More Information
Submitted: September 20, 2022 | Approved: September 29, 2022 | Published: September 30, 2022
How to cite this article: Sweety M, Biswas A, Rahman MM. Micropropagation and cytological studies of Aole vera Linn. J Plant Sci Phytopathol. 2022; 6: 126-132.
DOI: 10.29328/journal.jpsp.1001085
Copyright License: © 2022 Sweety M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Aloe vera; Explant; in vitro propagation; Chromosome; Ideogram; Karyotype
Micropropagation and cytological studies of Aole vera Linn
Sweety Majumder*, Animesh Biswas and Mohammad Mahbubur Rahman
Department of Botany, University of Chittagong, Chittagong-4331, Bangladesh
*Address for Correspondence: Sweety Majumder, Department of Botany, University of Chittagong, Chittagong-4331, Bangladesh, Email: majumdersweety01@cu.ac.bd
Aloe vera Linn. is an essential medicinal plant. In this present research work, a protocol of in vitro regeneration and karyomorphological analysis of Aloe vera was developed using different concentrations and compositions of media. Shoot apices of field-grown plants were used as explant and aseptically cultured on Murashige and Skoog (MS) medium fortified with different concentrations and combinations of auxins (IAA and NAA) and cytokinins (BAP and Kn). The highest number of multiple shoot buds (4.36 ± 0.07) was obtained from MS + 2.0 mg/l BAP + 1.0 mg/l IAA and induced shoot buds underwent rapid elongation (4.24 ± 0.06 cm) on the same medium composition. Half strength MS media with 2.0 mg/l IBA was suitable for induction and proliferation (6.31 ± 0.05) of roots and 95% of plantlets were acclimatized to field conditions successfully. Somatic chromosome numbers of mother and in vitro grown plants were confirmed to be 2n = 14. Chromosome length ranged from 4.28 - 13.74 µm in the naturally grown plants and 4.46 - 14.1 µm for in vitro grown plants. The total form percent (TF%) of mother and in vitro grown plants was 41.69% and 42.23%, respectively. The karyotype formula of in vivo grown plants was 2n = 14 = 4Lsm + 6Mm + 4Sm, whereas that of the micropropagated plants was 2n = 14 = 4Lsm+ 4Mm + 6Sm. The frequency of the chromosome having arm more than 2:1 was 0.08 for mother plants and 0.15 for in vitro grown plants. Therefore, the karyotype of both plants falls into the 2B symmetrical type based on Stebbins classification (1971).
Aloe vera Linn., family Liliaceae, is a spiky cactus-like medicinal plant The Plant is a commonly looking perpetual with small, thick to some degree isolated stem 30 cm - 60 cm. The leaves have opaque green stalk less, gloat, lanceolate, erect spreading instead of inward, sharp-toothed at the edge, and with saw-like teeth along their margins, up to 0.5 m long and 8–10 cm across at the base. In a transverse section, the plant shows a slightly concave appearance on the adaxial surface and a distinctly convex appearance on the lower abaxial surface [1]. The leaves are also covered with thick cuticles, beneath which epidermis and mesophyll. Roots are 30 cm - 40 cm in length [2]. The smell is nauseous and bitter.
There are 200 different types of molecules identified in Aloe vera [3]. Leaf extract of Aloe vera leaf contains about 98% water [4]. Different types of vitamins including the important antioxidant vitamins A, C and E. Vitamin B1 (thiamine), niacin, Vitamin B2 (riboflavin), choline and folic acid are also present in the Aloe vera leaf gel [5]. A. vera has been used in pharmaceutical, food, and cosmetic industries, or in traditional medicine worldwide, [6]. Bioactive compounds of Aloe species have been known to have anti-inflammatory, antitumor, antiulcer, anticancer, antibacterial, and antiviral properties [7]. Leaves of this plant species are also used to treat bacterial and fungal skin diseases [8].
Traditional propagation methods of Aloe vera have many downsides such as a slow propagation rate in which each plant produces three to four sprouts every year, and male sterility to use its seeds as well [9]. To meet the high demand for A. vera in the pharmaceutical industries and also overcome the above-mentioned problems, in vitro propagation has been used for rapid micropropagation of many important plants [10,11]. Hence, there have been many attempts to rapid plant multiplication and genetic improvement of disease-free plants [12]. For the establishment of most of the plant tissue, culture protocols shoot apices were used as the explant. Cytological studies in many plant species have been used for resolving a plethora of taxonomic and evolutionary problems [13,14]. The present research work aimed at micropropagation and cytological studies of A. vera.
Preparation of plant materials
Shoot apices of field-grown plants were collected and thoroughly washed under running tap water. The plant materials were then cut into short pieces and surface sterilized with 1% savlon and liquid soap for 5 - 10 minutes with constant shaking. The plant parts were then washed 3 - 4 times with distilled water for complete removal of the trace of detergent and taken under a running laminar airflow cabinet and transferred to a 500 ml sterilized conical flask. After rinsing with 70% ethanol for less than 60 seconds, they were immersed in 0.1 ml HgCl2 for different duration of time and after that, the plant segments were washed 4 - 5 times with double distilled water.
Culture media and conditions for plant regeneration
The sterilized explants were cultured on an MS basal medium fortified with various concentrations and combinations of plant growth regulators (PGRs) for the induction of multiple shoot buds. The media were solidified with 0.8% (w/v) agar and PH was adjusted to 5.8 before autoclaving for 30 minutes at 121 oC under 1.1 kg/cm2 pressures. The culture vessels with inoculated explants were then taken to the culture room for incubation.
Subculture for multiple shoot buds
Induced multiple shoot buds were rescued very carefully in aseptic conditions and then these were subcultured on the same media or different media for further response at an interval of certain periods.
Rooting of in vitro shoot
The steps of rooting experiments were carried out in a half-strength MS medium with or without growth regulators. The elongated shoot buds at definite height (2 - 4 cm) were separated from the culture vessels and then separately moved to root media. After 2 - 3 weeks of inoculation on a suitable medium, the roots were produced from the cut ends of each micro shoot.
