More Information
Submitted: 24 March 2020 | Approved: 12 August 2020 | Published: 13 August 2020
How to cite this article: Brown-Martínez M, Hernández Z, Valdés Y, González E, Despaigne E, et al. Silent cerebrovascular disease in hypertensive adults is frequent and age-dependent. Ann Clin Hypertens. 2020; 4: 001-008.
DOI: 10.29328/journal.ach.1001021
ORCiD: orcid.org/0000-0003-1740-2359
Copyright License: © 2020 Brown-Martínez M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Cerebral small vessel disease; Extracraneal carotid disease; Hypertension; Cardiovascular risk; Cognitive impairment
Silent cerebrovascular disease in hypertensive adults is frequent and age-dependent
Marta Brown-Martínez1*, Zenaida Hernández2, Yamile Valdés3, Edilberto González4, Emelina Despaigne5 and Evelio Gonzalez6
1MD, Neurophysiology Department, Sport Medicine Institute. Calle 10 e/ 100 y 14, Boyeros, La Habana, 10400, Cuba
2MD, Radiology Department, International Center for Neurological Restoration, La Habana, Cuba
3MD, Research Department, University Hospital General Calixto García, La Habana, Cuba
4MD, Director University Hospital General Calixto García, La Habana, Cuba
5Nr, Research Department, University Hospital General Calixto García, La Habana, Cuba
6PhD, Neuroimaging Department, Cuban Neuroscience Center, La Habana, Cuba
*Address for Correspondence: Marta Brown-Martínez, Sport Medicine Institute, Calle 10 e/ 100 y 14, Boyeros, La Habana, 10400, Cuba, Tel: +53 52662291; Email: martabr@imd.inder.cu; martykabrown@gmail.com
Background: Cerebral small vessel disease and extracranial atherosclerotic carotid disease are manifestations of silent cerebrovascular disease (CVD). Information on these two pathologies in hypertensive population with low cardiovascular risk (CVR) is scarce.
Objective: To explore frequency and characteristics of silent CVD in hypertensive adults and cognitive repercussion of these alterations.
Methods: 39 hypertensive patients (mean age: 53.5 years) were studied. Cerebral magnetic resonance imaging (3T), doppler ultrasound of the carotid artery and neuropsychological studies were obtained.
Results: 79% of patients presented white matter lesions (WML), 18% showed only cerebral atrophy and/or enlarged perivascular spaces, 60% presented hyperplasia of intimal media complex (IMC) and/or atheroma plaques. In women, a significant correlation was observed between IMC thickness and bifrontal index, and WML was greater in patients with carotid plaques. A non-significant decrease in neuropsychological performance was observed in the groups of patients with intra and/or extracerebral injury and a negative correlation with the bifrontal index in men was found.
Conclusion: Frequency of intra and extracerebral silent CVD was high in hypertensive adults with low to moderate CVR. WML and brain atrophy were partially related with carotid lesions. Age significantly influenced the appearance of intra and extracerebral lesions. Cognitive performance did not decrease significantly due to the presence of these lesions.
Cardiovascular diseases account for about 48% of all deaths from chronic non-communicable diseases. It include cerebrovascular diseases (CVD), which are the second leading cause of death worldwide [1]. It is recognized that prevalence of silent CVD exceeds that of clinically manifest CVD and constitutes the most frequent incidental finding in brain imaging, especially in elderly patients [2]. These abnormalities generally represent signs of cerebral small vessel disease (CSVD), which comprises a group of lesions visible on magnetic resonance imaging (MRI) such as recent small silent infarcts, lacunar infarcts, white matter lesions (WML), microbleeds, cerebral atrophy, and enlarged perivascular spaces [3].
Similarly, Extracranial Carotid Atherosclerotic Disease (ECAD) develops asymptomatically over a long period of time, causing up to 20% of all ischemic strokes [4]. However, results of ECAD s studies mainly focus on the significant carotid stenosis (≥ 50% -70%) identification and on the increase in intima-media complex (IMC) thickness. Only recent investigations begin to consider subclinical morphological and hemodynamic changes present in ECAD [5-9].
Several studies have reported a high predictive value of these pathologies, which is why they are considered potential biomarkers useful to improve the algorithms for predicting vascular events [10,11]. However, the cost and availability of neuroimaging techniques necessary for identification of silent CVD does not allow its massive application. On the other hand, identification of ECAD by doppler ultrasound as the most affordable technique, is currently recommended only for evaluation of patients with high cardiovascular risk [4].
Therefore, the information on these two pathologies is not sufficient, especially in the hypertensive population of low to moderate risk. In this work we explore the frequency and characteristics of silent intra and extracerebral CVD in a group of adult hypertensive patients. Additionally, we evaluated the relationship between carotid morphological changes with asymptomatic brain injury, and the cognitive impact of these injuries in these patients.
Subjects
A cross-sectional study was carried out in the period from October 2015 to July 2016 in neurologically asymptomatic hypertensive patients, who came consecutively to the service of Vascular Risk Group at Calixto García Hospital. The inclusion criteria included a history of essential arterial hypertension (HT), of any time of evolution and age under or equal to 65 years. Exclusion criteria included a history of cerebrovascular disease or cerebral disease from any other cause, diagnosis of cognitive impairment or dementia, claustrophobia and incompatibility with the study of resonance. Variables such as arterial hypertension control and treatment were not taken into account to participate in the study. All patients signed the informed consent for inclusion in the study, which was approved by Institutional Ethics Committee and was in accordance with the principles of the Helsinki´s Declaration for research in humans.
Global cardiovascular risk of patients was calculated using the online application from Pan American Health Organization (PAHO) web, available at https://www.paho.org/cardioapp/web/. It allows to estimate the risk of a relevant cardiovascular disease for first time in ten years, such as myocardial infarction, angina, stroke, in individuals over 40 years, taking into account the following variables: gender, age, smoking, systolic pressure, cholesterol levels and the presence of Diabetes mellitus.
