More Information
Submitted: 14 June 2020 | Approved: 01 July 2020 | Published: 02 July 2020
How to cite this article: Halwani C, Zribi S, Bouomrani S. An exceptional case of bilateral vestibular areflexia complicating acute otitis media. Heighpubs Otolaryngol Rhinol. 2020; 4: 008-011.
DOI: 10.29328/journal.hor.1001019
Copyright License: © 2020 Halwani C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Bilateral vestibular ataxia; Bilateral vestibulopathy; Otitis media; Peripheral vertigo
An exceptional case of bilateral vestibular areflexia complicating acute otitis media
Chiraz Halwani1,2, Sarra Zribi2 and Salem Bouomrani3,4*
1Department of Otorhinolaryngology, Military Hospital of Tunis, Mont Fleury 1008, Tunisia
2Tunis Faculty of Medicine, Tunis El Manar University, Tunis 1007, Tunisia
3Department of Internal Medicine, Military Hospital of Gabes, Gabes 6000, Tunisia
4Sfax Faculty of Medicine, University of Sfax, Sfax 3029, Tunisia
*Address for Correspondence: Dr. Salem Bouomrani, Department of Internal Medicine, Military Hospital of Gabes, Gabes 6000, Tunisia, Tel: +00216 98977555; Email: salembouomrani@yahoo.fr
Introduction: Bilateral vestibular areflexia is a rare pathological entity whose most frequent etiology is drug ototoxicity. We report an unusual case of bilateral vestibular areflexia complicating acute otitis media through which we raise the difficulties of diagnosis and therapeutic management of this pathology.
Case Report: 57-year-old Tunisian patient who consults for a loss balance associated with earache and hearing loss. Initial clinical examination revealed bilateral acute otitis media with a right harmonious vestibular syndrome and normal neurological examination. The diagnosis of post-otitis labyrinthitis was retained. The patient was put on antibiotics and corticosteroids. The evolution was marked by the persistence of instability in darkness and oscillopsia; vestibular explorations concluded with bilateral vestibular areflexia. MRI concluded to posterior labyrinthitis and eliminated central neurological involvement. The patient was kept under betahistine. The tympanic cavity was drained by a tympanic aerator on both sides. Vestibular rehabilitation was started quickly. Gradual improvement was obtained of autonomy with persistent oscillopsia.
Conclusion: Bilateral vestibular areflexia poses diagnostic problems based on anamnestic and clinical arguments and vestibular explorations. The therapeutic management is delicate, vestibular reeducation occupies a primordial place.
Bilateral vestibular areflexia (BVA), also known as bilateral vestibulopathy, bilateral vestibular weakness or bilateral vestibular dysfunction is a rare pathological entity of recent individualization [1,2]. Its prevalence is globally estimated at 28/100,000H by Ward, et al [3], and it represents only 0.7% of all consultations in an otolaryngology department [2].
The diagnosis of BVA is a real challenge for clinicians [4] because various systemic and neurologic diseases may present with bilateral vestibular loss [2,5].
The most common etiologies for BVA are: ototoxicity/ototoxic drugs (particularly aminoglycoside antibiotics), bilateral Ménière’s disease, bilateral vestibular neuritis, meningitis, autoimmune inner ear disease, and bilateral vestibular schwannomas (particularly in patients with neurofibromatosis type 2) [2,4,5]. Rarer entities such as neurosyphilis, superficial siderosis, neurosarcoidosis, congenital malformations of the vestibular end organs, head injuries and trauma, “cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS)” can be listed as possible causes of BVA [2,4,5], and the etiology of these dysfunctions remains unknown in approximately 50% of cases [2,4,5].
Acute otitis media remains an exceptional and often overlooked etiology of BVA [1-5].
We report an unusual case of BVA complicating acute otitis media in a 57-years-old Tunisian woman.
