Introduction
Understanding the biogeochemistry of perennially ice-covered aquatic systems has become an important prerequisite to understanding a number of current scientifically intriguing problems, including the documentation of life on ancient Mars (Reference Doran, Wharton, DesMarais and McKayDoran and others, 1998), in subglacial lakes in Antarctica (e.g. Vostok lake) (Reference PriscuPriscu and others, 1999a; Reference Siegert, Tranter, Ellis-Evans, Priscu and LyonsSiegert and others, 2003) and the biological consequences of a Neoproterozoic ‘snowball Earth’ (Reference Fairchild and WrightFairchild, 1993; Reference Hoffman and SchragHoffman and Schrag, 2002). The perennially ice-covered closed-basin lakes of the McMurdo Dry Valleys (MCM) region of Antarctica (∼78˚ S) offer a unique opportunity to compare biological processes to the imprint that they produce via the transfer of carbon within the lakes. Although the MCM lakes are clearly very different from the subglacial lakes on the continent, the investigation of these lakes offers some important insights into the role that perennial ice cover has in the biogeochemistry of aquatic systems. These MCM lakes are photosynthetic in nature, with photosynthesis being the primary driver of carbon fixation in the ecosystem, yet, in contrast to temperate systems, the lack of direct contact with the atmosphere minimizes gas exchange, wind-driven mixing, temperature-driven density change and lake turnover. The δ13C composition of either the solutes or the inorganic materials produced within the waters provides important clues to the biological processes that have occurred within these ice-covered systems Reference McKenzie and Stumm(McKenzie, 1985). Because biological activity is closely connected to the carbon cycle, both photosynthesis and respiration change the δ13C distribution of dissolved inorganic carbon (DIC) in aquatic systems (Reference Quay, Emerson, Quay and DevolQuay and others, 1986). In this paper, we report for the first time, the δ13C ratios of DIC in five lakes from the MCM region (Lakes Miers, Joyce, Vanda and both the east and west lobes of Lake Bonney). The data, when compared and contrasted to previously published δ13C–DIC profiles of Lakes Fryxell and Hoare (Reference Neumann, Lyons and MaraisNeumann and others, 1998), allow for a much clearer indication of how biological processes affect the isotopic composition in these ice-covered systems. The interpretation of these data from the MCM lakes should aid in the interpretation of δ13C data collected from other ice-covered systems.
Site Description
The MCM region is the largest ice-free area in Antarctica. The perennially ice-covered lakes in MCM have been investigated since the International Geophysical Year 1957/58, but only since a US National Science Foundation (NSF)-sponsored long-term ecological research (LTER) site was established there in 1993 have they been studied in an interdisciplinary manner (http://huey.colorado.edu). Since this time, biological, chemical, glaciological and meteorological measurements have been made on a routine basis. The primary site of the MCM-LTER is Taylor Valley, although measurements are also made less frequently in a number of the nearby valleys (Fig. 1). The MCM region is a polar desert with water equivalent precipitation rates ≤100mma–1 (Reference Clow, McKay, Simmons and WhartonClow and others, 1988). Temperatures vary by location, but all of the MCM-LTER meteorological station locations have mean annual temperatures at or below –16.8˚C. The lakes in MCM gain water in the austral summer during a few weeks of glacier meltwater production, and, with the exception of Lake Miers, only lose water through freeze-on to the ice cover and eventual sublimation at the surface. Lake Miers is the only lake in this group that has an outlet stream; therefore it also loses water via outflow. The other lakes are closed-basin lakes and represent a terminus of water flow in each of their basins. Much has been written on the hydrology of these systems, especially the Taylor Valley systems (Reference McKnight, Niyogi, Alger, Bomblies, Conovitz and TateMcKnight and others, 1999). The physical description of these lakes is provided in detail in Reference Spigel, Priscu and PriscuSpigel and Priscu (1998) and will not be repeated here. With the exception of Lake Miers, these lakes are chemically stratified, with the hypolimnia of Lakes Fryxell and Joyce being brackish and those of Lakes Bonney and Vanda being hypersaline. The density stratification in these lakes (with the exception of Lake Hoare) is due to previous changes in climate, and their hypolimnetic waters represent ‘older’ water introduced to the lakes in the past (Reference Lyons, Tyler, Wharton, McKnight and VaughnLyons and others, 2000). The stable density structure of Lakes Bonney, Fryxell, Vanda and Joyce and lack of wind-driven circulation minimize water exchange between the surface and deep waters. Lakes Bonney, Fryxell and Hoare have been sampled at least twice during the austral summer since the beginning of the LTER effort in 1993, while Lakes Vanda, Miers and Joyce have been sampled much less frequently. Ice thicknesses of these lakes from the 1960s through the 1980s varied from 2.5 to 6.0 m depending on season and year, with Lake Joyce generally having the thickest ice (Reference Wharton, McKay, Clow, Andersen, Green and FriedmannWharton and others, 1993). Annual ablation of ice from the surface of the lakes is on the order of 35 cma–1 (Reference Clow, McKay, Simmons and WhartonClow and others, 1988), and the ice cover is maintained by refreezing lake surface water onto the bottom of the ice cover during winter.
Fig. 1. Location of study area and McMurdo Dry Valleys lakes sampled in this investigation.