Hemochemical results were obtained from peripheral venous blood in the morning with standardized procedures in the hospital laboratory.
Brain magnetic resonance
Brain images were obtained with high-resolution (3T) MRI equipment at Cuban Neuroscience Center (Siemens, MAGNETOM Concerto, Germany). Three-dimensional T1 and T2 sequences were used in the axial, sagittal and coronal planes (1 mm cuts); FLAIR sequences (2 mm cuts) and T2 * sequences (4 mm cuts), both in axial plane [12].
The images obtained were evaluated by an observer and the Scheltens visual semi-quantitative scale was applied for quantification of cerebral WML, using FLAIR sequence images. The same observer also performed linear measurements of cerebral atrophy including bifrontal index and frontal interhemispheric fissure, using images of T1 sequences [13]. In one case, it was not possible to obtain these indices due to image sharpness defects because of artifacts produced by patient movements.
Carotid doppler ultrasound
Both carotid axes (common carotid artery, bulb) were studied by color echo-doppler in the ALOKA equipment (PROSOUND alpha 10 model, Japan). The same image expert performed all the procedures, following the standardized methodology for it. Thickness of Intima-Media Complex (IMC) in the posterior wall of the distal third of common Carotid artery was determined taking as normal value ≤ 0.9 mm. The presence of carotid atherosclerotic plaque was evaluated by the Mannheim consensus (focal structure that invades the arterial lumen at least 0.5 mm or 50% of the value of the adjacent IMC; or IMC > 1.5 mm measured from the adventitia interface to the intimate interface) [14]. The hemodynamic flow parameters of the explored vessels were evaluated, but were not taken into account in this study. In one case it was not possible to perform the test due to insufficient visualization due to a poor acoustic window. In other 3 cases it was not possible to adequately define the IMC thickness.
Neuropsychological test
The Digit Symbol-Coding test was applied to all participants. It is one of the most sensitive test for cognitive deterioration secondary to vascular damage and allows evaluation of processing speed and executive function [15]. It was done by one examiner at a desk in a quiet room, with privacy, in the morning session.
Statistical analysis
Statistical analysis was performed using the Statistic program (Statsoft Inc.). Continuous variables with normal distribution were evaluated using mean and range parameters, and categorical variables using frequency and percent parameters. Continuous variables were compared using the non-parametric statistical tests Mann-Whitney and Kruskal Wallis test, to assess the differences between groups, and Spearman’s correlation coefficients were obtained to assess the relationship between variables. To evaluate the association between qualitative variables, the X2 test was used. In all statistical tests, the significance level was set less than 0.05.
Thirty-nine neurologically asymptomatic hypertensive patients were studied, five patients were withdrawn from the study due to the impossibility of performing the MRI (claustrophobia and unknown metals in the body). The final sample comprised 34 patients, 9 men and 25 women, with mean age of 53,5 years (SD: 6.7) and mean years of HT for 15.6 years (SD: 9.4). 8 patients (1 man and 7 women) also suffered from Type 2 Diabetes Mellitus. Clinical and demographic data are shown in table 1.
| Table 1: Demographic,clinical and laboratory data from patients and statistical significance ofthe difference. | |||
| Parameters (mean, range) |
Women n = 25 |
Men n = 9 |
p level |
| Age | 53.4 (41-65) | 53.7 (40-64) | 0,81 |
| Years ofHT | 14.5 (1-37) | 18.6 (1-31) | 0,17 |
| BMI | 28.8 (20-42) | 29 (21-33) | 0,70 |
| SystolicBP | 155 (117-214) | 144(115-167) | 0.29 |
| DiastolicBP | 95 (71-134) | 90(66-105) | 0.73 |
| Glycaemia(mmol/L) | 6.07 (4.6-12.7) | 6.24(4.7-9.3) | 0,56 |
| Cholesterol(mmol/L) | 5.4 (3.5-8.2) | 5.04 (4.27-6.19) | 0,55 |
| Triglycerides(mmol/L) | 1.61 (0.69-3.21) | 2 (0.96-4.55) | 0,42 |
| Uric acid(µmol/L) | 318(157-486) | 443 (277-607) | 0,01 |
| IMCthickness (mm) | 0.89 (0.6-1.3) | 0.92 (0.6-1.3) | 0,67 |
| Digits-symbols score | 39 (20-59) | 40(27-63) | 0,87 |
The application of the PAHO cardiovascular risk calculator revealed 58.8% of patients with low risk, 14.7% with moderate risk and 26.5% with high risk; all diabetic patients were included in this last category.
Intracerebral injury
Subcortical and periventricular white matter lesions were observed in 79% of these patients (27 cases), one of them presented a lacunar infarction. 18% (7 cases) showed only signs of cerebral atrophy and/or enlarged perivascular spaces (EPS), the latter appeared in 82% of the cases, located globally, mainly in the basal ganglia. Only 3% of the patients (1 case) were free of hypertensive-induced brain disorders. Based on these findings, the patients were divided into 2 groups, one group with non-ischemic injury (no lesion, brain atrophy and/or EPS) and another group with ischemic injuries (white matter injury or lacunar infarction). The application of Scheltens semi quantitative scale reflected predominance of subcortical white matter injury in frontal and parietal regions. Periventricular injuries were less frequent and predominantly at frontal horns. Women presented a higher frequency of subcortical frontal and parietal lesions than men, but these differences were not statistically significant (Table 2).
