More Information
Submitted: April 20, 2022 | Approved: May 03, 2022 | Published: May 04, 2022
How to cite this article: Chairi MHM, Peña FJH, Zurbano MS, Alcalá TT, del Moral JMV. Treatment of perianal fistulae in crohn's disease with mesenchymal stem cells. Ann Clin Gastroenterol Hepatol. 2022; 6: 006-020.
DOI: 10.29328/journal.acgh.1001033
Copyright License: © 2022 Chairi MHM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Treatment of perianal fistulae in crohn's disease with mesenchymal stem cells
Mohamed Hassin Mohamed Chairi*, Francisco José Huertas Peña, Marta Santidrián Zurbano, Tomás Torres Alcalá and Jesús María Villar del Moral
Department of Surgery, Coloproctological Surgery Division, Virgen de las Nieves University Hospital, Granada, Spain
*Address for Correspondence: Mohamed Hassin Mohamed Chairi, Department of Surgery, Virgen de las Nieves University Hospital. Avenida de las Fuerzas Armadas, 2, 18014, Granada, Spain, Email: yasinmc1994@gmail.com
Crohn's disease is a chronic syndrome of the gastrointestinal tract that produces idiopathic inflammation. Approximately half of the patients develop abscesses and/or fistulas throughout their history that are located, mainly, in the perianal region. Current treatments are based on individualized plans that generally use combined pharmacology for symptomatic relief based on glucocorticoids, immunosuppressants or immunomodulators, antibiotics, anti-inflammatories, probiotics, and antibodies, or surgical therapies such as intestinal resections or ostomizations (colostomy and ileostomy) that tend to cause notable side effects in a considerable percentage of patients and a significant decrease in their quality of life.
Perianal fistulas consist of abnormal tracts, inflammatory tunnels, or chronic tracts of granular tissue that connect two surfaces lined with epithelium, have an external hole in the skin that borders the anus, and an internal hole located inside it around the anal canal, rectus and sphincters.
Treatment is a complex process that requires a multidisciplinary approach and the combination of several treatments. In the short term, the goal is to drain abscesses, reduce inflammatory and infectious processes, guard the fistulous tract with seton or lax lines, facilitate patency, and hinder new formations. In the long term, a total cure and the avoidance of complications that require surgery or the creation of intestinal stomas are pursued.
For this reason, new effective remedies with fewer adverse effects continue to be investigated, one of the most promising being the use of mesenchymal stem cells for the regeneration and cure of perianal fistulas and the remission of symptoms. The present bibliographic review delves into this new therapy and analyzes the current state of the situation regarding its efficacy and safety.
Crohn´s disease
Crohn's disease (CD) is one of the main chronic inflammatory bowel diseases alongside ulcerative colitis [1,2]. Its discoverer, Burril Bernard Crohn, named it terminal ileitis in 1932 because it is most prevalent in the lower end of the small intestine (ileum), although it is also common in the proximal colon, producing ileitis in one in four patients, colitis in an equal proportion and ileocolitis in the remaining half [1]. However, we now know that it can affect any segment and layer of the digestive tract in a discontinuous manner and with variable extension (from the mouth to the perianal area), with a greater gradient of distal-proximal involvement [2], and can cause intestinal transmural inflammation and luminal stenosis (luminal CD) and even ulcerations, fistulas and/or abscesses (fistula CD) that have a very negative effect or partially disable the most affected patients [1-3].
Its relapsing or recurrent pathophysiology, alternating asymptomatic cycles with stages of activity (flares or relapses) [2,3], significantly diminish patients' quality of life. It manifests as diarrhea, intestinal spasms, bloating and severe abdominal pain, nausea, fever, loss of appetite and weight, obstructive vomiting, dyspepsia, and/or rectorrhagia [3,4]. In addition, it may be accompanied by malabsorption syndrome, which adds nutritional deficiencies, anemia, anorexia, or asthenia to the above symptoms. Often, the patient requires hospitalization for treatment of flare-ups or side effects of medication [3] and, in the most severe cases, intestinal strictures and occlusions develop, requiring surgical interventions: stricturoplasty, intestinal resection with or without anastomosis, ostomies, etc., which can lead to various complications or incapacitation [1].
Extra-intestinal joint manifestations such as arthritis, granulomatous lesions, arthralgias, tendonitis, enthesitis, periostitis, or hypertrophic osteopathy may occur; dermatological as erythema nodosum, pyoderma gangrenosum, aphthous stomatitis or Sweet's syndrome; ocular as scleritis, episcleritis or uveitis; hepatobiliary as sclerosing cholangitis; metabolic as osteoporosis or osteopenia and, finally, thromboembolic [3].
Epidemiologically, its incidence has been steadily increasing worldwide for the last 50 years but remains higher in more developed Western countries and certain ethnic groups, especially Jews and Caucasians [1]. It exhibits, with some exceptions, a north-south gradient of incidence [1], with average annual incidence rates ranging from 10-200/100,000 inhabitants depending on these factors [5]. Thus, Australia (29.3), Canada (20.2), New Zealand (16.5), North America (16.1), and Northern Europe (10.6) are the regions with the highest incidence, while Europe (322/100,000 inhabitants), Canada (319) and the USA (201-214) have the highest prevalence worldwide [3]. However, these differences have been reduced by the marked increase of cases in Spain and Greece [2], with the Spanish average annual incidence rate between 3.6 and 10.6 [5]. In terms of age, the disease usually begins between the second and fourth decade of life and occurs less frequently after the age of 60 [2,4]. In Spain, it usually begins between 15 and 35 years of age, with an increased incidence in children of between 7 and 20% of diagnosed cases [5]. In terms of gender, no significant differences have been found between men and women in most studies [1,2].
