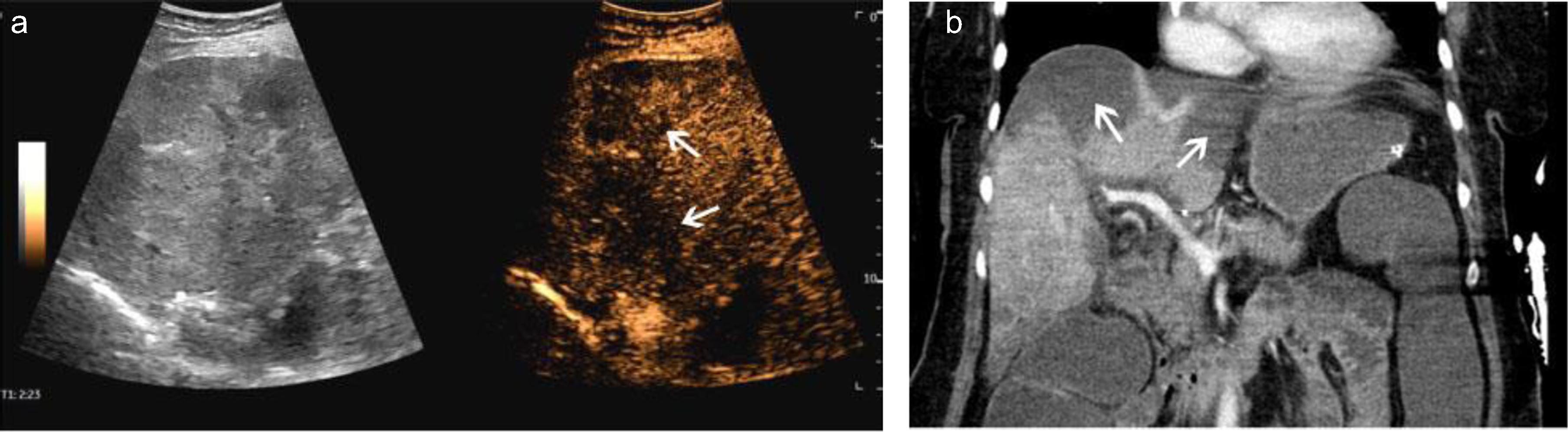
Fig. 5
A case of scan Covid 19 infection. After thrombosis of the portal vein and liver veins, CEUS showed scattered multiple hypoenhanced lesions with necrosis (a, arrow). CT showed the same low-density lesions in the liver (b, arrow).
4Discussions
There is growing evidence that COVID-19 is associated with an increased risk of coagulopathy [9]. The immune dysregulation characteristic of severe COVID-19 infection cause massive inflammation and cytokine release, which increase the production of clotting factors leading to an increased risk of arterial and venous thrombosis [10]. In addition, hypoxia, endothelial dysfunction, immobility, the use of mechanical ventilation and extracorporeal circuits all contribute to the increased risk thrombosis [11, 12]. The association of COVID-19 with vascular thrombosis is increasingly being recognized. The effect of SARS-CoV-2 on different organs other than the lungs is not yet fully understood, several studies reported that the coagulation pathway may be adversely affected resulting in acute venous and arterial thrombotic events, as venous thromboembolism, acute stroke, acute myocardial infarction, and clotting of the ECMO (extracorporeal membrane oxygenation) circuit [13, 14]. The occurrence of macrovascular thrombosis in COVID-19 has been reported in a few studies [15]. Both small and large vessel thrombosis has been reported in patients with COVID-19 [16, 17]. Published reports have mentioned VSM, PV, aortic, hepatic artery, splanchnic vein, portomensenteric and ovarian vein thrombosis as a result of the hypercoagulable state due COVID-19 [2, 16, 18–26]. The digestive manifestations of a SARS-CoV-2 infection are varied and nonspecific. The appearance of portal thrombosis in these patients is very rare [27]. Klok, et al. reported an incidence of 31% venous thrombosis and thromboembolism in ICU patients [5]. In our study 13 patients had abdominal thrombosis, 5 of them required extracorporeal membrane oxygenation (ECMO).
On various imaging methods, the presence of a filling defect within a vessel, with or without occlusion, is the characteristic finding of venous thrombosis [3, 10]. CT angiography/venography helps for adequate evaluation of thrombus extension into central veins. To date, only a few CEUS imaging findings of abdominal thrombosis related to COVID-19 have been reported [28]. For optimizing flow detection of low venous flow combination of HR-Flow and Glazing flow could be helpful [28]. Many venous thromboembolic events have been documented in association with COVID-19, whereas arterial thrombosis has been described in relatively fewer cases and could possibly be underestimated [29]. COVID-19 associated arterial thrombosis has been frequently observed in non-atherosclerotic vessels on CT imaging, suggesting that patients with a severe inflammatory reaction as indicated by elevation of D-dimer fibrin degradation products and platelet count coupled with decreased antithrombin levels are at greater risk for thrombo embolic comlications.