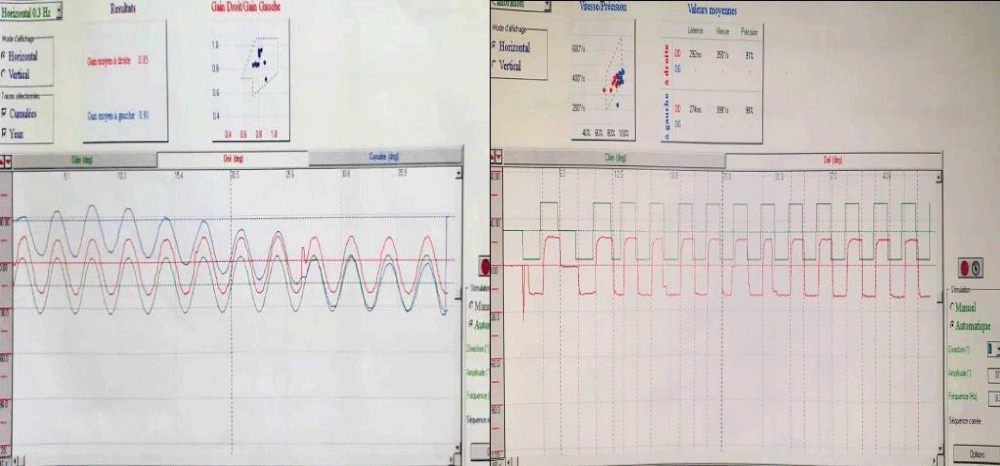
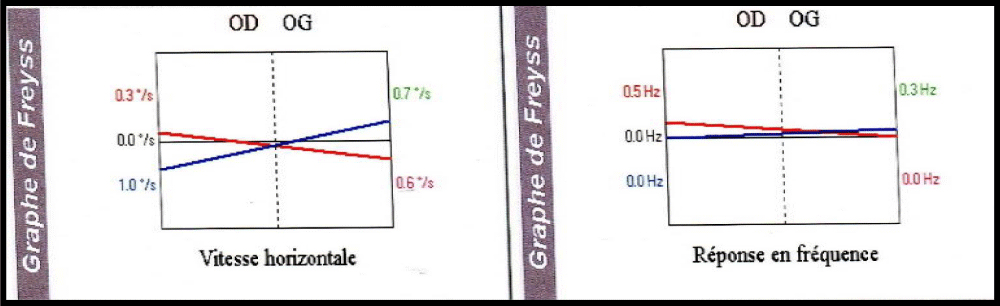
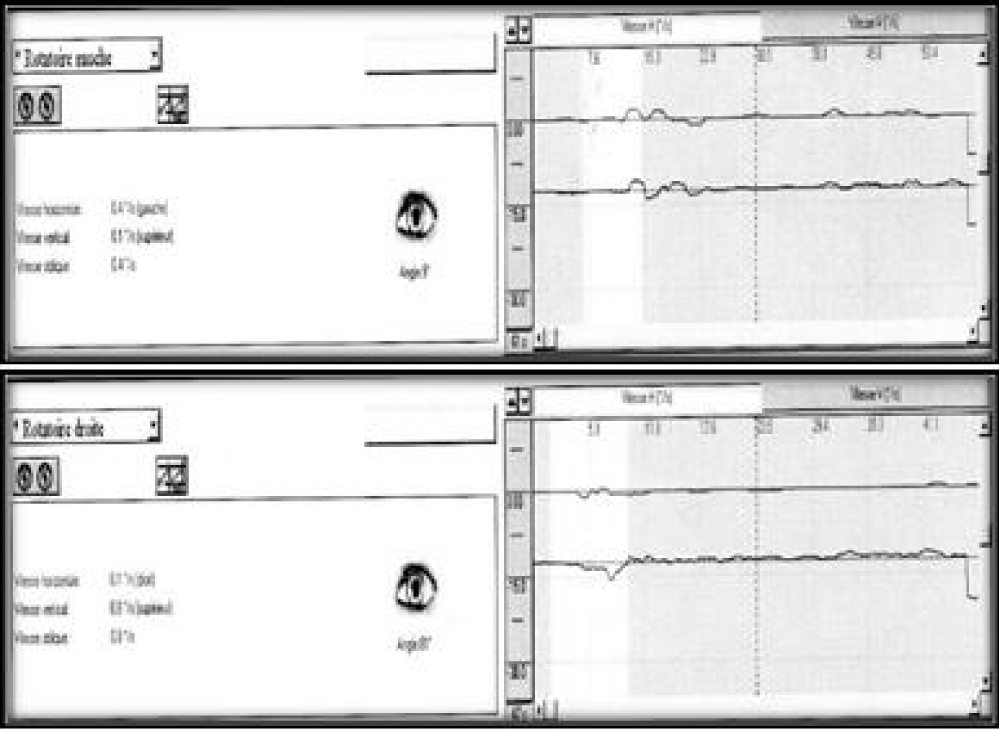
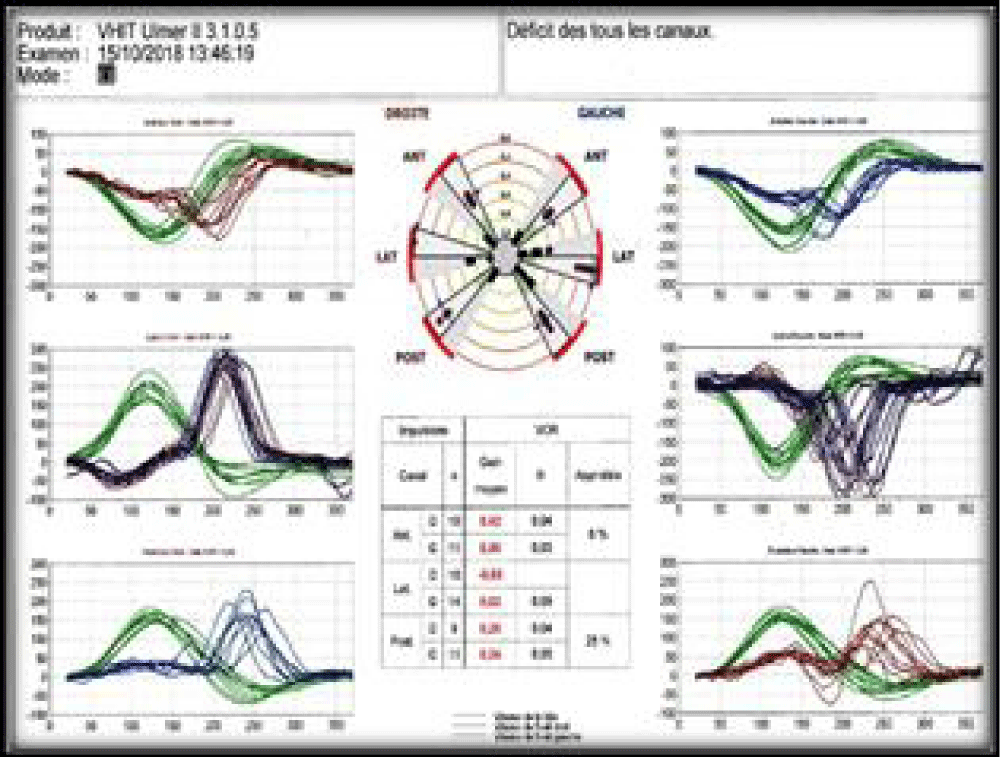
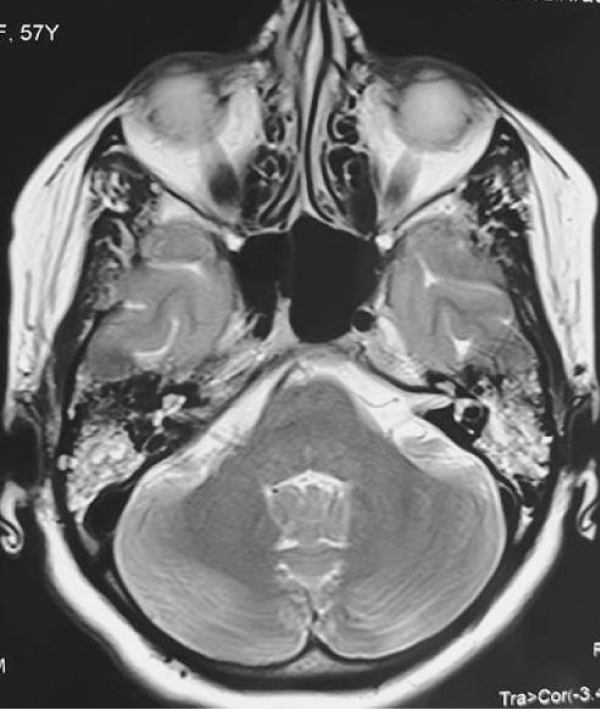
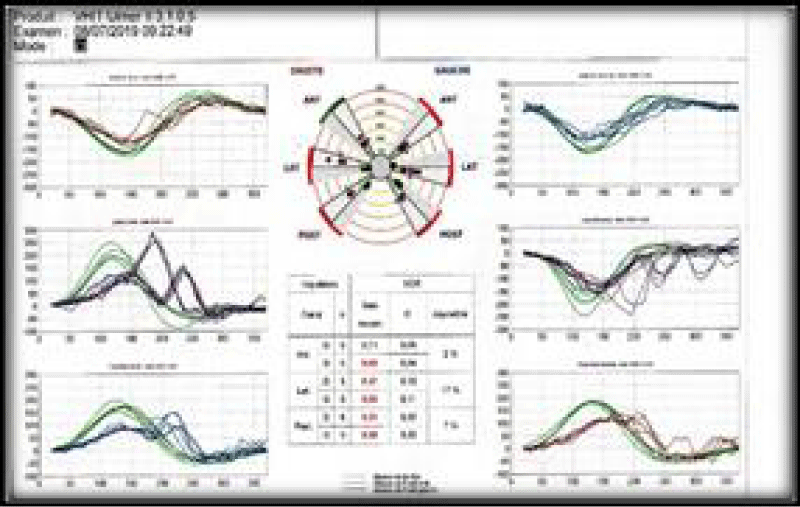
A 57-years-old Tunisian woman, presented with a sudden loss of balance with nausea and vomiting, earache, tinnitus and deafness. Vestibular examination found a horizontally rotational left nystagmus (grade III), with index fingers deflected to the right. The neurological examination was normal. The otoscopic examination showed bilateral eardrum congestion without perforation. The diagnosis of post otitic labyrinthitis was accepted, the patient was treated with cefpodoxime, corticosteroids and acetyl-leucine. After a week, the patient described oscillopsies and could not feel the