Methods
Water samples were collected by standard limnological techniques using 5 L Niskin bottles during the 1994/95 and 1996/97 austral summers and represent a one-time sampling effort. Although long-term data exist for Lakes Bonney, Fryxell and Hoare for Cl– and DIC, the data presented are only from the sampling times when the δ13C samples were collected. The long-term data for the lakes can be seen at http://huey.colorado.edu. Samples were collected through the water column over the deepest part of each lake. Samples for major ions were filtered within hours of collection through 0.4 μm Corning NucleoporeTM filters in precleaned plastic filtering units. DIC samples were collected in 60 mL serum vials, stabilized with chloroform, capped and sealed, and stored in the dark at 4˚C. Within 2weeks, these samples were analyzed using a MSA Lira infrared gas analyzer after acid sparging with 6N H2SO4. Chloride was measured on filtered samples using a Dionex DX-300 ion chromatograph (Reference Wang and VeizerWelch and others, 1996). Precision of the Cl– ranged between 0.7% and 0.9%, and that of DIC ranged from 1.8% to 2.8% for the Taylor Valley lakes. Samples for δ13C analysis were filtered into pre-evacuated 60 mL serum bottles using Whatman GF/F filters and stabilized by the addition of 0.2 mL saturated HgCl2 solution. Samples were analyzed at the NASA Ames Research Center on a modified Nuclide 6-6ORMS mass spectrometer. Precision of these measurements was 1.5%.
Results
The Cl– profiles for the seven lakes (the two lobes of Lake Bonney are considered to be distinct lakes) are shown in Figure 2. Lake Miers, the lake with an outflowing stream, has the lowest Cl– concentrations. Of the closed-basin systems, Lake Hoare is the freshest, followed by Lakes Joyce and Fryxell, which are considered to be brackish water lakes. As mentioned above, Lakes Bonney and Vanda have hypersaline hypolimnia (Fig. 2). The high Cl– concentrations in the bottom waters of these lakes reflect, in part, past climatic variation in the MCM, including the loss of the ice covers of these lakes, the regaining of those ice covers and the subsequent refilling of the lakes. This event occurred ∼1 kyr ago for most of these lakes, although the timing of this event in Lake Joyce is unknown (Reference Matsubaya, Sakai, Torii, Burton and KerryMatsubaya and others, 1979; Reference Lyons, Fountain, Doran, Priscu, Neumann and WelchLyons and others, 1998b). Therefore the hypolimnia of these lakes represents older waters that have been removed from contact with the atmosphere. The Cl– concentrations increase with depth in all lakes, other than Lake Miers (Fig. 2). The DIC data for the lakes are plotted in Figure 3. The DIC concentrations range from a low of 0.28 mM in the surface waters of Lake Joyce to values of 84.7 mM at 35 m in the west lobe of Lake Bonney (Fig. 3). With the exception of the east lobe of Lake Bonney, the concentrations generally increase with depth, reflecting the evaporative concentration from the drawdown event outlined above, mineralization of organic matter and dissolution of CaCO3, depending on the lake (Reference NeumannNeumann, 1999). The δ13C ratios of the DIC are plotted vs depth in Figure 4. Four of the lakes have δ13C ratios in the surface waters of 2–4‰, one of the lakes has more negative δ13C ratios, while Lake Joyce has a much higher ratio at ∼10.5‰. All the lakes have upper- to mid-depth maxima of δ13C (Fig. 4). Chlorophyll-a (Chl-a) samples were taken at the same time as the geochemistry samples, with the exception of Lake Vanda, which was sampled for Chl-a a few days later; all the data are shown in Figure 5. Lakes Fryxell, Hoare and the east lobe of Lake Bonney have distinct upper-layer (directly below the ice cover) maxima, while the west lobe of Lake Bonney and Lake Joyce have deeper maxima. Lake Miers has the most unusual Chl-a profile, with a mid-depth minimum and a bottom-water maximum (Fig. 5). Primary productivity rate (PPR) data only exist for four of the lakes and were obtained at the same time as the geochemical and Chl-a profiles (Fig. 6). Lake Hoare and both lobes of Lake Bonney have mid-water maxima, while Lake Fryxell has an upper-layer maximum. The highest PPR values approach 12 μg CL–1 d–1 (Fig. 6). These are low-productivity systems that are greatly influenced by mixotrophy (Reference Laybourn-Parry, Lyons, Howard-Williams, Hawes and BalkemaLaybourn-Parry, 1997; Reference PriscuPriscu and others, 1999b).