Hardening and acclimatization of plantlets
The induced plantlets were taken out of the culture vessels and then washed thoroughly with running tap water for the removal of agar attached to the roots. After that, the rooted plantlets were transplanted to small plastic pots containing garden soil and compost in the ratio of 1:1 and kept at room temperature for 3 - 5 days. The soil was treated with a 0.1% agrosan (fungicide) solution.
Statistical analysis of data
All experiments were carried out with three replicates in each treatment. Observations were recorded on the basis of percentage of response, number of shoots per explant, and number of roots per shoot bud. Means and standard deviations (mean ± SD) were calculated for each treatment. The mean values of the parameters were compared by using the SPSS (Ver. 21) software.
Cytological studies
Root tip collections: The root tips of in vivo and in vitro grown plants were collected cautiously, in the early morning (9.30 a.m to 10.30 a.m) and when lengths of roots become 1 cm - 1.5 cm in size.
Pre-treatment: 1,4-Para-dichlorobenzene (PDB) was used for the pretreatment of root tips for 2.00 - 3.00 hours at room temperature.
Preservation of roots: After the above stage, the pretreated roots were washed with distilled water and kept in 1:3 (v/v) aceto-alcohol for 24 hours. And then the collected roots were transferred in 70% alcohol (v/v) for long time preservation.
Staining of chromosomes: The preserved roots were thoroughly washed with distilled water after that hydrolyzed in 50% HCl for 6-8 minutes at room temperature. Then treated roots were washed 4-5 times with distilled water. After that, the roots were kept in a 2% (w/v) aqueous solution of iron alum for 8-10 minutes and then the roots were stained with an aqueous solution of Haematoxylin and kept for 30 minutes. The darkly colored portions of root tips were squashed in a drop of 0.2% (w/v) acetocarmine. Then set a cover glass on the squashed root material, then the slide was warmed gently over a flame of a spirit lamp. Finally, the slides were prepared for observation under a microscope.
Chromosome characterization: Chromosomes were classified based on Centromeric Type (CT), Relative Length (RL), Centromeric Index (CI) and Karyotypic similarity (TF%).
Centromeric type (CT): The procedure proposed by Levan [15] for determining the centromeric type of chromosomes was followed. Here the l/s ratio was used as an index of centromeric type. Here, l = Length of the longer arm and s = Length of the shorter arm.
Relative Length (RL): Relative Length (RL)
Centromeric index (CI): Centromeric Index (CI)
Karyotypic symmetry: The symmetry/asymmetry of karyotype was determined by
a) TF%: According to Huziwara [16].
TF%
b) TF% and Stebbins qualitative classification for the determination of asymmetry: The formula of Stebbins [17] qualitative classification is given below.
| Ratio of longest/shortest chromosome (l/s ratio) | The proportion of chromosomes with an arm ratio lessthan (< 2: 1) | |||
| 0 | 0.01-0.50 | 0.51-0.99 | 1.00 | |
| <2:1 | 1A | 2A | 3A | 4A |
| 2:1 – 4:1 | 1B | 2B | 3B | 4B |
| >4:1 | 1C | 2C | 3C | 4C |
Ideogram: For an accurate measurement of lengths at least three metaphase plates were measured. Their average arm length was used to prepare the ideogram. Karyotype XY and Image J software were used for preparing Ideogram.
Micropropagation of Aloe vera
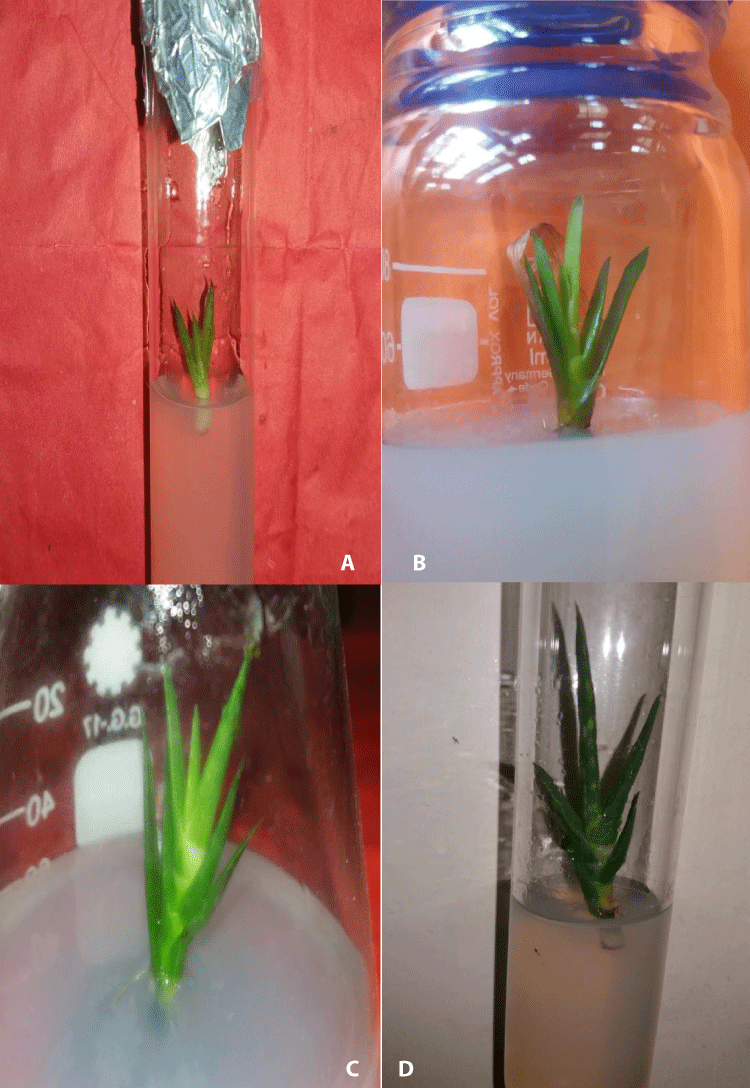
The shoot apices of Aloe vera were cultured on the MS basal concentrations of Kn and BAP in combination with NAA or IAA. After 30 days of culture, data were collected and also presented in Table 1 and Table 2. Shoot apices of field-grown plants were cultured on MS medium supplemented with BAP and Kn (0.5-3.0 mg/l). The explants started producing MSBs within 10-25 days of inoculation. Among the different combinations tested, the maximum explants (91%) produced MSBs in the medium containing 2.0 mg/l BAP + 1.0 mg/l IAA with the highest number of MSBs 4.36 ± 0.07 per explant (Figure 1a,b). Whereas, On the contrary, the minimum number of MSBs (30%) per explant was found in 3.0 mg/l Kn and the lowest length (1.01 ± 0.09) was observed in the same medium within 12 - 18 days of culture. The effect of BAP with IAA was superior to that of BAP alone and in a combination of Kn with IAA and NAA. Different growth regulators, especially auxins and cytokinins played a notable role in the micropropagation of many important medicinal plants species such as Pimpinella anisum [18], Scrophularia kakudensis [19], Euonymus verrucosus [20]; Valeriana jatamansi [21].