| Table 2: Mean values of Scheltens scale score (global and by brain regions) and linear measurements of brain atrophy and statistical significance of the difference. | |||
| Variables | Men | Women | p level |
| Scheltens Score mean (range) |
6 (0-21) | 6.92(0-24) | 0.70 |
| Frontal Subcortical | 2 | 2.24 | 0,81 |
| Parietal Subcortical | 1 | 1.36 | 0,64 |
| Frontal Periventricular | 1 | 0.80 | 0,50 |
| Occipital Subcortical | 0,78 | 0,68 | 0,94 |
| Temporal Subcortical | 0,78 | 0,56 | 0,53 |
| Occipitals Periventricular | 0,55 | 0,48 | 0,99 |
| Lateral Periventricular | 0,44 | 0,48 | 0,84 |
| Insula Subcortical | 0,11 | 0,16 | 0,92 |
| Bifrontal index mean (range) |
0.32(0.28-0.35) | 0.29(0.26-0.34) | 0.00 |
| Frontal interhemispheric fissure mean (range) | 3.83(3-5) | 3.82 (1.9-6.10) | 0.98 |
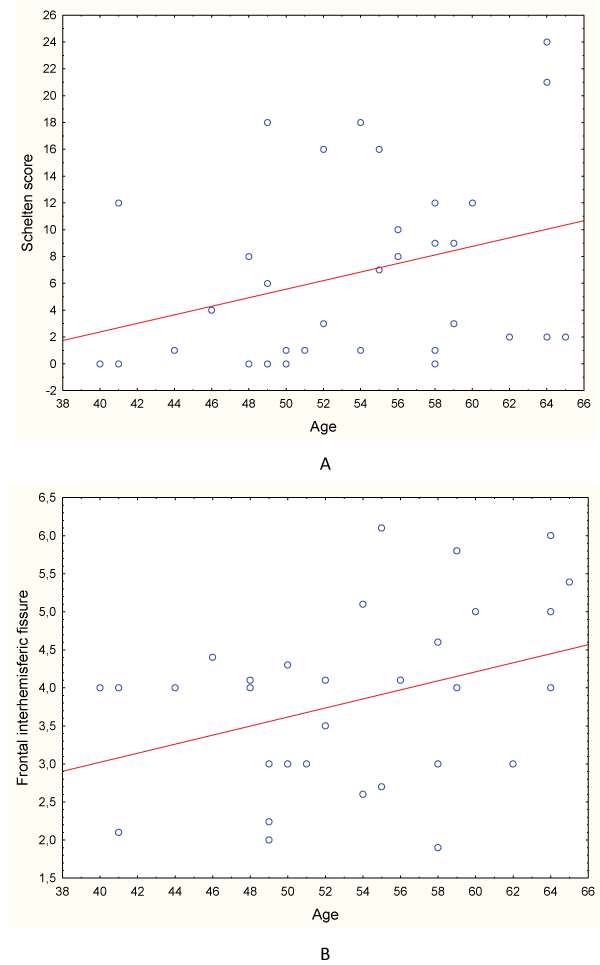
A positive significant correlation was observed between age and WML quantification (R = 0.36; p = 0.03) (Graph 1A).
Graph 1: Correlation between age and the severity of white matter lesion (A) and frontal interhemispheric fissure (B) in all patients.
68% of patients presented cerebral atrophy isolated or in combination with other lesions. The most frequent presentation was generalized atrophy (87%), followed by frontally located atrophy (9%). These patients had a significantly higher mean age (55 vs. 50 years; p = 0.02).
In addition, a weak positive correlation was observed between age and interhemispheric fissure values (R = 0.33; p = 0.06) (Graph 2), while bifrontal index values were significantly higher in men than in women (0.32 vs 0.29; p = 0.002), which forced subsequent analyzes taking into account gender.
Individuals with ischemic brain injuries presented similar bifrontal index (0.30 vs. 0.30, p = 0.94,) and higher frontal interhemispheric fissure (3.98 vs. 3.22, p = 0.12) compared with those with atrophy and/or EPS, without showing significant differences for both men and women. No significant correlation was obtained between Scheltens score and the bifrontal index in men (R = 0.02; p = 0.95) and women (R = 0.06; p = 0.77). A positive but non-significant correlation between Scheltens score and interhemispheric fissure was observed in men (R = 0.66; p = 0.08), but not in women (R = -0.03; p = 0.87). Subjects with cerebral atrophy presented more Scheltens score (8.34 vs. 2.37; p = 0.005), indicating a relationship between brain atrophy and white matter injury.
Extracerebral injury
The study of the common Carotid artery, revealed 60% of the patients with alterations, either an increase in IMC thickness or the presence of atheroma plaques. The IMC thickning isolated was observed in 15% of patient, presence of atheroma plaques isolated was observed in 21%, and 24% of the patients presented both lesions, without gender differences. There were no significant differences in the IMC thickness between sides (D/I: 0.90 vs. 0.79 mm, p = 0.18), so right-side values were considered for analysis. There were also no differences between men and women regarding this variable, and a weak and non-significant positive correlation was observed between age and the IMC thickness (R = 0.23; p = 0.21). Patients with plaques were significantly older than those without plaques (57 vs. 50, p < 0.001). In any case was significant carotid lumen stenosis, an irregular surface plaque with intraplate flow, classified as unstable, was observed in one 42 years old patient. According to this findings, patients were classified into 4 groups: normal, with IMC hyperplasia, with atheroma plaques only, and with IMC hyperplasia associated with plaques.
Relationship between intra and extracerebral injuries
Values of the mean IMC thickness were similar in patients with ischemic brain injury and with atrophy and/or EPS (0.90 vs. 0.89; p = 0.89). We observe no correlation between IMC thickness and the total Scheltens score (R = 0.02, p = 0.88).