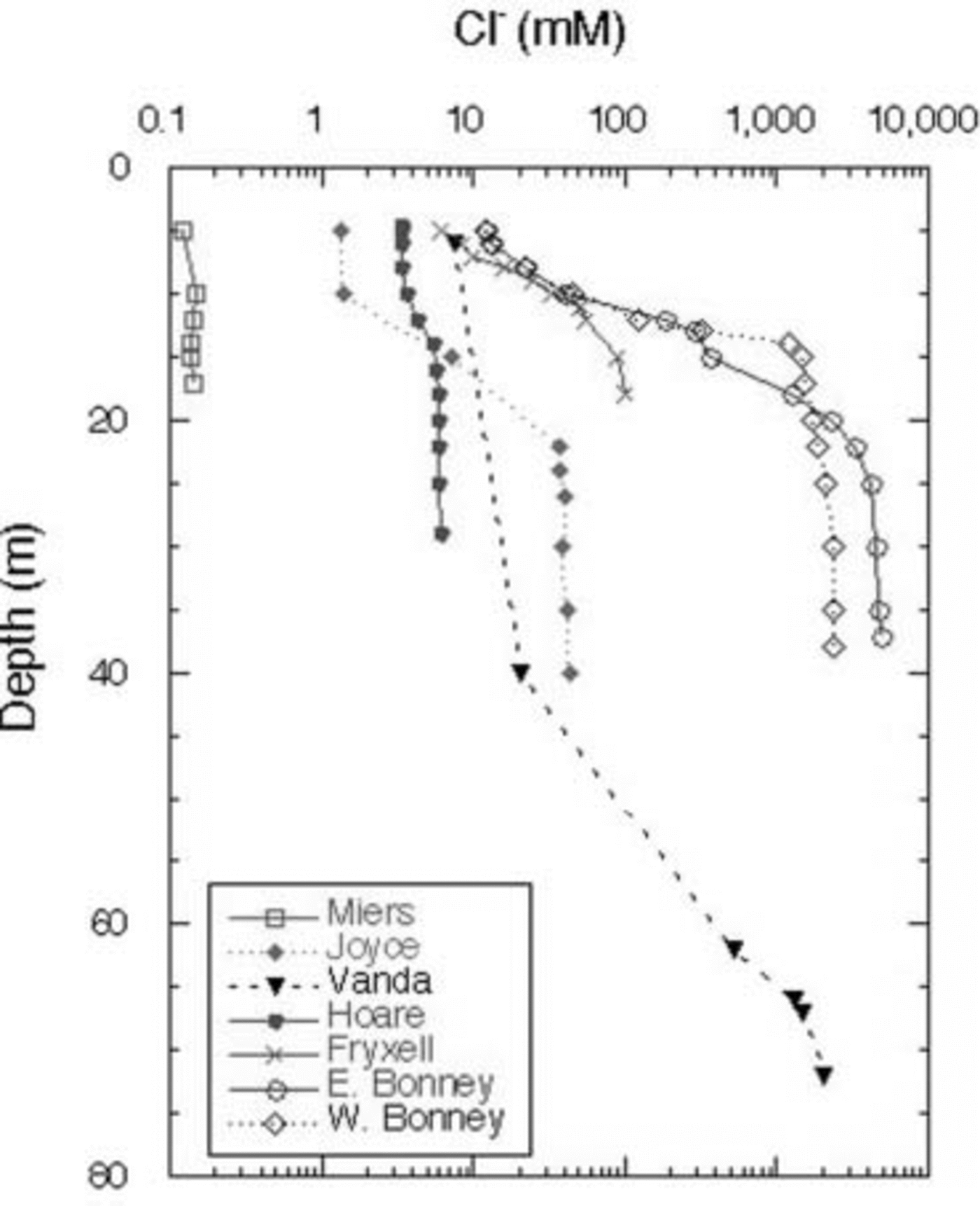
Fig. 2. Chloride concentrations vs depth in MCM lakes.

Fig. 3. DIC concentrations vs depth in MCM lakes.

Fig. 4. δ13C of DIC vs depth in MCM lakes.
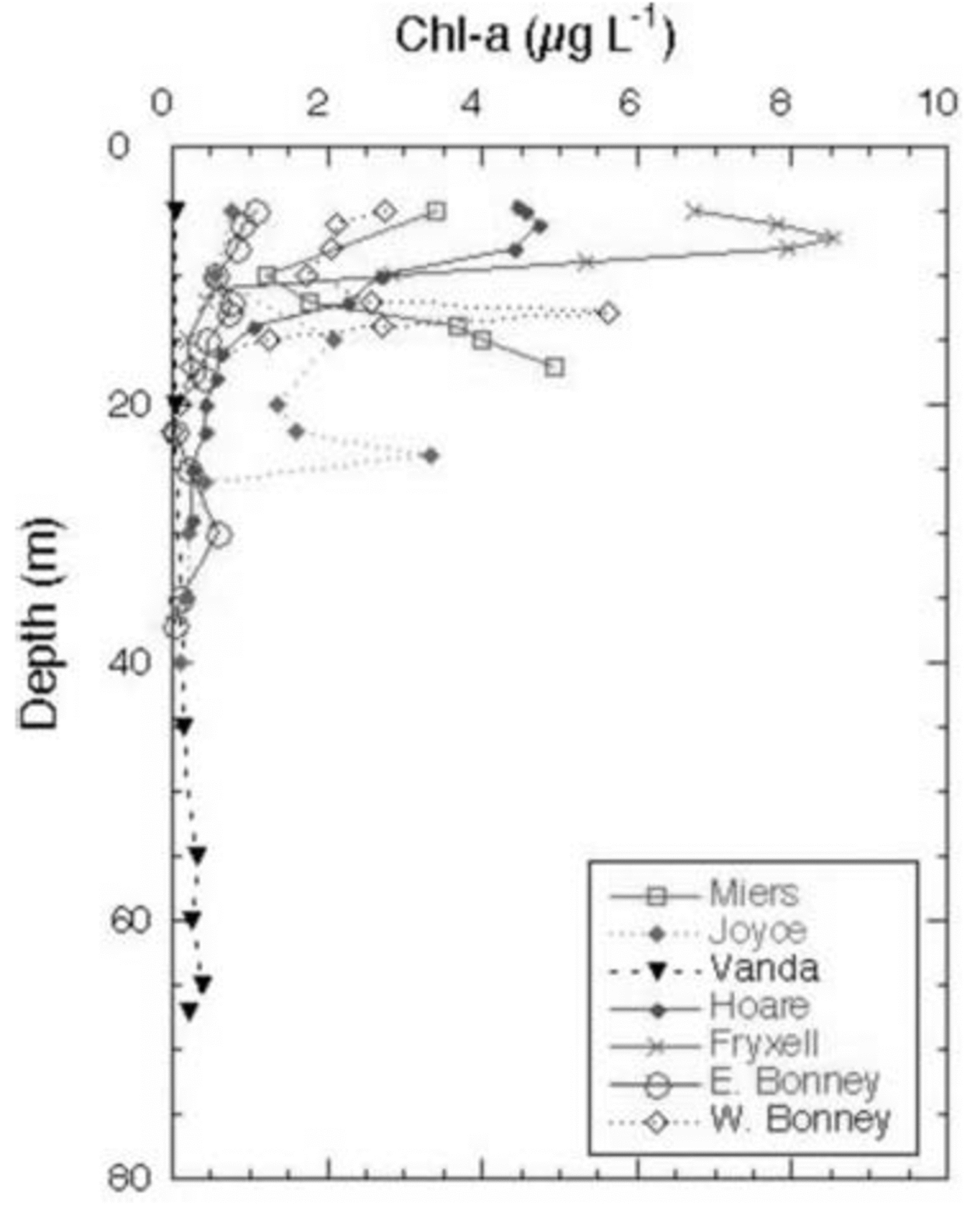
Fig. 5. Chl-a concentrations vs depth in MCM lakes.