The etiology of the disease is still unknown, although we do know of the interactive influence of genetic and environmental risk factors in its onset and development, as well as its autoimmune nature, as the inflammation is induced by Th-1 lymphocytes and associated, in part, with immune dysfunction intolerance to intestinal mucosal antigens [1,5]. Since the completion of the human genome in 2003, genetic epidemiology studies (ethnic, familial, twin, animal, or related syndromes), genetic mutation studies, and candidate gene linkage and association studies have led to the identification of numerous genetic markers and polymorphisms associated with CD. In this regard, the NOD2/CARD15 gene mutation (IBD1 locus on chromosome 16), is related to the immune attack on ileal bacteria, fibrostenosis, with an early onset of the disease and with having a family history, with carriers having a higher risk of suffering from it in a 2 to 4 ratio with heterozygous alleles and ten times more (20 to 40) if they are homozygous (1.5). Other markers in the genes NFKB1, PTPN22, FOXP3, NOS2A and NOS3, MIF, MYO9B, ATG16 or IL-23R, among others, are also relevant [5], the latter being involved in a dysfunctional differentiation of Th-17 lymphocytes that deregulates the production of cytokines, involved in the pathogenesis of CD. It is also highly heritable (10% - 20% of sufferers have a family history), which increases the risk of suffering from CD by a factor of thirty [1].
In terms of environmental factors, smoking almost doubles the likelihood of contracting the disease earlier. It is also associated with a higher occurrence of fistulas, abscesses, skin ulcerations, relapses, and surgical interventions, with a higher number of post-surgical recurrences and a greater need for steroid (corticosteroid) and immunosuppressive treatment [1,4]. On the other hand, subjects with a diet poor in dietary fiber (from fruit) and vitamin D, or rich in saturated fats and carbohydrates, are also at increased risk [1]. The same applies to sedentary lifestyles, high stress, sleep problems, or psychological factors such as anxiety and depression, which can also intensify symptoms, increase relapses and hospitalizations and/or reduce the effects of drug treatments [3]. At the bacterial level, the presence of enteroinvasive Escherichia coli and Faecalibacterium prausnitzii increase and decrease the risk, respectively. Also, individuals with low diversity in the gut microbiota, or long-term users of certain drugs (such as oral contraceptives, non-steroidal anti-inflammatory drugs, aspirin, or early antibiotics) are at increased risk [1].
The main diagnostic tests focus, firstly, on laboratory tests to detect secondary indicators of inflammation such as hemoglobin and hematocrit levels, C-reactive protein, vitamin B12, folic acid, erythrocyte sedimentation rate, and stool studies to assess fecal calprotectin and lactoferrin levels, as well as the presence of parasites and eggs [3,4]. Secondly, radiological tests for the evaluation of signs of strictures, thickening, or typical cobblestones. Among the various tests are intestinal transits, X-rays, ultrasound, MRI, and abdominal CT scans (the latter being the most commonly used). Finally, we have endoscopic tests, which in addition to evaluating the characteristic signs of the disease, allow biopsies to be taken to reach a definitive diagnosis, as well as to detect complications such as fistulas, stenosis, etc. [1.4]. The differential diagnosis of CD, given the variety of symptoms, is complex and is made by exclusion of other similar pathologies, such as ulcerative colitis.
Current treatments are based on individualized plans that generally use combined pharmacology for symptomatic relief based on glucocorticoids, immunosuppressants or immunomodulators, antibiotics, anti-inflammatory drugs, probiotics, and antibodies, although patients sometimes suffer from significant side effects that reduce their quality of life [4]. On the other hand, supplementary and protein-rich dietary treatments compensate for malnutrition caused by intestinal malabsorption and sometimes require direct probing of the intestinal tract (enteral nutrition) or intravenous (parenteral nutrition). When the above treatments are insufficient or ineffective, surgical therapy is resorted to using intestinal resections of the most affected segments and ostomisations (colostomy and ileostomy) to allow the elimination of feces when the pathophysiology prevents it through the anus [1,4].
Perianal fistulas
Perianal fistulas (PF) consist of abnormal tracts, inflammatory tunnels, or chronic tracts of granular tissue connecting two epithelium-lined surfaces. Specifically, they have an external orifice in the skin bordering the anus and an internal orifice located inside the anus around the anal canal, rectum, and sphincters [3]. One can speak of perianal disease (PAD) when some of the complications of CD are located in these parts of the digestive tract in the form of surface erosions, papillomas and skin folds, ulcers, strictures, fissures, abscesses, anal strictures or rectovaginal and perianal fistulae, mainly [2,4,6].
To the latter, the subject of this review, they were first described in 1938 by Penner and Crohn, which later gave rise to the so-called fistulising CD. As with CD, the exact pathogenesis has not been confirmed, although some studies point to deep ulcers of the intestinal mucosa as the starting point from which the fistula spreads from the mucous membrane to other areas of the skin or organs [7]. It seems to be due to an alteration in the intestinal barrier, together with a dysfunction of the microbiota, which activates an inadequate immune response with increased concentrations of leukocytes and inflammatory cytokines, thus perpetuating local tissue inflammation and leading to the development of fistulas [8].
In terms of epidemiological data, it affects at least 20% - 30% of Crohn's patients at least once in their lifetime [1]. Other studies place these percentages between 15% and 50% throughout CD [7]. The risk increases over time: 33% after 10 years of clinical history and 42% after 20 years. In patients with proctitis, this risk increases to 90% [6,8,9]. Patients younger than 40 years with CD have a higher incidence, which increases for each year of clinical history by about 6.1%. Finally, higher activity values (CDAI, PDAI, etc.) also increase the risk of formation and proliferation of perianal fistulas and abscesses [9].
Symptomatically, perianal fistulas cause incontinence, bleeding, pain during defecation, perianal inflammation, general malaise, fever, fatigue, and recurrent abscesses which, logically, significantly impair the quality of life of affected patients and increase the risk of undergoing surgery if the fistulas proliferate, persist or do not respond to conventional treatments [1,7].
The ASCRS taxonomy classifies them as simple or complex: while the former affect 78% of cases and are characterized by one or a few short, straight, superficial, easily identifiable tracts with only two clear primary orifices, the latter is less frequent (22%) and present multiple, deep, winding tracts with several primary orifices that are difficult to detect [2,3]. On the other hand, at the anatomical level, the PARKS classification distinguishes between intersphincteric, transsphincteric, suprasphincteric, extrasphincteric, and horseshoe-shaped PFs [3].