ground under her feet. Tonal audiometry (audiogram) noted pure transmissive deafness with a threshold at 30 dB on both sides. Videonystagmography (VNG) noted a spontaneous left nystagmus with vestibular diplegia on caloric and rotary tests (Figures 1-3). At video head impulse testing (VHIT), a significant drop in all earnings was noted and concluded that all channels had been reached (Figure 4). Magnetic resonance imaging (MRI) with T1, T2, and FLAIR sequences noted filling of the posterior cavities of the middle ear with a decrease in signal of the posterior labyrinth without signs of a labyrinthine fistula (Figure 5). There were no other abnormalities in the cerebral, cerebellar, or acousto-facial bundle on either side. The patient was kept on betahistine. The tympanic cavity was ventilated by the installation of a transtympanic aerator (T-Tube) on both sides. Vestibular rehabilitation was initiated after 15 days in the face of persistent discomfort at the rate of 3 sessions per week for a period of 6 months. The vertical optokinetics technique and dynamic visual acuity were used to decrease visual dependence and the active dynamic movement technique on a soft plane to decrease oscillopsies. A gradual improvement in autonomy was noted with a decrease in oscillopsia and perfect autonomy during ambulations at home and outside. After 15 months, the patient resumed an almost normal activity with driving a car, daily walk swimming in the pool without incidents. However, she describes a hint of instability. The control VNG and VHIT (Figure 6) did not show improvement in vestibular function, only the gain of the right upper channel was improved.