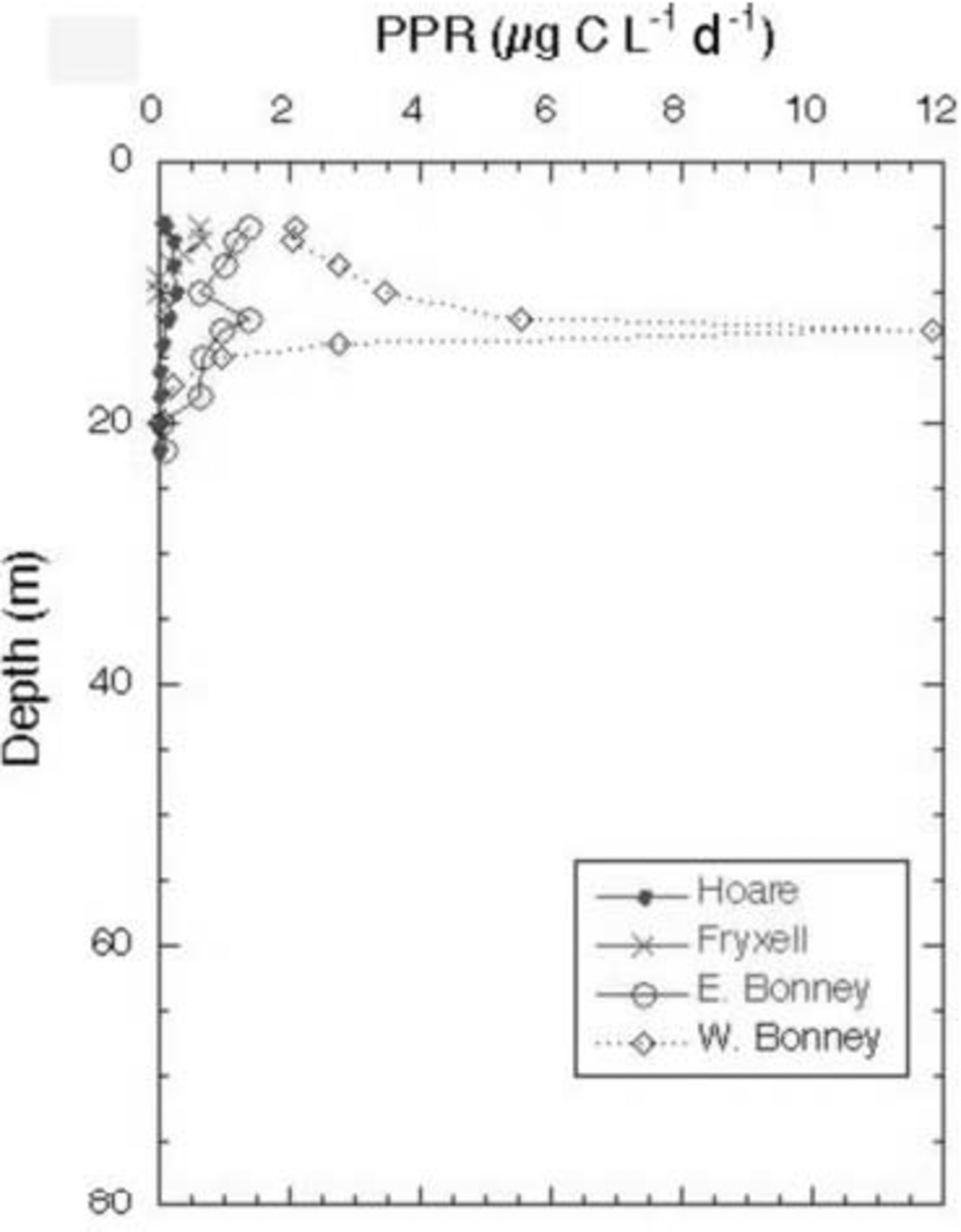
Fig. 6. Primary production rates as measured through 14C uptake during a 24 hour incubation vs depth in MCM lakes.
