Abstract
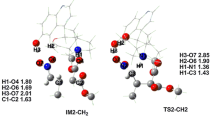
A quantum chemical study of the mechanism and determination of the activation barriers of intramolecular η6,η6-inner-ring haptotropic rearrangements (IHR), consisting in moving a chromium tricarbonyl group Cr(CO)3 from one six-membered aromatic ring to another, are carried out using the density functional theory (DFT) for the respective η6-complexes of coronene I and kekulene II. The stationary states on the potential energy surface, determining the mechanism of η6,η6-IHR, have a lower hapticity, which is of interest for catalysis because of the possibility of coordinating an additional substrate and reagent around the transition metal during the rearrangement. The processes of η6,η6-IHR complexes I and II occur with similar energy barriers (ΔG≠ ≈ 20–25 kcal/mol) that are lower than the barriers (ΔG≠ ≈ 30 kcal/mol) of similar transformations previously calculated or measured for naphthalene complexes and a number of small polyaromatic hydrocarbons.
Similar content being viewed by others
References
Gridnev, I.D. and Tok, O.L., in Physical Organometallic Chemistry, vol. 4: Fluxional Organometallic and Coordination Compounds, Gielen, M., Willem, R., and Wrackmeyer, B., New York: Wiley, 2004, p. 41.
Bartholomew, C.H. and Farrauto, R.J., in Fundamentals of Industrial Catalytic Processes, Hoboken, NJ: Wiley, 2006, p. 597.
Weissermel, K. and Arpe, H.-J., Industrial Organic Chemistry, Weinheim: Wiley, 2003.
Maiorana, S., Baldoli, C., Licandro, E., Casiraghi, L., de Magistris, E., Paio, A., Provera, S., and Seneci, P., Tetrahedron Lett., 2000, vol. 41, p. 7271.
Oprunenko, Y.F., Russ. Chem. Rev., 2000, vol. 69, p. 2237.
Oprunenko, Yu., Gloriozov, I., Lyssenko, K., Malyugina, S., Mityuk, D., Mstislavsky, V., Günther, H., von Firks, G., and Ebener, M., J. Organomet. Chem., 2002, vol. 656, p. 27.
Zabalov, M.V., Gloriozov, I.P., Oprunenko, Yu.F., and Lemenovskii, D.A., Russ. Chem. Bull., 2003, vol. 52, p. 1567.
Gridnev, I., Coord. Chem. Rev., 2008, vol. 252, p. 1798.
Oprunenko, Yu.F., Doctoral (Chem.) Dissertation, Moscow, 1999.
Jiménez-Halla, J.O.C., Robles, J., and Solá, M., Organometallics, 2008, vol. 27, p. 5230.
Gloriozov, I.P. and Oprunenko, Yu.F., Russ. J. Phys. Chem. A, 2004, vol. 78, p. 244.
Gloriozov, I.P., Oprunenko, Yu.F., and Saillard, J.-Y., Eur. J. Inorg. Chem., 2015, vol. 2015, p. 250.
Nunzi, F., Mercuri, F., De Angelis, F., Sgamellotti, A., Re, N., and Giannozzi, P., J. Phys. Chem. B, 2004, vol. 108, p. 5243.
Oprunenko, Y.F. and Gloriozov, I.P., J. Organomet. Chem., 2009, vol. 694, p. 1195.
Oprunenko, Y.F. and Gloriozov, I.P., Russ. Chem. Bull., 2010, vol. 59, p. 2061.
Oprunenko, Y.F. and Gloriozov, I.P., Russ. Chem. Bull., 2011, vol. 60, p. 213.
Fetisov, E.O., Gloriozov, I.P., Oprunenko, Y.F., Saillard, J.-Y., and Kahlal, S., Organometallics, 2013, vol. 32, p. 3512.
(a) Kündig, E.P., Top. Organomet. Chem., 2004, vol. 7, p. 3
(b) Sodeoka, M. and Shibasaki, M., Synthesis, 1993, vol. 7, p. 643
(c) Sodeoka, M. and Shibasaki, M., Org. Chem., 1985, vol. 50, p. 1147.
Perdew, J.P., Burke, K., and Ernzerhof, M., Phys. Rev. Lett., 1996, vol. 77, p. 3865.