| Table 1: Effects of BAP individually and in combination with NAA or IAA on induction of MSBs in shoot segment of A vera L. | ||||
| PGRs supplement in the media (mg/l) | % of explants gave response | Time (d) requires for induction of MSBs Average* no. of MSBs/explant 𝐱̅ ± SE) | ||
| BAP | 0.5 | 47 | 12 - 22 | 2.03 ± 0.03m |
| 1.0 | 56 | 12 - 20 | 2.05 ± 0.071 | |
| 2.0 | 68 | 12 - 17 | 2.08 ± 0.08k | |
| 3.0 | 33 | 13 - 25 | 2.09 ± 0.04k | |
| BAP + NAA | 2.0 + 0.2 | 50 | 12 - 18 | 3.02 ± 0.09d |
| 2.0 + 0.5 | 73 | 10 - 16 | 2.98 ± 0.05e | |
| 2.0 + 1.0 | 61 | 12 - 14 | 2.42 ± 0.07i | |
| 3.0 + 0.2 | 56 | 12 - 14 | 2.78 ± 0.06f | |
| 3.0 + 0.5 | 43 | 11 - 20 | 2.27 ± 0.05j | |
| 3.0 + 1.0 | 42 | 12 - 20 | 2.01 ± 0.05n | |
| BAP + IAA | 2.0 +0.2 | 57 | 10 - 12 | 2.84 ± 0.08e |
| 2.0 + 0.5 | 72 | 10 - 14 | 3.65 ± 0.09b | |
| 2.0 + 1.0 | 91 | 10 - 14 | 4.36 ± 0.07a | |
| 3.0 + 0.2 | 61 | 11 - 14 | 3.27 ± 0.10c | |
| 3.0 + 0.5 | 55 | 10 - 14 | 2.77 ± 0.11g | |
| 3.0 + 1.0 | 49 | 12 -16 | 2.51 ± 0.04h | |
| d = days, MSBs = Multiple shoot buds.*values are the means of three replicates with 15 explants. Figures in the column having the same letter(s) within a location did not differ significantly according to DMRT≤ 0.05. | ||||
| Table 2: Effects of Kn individually and in combination with NAA or IAA on induction of MSBs in shoot apices of A. vera L. | ||||
| PGRs supplement in the media (mg/l) | % of explants gave a response | Time (d) requires for induction of MSBs | Average* no. of MSBs/explant 𝐱̅ ± SE) | |
| Kn | 0.5 | 35 | 15 - 20 | 1.95 ± 0.04i |
| 1.0 | 37 | 14 - 16 | 1.21 ± 0.11k | |
| 2.0 | 43 | 14 - 15 | 2.13 ± 0.07g | |
| 3.0 | 30 | 12 - 18 | 1.01 ± 0.091 | |
| Kn + NAA | 2.0 + 0.2 | 54 | 10 - 14 | 1.95 ± 0.08i |
| 2.0 + 0.5 | 82 | 10 - 14 | 3.12 ± 0.15a | |
| 2.0 + 1.0 | 63 | 12 - 14 | 2.79 ± 0.08c | |
| 3.0 + 0.2 | 65 | 10 - 12 | 2.35 ± 0.07e | |
| 3.0 + 0.5 | 43 | 11 - 14 | 2.61 ± 0.06d | |
| 3.0 + 1.0 | 38 | 10 -14 | 2.13 ± 0.05g | |
| Kn + IAA | 2.0 + 0.2 | 36 | 15 - 18 | 1.18 ± 0.04m |
| 2.0 + 0.5 | 35 | 13 - 15 | 2.95 ± 0.11b | |
| 2.0 + 1.0 | 51 | 12 - 17 | 2.27 ± 0.07f | |
| 3.0 + 0.2 | 40 | 12 - 15 | 2.04 ± 0.06h | |
| 3.0 + 0.5 | 32 | 12 - 18 | 1.50 ± 0.14j | |
| 3.0 + 1.0 | 32 | 11 - 15 | 1.25 ± 0.13k | |
| d = days, MSBs = Multiple shoot buds.*values are the means of three replicates with 15 explants. Figures in the column having the same letter(s) within a location did not differ significantly according to DMRT≤ 0.05. | ||||
Figure 1: Induction and development of multiple shoot buds in soot apices of A. vera L. a) Initiation of MSBs formation after 10 days of culture, b) Development of MSBs on MS medium with 2.0 mg/l BAP + 1.0 mg/l IAA, c-d) Elongation of MSBs on MS medium containing 2.0 mg/l BAP + 1.0 mg/l IAA.