Discussion
DIC profiles
The profiles of DIC concentration in the lakes have been influenced by a number of processes, including past lake history (i.e. the accumulation of carbon via lake drawdown, or what we have termed legacy events), stream input (i.e. landscape position) and in-lake processes, both biochemical and geochemical. Therefore the DIC and its δ13C signature in the hypolimnia of these lakes represent the consequences of both current and past geochemical and biogeochemical processes occurring in the lakes. This is the case because, with the exception of Lake Miers, the closed-basin nature of the lakes negates the loss of carbon through water outflow. Lake Fryxell receives a very large percentage of its water input from streams, as opposed to direct runoff from glaciers. Stream-waters are enriched in DIC through the dissolution of CaCO3 and the weathering of silicate minerals (Reference Nezat, Lyons and WelchNezat and others, 2001). Lakes Bonney and Miers also have higher inputs of DIC than the other lakes due to a higher percentage of stream input (Reference Neumann, Lyons and MaraisNeumann and others, 1998), which leads to higher DIC concentrations in the surface waters of these lakes (Fig. 3). The proglacial lakes, Lakes Hoare and Joyce, which receive their recharge directly via glaciers, have lower concentrations of DIC in the surface. Lake Vanda, which is fed by the longest river on the continent, the Onyx River, has intermediate surface water DIC concentration. This may be due, in part, to uptake by the abundant photosynthetic cyanobacteria in the river bed. The lakes with known historic drawdown events, such as Lakes Bonney, Fryxell and Vanda (Reference WilsonWilson, 1964; Reference Matsubaya, Sakai, Torii, Burton and KerryMatsubaya and others, 1979; Reference Matsubaya, Sakai, Torii, Burton and KerryLyons and others, 1998b), have much higher DIC concentrations in their hypolimnia. The highest values are in the west lobe of Lake Bonney, in part because this lake has not lost its ice cover over the past few millennia (based on δ18O and δD profiles of the water column (Reference Matsubaya, Sakai, Torii, Burton and KerryMatsubaya and others, 1979)), as have Lakes Vanda and Fryxell and the east lobe of Lake Bonney. The loss of the ice cover allowed for the loss of DIC both through CO2 degassing and through concentration of the lake waters and the precipitation of carbonate minerals. It is possible that Lake Joyce proceeded through a similar drawdown event prior to ∼1000 years BP, which would explain the higher DIC concentrations in its hypolimnion. The DIC profiles in Lakes Miers and Hoare represent more modern (∼1000 year) accumulation of DIC at depth.
Thermodynamic mineral solubility calculations using PHRQPITZ indicate that the deep waters of Lake Hoare and the west lobe of Lake Bonney are undersaturated with respect to calcite, while the deep waters of Lakes Fryxell, Joyce, Vanda and the east lobe of Lake Bonney are supersaturated with respect to calcite (Reference Lyons, Fountain, Doran, Priscu, Neumann and WelchLyons and others, 1998a; Neumann, unpublished information). Lake Miers is slightly supersaturated with respect to calcite from 10 to 14 m, but is slightly undersaturated elsewhere. The surface waters of both lobes of Lake Bonney and of Lake Fryxell proceed from supersaturated to undersaturated with respect to calcite as the austral summer proceeds and stream-water enters the lakes. In addition to the potential carbon uptake and input from calcium carbonate dynamics within the lakes, carbon uptake through photosynthesis in the mid- to upper portions of the lakes and input through mineralization of organic matter at depth also play a potentially important role in carbon geochemistry in these systems (Reference NeumannNeumann, 1999). Early-season photosynthesis decreases the CO2 concentrations in the surface waters of many of these lakes, and because their ice covers limit atmospheric exchange, CO2 may become a limiting nutrient (Reference Neumann, Lyons, Priscu and DonahoeNeumann and others, 2001). Based on the PPR and Chl-a data alone, we hypothesize that DIC uptake in the euphotic zone would be greatest in the west lobe of Lake Bonney and in Lake Fryxell.
δ13C profiles
Studies utilizing stable isotopes have proven to be very effective for investigating carbon cycling in aquatic systems (e.g. Reference McKenzie and StummMcKenzie, 1985; Reference Quay, Emerson, Quay and DevolQuay and others, 1986; Reference Rau, Riebesell and Wolf-GladrowRau and others, 1997). The fractionation of inorganic carbon during carbonate mineral formation at the temperatures of these waters is 1–2‰ at 0˚C. Therefore the precipitation of CaCO3 in surface waters and its subsequent dissolution at depth could increase the bottom-water δ13C, while the precipitation of CaCO3 could potentially decrease the δ13C values in the water column. Conversely, if abundant CO2 is available, photosynthetic activity preferentially utilizes 12C, leaving the residual DIC enriched in 13C. As noted above, because of the ice covers on these lakes, surface waters can become very depleted in CO2 in the earliest part of the growing season (Reference Neumann, Lyons, Priscu and DonahoeNeumann and others, 2001), which in turn produces organic carbon more enriched in 13C (i.e. less fractionated), and DIC enriched in 13C in the water column. The mineralization of this organic matter produced via photosynthesis would lead to DIC enriched in 12C.