Dyall, K.G.J., Chem. Phys., 1994, vol. 100, p. 2118.
Laikov, D.N., Chem. Phys. Lett., 2005, vol. 416, p. 116.
Gonzalez, H.B. and Schlegel, J., J. Phys. Chem., 1990, vol. 94, p. 5523.
Schreckenbach, G. and Ziegler, T., Int. J. Quantum Chem., 1997, vol. 61, p. 899.
Laikov, D.N. and Ustynyuk, Yu.A., Russ. Chem. Bull., 2005, vol. 54, p. 820.
Fetzer, J.C., The Chemistry and Analysis of the Large Polycyclic Aromatic Hydrocarbons, New York: Wiley, 2000.
Yoshida, Y., Isomura, K., Kumagai, Y., Maesato, M., Kishida, H., Mizuno, M., and Saito, G., J. Phys.: Condens. Matter, 2016, vol. 28, 304001.
Robertson, J.M. and White, J.G., J. Chem. Soc., 1945, p. 607.
Staab, H.A., Diederich, F., Krieger, C., and Schweitzer, D., Chem. Ber., 1983, vol. 116, p. 3504.
Almenningen, A., Bastiansen, O., and Dyvik, F., Acta Crystallogr., 1961, vol. 14, p. 1056.
Cyvin, B.N., Cyvin, S.J., Brunvoll, J., and Brebdsdal, E., J. Mol. Struct., 1995, vol. 346, p. 21.
Haiujun Jiao and von Rague Schlyer, P., Angew. Chem., Int. Ed. Engl., 1996, vol. 35, p. 2383.
Diederich, F. and Staab, H.A., Angew. Chem., Int. Ed. Engl., 1978, vol. 17, p. 372.
Thonhauser, T., Cerresoli, D., and Marzari, N., Int. J. Quantum Chem., 2009, vol. 109, p. 3336.
Martin, J.M.L., Chem. Phys. Lett., 1996, vol. 262, p. 97.
Seiders, T.J., Baldridge, K., O’Conor, J.M., and Siegel, J.S., J. Am. Chem. Soc., 1997, vol. 119, p. 4781.
Rabaa, H., Lacoste, M., Delville-Desboise, M.-H., Gloaguen, J.R.B., Ardoin, N., Astruc, D., Le Beuze, A., and Saillard, J.-Y., Organometallics, 1995, vol. 14, p. 5078.
Sarkar, S., Zhang, H., Huang, J.W., Wang, F., Bekyarova, E., Lau, C.N., and Haddon, R.C., Adv. Mater., 2013, vol. 25, p. 1131.
Bekyarova, E., Sarkar, S., Wang, F., Itkis, M.E., Kalinina, I., Tian, X., and Haddon, R.C., Acc. Chem. Res., 2012, vol. 46, p. 65.
Fetzer, J.C. and Biggs, W.R., Polycyclic Aromat. Compd., 1994, vol. 4, p. 3.
Türker, L. and Gümüs, S., Acta Chim. Slov., 2009, vol. 56, p. 246.
Rees, B. and Coppens, P., Acta Crystallogr., 1973, p. 2515.
Almenningen, A., Bastiansen, O., and Dyvik, F., Acta Crystallogr., 1961, vol. 14, p. 1056.
Sato, H., Kikumori, C., and Sakaki, S., Phys. Chem. Chem. Phys., 2011, vol. 13, p. 309.
Oprunenko, Y.F., Russ. Chem. Bull., 2002, vol. 51, p. 907.
Lokshin, B.V., Borisova, N.E., Senyavin, B.M., and Reshetova, M.D., Russ. Chem. Bull., 2002, vol. 51, p. 1656.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.S. Zhulyaev, I.P. Gloriozov, Yu.F. Oprunenko, J.-Y. Saillard, 2017, published in Vestnik Moskovskogo Universiteta, Seriya 2: Khimiya, 2017, No. 5, pp. 211–222.
About this article
Cite this article
Zhulyaev, N.S., Gloriozov, I.P., Oprunenko, Y.F. et al. DFT study of chromium tricarbonyl complexes of coronene and kekulene. Moscow Univ. Chem. Bull. 72, 201–211 (2017). https://doi.org/10.3103/S0027131417050091
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0027131417050091