In terms of location, perianal fistulas are associated with the rectal area in 92% of cases, with colic EC in 41%, ileocolic EC in 15%, and ileal EC in 12% [9].
In terms of diagnosis, the gold standard test, due to its great precision and clarity of image, is pelvic MRI [1,3,6], which allows assessment of the sphincters, the pelvic floor, the route of the fistulous tracts, and their anatomical relationship, as well as the presence of abscesses. However, endoscopic tests such as sigmoidoscopy are also useful for taking biopsies, and detecting internal orifices, luminal inflammation, or secondary strictures [1].
So far, the treatment of PF is a complex process that often requires a multidisciplinary approach and the simultaneous combination of several treatments (medical and surgical). In the acute process, presenting as abscesses with or without external suppuration, the aim is to drain the abscesses, reduce inflammatory and infectious processes and try to tutor the fistulous tract with a seton to ensure better drainage and facilitate patency of the tracts, as well as to place lax lines to redirect the fistulous tracts and make new abscess formation more difficult [1,3,6,7]. In the long term, the aim is to completely cure it to avoid complications requiring complex surgery or the need for intestinal stomas [1,7]. Several treatment alternatives exist and are often used together to treat both the underlying disease and the fistula:
Biological therapy with TNF-α (tumor necrosis factor) inhibitors: such as infliximab, adalimumab, or certolizumab, which have shown great efficacy [1,3,4,7,10]. However, in case of failure with this therapy, new biological agents (antibodies, anti-integrins, or interleukin blockers) such as dolizumab, vedolizumab, or ustekinumab [3,6,7,10] are being tested without strong evidence of efficacy but with promising results for the future [3,6,7,10].
Immunosuppressive therapy with thiopurines: such as azathioprine or mercaptopurine [4] and immunomodulatory therapy with calcineurin inhibitors, such as cyclosporine or tacrolimus, or purine inhibitors such as methotrexate [6,10], which appear to have some efficacy, although there is still insufficient evidence and they are still being studied in clinical trials [7].
Combination drug therapy: such as metronidazole and/or ciprofloxacin in combination with azathioprine, infliximab with ciprofloxacin, and both with azathioprine, adalimumab with ciprofloxacin [7], or thiopurines with anti-TNF and antibiotics [6].
Aminosalicylate and glucocorticoid therapy: no conclusive studies have been published on the efficacy of these substances for the treatment of perianal fistulas and abscesses, as they are mainly used in cases of luminal CD, although they are often used in combination with anti-TNF therapies [3,10].
Surgical therapy: drains, placement of lax lines and harnesses, resections of fistulous tracts while maintaining the integrity of the anatomy, bowel resections with or without ostomies as well as other techniques such as advancement flap, fistula plug, fibrin glue, tract ligation, laser closure, video-assisted treatment -VAAFT- or local anti-TNF injections; and subsequent prophylactic therapies, make up the most common surgical spectrum for treating perianal disease [6,10].
Mesenchymal stem cell therapy: these types of therapies are currently showing promising and increasingly successful results and offer hope for patients affected by perianal CD who do not respond adequately to previous treatments, the efficacy of which this review will attempt to test. For this reason, it will be discussed in more detail in the following sections, as it is the main object of study.
Mesenchymal stem cells
The existence of non-hematopoietic stem cells in bone marrow was first demonstrated by the German-Jewish pathologist Julius Cohnheim in the late 1860s, who demonstrated the repair of wounds by cells from the distal bloodstream. Almost a century later, in the late 1960s, Travassoli and Crosby confirmed the existence of wound repair after reconstructing certain hematopoietic and adventitial structures in extramedullary areas of mice by transplanting intact parts of bone marrow [11]. In the 1970s, Dexter demonstrated the ability of stromal cells to maintain the growth and self-renewal of other cells by intercellular communication through the secretion of so-called trophic factors and even suggested the possible ability to regulate certain aspects of the immune system [12].
However, around the same time, between 1966 and 1970, the definitive discovery of these cells was attributed to Friedeinstein, who reported the presence of heterogeneous cell subsets, of probable mesodermal origin [12], similar to fibroblasts, with a fusiform shape and ubiquitous location [11]. After successfully isolating and culturing stem cells and using them for ectopic bone marrow transplantation in the renal capsule, he achieved proliferation of bone marrow cells and even bone formation [15]. Furthermore, these cells were able to form colonies and differentiate, both in vivo and in vitro, into a wide variety of mesenchymal tissue lineages such as osteoblasts, chondrocytes, and adipocytes, involved respectively in the formation, development, and maturation of bone, cartilage and adipose tissue [11,12]. Friedeinstein also detected in these cells the capacity to secrete inflammatory cytokines, proteins with a relevant role in hematopoiesis and other blood formation processes [14]. Finally, it was Caplan, in 1991, who definitively coined the term stromal or mesenchymal stem cells, synonymous with mesodermal embryonic connective tissue, given that they can form part of or surround certain types of connective tissue, blood vessels, lymphatic tissue, and/or adipose tissue [11,15]. The various sources consulted often refer to them by the initials MSCs or MSCs.
Since then, there has been an exponential proliferation of research, clinical trials, culture, and therapeutic application of these cells in many different pathologies, such as fractures and craniofacial trauma; degenerative or autoimmune diseases of the skeletal system (rheumatoid arthritis or osteoarthritis); glaucoma, retinitis pigmentosa and macular degeneration in the eye; pathologies in multiple systems such as ischaemic cardiomyopathy, acute kidney injury, diabetic complications (pancreas), systemic lupus erythematosus, fibrosis and inflammatory diseases of the lungs or other organs (sepsis); multiple sclerosis (brain and spinal cord); immune rejections in allogeneic transplants and gastrointestinal tract diseases such as CD [16].