Using stream-flow data and the δ13C ratios of DIC for the streams (Reference NeumannNeumann, 1999), we have calculated the δ13C input into each of the three Taylor Valley lakes during the 1996/97 season. The flow-weighted values for the δ13C of DIC entering Lakes Bonney, Fryxell and Hoare are –0.86‰, –0.33‰ and –0.04‰, respectively. If these values are representative of δ13C of DIC entering the lakes over time, it is clear that the surface waters have been greatly enriched via photosynthesis. The exception to this is Lake Hoare whose surface waters are more depleted than the incoming stream-water. However, it should be noted that because Lake Hoare is a proglacial lake, a high percentage of its surface inflow is suspected to come directly from glacial melt or from supraglacial streams (Reference House, McKnight and von GuerardHouse and others, 1995). These waters could have more negative δ13C ratios than the streams. The δ13C maximum at mid-depths clearly represents a biological signal as the lighter isotope is depleted due to photosynthesis. More negative values of δ13C at depth in the lakes suggest remineralization of 12C-enriched organic matter there.
These lakes undergo cryoconcentration of solutes as water is frozen onto the bottom of the ice cover every winter while the solutes remain in the liquid phase. Some of the variation of DIC is due to this effect. By normalizing DIC to Cl– (Fig. 7), a better assessment of non-conservative processes controlling DIC and hence the δ13C variation can be made. DIC .Cl– vs δ13C of the DIC is shown for all the lakes in Figure 8.
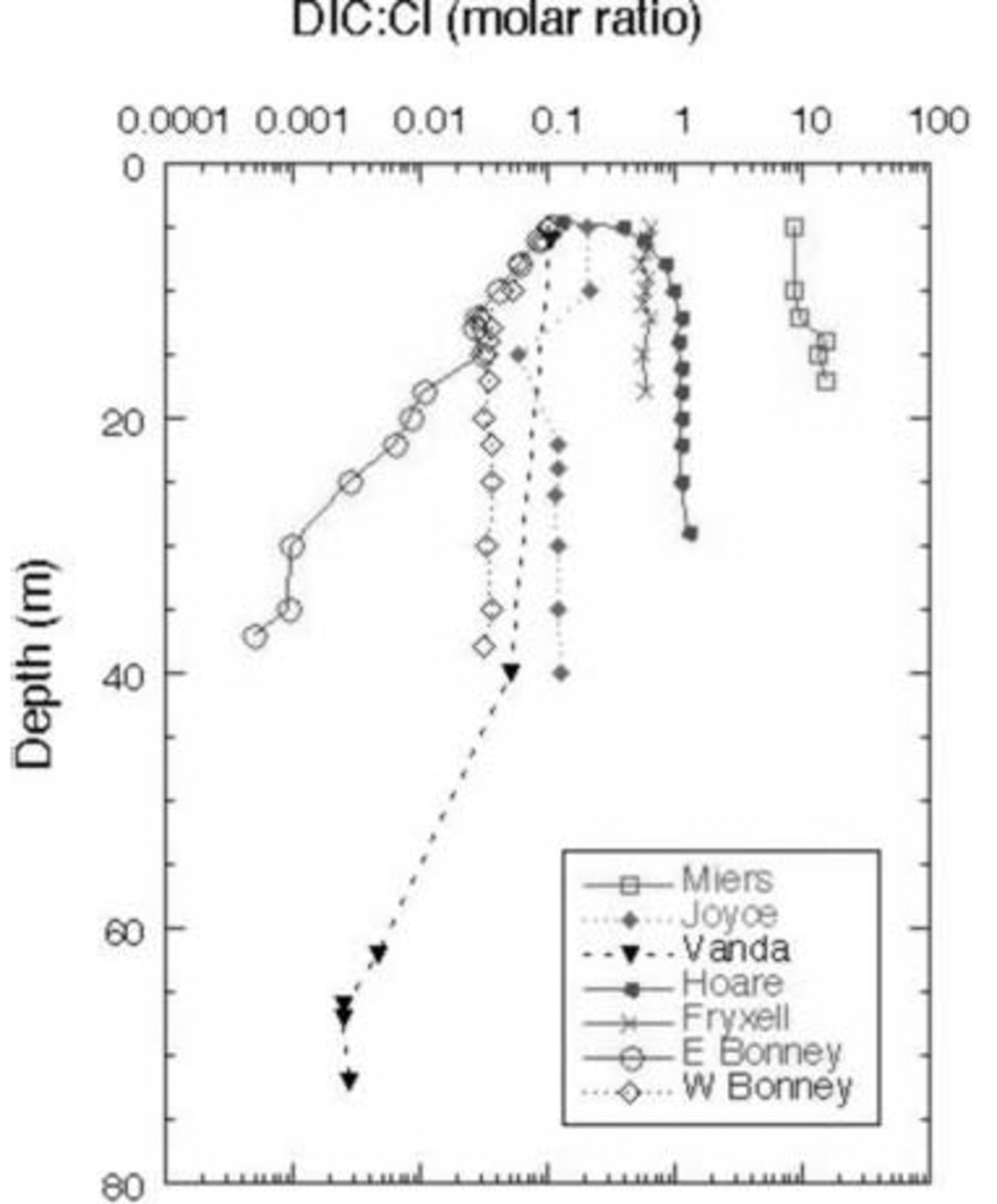
Fig. 7. DIC : Cl– ratio vs depth in MCM lakes.