Elongation of induced MSBs
The regenerated multiple shoot buds were individually grown in aseptic conditions on elongation media. It was found that BAP in combination with IAA was better for elongation of MSBs than that other combinations (Table 3). The MS medium fortified with 2.0 mg/l BAP + 1.0 mg/l IAA produced the maximum elongated shoot buds (5.21 ± 0.11 cm) after 30 days of culture. Figure 1c,d showed the highest elongation of MSBs. The lowest elongation (1.61 ± 0.12 cm) was found when shoot buds were cultured on the medium fortified with 3.0 mg/l BAP + 1.0 mg/l NAA. It was observed that BAP supplemented with MS media was better than Kn supplemented MS media for elongation of MSBs. The same type of enhancement of in vitro shoot buds elongation was also noted in many other medicinal plants including, Alpinia galangal [22], Lythrum salicaria [23], Rhinacanthus nasutus [24], Securidaca longipedunculata [25].
| Table 3: Effects of different concentrations and combinations of plant growth regulators (PGRs) on the elongation of directly produced multiple shoot buds ofA. vera L. when cultured on MS medium. | |||
| PGRs supplement in the medium (mg/l) | Initial* length (cm) of an individual shoot buds (𝐱̅ ± SE) | Length* (cm) of individual shoot buds after 30d of culture (𝐱̅ ± SE) | |
| BAP + NAA | 2.0 + 0.5 | 1.04 ± 0.10i | 2.20 ± 0.06v |
| 2.0 + 1.0 | 1.27 ± 0.08f | 2.62 ± 0.03q | |
| 3.0 + 0.5 | 1.01 ± 0.08ij | 2.54 ± 0.10r | |
| 3.0 + 1.0 | 0.78 ± 0.09k | 1.61 ± 0.12w | |
| BAP + IAA | 2.0 + 0.5 | 1.36 ± 0.10d | 2.60 ± 0.09q |
| 2.0 + 1.0 | 1.74 ± 0.05a | 5.21 ± 0.11l | |
| 3.0 + 0.5 | 1.31 ± 0.11e | 3.57 ± 0.10m | |
| 3.0 + 1.0 | 1.00 ± 0.10j | 2.35 ± 0.07t | |
| Kn+ NAA | 2.0 + 0.5 | 1.08 ± 0.11h | 2.22 ± 0.06u |
| 2.0 + 1.0 | 1.34 ± 0.09d | 3.09 ± 0.05o | |
| 3.0 + 0.5 | 1.15 ± 0.08g | 2.44 ± 0.18p | |
| 3.0 + 1.0 | 1.00 ± 0.11j | 1.74 ± 0.12v | |
| Kn + IAA | 2.0 + 0.5 | 1.25 ± 0.07f | 2.26 ± 0.07t |
| 2.0 + 1.0 | 1.58 ± 0.06b | 2.33 ± 0.8s | |
| 3.0 + 0.5 | 1.57 ± 0.05b | 2.33 ± 0.11s | |
| 3.0 + 1.0 | 1.41 ± 0.07c | 3.11 ± 0.08n | |
| d = days, MSBs = Multiple shoot buds.*values are the means of three replicates with 15 explants. Figures in the column having the same letter(s) within a location did not differ significantly according to DMRT≤ 0.05. | |||
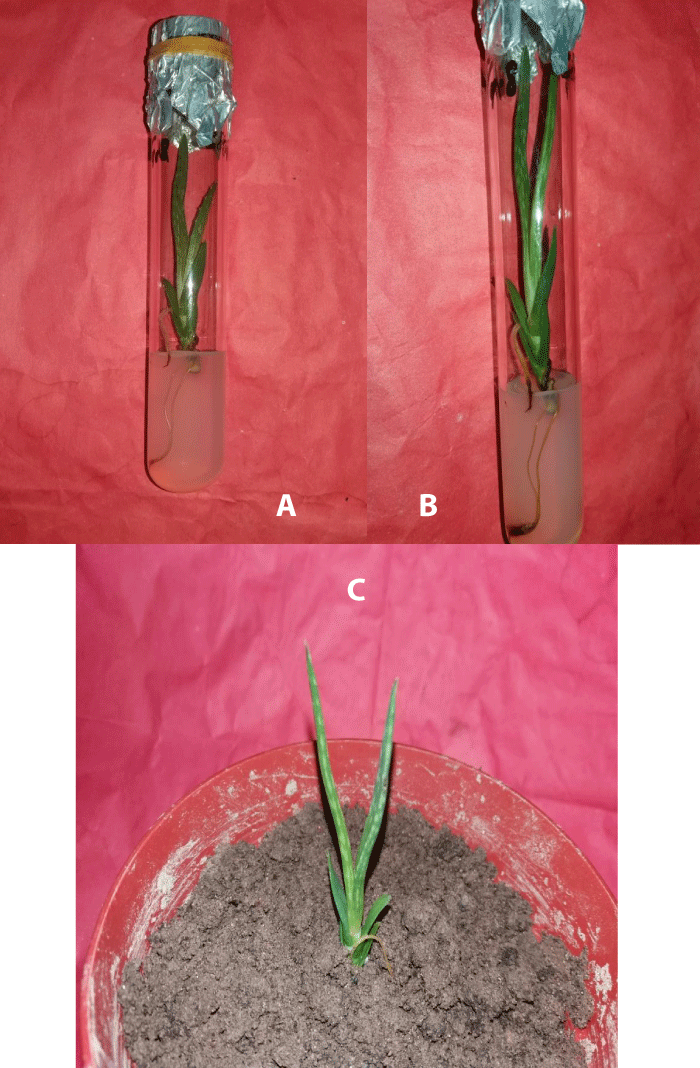
Results of induction of roots in elongated shoot buds of A. vera 3 cm - 4 cm long micropropagated shoot buds were individually transferred to rooting media (1/2 strength MS media) with and without PGR supplement. The maximum rooting (96%) was observed when 2.0 mg/l IBA was added in half strength MS medium within the 11-12 days of inoculation and the highest numbers of roots per micro shoots were 6.31 ± 0.05 and the average length of roots after 30 days of culture was 5.81 ± 0.17 in the same medium composition (Figure 2a,b)(Table 4).