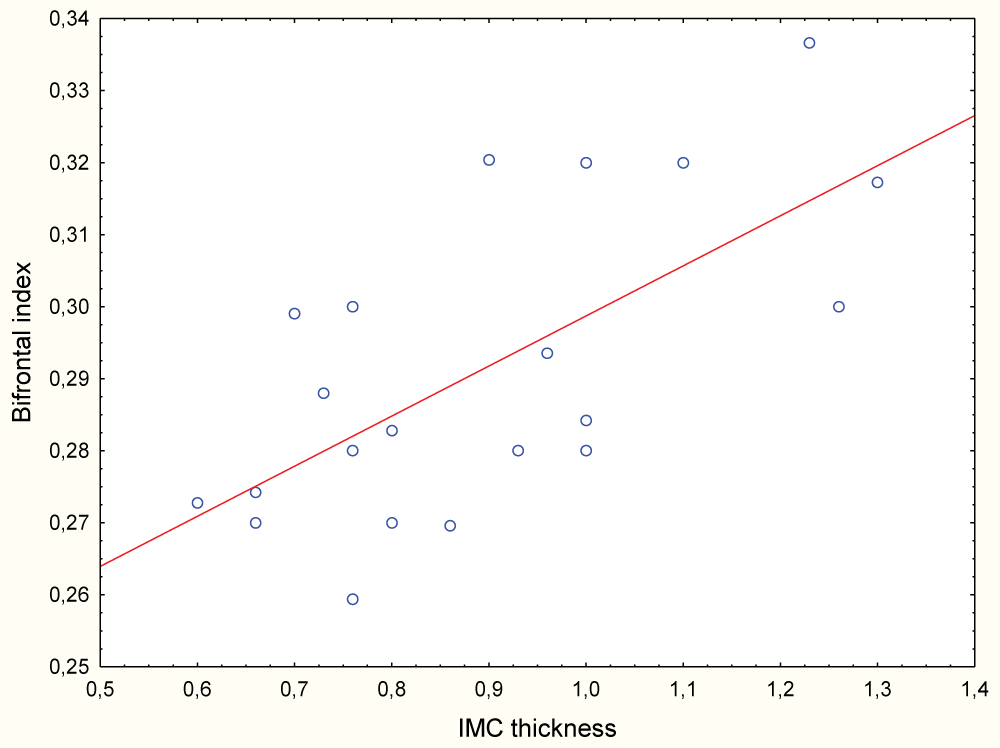
Patients with cerebral atrophy presented greater IMC thickness (0.91mm vs. 0.88 mm, p = 0.93) and in women a significant positive correlation between the IMC thickness and Bifrontal Index was observed (R = 0.59; p < 0.001) (Graph 2).
Graph 2: Correlation between the IMC thickness and the Bifrontal Index in women.
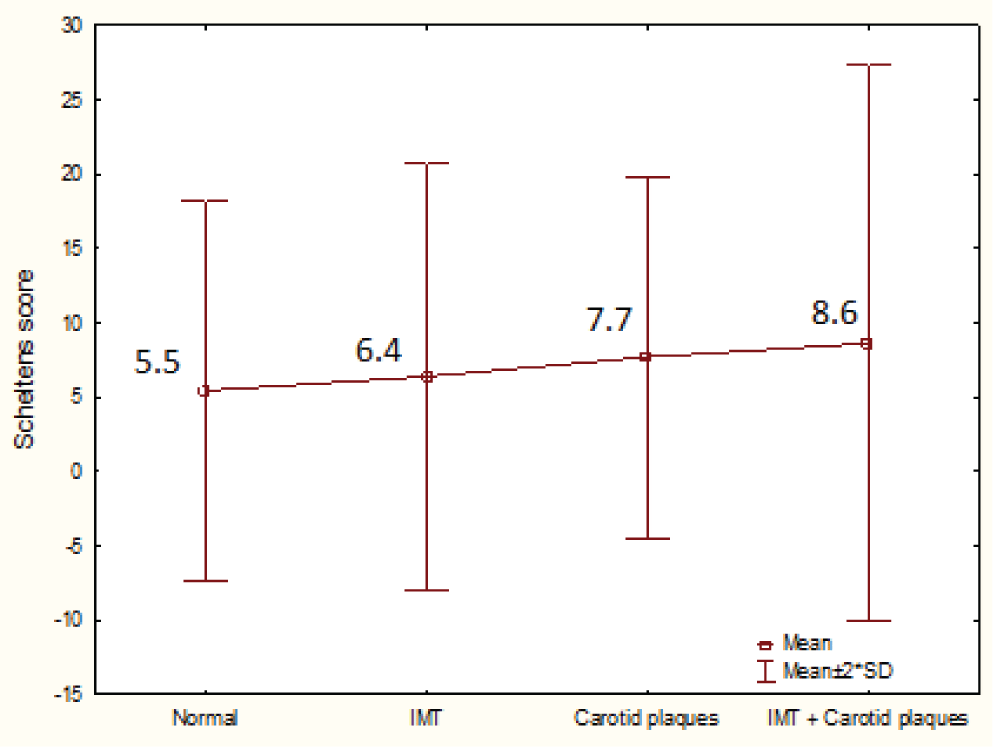
We also explore severity of WML in each group of patients according to findings in carotid examination. Progressively higher scores on Scheltens scale were observed, especially in those patients with carotid plaques (isolated or associated with IMC hyperplasia), without significant differences (p = 0.65) (Graph 3).
Graph 3: Mean values of total Scheltens score in 4 groups of patients according to carotid findings. IMT: intimal thickening.
Graph 4: Frequency of ischemic brain injury, cerebral atrophy, carotid plaques, and combined intra and extracerebral injuries in each group of patients according to the cardiovascular risk (CVR).
This led us to compare the severity of WML according to the presence or not of carotid plaques (isolated or associated with IMC hyperplasia), and higher mean values of total WML were observed (8.2 vs. 5.7, p = 0.24) in those with plaques. They also have higher mean values of WML in frontal subcortical regions (2.7 vs. 1.8, p = 0.19), but this differences were not statistically significant.