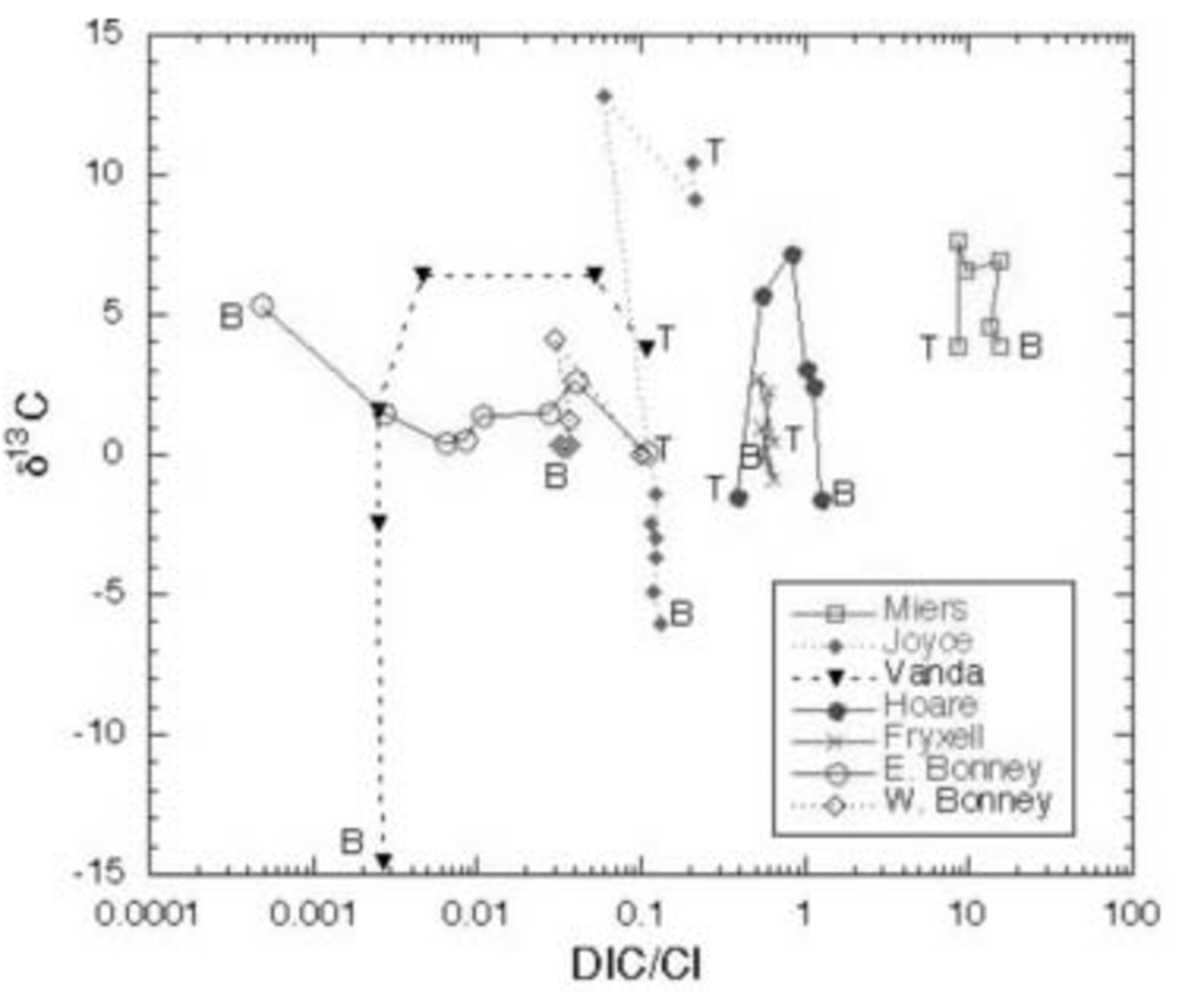
Fig. 8. δ13C of DIC vs DIC : Cl– ratio in MCM lakes. T and B represent the top and bottom of the water column, respectively.
In general, all the lakes except for the east lobe of Lake Bonney become depleted in δ13C from mid-depths to the bottom. DIC .Cl– in the west lobe of Lake Bonney and Lakes Joyce, Fryxell and Vanda either shows a decrease or remains constant with depth, coincident with an increase in δ13C from the surface to mid-depths, and a decrease in δ13C in the bottom of the water column. Biological processes discussed above can be used to explain this pattern (i.e. uptake of 12C in the euphotic zone and subsequent mineralization of this light carbon in the deep waters). There are notable exceptions to this general trend, however. For example, in Lakes Hoare and Miers, while the δ13C of the DIC is highest at mid-depths in the water column, which follows the general pattern described above, the DIC concentration increases relative to Cl– with depth. As the waters are not undersaturated with respect to carbonate mineral phases at these depths, our explanation for this is, in part, that the DIC : Cl– of this water must have been larger than that of the most recent water entering the lake. Lake Vanda shows only a slight decrease in DIC : Cl– with depth, although the δ13C becomes significantly more negative. This reflects the ultraoligotrophic nature of the lake, with very low primary production, biomass and respiration (Fig. 6). Lake Fryxell is more complex than the previously mentioned lakes, with more variation in δ13C with depth and fairly constant DIC : Cl– (Fig. 7 and 8). The heavier δ13C values at depth in the lake reflect methane production there (Reference Smith, Miller and HowesSmith and others, 1993). During methane production, bacteria preferentially reduce 12C, leaving the residual DIC enriched in 13C. Both Lakes Joyce and Vanda show dramatic depletions of 13C with depth, with little to no change in DIC : Cl– (Fig. 8). These profiles relate to past conditions where at lower lake levels there was more biological productivity. Reference Quay, Emerson, Quay and DevolPriscu and others (1999b) have speculated that at lower lake stands, these lakes were more autotrophic than they are now. Perhaps this change in trophic status of these lakes over time has led to the observed isotopic compositions.