Visceral organ infarction should be considered in the differential diagnosis among COVID-19 patients presenting with unexplained abdominal pain [30]. Solid organ infarction in COVID-19 could either be incidentally detected on CTA imaging or could manifest with abdominal pain or features of organ dysfunction. Clinicians need to be aware of the thrombotic manifestations of COVID-19 and radiologists should monitor patients for thrombosis to facilitate early diagnosis. In cases of portal vein thrombosis, early anticoagulation within the first week and its maintenance for 6 months promotes recanalization in > 60 % of patients, and in < 20 % of patients if this therapy is initiated after the first week [26].
Few reports of COVID-19 associated renal infarction have emerged, some of these manifesting with acute kidney injury. CECT including the arterial and venous phases is the study of choice for the recognition of kidney infarction. It is important to keep in mind that patients with COVID-19 may develop thrombosis of various vascular systems and there could be concomitant involvement of one or more venous or arterial vascular beds. Therefore, if evidence of thrombosis is detected, careful assessment of the entire imaged vascular system as well as correlating organ or structure is essential.
Previously ultrasound revealed arterial aneurysms were reported in young patients [26]. In our cohort, a case of aneurysm of the PV happened during severe COVID-19 infection. BMUS, B-flow, CCDS and power Doppler could clearly show enlargement of vascular lumen filled with blood flow. Therefore, the multimodality ultrasound features are helpful for diagnosis of aneurysm and suggest the involvement of the COVID-19 infection.
There are some limitations of this study. First, this was a single-center retrospective study, further multicenter studies are recommended. Second, the pathologic correlation with imaging abnormalities was not available for our patients.
5Conclusions
Thrombotic events are well-recognized complications of COVID-19. Our study highlights those thromboembolic complications can be seen in hospitalized patients with COVID-19. Multimodality ultrasound examinations are helpful for early and accurate diagnosis of these complications.
Grant Support
Supported by National Natural Science Foundation of China (Grant No. 82071942). Sponsored by Shanghai Pujiang Program (Grant No. 2020PJD008). Supported by Clinical Research Plan of SHDC (Grant No. SHDC2020CR1031B, SHDC2020CR4060).
References
[1] | Behzad S , Aghaghazvini L , Radmard AR , Gholamrezanezhad A . Extrapulmonary manifestations of COVID-19 Radiologic and clinical overview. Clin Imaging. (2020) ;66: :35–41. |
[2] | Bhayana R , Som A , Li MD , Carey DE , Anderson MA , Blake MA , Catalano O , et al. Abdominal Imaging Findings in COVID-19 Preliminary Observations. Radiology. (2020) ;297: :E207–E215. |
[3] | Abdelmohsen MA , Alkandari BM , Abdel Razek AAK , Tobar AM , Gupta VK , Elsebaie N . Abdominal Computed Tomography Angiography and Venography in Evaluation of Hemorrhagic and Thrombotic lesions in Hospitalized COVID-19 patients. Clin Imaging. (2021) ;79: :12–19. |
[4] | Cui S , Chen S , Li X , Liu S , Wang F . Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. (2020) ;18: :1421–4. |
[5] | Klok FA , Kruip M , van der Meer NJM , Arbous MS , Gommers D , Kant KM , Kaptein FHJ , et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. (2020) ;191: :145–7. |
[6] | Inal J . Complement-mediated Extracellular Vesicle release as a measure of endothelial dysfunction and prognostic marker for COVID-19 in peripheral blood - Letter to the Editor. Clin Hemorheol Microcirc. (2020) ;75: :383–6. |
[7] | Alzoughool F , Alanagreh L , Abumweis S , Atoum M . Cerebrovascular comorbidity, high blood levels of C-reactive protein and D-dimer are associated with disease outcomes in COVID-19 patients. Clin Hemorheol Microcirc. (2021) ;77: :311–22. |
[8] | Nugroho J , Wardhana A , Mulia EP , Maghfirah I , Rachmi DA , A’Yun M Q , Septianda I . Elevated fibrinogen and fibrin degradation product are associated with poor outcome in COVID-19 patients: A meta-analysis. Clin Hemorheol Microcirc. (2021) ;77: :221–31. |