Figure 2: Rooting of shoot buds of A. vera L. and their establishment in pots. a-b) Induction and proliferation of half-strength MS medium fortified with 2.0 mg/l IBA, c) in vitro development of complete seedling after transplantation in a plastic pot.
| Table 4: Effects of different concentrations and combinations of plant growth regulators (PGRs) on the development of roots in elongated multiple shoot buds of A. vera L. when cultured on half-strength MS medium. | |||||
| PGRs supplements in the media (mg/l) |
Days to root induction | % of micro Shoot Produced roots | Average*number of roots/macro shoot (𝐱̅ ± SE) | Average*length (cm) roots after 30d of culture (𝐱̅ ± SE) | |
| ½MS | 0 | 15 - 25 | 58 | 2.65 ± 0.11j | 2.13 ± 0.08y |
| ½MS + IBA | 0.5 | 15 - 22 | 61 | 3.80 ± 0.06f | 2.76 ± 0.04s |
| 1.0 | 12 - 18 | 70 | 4.50 ± 0.07c | 3.14 ± 0.07q | |
| 2.0 | 11 - 12 | 96 | 6.31 ± 0.05a | 5.81 ± 0.17n | |
| 3.0 | 12 - 17 | 65 | 4.01 ± 0.11e | 2.15 ± 0.11x | |
| ½MS + IAA | 0.5 | 12 - 18 | 63 | 2.24 ± 0.01k | 2.22 ± 0.03w |
| 1.0 | 13 - 17 | 79 | 5.05 ± 0.08b | 2.50 ± 0.04u | |
| 2.0 | 11 - 14 | 81 | 3.51 ± 0.05g | 3.18 ± 0.06g | |
| 3.0 | 12 - 115 | 53 | 3.50 ± 0.12g | 2.02 ± 0.04y | |
| ½MS + NAA | 0.5 | 18 - 22 | 50 | 2.05 ± 0.09l | 2.52 ± 0.11u |
| 1.0 | 18 - 20 | 54 | 2.58 ± 0.10j | 3.30 ± 0.02o | |
| 2.0 | 15 - 20 | 43 | 2.86 ± 0.04i | 2.53 ± 0.16t | |
| 3.0 | 18 - 20 | 34 | 3.07 ± 0.12h | 2.42 ± 0.24v | |
| ½MS + IBA + IAA | 1.0 + 0.5 | 10 - 14 | 85 | 4.35 ± 0.03d | 3.18 ± 0.05p |
| 1.0 + 1.0 | 13 - 16 | 82 | 5.12 ± 0.06b | 2.81 ± 0.05s | |
| ½MS + IBA + NAA | 1.0 + 0.5 | 11 - 14 | 76 | 3.89 ± 0.10f | 2.91 ± 0.05r |
| 1.0 + 1.0 | 14 - 18 | 49 | 1.76 ± 0.15m | 2.09 ± 0.20z | |
| d = days, MSBs = Multiple shoot buds.*values are the means of three replicates with 15 explants. Figures in the column having the same letter(s) within a location did not differ significantly according to DMRT≤ 0.05. | |||||
The lowest proportion (49%) of rooting was recorded in half-strength MS medium fortified with 1.0 mg/l IAA + 1.0 mg/l IBA within 14 - 18 days of inoculation. In this medium, the minimum numbers of roots per micro shoot were 1.79 ± 0.15 and the average length of roots after 30 days of culture was 2.09 ± 0.20.
Half-strength MS medium with different concentrations and combinations of auxins has also been reported for several plants including Greek oregano [26], Rosa hybrid [27], Momordica cymbalaria [28], Paulownia elongata [29], Heliotropium Indicum [30].
Acclimatization and establishment of in vitro grown plantlets of A. vera
For the establishment of in vitro raised complete seedlings, the culture vessels with well-developed rooted plantlets were taken out of the culture room and followed several steps for acclimatization. Then the plantlets were planted in small plastic pots (Figure 2c) containing garden soil and compost in the ratio of 1:1 and kept at room temperature for 3 - 5 days and the survival percentage of the regenerated plantlets under natural conditions was about 95%. in vitro, grown plantlets were morphologically uniform having a normal phenotype.
Karyomorphological analysis of A. vera
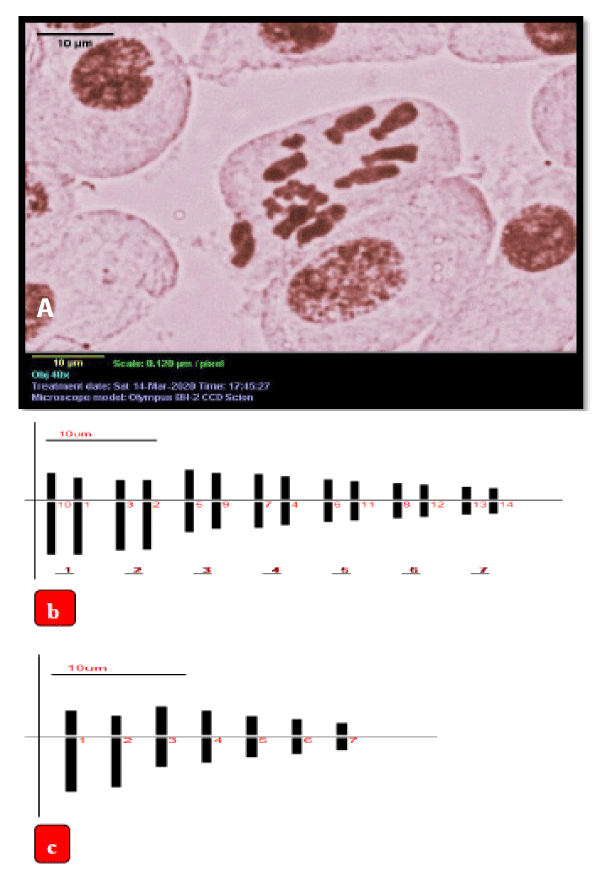
Karyotype of naturally grown plants of A. vera: The somatic cells had 14 chromosomes each and the chromosome length ranged from 4.46 to 14.10 µm (Table 5). Among the 7 basic chromosomes, 2 were submetacentric and 5 were metacentric (centromeric formula = 2sm + 5m). All the submetacentric were long (Figure 3a). Among the 5 metacentric chromosomes, 2 were medium and 3 were small (Figures 3b,c). For this chromosome characteristic, the appropriate karyotype formula was n = 7 = 2Lsm + 2Mm + 3Sm. The total length of haploid complement was 62.96 and TF% 41.69. The total length of long arms and that of shot arms were 36.73 µm and 26.26 µm, respectively. None of the chromosomes had secondary constrictions. The ratio of the longest and shortest chromosome was more than 2:1 and the frequency of the chromosome having arm more than 2:1 was 0.29 and the karyotype falls into the 2B category of Stebbins (1971) chart of chromosome asymmetry. A similar type of chromosome morphology and the karyotype is shown in different plant species like Santalum album [31], Plumbago auriculata [32], Thespesia lampas [33].