No significant association was obtained between the presence of cerebral atrophy and carotid plaques (X2 = 0.14, p = 0.71). Bifrontal index (0.30 mm vs. 0.29 mm, p = 0.23) and the frontal interhemispheric fissure (4.16 mm vs. 3.66 mm, p = 0.18) were also not significantly different between patients with and without plaques, in both sexes.
Relationship of intra and extracerebral injuries with cardiovascular risk
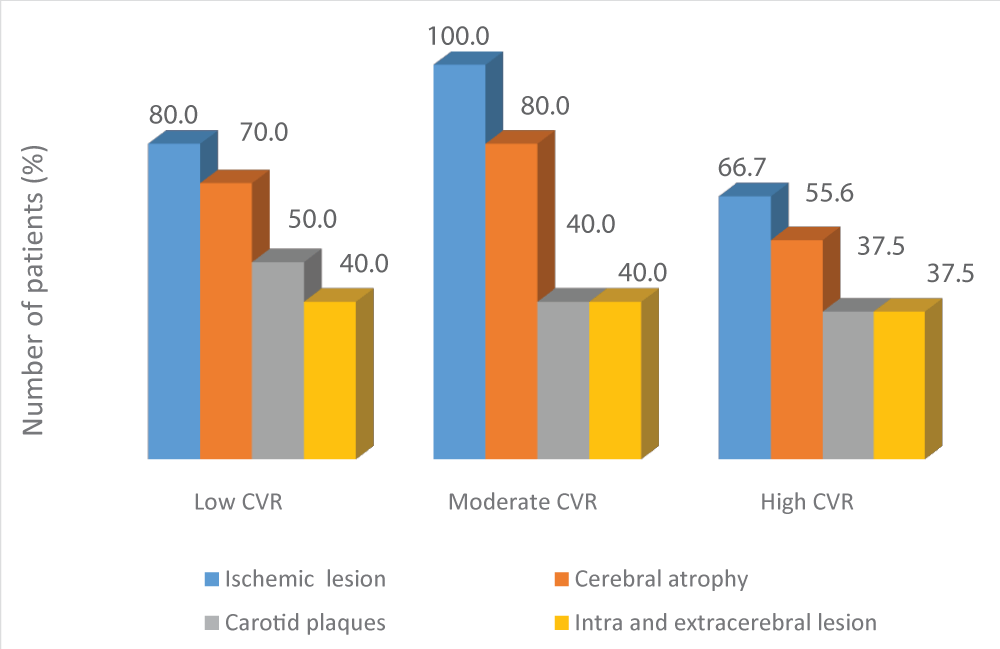
In general, patients with low and moderate CVR presented a higher frequency of intracerebral lesions (both ischemic and cerebral atrophy) and extracerebral lesions, than patients with high CVR. (Graph 4). However, no significant differences were found in total Scheltens score, linear measurements of atrophy, IMC thickness and cognitive performance in relation to the CVR of patients (Table 3).
| Table 3: Meanvalues of quantification of intra and extracerebral lesions and cognitiveperformance in each cardiovascular risk group and statistical significance ofthe difference. | ||||
| Variables | Cardiovascular Risk | |||
| Low n = 20 | Moderate n = 5 | High n = 9 | p level | |
| TotalScheltens Score | 7.5 | 4.40 | 6 | 0.96 |
| Bifrontalindex | 0.30 | 0.29 | 0.29 | 0.42 |
| Frontalinterhemispheric fissure | 3.78 | 4.78 | 3.36 | 0.22 |
| IMCthickness | 0.93 | 0.76 | 0.89 | 0.58 |
| Digits-symbols Test | 37.8 | 37 | 42.5 | 0.47 |
Neuropsychological findings
No statistic significant effects of age were observed on neuropsychological score of patients (R = -0.10; p = 0.55), nor differences between men and women (40 vs. 38, p = 0.76). However, a marked influence of the degree of education were observed, with significantly higher neuropsychological scores in those with higher education level (p = 0.01). This factor could not be considered in subsequent analysis because it caused inhomogeneity in the sample.
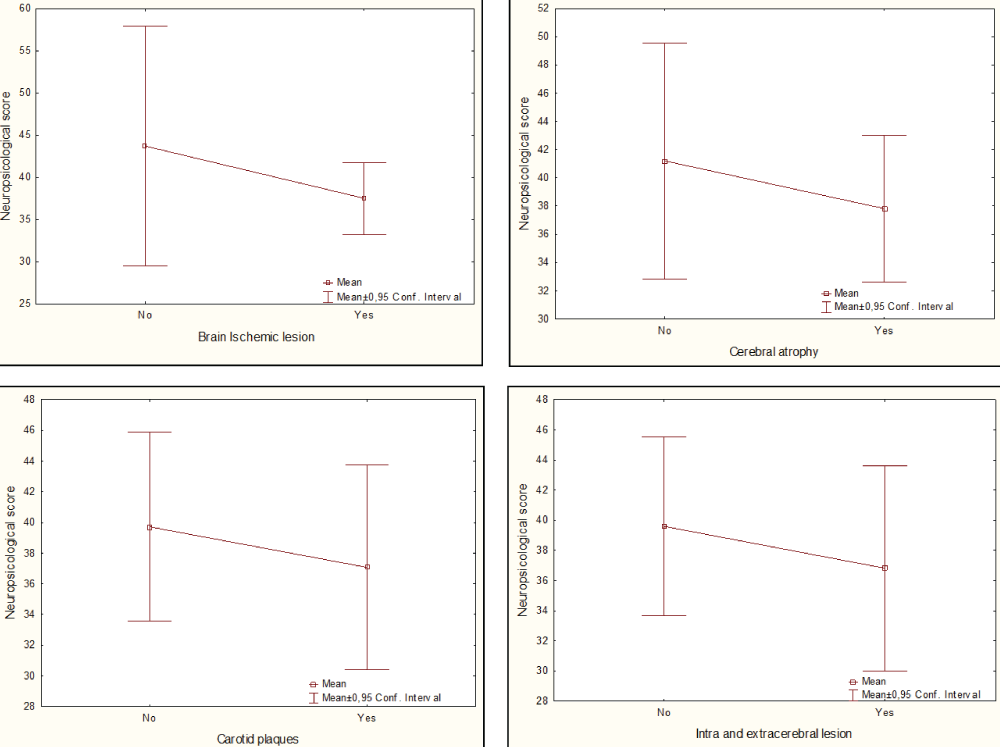
A non-significant decrease in neuropsychological score was observed in the groups of patients with ischemic brain injury compared with those without it (38 vs. 44, p = 0.24) (Graph 5A). A weak positive but not significant correlation was obtained between neuropsychological and total Scheltens scores (R = 0.35; p = 0.09) in these patients. A slight decrease in cognitive score was observed in patients with cerebral atrophy compared with those without it (38 vs. 41, p = 0, 56) (Graph 5B). A negative correlation was obtained between neuropsychological score and bifrontal index in men (R = -0.67; p = 0.07), but without statistical significance.