[9] | Silva Andrade B , Siqueira S , de Assis Soares WR , de Souza Rangel F , Santos NO , Dos Santos Freitas A , Ribeiro da Silveira P , et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses. (2021) ;13. |
[10] | Revzin MV , Raza S , Warshawsky R , D’Agostino C , Srivastava NC , Bader AS , Malhotra A , et al. Multisystem Imaging Manifestations of COVID-19, Part Viral Pathogenesis and Pulmonary and Vascular System Complications. Radiographics. (2020) ;40: :1574–99. |
[11] | Hafezi B , Chan L , Knapp JP , Karimi N , Alizadeh K , Mehrani Y , Bridle BW , et al. Cytokine Storm Syndrome in SARS-CoV-2 Infections: A Functional Role of Mast Cells. Cells. (2021) ;10. |
[12] | Connors JM , Levy JH . COVID-19 and its implications for thrombosis and anticoagulation. Blood. (2020) ;135: :2033–40. |
[13] | Oudkerk M , Buller HR , Kuijpers D , van Es N , Oudkerk SF , McLoud T , Gommers D , et al. Diagnosis, Prevention, and Treatment of Thromboembolic Complications in COVID-19 Report of the National Institute for Public Health of the Netherlands. Radiology. (2020) ;297: :E216–E222. |
[14] | O’Shea A , Parakh A , Hedgire S , Lee SI . Multisystem Assessment of the Imaging Manifestations of Coagulopathy in Hospitalized Patients With Coronavirus Disease (COVID-19). AJR Am J Roentgenol. (2021) ;216: :1088–98. |
[15] | Avila J , Long B , Holladay D , Gottlieb M . Thrombotic complications of COVID-19. Am J Emerg Med. (2021) ;39: :213–8. |
[16] | Abeysekera KW , Karteszi H , Clark A , Gordon FH . Spontaneous portomesenteric thrombosis in a non-cirrhotic patient with SARS-CoV-2 infection. BMJ Case Rep. (2020) ;13. |
[17] | Amaravathi U , Balamurugan N , Muthu Pillai V , Ayyan SM . Superior Mesenteric Arterial and Venous Thrombosis in COVID-19. J Emerg Med. (2021) ;60: :e103–e107. |
[18] | Hanif M , Ahmad Z , Khan AW , Naz S , Sundas F . COVID-19-Induced Mesenteric Thrombosis. Cureus. (2021) ;13: :e12953. |
[19] | Sinz S , Glaser-Gallion F , Steffen T . Portal vein thrombosis in COVID-19 infection. Surg Case Rep. (2021) ;7: :87. |
[20] | Sharma N , Shukla R , Kumar K , Arora S , Warrier R , Philip S . Portal Vein Thrombosis-a Rare Complication of SARS-CoV-2 Infection. SN Compr Clin Med. (2021) ;3: :1416–9. |
[21] | Baeza C , Gonzalez A , Torres P , Pizzamiglio M , Arribas A , Aparicio C . Acute aortic thrombosis in COVID-19. J Vasc Surg Cases Innov Tech. (2020) ;6: :483–6. |
[22] | Posada-Arango AM , Garcia-Madrigal J , Echeverri-Isaza S , Alberto-Castrillon G , Martinez D , Gomez AC , Pinto JA , et al. Thrombosis in abdominal vessels associated with COVID-19 Infection: A report of three cases. Radiol Case Rep. (2021) ;16: :3044–50. |
[23] | Badrawi N , Abdulghaffar S . Ovarian vein thrombosis as a first manifestation of COVID-19 infection. Radiol Case Rep. (2021) ;16: :3491–3. |
[24] | Tripolino C , Pizzini AM , Zaccaroni S , Cicognani C , Dapporto S , Cipollini ML , Giannone C , et al. Is SARS-CoV-2 infection an emerging risk factor for splanchnic venous thrombosis? Clin Hemorheol Microcirc. (2021) ;79: :347–55. |
[25] | Hussein MH , Alabdaljabar MS , Alfagyh N , Badran M , Alamiri K . Splanchnic venous thrombosis in a nephrotic patient following COVID-19 infection: a case report. BMC Nephrol. (2021) ;22: :420. |
[26] | Antunes de Brito CA , de Oliveira Filho JRB , Marques DT , Lencastre MDC , de Almeida JR , Lopes EP . COVID-19 and Hepatic Artery Thrombosis: A Case Report. Am J Case Rep. (2021) ;22: :e932531. |
[27] | Ortiz Lopez D , Puente Fernandez A , Ramos Gomez I , Godoy Diaz D . Portal thrombosis in a patient with SARS-CoV-2 infection. Rev Esp Enferm Dig. (2021) ;113: :840–1. |
[28] | Jung EM , Kammerer S , Brandenstein M , Putz FJ , Stroszczynski C , Jung F . High resolution flow (HR Flow) and Glazing Flow in cases of hepatic flow changes: Comparison to color-coded Doppler sonography (CCDS). Clin Hemorheol Microcirc. (2021) ;79: :3–17. |
[29] | Vaidya T , Nanivadekar A , Patel R . Imaging spectrum of abdominal manifestations of COVID-19. World J Radiol. (2021) ;13: :157–70. |
[30] | Agarwal L , Agarwal A , Advani S , Katiyar V , Chaturvedi A , Madhusudhan KS . The eyes see what the mind seeks: a systematic review of abdominal imaging findings in patients with COVID-19. Br J Radiol. (2021) ;94: :20201220. |