Figure 3: a) Microscopic photograph of somatic metaphase chromosomes of mother plant of A. vera L., b,c) Ideogram of the mother plant, scale bar = 10 µm, b) 2n = 14,c) n = 7.
| Table 5: Length, Arm ratio, Relative length, Centromeric index, Centromeric type, TF% and Centromeric formula of mitotic metaphase chromosome of in vivo grown plants of A. vera L. | |||||||||||
| Chromosome pair | Long arm (l) µm | Short arm (s) µm | Total length (I+s = T) µm | Arm ratio (l/s) | Relative length (RL) % | Centromeric Index (CI) % | Centromeric Type (CT) | Chromosome Type | TF% | Centromeric formula | |
| 1 | 9.65 | 4.45 | 14.10 | 2.17 | 22.4 | 31.54 | sm | L | 41.69 | 2sm + 5m | |
| 2 | 8.82 | 3.55 | 12.37 | 2.48 | 19.65 | 28.72 | sm | L | |||
| 3 | 5.18 | 5.18 | 10.36 | 1 | 16.44 | 50 | m | M | |||
| 4 | 4.45 | 4.45 | 8.90 | 1 | 14.14 | 50 | m | M | |||
| 5 | 3.52 | 3.52 | 7.04 | 1 | 11.17 | 50 | m | S | |||
| 6 | 2.88 | 2.88 | 5.76 | 1 | 9.13 | 50 | m | S | |||
| 7 | 2.23 | 2.23 | 4.46 | 1 | 7.08 | 50 | m | S | |||
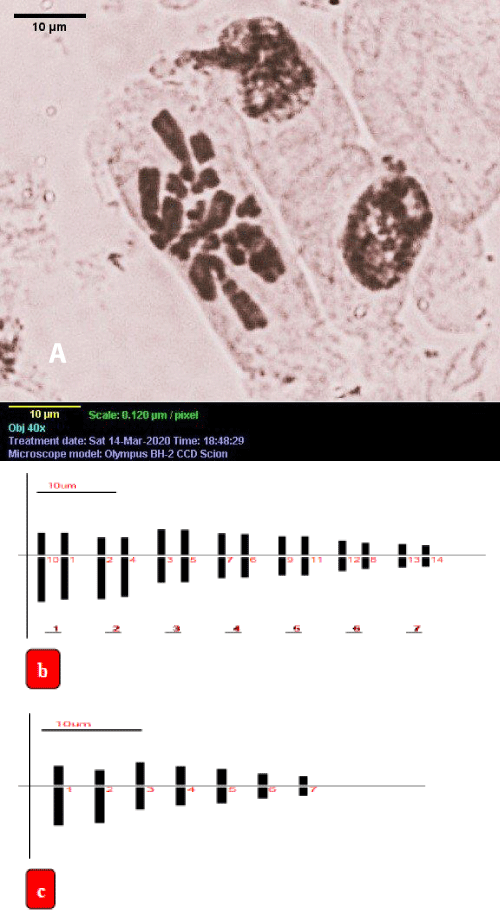
Karyotype of in vitro grown plants of A vera L: The chromosome number of in vitro raised plants was found to be 2n = 14 in the somatic cells (Figure 4a) and the individual chromosome length ranged from 4.28 µm to 13.74 µm (Table 6).
| Table 6: Characterization of chromosomes of in vivo grown plants of A. vera L. on the basis of Length, Arm ratio, Relative length, Centromeric index, Centromeric type and TF%. | ||||||||||
| Chromosome pair | Long arm (l) µm |
Short arm (s) µm | Total length (T) µm | Arm ratio (l/s) | Relative length (RL) % | Centromeric Index (CI) % | Centromeric Type (CT) | Chromosome type | TF% | Centromeric formula |
| 1 | 9.22 | 4.52 | 13.74 | 2.04 | 21.92 | 32.88 | sm | L | 42.23 | 2 sm + 5 m |
| 2 | 8.64 | 3.61 | 12.25 | 2.39 | 19.56 | 29.44 | sm | L | ||
| 3 | 5.27 | 5.27 | 10.54 | 1 | 16.83 | 50 | m | M | ||
| 4 | 4.41 | 4.41 | 8.82 | 1 | 14.07 | 50 | m | M | ||
| 5 | 3.86 | 3.86 | 7.72 | 1 | 12.33 | 50 | m | M | ||
| 6 | 2.66 | 2.66 | 5.32 | 1 | 8.48 | 50 | m | S | ||
| 7 | 2.14 | 2.14 | 4.28 | 1 | 6.82 | 50 | m | S | ||
Of the 7 genomic chromosomes, 2 were submetacentric and 5 were metacentric. The centromeric formula was 2sm + 5m (Figures 4b,c). All the submetacentric were long. Three of the metacentric chromosomes were medium and 2 were small. Thus the appropriate karyotypic formula was n = 2Lsm + 3Mm + 2Sm. TF% was 42.23 and the total length of the haploid complement set was 62.63. Chromosomes with secondary constrictions were absent. The total length of long arms was 36.20 µm and that of short arms was 26.47 µm. The ratio between longest and shortest chromosomes was more than 2:1 and the frequency of chromosomes with arm ratio was more than 0.29. Thus karyotype fell in the 2B type. The same types of results are shown in various types of plant sp species namely, Brachystemma and Craspedolobium [34], Lonicera [35].
Figure 4: a) Microscopic photograph of somatic metaphase chromosomes of in vitro grown plant of A. vera L., b,c) Ideogram of in vitro grown plant, scale bar = 10 µm, b) Somatic cell 2n = 14, c) n = 7.