Figure 1: VNG: normal slow and jerky eye tracking.
Figure 2: Vestibular diplegia on caloric test.
Figure 3: VNG: right rotary test no nystagmus neither per nor post rotary.
Figure 4: VHIT very low VOR gains on all channels.
Figure 5: T2-weighted axial brain and ponto-cerebellar angle (PCA) MRI: bilateral filling, especially on the left, of mastoid cells with APC integrity and labyrinthine hyper signal.
Figure 6: Control VHIT, improvement of the gain of the right anterior channel.
The post-otitis infectious etiology of BVA is rare [2,4,5], and is seen mainly in the course of chronic otitis media [6]. Indeed, only three cases of otitis was found in the small Chinese series of 42 patients with bilateral vestibular weakness [4], but no case secondary to otitis was noted in Hain, et al. series of 213 patients followed for BVP [2].
Its main symptoms are postural imbalance, instability in walking, deficits in spatial memory and navigation [7], aggravated in the dark and/or by walking on uneven ground. No symptoms appear under static conditions. Oscillopsies during head and body movements are also major symptoms [2,8]. The absence of an intense rotatory character of the vertigo as it would be during the brutal vestibular deafferentations is due to the concomitant involvement of the two vestibules [2,6-8]. In our case, the significant imbalance dominated the clinical picture. The patient described hearing loss which was of the transmissive type. The preservation of normal cochlear function and the purely vestibular involvement makes our original observation, to our knowledge unique in the literature.
Vestibulo-ocular (VOR) and vestibulo-spinal reflex deficits during BVA lead to impaired orientation, navigation and spatial memory during movements of the head at high acceleration, which results in an oscillopsia induced by head movement and reduced dynamic visual acuity [2,6-8]. In the absence of visual and proprioceptive signals, patients lose their sense of verticality and become disoriented. Spatial learning performance is delayed due to anatomical and functional changes in hippocampal formation [7]. For the diagnosis of BVA, vestibular explorations are essential [9]. According to Barany’s criteria, the diagnosis is made on reduced or absent gains on the VHIT affecting the horizontal channel (< 0.6), reduced responses on the caloric test with a sum of the responses less than 6°/sec and a reduction VOR with a gain < 0.1 during sinusoidal stimulation on a rotary chair (0.1Hz, Maximum speed of 50°/sec) [7]. All these criteria are met in the case of our observation.
Imaging is essential in the etiological assessment [10]. On the MRI, the FLAIR and particularly delayed 3D-FLAIR sequence allows an analysis of the brain parenchyma and the inner ear [10-12]. In addition, functional MRI (fMRI) would be very useful for the diagnosis of BVA by showing increased brain responsivity to galvanic vestibular stimulation [11].
The management of BVA is delicate. Vestibular rehabilitation has an important role [2,5]. Bilateral vestibular involvement justifies optokinetic stimulation. Exercises on a rotary chair are irrelevant [13]. Rehabilitation is based on the use of the optokinetic stimulation generator, the work of vestibulo-spinal reflexes with eyes open and closed on a stable and unstable plane, the work of walking on more or less stable ground with decrease in the lift polygon and the work of dynamic visual acuity [14,15]. Recently, new techniques have been proposed for the management of this disease: cognitive rehabilitation/training and noisy galvanic vestibular stimulation are two emerging treatment options for BVA which seem to be very promising, particularly for improving gait performance [16,17].
BVA poses diagnostic problems which are based on currently well-defined arguments and must be sought by history, clinical examination and especially vestibular explorations.
Therapeutic management is delicate. Vestibular rehab-
ilitation occupies a primordial place and allows resolution of symptoms and improvement of functioning in the medium term.
Acute otitis media remains an exceptional and unusual etiology of this bilateral vestibulopathy. It deserves to be known by any healthcare professional to avoid delayed diagnosis. Early diagnosis is the only guarantee of a good prognosis since this etiology is one of the rare treatable causes of this disease.