In patients with carotid plaques, isolated or combined with IMC hyperplasia, a non-significant decrease in neuropsychological score was observed compared with those without it (37 vs. 40, p = 0.70) (Graph 5C). No correlation was obtained between cognitive performance and IMC thickness (R = -0.07, p = 0.72).
Considering the effect of the concurrence of both types of injuries (intra and extracerebral) on cognitive performance we observed a non-significant decrease in patients with both injuries compared with those who just have one type of injury (37 vs. 40, p = 0.69) (Graph 5D).
Graph 5: Frequency of ischemic brain injury, cerebral atrophy, carotid plaques, and combined intra and extracerebral injuries in each group of patients according to the cardiovascular risk (CVR).
Intracranial vascular injury
CSVD is very common in elderly patients [16], however, in our study we found a high frequency of these lesions in a group of hypertensive patients with a mean age below 65 years, most of them with low to moderate CVR. Few studies have reported these injuries in non-elderly individuals [-2017], in which WML is the most common injury, as in our study. Nonetheless, our results showed a positive correlation between age and WML, and cerebral atrophy, reflecting the aging process underlying these lesions. In particular, WML were more frequent in frontal and parietal subcortical regions, which could affect the cognitive performance of patients. It has been pointed out that white matter lesions contribute to cerebral atrophy, which agrees with the relationship between these lesions obtained in our study, specifically in men, although it wasn’t statistically significant [21,22].
Other frequent alterations found were cerebral atrophy and enlarged perivascular spaces. They are also commonly reported in elderly individuals and associated with cognitive decline and dementia [18]. Enlarged perivascular spaces are part of the changes seen in the CSVD, although its diagnostic and prognostic significance has not yet been defined. Its pathophysiological mechanism is not precise and its appearance becomes more frequent with age and with chronic hypertension [23]. In agreement, our results reflect a high frequency of enlarged perivascular spaces in this group of patients related to the chronic effect of HT.
Other CSVD markers visible on MRI such as recent small subcortical infarcts and microbleeds were not observed in this group of patients. Frequency of these injuries is less than that of WML and brain atrophy. A 3% - 7% prevalence of microbleeds has been reported in people with no history of cerebrovascular disease and its consistent association with age and hypertension has been reported [24]. The absence of these brain lesions in our study possibly is related to the mean age of this group under 65 years or based in ethnic differences.
Extracranial vascular injury
Atherosclerosis is the most frequent cause of extracranial carotid artery disease and these patients have a higher risk of suffering from adverse vascular events such as heart attacks, peripheral arterial disease and death. It is recognized that it is responsible for 15% - 20% of ischemic strokes [4]. However, most studies have focused on the relationship between carotid stenosis and the occurrence of fatal vascular events, while few investigations have explored the prevalence of earlier stages of this disease in hypertensive patients with low or moderate CV risk, and the association between ECAD and CSVD.
Presence of carotid plaques is common in asymptomatic patients, reaching up to 40% in the general population and mostly in the elderly [25]. Few studies have reported data on the prevalence of these lesions in non-elderly adult patients. In PESA study [26], 31% of subjects with carotid plaques was detected in a group of asymptomatic persons with a mean age of 45 years. In another group of asymptomatic subjects with a mean age of 47 years, Varleta, et al. detected 15% of patients with carotid plaques and 33% of subjects with IMC hyperplasia, these findings were more frequent in patients with HT [27]. In our study, a higher frequency of carotid plaques and/or IMC hyperplasia was found, which can be explained by the presence of hypertension in all the patients studied. As with intracranial lesions, age was related to these injuries, especially the presence of carotid plaques. Also, it’s worthwhile to note the presence of a risky and unstable carotid plaque in a young patient in whom other cardiovascular risk factors must be present.
Regarding the relationship between intra and extracerebral vascular lesions, a meta-analysis carried out based on 7 studies evaluating IMC thickness in 6,571 subjects, and on 11 studies, evaluating carotid stenosis in 12,347 subjects, revealed that both forms of carotid disease were significantly associated with the presence of silent cerebral infarction [28]. Another meta-analysis including 9 systematic reviews showed that ECAD was significantly associated with presence of WML, but could not demonstrate the relationship with cerebral atrophy due to lack of adequate studies [29]. In that study, only the presence of fully developed atherosclerotic lesion was considered, without including IMC hyperplasia.