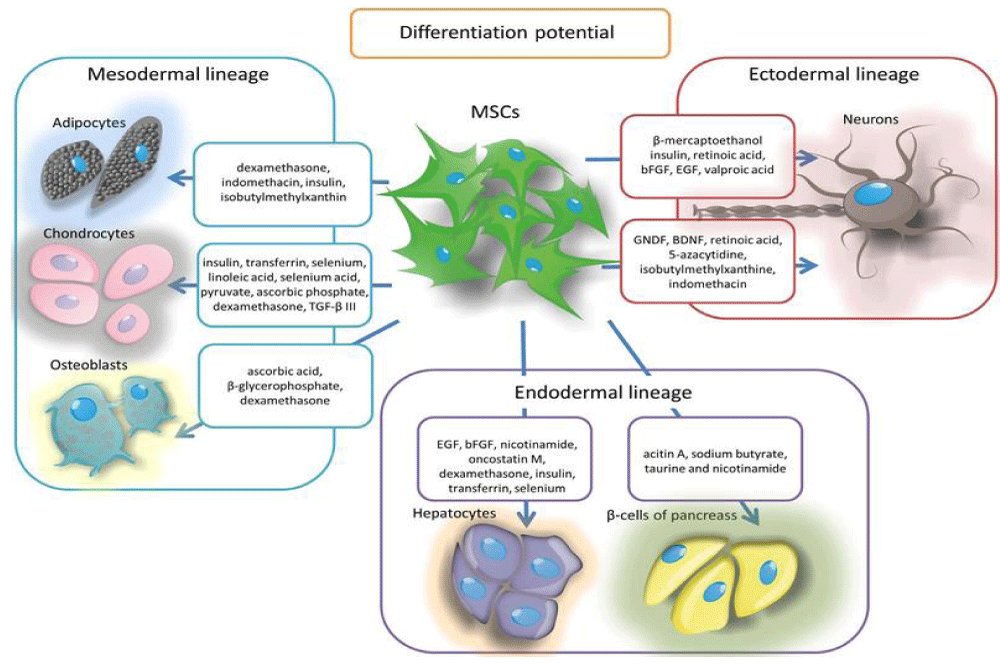
As a result, we now know that MSCs are capable of self-renewal, differentiating into different cell types (multipotentiality), and repairing tissues by direct contact with other damaged cells or by sending paracrine signals (release of proteins, mRNA, microRNAs, and other substances through exosomes or extracellular vesicles) [11]. Furthermore, depending on their origin (umbilical, ossicular-medullary, or adipose), they can inhibit B and T lymphocytes and have anti-inflammatory, immunosuppressive and immunomodulatory effects [17]. We also know that they can be extracted not only from bone marrow but also from endometrial, peripheral, uterine, and menstrual blood; placenta, umbilical cord wall, breast milk, and cervix; fatty, dermal, hepatic, and pulmonary tissues; synovial membrane and periosteum; dental pulp, nasal mucosa, and scalp tissue; and even muscle and corneal limbus [13,14]. Another property is their ability to migrate and repair damaged tissues by the differentiation and replacement of diseased cells [11]. The figure below illustrates the main differentiations (multipotentiality) of MSCs (Figure 1):
Figure 1: A pre-incisional kaolin-activated thromboelastogram. All parameters are within the normal range, suggesting a balance of coagulation between the simultaneously diminished pro-, and anticoagulation drivers.
In the view of these properties discovered in the multiple and successive studies carried out over the last five decades, it was in 2006 that the International Society for Cell Therapy (ISTC) established three criteria to characterize human mesenchymal stem cells [11,18]:
Ability to plastically adhere to the tissue culture flask under standard culture conditions.
Cell differentiation of mesodermal lineage, such as chondrocytes, osteoblasts, or adipocytes in vitro, under standard differentiation conditions.
More than 95% of the MC population must express specific surface antigens (phenotypic markers) such as CD14-, CD19-, CD34-, CD45-, CD73+, CD90+, CD105+ and human leukocyte antigen HLA-DR-, but not less than 2% positive for CD14 or CD11, CD19 or CD79α, CD34, CD45 or HLA-DR-, i.e. these markers have been identified as CD14 or CD11, CD19 or CD79α, CD34, CD45 or HLA-DR. In other words, these markers must be expressed, most importantly, in the absence of hematopoietic markers [16,18,19].
As the topic is complex and is still under investigation, some discrepancies or updates have emerged regarding the mesodermal origin of the MSC lineage, their nomenclature, their immunosuppressive and immunomodulatory capa-cities, and the identification of markers established by the ISTC (2006). In the first case, the initial and predominant hypothesis of MSC differentiation into connective tissues only is called into question by the findings reported in some studies, in which MSCs differentiated into functional cells not derived from the mesodermal germ layer, but endodermal (endothelial, cardiomyocytes, hepatocytes, etc.) [12,13]. Other research associates the origin of MSCs to the neuroectoderm and neural crest during embryonic development (neurocytes), ontogenetically originated by precursor cells of the Sox1+ gene located in the SRY chromosomal region, which defines the sex of the fetus, ending up in the bone marrow of the fetus [15,20]. And the third group of studies points to methodological limitations or cell fusions as the cause of germline crossing over, so the debate continues on this issue [12].
Secondly, there is a discrepancy between calling them stromal cells (as part of connective tissue with supportive functions), signaling cells (because of their local secretory function in diseased, damaged, or inflamed areas) or mesenchymal cells. This question is resolved by the fact that stromal cells are also involved in the secretion of lipid substances from fatty acids, such as prostaglandin E2 (PGE2), which has anti-inflammatory, regenerative, and immunomodulatory capacity by suppressing T-cell receptor signaling. They are therefore more appropriately termed mesenchymal stem cells [20].
Thirdly, on immunosuppressive or immunomodulatory capacity, several studies support the different capacities of MSCs extracted from bone marrow, adipose tissue, and umbilical matrix to inhibit peripheral blood killer cells and B and T lymphocytes by secreting inflammatory cytokines into the tissue environment. They may also exert immunosuppression on immunocompetent cells such as dendritic cells, but the secretion of immunosuppressive cytokines (such as interferon-gamma -IFN-γ- and tumor necrosis factor-alpha -TNF-α-) may impair innate and adaptive immunity. Therefore, their therapeutic benefits are also currently not sufficiently clarified and are still under study [17].