- Ulmer E, Magnan J, Chays A. Bilateral vestibular areflexia: quantification is required. Ann Otolaryngol Chir Cervicofac. 2002; 119: 216-226. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12410118
- Hain TC, Cherchi M, Yacovino DA. Bilateral Vestibular Weakness. Front Neurol. 2018; 9: 344. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29904366
- Ward BK, Agrawal Y, Hoffman HJ, Carey JP, Della Santina CC. Prevalence and impact of bilateral vestibular hypofunction: results from the 2008 US National Health Interview Survey. JAMA Otolaryngol Head Neck Surg. 2013; 139: 803‐810. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23949355
- Lin Y, Gao LX, Li L, Wang JL, Shen JJ, et al. Etiology Analysis and Vestibular Assessment of Bilateral Vestibular Vestibulopathy. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018; 32: 379-382. PubMed: https://pubmed.ncbi.nlm.nih.gov/29798299/
- Lee SU, Kim HJ, Kim JS. Bilateral Vestibular Dysfunction. Semin Neurol. 2020; 40: 40‐48. PubMed: https://pubmed.ncbi.nlm.nih.gov/31935769
- Taki M, Nakamura T, Matsuura H, Hasegawa T, Sakaguchi H, et al. Cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS). Auris Nasus Larynx. 2018; 45: 866-870. PubMed: https://pubmed.ncbi.nlm.nih.gov/30606994
- Strupp M, Kim JS, Murofushi T, Straumann D, Jen JC, et al. Bilateral vestibulopathy: diagnostic criteria consensus document of the Classification Committee of the Bárány Society. J Vestib Res. 2017; 27: 177-189. PubMed: https://pubmed.ncbi.nlm.nih.gov/29081426/
- Fujimoto C, Yagi M, Murofushi T. Recent advances in idiopathic bilateral vestibulopathy: a literature review. Orphanet J Rare Dis. 2019; 14: 202. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31426838
- Rosengren SM, Welgampola MS, Taylor RL. Vestibular-Evoked Myogenic Potentials in Bilateral Vestibulopathy. Front Neurol. 2018; 9: 252. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29719527
- Craighero F, Casselman JW, Safronova MM, De Foer B, Delanote J, EF Officers. Sudden onset vertigo: imaging work-up. J Radiol. 2011; 92: 972-986. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3508265/
- Helmchen C, Rother M, Spliethoff P, Sprenger A. Increased brain responsivity to galvanic vestibular stimulation in bilateral vestibular failure. Neuroimage Clin. 2019; 24: 101942. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6690736/
- Eliezer M, Hautefort C, Van Nechel C, Duquesne U, Guichard JP, et al. Electrophysiological and inner ear MRI findings in patients with bilateral vestibulopathy. Eur Arch Otorhinolaryngol. 2020; 277: 1305-1314. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32036409
- Sulway S, Whitney SL. Advances in Vestibular Rehabilitation. Adv Otorhinolaryngol. 2019; 82: 164-169. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30947180
- Tsuzuku T, Vitte E, Sémont A, Berthoz A. Modification of parameters in vertical optokinetic nystagmus after repeated vertical optokinetic stimulation in patients with vestibular lesions. Acta Otolaryngol Suppl. 1995; 520: 419‐422. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8749178
- Jahn K, Saul AK, Elstner M, Sapa K, Kellerer S. Vestibular rehabilitation therapy and Nintendo Wii balance board training both improve postural control in bilateral vestibulopathy. J Neurol. 2018; 265: 70-73. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29725843
- Ellis AW, Schöne CG, Vibert D, Caversaccio MD, Mast FW. Cognitive Rehabilitation in Bilateral Vestibular Patients: A Computational Perspective. Front Neurol. 2018; 9: 286. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29755404
- Wuehr M, Decker J, Schniepp R. Noisy galvanic vestibular stimulation: an emerging treatment option for bilateral vestibulopathy. J Neurol. 2017; 264: 81-86. PubMed: https://pubmed.ncbi.nlm.nih.gov/28391373/