The east lobe of Lake Bonney shows a very different pattern than the other lakes (Fig. 7). Its DIC concentration relative to Cl– decreases with depth, while the deepest waters have the heaviest δ13C. As mentioned above, this pattern represents a legacy of past climate change, when this lobe of Lake Bonney had no ice cover. The work of Reference Matsubaya, Sakai, Torii, Burton and KerryMatsubaya and others (1979) and some of our own (R. J. Poreda and others, unpublished information) indicate that the east lobe has only had its current ice cover for ∼300 years. The DIC : Cl– pattern in the east lobe of Lake Bonney represents the influence of extensive water loss and concentration of solutes, leading to the precipitation and possible subsequent dissolution of carbonate minerals, mixing with west lobe waters, and not to any biological processes as the DIC: Cl– patterns in the other lakes do. Clearly for the majority of these lakes the primary influences on the δ13C of the DIC are biological: photosynthesis and organic-matter decomposition.
Comparison of MCM lakes to other lacustrine systems
The stable-isotopic dynamics of DIC in the MCM lakes have both similarities and differences with lakes that do not maintain a permanent ice cover. Clearly, current biological– physical coupled processes influence the δ13C signature of the DIC in the MCM lakes. For example, the uptake of 12C relative to 13C by photosynthetic organisms leads to heavier δ13C-DIC values in the water column where photosynthesis is occurring, i.e. at the location of the chlorophyll maxima, and lighter δ13C values where this organic matter is being decomposed. This is similar to the distribution observed in temperate lakes (see Reference Quay, Emerson, Quay and DevolQuay and others, 1986). The influence of methanogenesis such as observed in the bottom waters of Lake Fryxell, and the subsequent production of heavier δ13C-DIC values is also similar to what is observed in temperate systems. Therefore biological processes imprint the δ13C signature and affect the δ13C distribution in both ice-covered lakes and other types of lake systems. The difference with the MCM lakes is that they do not turn over, mix or exchange CO2 with the atmosphere to any great extent. Therefore, DIC that accumulates in the hypolimnia of the MCM lakes is maintained there. As the hypolimnetic waters age, more old carbon accumulates. So although present-day biological processes affect the δ13C, they probably exert less influence on the deep waters of the MCM lakes than in lacustrine environments that mix on a seasonal, annual or even longer basis. The isolation of carbon into shallow and deep pools in the MCM lakes makes them unusual lakes in a global sense. However, this occurrence of carbon stratification also occurs in other polar lakes where fresh water has flowed into a depression where sea water had been trapped (Reference Jeffries, Krouse, Shakur and HarrisJeffries and others, 1984; Page and others, 1984).
The other major difference with the MCM lakes is that, in contrast to temperate systems, there is little DIC introduced to the lakes produced by organic-matter decomposition in the terrestrial realm. The δ13C values of ∼0‰ for the DIC entering the MCM lakes contrasts dramatically with values of –4‰ to –10‰ for Meech Lake and –10‰ to –14‰ for Lake Washington, for example (Reference Quay, Emerson, Quay and DevolQuay and others, 1986; Reference Wang and VeizerWang and Veizer, 2000). This lack of input of biologically derived light δ13C into the surface waters of the MCM lakes helps maintain their surface waters in a state of heavier δ13C-DIC. As mentioned above, biological fractionation via photosynthesis causes the surface water to become even more enriched in 13C. The importance of the ice covers, the stratification and the lack of abundant biologically derived DIC helps to make these systems different from more temperate lakes.
Implications for subglacial lakes
Our work on the perennially ice-covered lakes of the MCM clearly indicates that a ‘biological signal’ or an imprinting of lake biological processes occurs in the δ13C profiles of DIC. In this way, MCM lakes are similar to more temperate systems. What might we expect to observe in subglacial lakes such as Vostok lake? Noteworthy here is the obvious: the lack of photoautotrophic organisms in these subglacial lakes would clearly negate the DIC decrease, with subsequent enriched δ13C values as seen in the MCM lakes (assuming that chemoautotrophy is negligible). However, if organic-matter decomposition is occurring in specific loci (e.g. aggregates) within the subglacial lake system, increases in DIC with depleted δ13C values might be expected. If chemosynthesis, methanogenesis, etc., were occurring, δ13C analysis would aid in sorting out the location and the extent of these processes as well. The use of δ13C will clearly aid in our overall understanding of the nature and the impact of metabolic activity on the subglacial lake waters. Importantly, δ13C analysis will also aid in discerning a possible/potential lack of biological activity in these systems.
Conclusions
The δ13C values of the DIC in the MCM lakes demonstrate the usefulness of carbon isotope analysis in describing biological activity within these systems by showing that the variation of δ13C in the lakes is primarily controlled by photosynthesis and decomposition activities. Landscape position (i.e. proglacial, closed-basin), legacy effects and carbonate mineral dynamics also play a role in the overall δ13C balance and depth profiles of the lakes. The analysis of δ13C in lake waters remains a powerful tool in the study of biogeochemical dynamics of aquatic systems.
Acknowledgements
We thank our MCM-LTER colleagues, especially R. Edwards and C. Takacs, for help with the collection of these samples. Discussions through the years with P. T. Doran regarding carbon isotope dynamics are also gratefully acknowledged. This work was supported by funds to K.N. from the University of Alabama and by US National Science Foundation grants OPP 9211773 and OPP 9810219. We gratefully acknowledge two anonymous reviewers of the original manuscript and the thoughtful editorial help of R. Mulvaney.