Our results partially agree with these reports, pointed to a relationship between IMC thickness and cerebral atrophy in women. Also the presence of carotid plaque was associated with more WML although in a non-significant way. This could be the expression of atherosclerosis process affecting large and, more extensively, the small vessel of cerebral circulation.
Several studies have reported the impact of carotid plaques and the increase of IMC thickness in the prognosis of adverse vascular events [7-9,11,28-30], which indicates the importance of considering these injuries in refinement of cardiovascular risk stratification. The results of our study are in line with this opinion, especially in hypertensive patients in whom the treatment strategy involves the detection of target organ injury to define CVR and, accordingly, to impose the most appropriate treatment regimen.
Cognitive impact of vascular lesions
Vascular cognitive impairment is a wide term including all forms of cerebrovascular disease with cognitive consequences. CSVD is possibly its more frequent cause and is associated with specific cognitive deficits such as slowed information processing, mild memory and attention deficits and a dysexecutive syndrome [31]. The later includes impairment in goal formulation, initiation, planning, organizing, sequencing, executing, set-shifting and set-maintenance. Therefore, it is recommended that neuropsychological evaluation in vascular cognitive impairment assess especially executive functions [15]. In our study, a non-significant decrease in Digit Symbol-Coding test score was observed in patients with both intra and extracerebral lesions, which could be an expression of the beginning of cognitive deficit in these relatively young patients. However, is important to note that this test just provide a direct measure of processing speed, so alterations in memory, attention and executive functions could not be explored, which constitute a limitation of this study.
However, information processing speed is recognized as an essential cognitive resource underlying the basic cognitive mechanisms and is correlated with the performance in a wide range of cognitive domains [32]. There are several evidences about the relationship between cognitive processing speed and the structural integrity of white matter tracts, reflecting that appropriate connections of white matter tracts allow a more efficient information processing in neural networks responsible for cognitive functions [33-35]. However, our result doesn’t show a significant and inverse relation between quantitative measure of WML and neuropsychological score. This could be related direct or indirectly with the effect of higher education´s degree in those individuals with higher neuropsychological score, but with higher WML score. So is necessary to increase the sample size and make more homogenous the education´s degree factor in the groups of patients. Also we must consider other risk factors such as arterial hypertension control and treatment.
Finally, it is important to highlight the higher frequency of injuries found in patients with low and moderate CVR, compared to those with high CVR. This could have been influenced by the fact that the CVR stratification method used does not take into account the presence of target organ lesions from cerebral vascular territory, as frequently happens in clinical practice, since these studies are not applied to all patients with HT. Asymptomatic cerebrovascular injuries often occur earlier than in other structures such as heart and kidney, which have most available and least expensive markers of damage. In this way, a discordance could be established between the real CVR and that calculated in hypertensive patients, making it important to search for markers of silent cerebrovascular injury.
Findings of this study point to the high frequency of silent intra and extra-cerebral cerebrovascular lesions in low to moderate risk hypertensive patients. WML in frontal and parietal regions and generalized cerebral atrophy were the most frequent brain lesions and were related, in non-significant way, with carotid IMC hyperplasia and atheroma plaques. Age significantly influenced the appearance of intra and extracerebral lesions, which points to the aging process as an important underlying factor. A non-significant decrease in cognitive performance was observed related to intra and extra-cerebral lesions. More comprehensive future studies may provide more information on the presence and impact of silent cerebrovascular injury in hypertensive patients with low and moderate CVR.
- Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, et al. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019; 18: 439-458. PubMed: https://pubmed.ncbi.nlm.nih.gov/30871944/
- Smith EE, Saposnik G, Biessels GJ, Doubal FN, Fornage M, et al. Prevention of Stroke in Patients With Silent Cerebrovascular Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2017; 48: e44-e71. PubMed: https://pubmed.ncbi.nlm.nih.gov/27980126/
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010; 9: 689-701. PubMed: https://pubmed.ncbi.nlm.nih.gov/20610345/
- Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients With Extracranial Carotid and Vertebral Artery Disease. Circulation. 2011; 124: e54-e130. PubMed: https://pubmed.ncbi.nlm.nih.gov/21282505/
- Amato M, Veglia F, de Faire U, Giral P, Rauramaa R, et al. Carotid plaque-thickness and common carotid IMT show additive value in cardiovascular risk prediction and reclassification. Atherosclerosis. 2017; 263: 412-419. PubMed: https://pubmed.ncbi.nlm.nih.gov/28602434/
- Knight-Greenfield A, Quitlong Nario JJ, Vora A, Baradaran H, Merkler A, et al. Associations Between Features of Nonstenosing Carotid Plaque on Computed Tomographic Angiography and Ischemic Stroke Subtypes. JAHA. 2019; 8: e014818. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6951053/
- Loizou CP, Kyriacou E. Ultrasound Asymptomatic Carotid Plaque Image Analysis for the Prediction of the Risk of Stroke. In: Golemati S, Nikita KS, editors. Cardiovascular Computing Methodologies and Clinical Applications. Singapore: Springer Singapore. 2019; 317-329.
- Masson W, Huerin M, Vitagliano L, Zeballos C, Lobo M, et al. Estimation of Cardiovascular Risk and Detection of Subclinical Carotid Atheromatosis in Middle-aged Postmenopausal Women. Arg J Cardiol. 2013; 81: 322-328.