Comparative analysis of karyotype of mother plants and micropropagated plants: The somatic chromosome number, chromosome size and details of the karyotype of in vivo and in vitro grown plants of A. vera were presented in (Figures 3,4) (Table 7). Chromosome sizes of the mother plant ranged from 14.1 µm to 4.46 µm long with a gradual decrease in length from the longest to the shortest chromosome. Whereas, chromosome sizes of in vitro raised plants ranged from 13.73 µm to 4.28 µm long with a gradual decrease in length from the longest to the shortest chromosome. Short arm and long arm chromosomes of naturally grown plants and in vitro grown plants were almost similar. The longest short arm chromosomes of in vivo and micropropagated plants were 4.45 µm and 4.52 µm, respectively, while the shortest short arm chromosomes of both plants were 2.23 µm and 2.14 µm, respectively. In addition, the shortest long arm chromosomes of in vivo and in vitro grown plants were 2.23 µm and 2.18 µm respectively, whereas the longest long arm chromosomes of mother plants and in vitro grown plants were 9.65 µm and 9.22 µm respectively.
| Table 7: Comparison of chromosome characters between in vivo and in vitro grown plants of A. vera L. | |||
| Chromosome characters | in vivo grown plant | in vitro raised plant | |
| Somatic chromosome number | 2n = 14 | 2n = 14 | |
| Range of Individual Chromosome Length (µm) | 4.46 - 14.1 | 4.28 - 13.74 | |
| Total Haploid Chromatin Length (TCL) | 62.96 | 62.63 | |
| The total length of haploid complement | Long arm(µm) | 36.73 | 36.20 |
| Short arm(µm) | 26.26 | 26.47 | |
| The ratio of longest/Smallest Chromosome | 3.16:1 | 3.21:1 | |
| Range of Relative Length (µm) | 7.08 - 22.40 | 6.82 - 21.92 | |
| The Total Form Percent (TF%) | 41.69 | 42.23 | |
| Centromeric Index (CI) | 31.54 - 50 | 32.88 - 50 | |
| Karyotype Formula | n = 7 = 2Lsm + 2Mm + 3Sm 2n = 14 = 4Lsm + 4Mm + 6Sm |
n = 7 = 2Lsm + 3Mm + 2Sm 2n = 14 = 4Lsm + 6Mm + 4Sm |
|
| Karyotype class | 2B | 2B | |
The total length of the haploid complement of field-grown plants and in vitro grown plants was 62.96 and 62.63 respectively. The centromeric index of mother plants and in vitro grown plants was 31.54 to 50 and 32.88 to 50 respectively. The total form percent of mother and in vitro grown plants was 41.96 and 42.23, respectively. The chromosome complement of both plants consisted of four submit centromeric and 10 metacentric chromosomes, (Figures 3,4) (Table 7). Therefore, the karyotype formula of in vivo grown plants was 2n = 14 = 4Lsm + 4Mm + 6Sm. Whereas, the karyotype formula of in vitro grown plants was 2n = 14 = 4Lsm + 6Mm + 4Sm. As shown in Figures 3,4, it can be seen that no satellite was found in both karyotypes. The result also showed that the proportion of the largest chromosome total length and the smallest chromosome total length (R) of the mother plant and in vitro plants were 3.16 and 3.21, respectively. The karyotype of both plants fell in the 2B type.
The present investigation has shown that the response of A. vera seems to be dependent more precisely on the types of explant as well as on the PGRs treatments. MS medium fortified with 2.0 mg/l BAP + 1.0 mg/l IAA gave the best response to shoot induction. The highest elongation (5.21 ± 0.11 cm) of multiple shoot buds was noted on MS medium with 2.0 mg/l BAP + 1.0 mg/l IAA and half strength MS medium with 2.0 mg/l IBA was most suited for root initiation. The plantlets obtained survived and grew normally in the outside environment. The results of the karyomorphological analysis showed that growth medium compositions, plant growth regulators, light length, culture time and subcultures did not affect the in vitro grown plants. Their generated plants were morphologically similar to the mother plants of A. vera. There were very minute karyotypic variations between selected in vivo and in vitro plant species. The Karyomorphological study of the present investigation will be helpful to understand the number and morphology of chromosomes which are essential for future research in cytogenetics.
- Grindlay D, Reynolds T. The Aloe vera phenomenon: a review of the properties and modern uses of the leaf parenchyma gel. J Ethnopharmacol. 1986 Jun;16(2-3):117-51. doi: 10.1016/0378-8741(86)90085-1. PMID: 3528673.
- Singh RP, Dhanalakshmi S, Rao AR. Chemomodulatory action of Aloe vera on the profiles of enzymes associated with carcinogen metabolism and antioxidant status regulation in mice. Phytomedicine. 2000 Jun;7(3):209-19. doi: 10.1016/S0944-7113(00)80006-9. PMID: 11185732.
- Davis RH. Aloe vera- A scientific approach. VantagePressInc, New York. 1997; 290–306.
- Bozzi A, Perrin C, Austin S. “Quality and authenticity of commercial Aloe vera gel powders. Food Chemistry. 2007; 103(1): 22–30.
- Lawless J, Allan J. The Clinical Composition of Aloe vera, In: Aleo vera natural wonder cure. Thorsons, Publishing Ltd, London, United Kingdom. 2000; 161-171.
- Campestrini LH, Kuhnen L, Lemos PMM, Bach DB, Dias PF, Maraschin M. Cloning protocol of Aloe vera as study case for tailormade biotechnology to small farmers. Journal of Technology Management and Innovation. 2000; 1:76-79.
- Reynolds T, Dweck AC. Aloe vera leaf gel: a review update. J Ethnopharmacol. 1999 Dec 15;68(1-3):3-37. doi: 10.1016/s0378-8741(99)00085-9. PMID: 10624859.
- Yadav K, Singh N. Micropropagation of Spilanthes acmella Murr. An important medicinal plant. National Sciences. 2010; 8: 4-11.
- Bhojwani SS, Razdan MK. Plant tissue culture: theory and practice. Elsevier. 1986.
- Baksha R, Jahan MAA, Rahima K. Micropropagation of Aloe barbadensis Mill. through In vitro culture of shoot tip explants. Plant Tissue Culture and Biotechnology. 2005; 152:121-126.