Finally, different sets of phenotypic cell surface markers, both positive and negative, as well as different lineage differentiation, properties, and functions have been identified, depending on the source from which MSCs are extracted, but also determined by the mode of culture. The following table (Table 1) summarises the variability of this issue based on the results of two extensive updated reviews of clinical trials in rats, mice, and humans, where transplants could in some cases be autogenic and allogeneic [18,20].
| Table 1: Biological characteristics of MS according to the origin of extraction. | ||||
| Collection source | Phenotypic surface markers | Differentiation | Functional potential | |
| + | - | |||
| Umbilical cord and umbilical cord blood | CK8, CK18, CK19, CD10, CD13, CD29, CD44, CD73, CD90, CD105, CD106, HLA-I, HLA-II | CD14, CD31, CD33, CD34, CD45, CD38, CD79, CD133, vWF, HLA-DR | adipocytes, chondrocytes osteoblasts, hepatocytes, endothelial cells, neuronal cells, pancreatic cells, endothelial cells, neuronal cells, pancreatic cells |
Immunomodulation, immunosuppression, anti-inflammatory |
| Placenta | CD29, CD44, CD73, CD90, CD105 | CD45, CD34, HLA-DR | Adipocytes, osteoblasts, endothelial-like cells, neuronal-like cells, adipocytes neuronal type cells |
|
| Bone marrow | SH2, SH3, CD29, CD44, CD49e, CD71, CD73, CD90, CD105, CD106, CD166, CD120a, CD124, STRO-1 | CD14, CD34, CD45, CD19, CD3, CD31, CD11b, HLA-DR | adipocytes, chondrocytes osteoblasts, hepatocytes, cardiomyocytes, pancreatic cells, neuronal type cells. neuronal cells. |
Immunomodulation, immunosuppression, anti-inflammatory, cloning, MSC proliferation, and wound healing. |
| Adipose tissue | CD13, CD29, CD44, CD71, CD73, CD90, CD105, CD166, HLA-I, HLA-ABC, STRO-1 | CD10, CD14, CD24, CD31, CD34, CD36, CD38, CD45, CD49, CD117, CD133, SSEA4, CD106, HLA-II, HLA-DR | adipocytes, chondrocytes osteoblasts, hepatocytes, cardiomyocytes, pancreatic cells, neuronal type cells. neuronal cells. |
Immunomodulation, immunosuppression, antimicrobial, anti-infective, healing of knee injuries. |
| Peripheral blood | CD44, CD90, CD105, HLA-ABC, CD29, CD73, CD90.1, CD106, CD140α | CD45, CD133, CD34, CD19, CD11b, c-kit | Adipocytes, osteoblasts, chondrocytes, neuronal type cells. |
Anti-tumour, immunomodulation, metabolic regulation. |
| Endometrium | CD73, CD90, CD105, CD146 | CD34, CD45 | Adipocytes, chondrocytes, osteoblasts. |
Immunomodulation, nerve tissue regeneration, and tissue engineering. |
| Salivary gland | CD13, CD29, CD44, CD49f, Thy-1, CD90, CD104, p75NGFR, β2-microglobulina, CD130, STRO-1 | CD34, CD38, CD45, CD133 | Adipocytes, chondrocytes, osteoblasts, pancreatic endocrine. |
Regeneration of salivary glands. |
| Wharton's jelly | CD13, CD29, CD44, CD73, CD90, CD105, HLA-I | CD14, CD34, CD45, CD31, CD79, HLA-II, HLA-DR | Adipocytes, osteoblasts, chondrocytes, hepatocytes, neuronal type cells. |
Immunosuppression in combination with bone marrow, adipose tissue, umbilical cord, or modified cells. |
| Amniotic fluid | SH2, SH3, SH4, CD, CD29, CD44, CD49, CD54, CD58, CD71, CD73, CD90, CD105, CD123, CD166, HLA-ABC, HLA-DR | CD10, CD11, CD14, CD31, CD34, CD49, CD50, CD117, HLA-DR, DP, DQ, EMA | Adipocytes, osteoblasts, neuronal type cells. |
Regeneration of bone, muscle, and nerve tissue. |
| Skin and foreskin | CD44, CD90, CD73, CD105, CD166, SSEA4, Vimentin | CD14, CD45, CD34, c-kit, CD133, SSEA3, OCT-4, TRA 1–60, TRA 1–81, HLA-DR | Adipocytes, osteoblasts, chondrocytes, myoblasts. |
Immunomodulation and regeneration of dermal tissue. |
| Nasal polyp tissues | CD105, CD90, CD73, CD54, CD44 | CD34, CD45, CD117, HLA-DR, PDL-1, PDL-2, CTLA-4, CD106, CD146, CD31 | Adipocytes, osteoblasts, chondrocytes, neuronal cells. |
Regeneration of nerve tissue. |
| Dental tissues | CD29, CD44, CD90, CD105, SH2, SH3, CDHLA-DR, CD117, CD46, DPSC-EZ, DPSC-OG | CD10, CD14, CD34, CD45, HLA-DR, Stro-1, NGFR | adipocytes, chondrocytes osteoblasts, pancreatic cells, melanocytes, neuronal cells. |
Engineering of dental and craniofacial tissues by mixing with pulp cells. |
| Synovial tissues | CD4, CD34, CD45 | CD44, CD73, CD90, CD105 | Adipocytes, chondrocytes, osteoblasts. |
Regeneration of the locomotor system (tendons and joints). |
| Synovial fluid | CD10, CD166, CD44, CD54, CD90, CD105, CD147, D7-FIB, STRO-1 | CD31, CD34, CD45, CD106, CD117, CD166, VEGFR2, Flk-1, CXCR4, BMPR-1A, NGFR | Adipocytes, chondrocytes, osteoblasts. |
|
| Limb bud | CD13, CD29, CD90, CD105, CD106 | CD3, CD4, CD14, CD15, CD34, CD45, HLA-DR | Osteoblasts, adipocytes, hepatocytes, neuronal cells. |
Regeneration of the relevant tissues, immunomodulation. |
| Source: Ryu, et al. (2020) (18), Mishra, et al. (2020) (20). Own elaboration. | ||||
With regards to the properties and mechanisms of MSCs for the treatment of PF derived from perforating CD (B3), thus entering the main object of study, we focus on the ability of these cells to reduce intestinal inflammation, proliferate and repair or regenerate damaged tissues (mucosal epithelial and conjunctival tissues of the intestinal submucosa) and to heal or close perianal wounds caused by fistulas and abscesses.