- Perez HA, Garcia NH, Spence JD, Armando LJ. Adding carotid total plaque area to the Framingham risk score improves cardiovascular risk classification. Arch Med Sci. 2016; 2016: 513-520. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4889685/
- Chambless LE, Heiss G, Shahar E, Earp MJ, Toole J. Prediction of Ischemic Stroke Risk in the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2004; 160: 259-269. PubMed: https://pubmed.ncbi.nlm.nih.gov/15257999
- Xie W, Wu Y, Wang W, Zhao D, Liang L, et al. A Longitudinal Study of Carotid Plaque and Risk of Ischemic Cardiovascular Disease in the Chinese Population. J Am Soc Echocardiogr. 2011; 24: 729-737. PubMed: https://pubmed.ncbi.nlm.nih.gov/21440416/
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013; 12: 822-838. PubMed: https://pubmed.ncbi.nlm.nih.gov/23867200/
- Frisoni GB, Beltramello A, Weiss C, Geroldi C, Bianchetti A, et al. Linear measures of atrophy in mild Alzheimer disease. Am J Neuroradiol. 1996; 17: 913-923. PubMed: https://pubmed.ncbi.nlm.nih.gov/8733967/
- Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, et al. Mannheim Carotid Intima-Media Thickness Consensus (2004-2006). Cerebrovas Dis. 2007; 23: 75-80. PubMed: https://pubmed.ncbi.nlm.nih.gov/17108679/
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards. Stroke. 2006; 37: 2220-2241. PubMed: https://pubmed.ncbi.nlm.nih.gov/16917086
- Caunca MR, De Leon-Benedetti A, Latour L, Leigh R, Wright CB. Neuroimaging of Cerebral Small Vessel Disease and Age-Related Cognitive Changes. Front Aging Neurosci. 2019; 11: 145. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6610261/
- Consoli D, Di CA, Inzitari D, De LD, Lamassa M, et al. Subcortical ischaemic changes in young hypertensive patients: frequency, effect on cognitive performance and relationship with markers of endothelial and haemostatic activation. Eur J Neurol. 2007; 14: 1222-1229.
- Meissner A. Hypertension and the Brain: A Risk Factor for More Than Heart Disease. Cerebrovasc Dis. 2016; 42: 255-262.
- Uiterwijk R, Huijts M, Staals J, Rouhl RPW, De Leeuw PW, et al. Endothelial Activation Is Associated With Cognitive Performance in Patients With Hypertension. Am J Hypertens. 2015; 29: 464-469. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4886483/
- Wang T, Li Y, Guo X, Huang D, Ma L, et al. Reduced perfusion in normal-appearing white matter in mild to moderate hypertension as revealed by 3D pseudocontinuous arterial spin labeling. J Magn Reson Imaging. 2016; 43: 635-643. PubMed: https://pubmed.ncbi.nlm.nih.gov/26256700/
- Kloppenborg RP. 7 Tesla MRI for visualisation of small cerebral vessels. Ned Tijdschr Geneeskd. 2017; 161: D552. PubMed: https://pubmed.ncbi.nlm.nih.gov/28537536/
- Ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, et al. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol. 2018; 14: 387-398. PubMed: https://pubmed.ncbi.nlm.nih.gov/29802354
- Hurford R, Charidimou A, Fox Z, Cipolotti L, Jager R, et al. MRI-visible perivascular spaces: relationship to cognition and small vessel disease MRI markers in ischaemic stroke and TIA. J Neurol Neurosurg Psychiatry. 2014; 85: 522-525. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3995332/
- Kim BJ, Lee SH. Cerebral microbleeds: their associated factors, radiologic findings, and clinical implications. J Stroke. 2013; 15: 153-163. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3859003/
- Zhan C, Shi M, Yang Y, Pang H, Fei S, et al. Prevalence and Risk Factors of Carotid Plaque Among Middle-aged and Elderly Adults in Rural Tianjin, China. Sci Rep. 2016; 6: 23870. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4814923/
- Fernández-Friera L, Peñalvo JL, Fernández-Ortiz A, Ibañez B, López-Melgar B, et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort. Circulation. 2015; 131: 2104-2113. PubMed: https://pubmed.ncbi.nlm.nih.gov/25882487/
- Varleta P, Concepcion R, Julio P, Casanova H, Navarrete C. Ateroesclerosis subclinica en poblacion de riesgo cardiovascular bajo y moderado por Framingham chileno. Revista medica de Chile. 2016; 144: 30-38.
- Finn C, Giambrone AE, Gialdini G, Delgado D, Baradaran H, et al. The Association between Carotid Artery Atherosclerosis and Silent Brain Infarction: A Systematic Review and Meta-analysis. J Stroke Cerebrovasc Dis. 2017; 26: 1594-1601. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5474126/
- Moroni F, Ammirati E, Magnoni M, D'Ascenzo F, Anselmino M, et al. Carotid atherosclerosis, silent ischemic brain damage and brain atrophy: A systematic review and meta-analysis. Int J Cardiol. 2016; 223: 681-687. PubMed: https://pubmed.ncbi.nlm.nih.gov/27568989/
- Baber U, Mehran R, Sartori S, Schoos MM, Sillesen H, et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol. 2015; 65: 1065-1074. PubMed: https://pubmed.ncbi.nlm.nih.gov/25790876/
- Wahlund LO, Erkinjuntti T, Gauthier S, editor. Vascular Cognitive Impairment in Clinical Practice. New York: Cambridge University Press; 2009.
- Kail R, Salthouse TA. Processing speed as a mental capacity. Acta Psychol. 1994; 86: 199-225. PubMed: https://pubmed.ncbi.nlm.nih.gov/7976467/
- Neubauer A, Fink A. Intelligence and neural efficiency. Neurosci Biobeh Rev. 2009; 33: 1004-1023. PubMed: https://pubmed.ncbi.nlm.nih.gov/19580915/
- Penke L, Muñoz Maniega S, Bastin ME, Hernandez M, Murray C, et al. Brain white matter tract integrity as a neural foundation for general intelligence. Mol Psychiatr. 2012; 17: 1026-1030.
- Turken U, Whitfield-Gabrieli SL, Bammer R, Baldo JV, Dronkers NF, et al. Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. NeuroImage. 2008; 42: 1032-1044. PubMed: https://pubmed.ncbi.nlm.nih.gov/18602840/