- Singh B, Sood N. Significance of explant preparation and sizing in Aloe vera L.-A highly efficientmethod for in vitro multiple shoot induction. Scientia Horticulturae. 2009; 122:146-151, 2009.
- Natali L, Sanchez I, Cavallini A. in vitro culture of Aloe barbadensis Mill: micropropagation from vegetative meristems. Plant Cell, Tissue and Organ Culture. 1990; 20:71-74.
- Samadder T, Nath S, Halder M, B. Sil, Roychowdhury D, Sen S, Sumita S. Karyotype analysis of three important traditional Indian medicinal plants Bacopa monnieri, Tylophora indica, and Withania somnifera. Nucleus; 55:17-20.
- Young HA, Sarath G, Tobias CM. Karyotype variation is indicative of subgenomic and ecotypic differentiation in switchgrass. BMC Plant Biol. 2012 Jul 26;12:117. doi: 10.1186/1471-2229-12-117. PMID: 22834676; PMCID: PMC3492167.
- Levan A, Fredga K, Sandberg AA. Nomenclature for Centromeric Position on Chromosomes. Hereditas. 1964; 52:201-220.
- Huziwara Y. Karyotypic analysis in some genera of compositae VIII. Further studies on the chromosomes of Aster.American Journal of Botany. 1982; 49:116-119
- Stebbins GL. Longevity, habitat. and release of genetic variability in the higher plants. Cold Spring Harb Symp Quant Biol. 1958;23:365-78. doi: 10.1101/sqb.1958.023.01.035. PMID: 13635568.
- Ahmed A, Omar H. In-vitro propagation of the multipurpose Egyptian medicinal plant Pimpinella anisum. Egyptian Pharmaceutical Journal. 2019; 18( 3):254-262.
- Manivannan A, Soundararajan P, Park YG, Jeong BR. in vitro Propagation, Phytochemical Analysis, and Evaluation of Free Radical Scavenging Property of Scrophularia kakudensis Franch Tissue Extracts. Biomed Res Int. 2015;2015:480564. doi: 10.1155/2015/480564. Epub 2015 Nov 16. PMID: 26649304; PMCID: PMC4663745.
- Kirillov V, Pathak A, Stikhareva T, Ercisli S, Daulenova M, Kazangapova N, Rakhimzhanov A. “in vitro propagation and ex vitro rooting of Euonymus verrucosus Scop. (Celastraceae) - a rare species of Kazakhstan flora on the southern border of its areal. Journal of Forest Research. 2022; 27(4):289-296.
- Nazir U, Gul Z, Shah G, Khan N. Interaction Effect of Auxin and Cytokinin on in vitro Shoot Regeneration and Rooting of Endangered Medicinal Plant Valeriana jatamansi Jones through Tissue Culture. American Journal of Plant Sciences. 2022; 13:23-240.
- Singh NM, Chanu LA, Devi YP, Singh WRC, Singh HB. Micropropagation-an in vitro technique for the conservation of Alpinia galangal. Advances in Applied Science Research. 2014; 5(3):259-263.
- Akın B, Çetin B, Bingöl NA. in vitro propagation of wetland medicinal plant Lythrum salicaria L. Celal Bayar University Journal of Science. 2018; 14(4): 369-372.
- Reshi KNA, Sudarshana MS, Girish HV. in vitro micropropagation of Rhinacanthus nasutus L. International Journal of Biodiversity and Conservation. 2018; 10(9):357-364.
- Lijalem T, Feyissa T. in vitro propagation of Securidaca longipedunculata (Fresen) from shoot tip: an endangered medicinal plant. J Genet Eng Biotechnol. 2020 Jan 20;18(1):3. doi: 10.1186/s43141-019-0017-0. PMID: 31956941; PMCID: PMC6970091.
- Zayova EG, Geneva MP, Georgieva KDM, Hristozkova MG, Stancheva IV. Impact of plant growth regulators on Greek oregano micropropagation and antioxidant activity. Biosciences Biotechnology Research Asia. 2019; 16(2):297-305.
- Aggarwal D, Upadhyay SK, Kumar K, Sehrawat N, Tuli HS, Singh R. Effects of plant growth regulators on in vitro propagation of economically important ornamental plant Rosa hybrida L. Asian Journal of Biological and Life Sciences. 2020; 9(2):227-233.
- Gopu C, Chakilam CS, Chirumamilla P, Vankudoth S, Taduri S. Rapid in vitro adventitious rooting and proliferation by leaf and nodal cultures of Momordica cymbalaria Fenzl. Journal of Applied Biology and Biotechnology. 2020; 8(02):103–107.
- Zayova E, Petrova M, Dimitrova L, Vasilevska-Ivanova R, Stoeva D. Effectof different auxins on in vitro rooting of Paulownia elongata propagated plants. Genetics and Plant Physiology. 2014; 4(3–4):155–162.
- Priyadarshni M, Arunima M, Kumara R, Shukla LN. Tissue culture studies of Heliotropium Indicum an important medicinal herb for callus induction and micro propagation. International Journal of Research and Analytical Reviews. 2014; 1(4):618-625.
- Xin-Hua Z, Silva AT, Ma G. Karyotype analysis of Santalum album L. Caryologia. 2010; 63(2):142-148.
- Zhao C, Li F, Gao S. Research on Chromosome Karyotype Analysis of Plumbago auriculata. Open Access Library Journal. 2014; 1:1-7.
- A. Biswas and M. M. Rahman, “Karyotype analysis of Thespesia lampas (Cav.) Dalz. & Gibs. from Bangladesh,” Journal of Pharmacognosy and Phytochemistry.2017; 6: 4;1316-1317.
- Xu J, Yin Z, Funamoto T, Peng H. First report of chromosome numbers and karyotypes of two monotypic genera endemic to eastern Asia: Brachystemma (Caryophyllaceae) and Craspedolobium (Fabaceae). Nordic Journal of Botany.2011; 29:200-203.
- Yan QJ, Zou LJ, Tian TT, Wang L, Shen XL, Luo MH. [Karyotype analysis of three Lonicera species growth in Sichuan]. Zhong Yao Cai. 2014 Mar;37(3):384-7. Chinese. PMID: 25174099.