The anti-inflammatory property of locally or systemically injected MSCs, especially those from adipose (AT-MSCs) and human embryonic (hUESC-MSCs) tissue, is due to their ability to regulate immune tolerance by decreasing the cellular secretion of a variety of mediators in intestinal inflammatory reactions [19].
On the one hand, intravenous injection of AT-MSC has demonstrated in some animal clinical trials its potential to reduce autoimmune and inflammatory reactions induced by Th1 lymphocytes, in collaboration with Th17, and to activate the action of regulatory and helper Tregs cells, thereby achieving a decrease in the disease activity index (CDAI), maintenance of body weight, containment of diarrhea and prolongation of survival. The underlying therapeutic mechanism appears to be epithelial cell proliferation, Wnt signaling pathway, and T-cell immunization, thereby accelerating wound healing. This improves the condition of refractory CD patients unresponsive to other treatments by expanded allogeneic administration of AT-MSC, lowering CDAI, although some adverse effects have also been found [20].
Moreover, intralesional hESC-MSC injection decreased serum levels of CD-associated inflammatory cytokines, such as IL-2 and IL-6. Intraperitoneal injection of cells from both sources also improved inflammatory histopathology and clinical severity [19].
As for the ability of MSCs to repair tissues, they migrate to the injured area thanks to molecules that regulate trafficking and facilitate localization, such as CXCR4, MMP2, CCR2, and VCAM1, or reactants such as TNF-α, IFN-γ, or nitric oxide. They then differentiate into myofibroblasts, stimulate local tissue proliferation and repair, and inhibit fibrosis and apoptosis through the secretion of angiogenic factors and proteolytic enzymes. In these cases, contact-dependent or intrinsic immunotolerogenic processes are activated. Immunomodulators are secreted by surface markers such as HLA-G1, CD40, CD80, CD86, CD154, and, to a lesser extent, ICAM-1 and VACM-1, and T-cell apoptosis is inhibited by expressing high levels of surface FasL. Immunomodulatory mediators (trophic factors) essential in immunosuppression and tissue regeneration (TGFβ1, PGE2, tryptophan degrading enzyme, indolamine-2,3-dioxygenase, IFN-γ, STAT1, TNFα 6 and hepatocyte growth factor) are also secreted, reducing T-cell proliferation and inhibiting macrophage and dendritic cell activity. Bioactive materials (proteins such as cytokines, lipids, hormones, nucleic acids such as miRNAs and transcription factors) are transferred by phospholipid extracellular vesicles, which are classified from small to large into exosomes, microvesicles, and apoptotic bodies that, by releasing substances into injured organs and tissues, increase tissue proliferation, limit inflammation and reduce cell death. Finally, mitochondria are transferred by microtubule tunneling (MTT) using MSCs expressing high levels of the RHOT1 protein, a molecular switch that facilitates mitochondrial transport [11,12,14].
Finally, in wound healing, related to the above capacity and paracrine and autocrine secretions, the benefits of uterine stem cells (UCSC-MSCs) on epithelial repair have been demonstrated, as the MSC secretome has an anti-fibrotic and angiogenic capacity that decreases scar formation and improves ejection fraction. MSCs also have the virtue of promoting angiogenesis, thanks to an inhibitor of metalloproteinase-1 (TIMP-1), which is primarily responsible for the anti-angiogenic effects, as well as endothelial cell proliferation and migration that promote tube formation and prevent endothelial cell apoptosis. These processes are also assisted by the antibacterial effects of UCSC-CM against E. coli and Staphylococcus through the chemokines CXCL10, CXCL8, CXCL1, CXCL6, CCL20, and CCL5 [14].
Methodology
Sourcing strategy: For the search and selection of accredited sources that met the needs of the work, we mainly consulted the specialized databases Cochrane Plus, PubMed, and Scopus, using the descriptors of the Health Sciences (DCS) and Medical Subject Headings (MeSH), in association with the Boolean operator AND and OR. In some cases, open-access databases such as Google Scholar, and some scientific journal databases such as ScienceDirect (Elsevier), MedlinePlus, Redalyc, and Scielo, among others, were also consulted. In these databases, the keywords or descriptors of the topics to be developed were entered and the results were filtered according to the following inclusion/exclusion criteria:
- Only articles from scientific journals published in the last five years (2017-2021) were selected.
- Only articles with free access to the full text were selected.
- Only articles published in English and Spanish were selected.
- Only articles contrasted with other accredited sources were selected, thus meeting the criterion of reliability or stability among the results of articles that address the same subject matter.
- Only articles whose titles and abstracts specified the subject studied at each point in the development of the work were selected.
Database search process:
Table 2.
Table 2: Selection of sources in the different databases consulted after application of the inclusion/exclusion criteria. |
|||||||
| Databases | Descriptors (MeSH) | Results without filters | Filtering (date) | Filtering (access) | Filtering (title) | Filtering (abstract) |
Sources |
| COCHRANE PLUS | Crohn AND disease | 64 | 29 | 18 | 0 | 0 | 0 |
| Perianal AND fistulas | 5 | 3 | 3 | 0 | 0 | 0 | |
| Mesenchymal AND stem AND cells | 3 | 2 | 2 | 0 | 0 | 0 | |
| Perianal fistulas AND mesenchymal stem cells AND Crohn's disease | 0 | 0 | 0 | 0 | 0 | 0 | |
| PUBMED | Crohn AND disease | 13.787 | 8.029 | 3.267 | 41 | 8 | 3 |
| Perianal AND fistulas | 621 | 284 | 124 | 10 | 6 | 4 | |
| Mesenchymal AND stem AND cells | 32.506 | 18.679 | 8.280 | 190 | 14 | 8 | |
| Perianal fistulas AND mesenchymal stem cells AND Crohn's disease | 65 | 56 | 22 | 13 | 10 | 7 | |
| SCOPUS | Crohn AND disease | 158.394 | 397 | 54 | 1 | 0 | 0 |
| Perianal AND fistulas | 15 | 7 | 7 | 1 | 1 | 0 | |
| Mesenchymal AND stem AND cells | 52 | 16 | 8 | 2 | 0 | 0 | |
| Perianal fistulas AND mesenchymal stem cells AND Crohn's disease | 25 | 9 | 5 | 3 | 1 | 1 | |
| GOOGLE SCHOLAR | Crohn AND disease | 18.700 | 7000 | 4.010 | 32 | 5 | 2 |
| Perianal AND fistulas | 13.200 | 4.260 | 300 | 23 | 3 | 1 | |
| Mesenchymal AND stem AND cells | 19.200 | 7.030 | 4.690 | 320 | 7 | 2 | |
| Perianal fistulas AND mesenchymal stem cells AND Crohn's disease | 382 | 126 | 116 | 17 | 3 | 1 | |
Synthesis of the search by descriptors
Tables 3-6.
| Table 3: Sources and content summary for DeCS "Crohn's disease". | |||
| Descriptors (DeCS) | Reference | Article | Contents |
| Crohn's disease | 1 | Review | Comprehensive update on CD (conceptualization, epidemiology, etiology, risk factors, classification, clinical manifestations, diagnosis, and treatment). |
| 2 | Review | It focuses in particular on the incidence and prevalence of CD according to ethnic, geographic, socio-demographic, and lifestyle factors. | |
| 3 | Review | The main focus is on the clinical and extraintestinal manifestations of CD, its diagnosis, and current and future treatments. | |
| 4 | Review | A comprehensive overview of the different diagnostic and therapeutic pathways for CD. | |
| 5 | Review | Overview of CD (conceptualization, epidemiology, risk factors, clinical manifestations, diagnosis, and treatments). | |
| Table 4: Sources and summary of contents for the DeCS "Perianal fistulas". | |||
| Descriptors (DeCS) | Reference | Article | Contents |
| Perianal fistulas | 6 | Review | A medical guide to the latest advances in the treatment of perianal fistula CD, including mesenchymal stem cell therapy. |
| 7 | Review | Analysis of clinical studies on the efficacy of the most commonly used therapies currently used to treat perianal fistulas and abscesses in CD. | |
| 8 | Review | It provides an in-depth look at the new substances used in the treatment of perianal CD, providing some figures on their efficacy. | |
| 9 | Empirical study | Comparative and predictive analysis of risk factors for the occurrence and severity of perianal fistulas and abscesses. | |
| 10 | Review | Comprehensive update on treatments for perianal CD, including mesenchymal stem cell therapy. | |
| Table 5: Sources and content summary for the DeCS "Mesenchymal cells". | |||
| Descriptors (DeCS) | Reference | Article | Contents |
| Mesenchymal cells | 11 | Review | It delves into the therapeutic applications of MSCs in inflammatory bowel disease. |
| 12 | Review | It analyses the mechanisms of the main functions of the MSCs. | |
| 13 | Review | Describe the dynamics of MSC cell differentiation. | |
| 14 | Review | It focuses the object of study on the secretome property of MSCs involved in tissue regenerative capacity. | |
| 15 | Review | Explain the main characteristics and biological properties of MSCs. | |
| 16 | Review | It addresses the biological functions of MSCs and their clinical applications. | |
| 17 | Review | It outlines the current clinical applications of MSCs. | |
| 18 | Review | It deals with MSC treatments in inflammatory and fibrotic diseases. | |
| 19 | Review | Study on immune-mediated disorders and the role of MSCs in their treatment. | |
| 20 | Review | It highlights the therapeutic importance of MSCs. | |
| Table 6: Sources and summary of contents for the DeCS "Treatment of PF with CMM". | |||
| Descriptors (DeCS) | Reference | Article | Contents |
| Treatment, perianal fistulas, mesenchymal cells | 21 | Clinical trial | To determine the efficacy and safety of intralesional administration of MSC (eASC) in the treatment of perianal fistula CD. |
| 22 | Clinical trial | Evaluate the feasibility, effectiveness, and safety of local injections of micro fragmented adipose tissue in refractory complex fistulizing perianal CD | |
| 23 | Clinical trial | Analyses the combined remission after 24 weeks of treatment of fistulising CD with Darvadstrocel. | |
| 24 | Clinical trial | Investigates the efficacy and safety of umbilical cord MSCs (UC-MSCs) for the treatment of fistulizing CD. | |
| 25 | Meta-analysis | Systematically reviews the literature to determine the safety and efficacy of MSCs for the treatment of refractory fistulising perianal CD. | |
| 26 | Clinical trial | It studies the long-term efficacy and safety of allogeneic expanded adipose-derived stem cells (C x 601) in patients with CD and PF. | |
| 27 | Clinical trial | Provides a clinical evaluation of 4 years of allogeneic BM-MSC treatment of perianal CD fistulas | |
| 28 | Clinical trial | Investigates the effects of injecting MSCs from autologous adipose tissue into the PF of CD patients. | |
| 29 | Clinical trial | It measures the effects of local administration of bone marrow-derived MSCs (BM-MSCs) from healthy donors in patients with fistulizing CD. | |
| 30 | Systematic review | Updates a previously published systematic review on the efficacy and safety of treatments. | |
Results of the reviewed studies
The following is a systematic analysis of the sample of 10 articles reporting on the efficacy and safety of the use of MSCs to treat PF in CD. All of them specify the title, authorship and year of publication, objectives, type of sampling, methodology used, main results obtained, and conclusions.
Study 1 [21]
• Title: Therapeutic Positioning Report of Darvadstrocel (Alofisel®) in the treatment of complex perianal fistulas in Crohn's disease.
• Author and year: Ministry of Health, Consumer Affairs and Social Welfare (2019).
• Objectives: To demonstrate the efficacy and safety of allogeneic, adipose, expanded MSCs (eASCs) in the treatment of fistulizing CD with Darvadstrocel C x 601 (Alofisel®).
• Sampling: 212 patients aged 38 years on average with mild or inactive CD (CDAI < 220) and presence of complex FPs with 2 internal and maximum 3 external orifices, who did not respond adequately to any of the biological or conventional treatments with antibiotics (ciprofloxacin and metronidazole for 4 weeks), immunosuppressants (azathioprine and mercaptopurine for 12 weeks) and/or anti-TNF during the same period.
• Methods: Two groups were formed. A control group administered a placebo (n1 = 105 subjects) and an experimental group (n2 = 107 subjects) treated with Alofisel® in 4 intralesional vials of 6ml suspension each, over 24 weeks and three treatment phases.
• Variables: As the primary variable, combined remission of fistula CD (clinical closure of orifices and absence of abscesses > 2 cm). As secondary variables, clinical remission (closure of external orifices draining at baseline), clinical response (closure ≥ 50% of external orifices) at week 24 of treatment, time of remissions, presence and time of relapse, Van Assche indices (radiological activity) and CDAI, possible changes in PDAI and results of the quality of life questionnaire (IBDQ).
• Results:
o On efficacy: combined remission was significantly higher (p = .012) in the experimental group than in the control (54.2% vs. 37.1%), as was the case for clinical remission (p = .016, 57% vs. 40%) and clinical response (p = .014, 64% vs. 53%). On the other hand, relapses were lower in the experimental group (p = 38% vs. 50% in the control group).
o On safety: adverse outcomes were statistically similar in both groups (76.7% and 72.5%) and the most common were proctalgia (14.5% and 11.8%) and anal abscess (19.4% and 14.7%). The results, in this case, are unclear and further findings are awaited in ongoing clinical trials.
• Conclusion:
o The efficacy of Darvadstrocel has allowed it to be licensed for the treatment of PF in CD.
o The safety of Darvadstrocel is acceptable but limited and further studies are needed to rule out the immunogenicity of this treatment.
o The administration should be limited to specialist physicians to avoid errors leading to potentially adverse effects.
Study 2 [22]
• Title: Refractory complex perianal Crohn's fistulas: the role of autologous injection of micro fragmented adipose tissue.
• Author and year: Laureti, et al. (2020).
• Objectives: To evaluate the effectiveness, safety, and feasibility of local injections of micro fragmented adipose tissue in patients with refractory complex fistulizing perianal CD.
• Sampling: 15 patients aged at least 18 years (mean age = 40.1 years) with failed surgical approach using a combination of drainage and anti-TNF-alpha administration for at least 12 months, with a subsequent mucosal flap or seton implantation. They have more than 1 internal and 3 external orifices, anovaginal and rectovaginal fistulas (PF), HIV, HCV, HBV, tuberculosis, and/or uncontrolled septic diseases.
• Method: a prospective pilot study performed with a single administration of autologous micro fragmented adipose tissue prepared by a minimal manipulation technique (Lipogems) in a closed system, where subjects are measured before and after treatment on the variables analyzed.
• Variables: success rate, operationalized by combined remission (closure of treated external openings > 2cm) at week 24 of treatment (primary variable), combined clinical and radiological remission at the same week, PDAI index, IBDQ, and Short-Form 36 Health Survey -SF-36- and IBDQ results (secondary variables).
• Results:
o On efficacy: 10 patients (67%) achieved combined (clinical and radiographic) remission, 4 patients (27%) showed improvement and 1 patient (6%) failed. There were significant decreases in pre-therapy values in CRP, ESR, and PDAI. There was a trend of significant improvement in combined remission. Anal stenosis was a relevant factor for lower efficacy, occurring in all 3 patients with anal stenosis (p = .018).
o On safety: no relevant postoperative complications or adverse events were reported.
• Conclusion:
o Local injection of autologous micro fragmented autologous adipose tissue is a feasible, simple, safe, and promising salvage therapy to treat patients with complex multidrug-resistant perianal fistulizing CD.
o It is minimally invasive, can be performed on an outpatient basis, and carries a lower risk of sphincter damage.
Study 3 [23]
• Title: Mesenchymal stem cells for perianal Crohn's disease.
• Author and year: Carvello, et al. (2019).
• Objectives: to analyze combined remission (clinical closure of fistulas) after 24 weeks of treatment of fistulizing CD with eASC-MSCs, Darvadstrocel C x 601 (AlofiselTM).
• Sampling: 212 patients with inactive or mild luminal fistulising CD (CDAI ≤ 220) who had complex perianal fistulas.
• Method: a randomized, double-blind, placebo-controlled study. Sample divided into two groups: experimental (n1 = 107 subjects treated with Darvadstrocel) and control (n2 = 105 subjects treated with saline placebo).
• Variables: as the primary variable, combined remission of fistula CD (clinical closure of orifices and absence of abscesses > 2 cm). As secondary, clinical remission (closure of external orifices draining at baseline), clinical response (closure = 50% of external orifices) at 6 months after treatment (week 24).
• Results:
o On efficacy: combined remission was significantly higher (p = .024) in the experimental group than in the control (53% vs. 36%), as was the case for clinical remission (p = .064, 57% vs. 41%), but no significant differences were obtained in clinical response (p = .054, 71% vs. 53%). On the other hand, relapses were lower in the experimental group (p = 38% vs. 50% in the control group). These results were confirmed one year after treatment.
o On safety: adverse effects occurred in 66% of the experimental group and 65% of the control group, especially proctalgia, perianal abscess (5% of the total), and/or nasopharyngitis in 17% and 29% respectively. One year after treatment, anal abscesses and fistulae were similar in both groups, and abscesses were found in 6.8% and 4.9% respectively, but the risk of infection and tumor were low.
• Conclusion: eASC-MSC administration is effective in healing fistulas, but the mechanisms that induce healing have not yet been fully explored, such as the protumorigenic effect on cancers (neoplastic cell proliferation and angiogenesis).
Study 4 [24]
• Title: Umbilical cord mesenchymal stem cell therapy for Crohn's disease: a randomized controlled clinical trial.
• Author and year: Zahng et al. (2018)
• Aims: To investigate the efficacy and safety of umbilical cord MSCs (UC-MSCs) for the treatment of fistulizing CD.
• Sampling: 82 patients, mean age 33.5 years, with moderate to severe CD (220 ≤ CDAI ≤ 450) who received steroid therapy (> 6 months). Of these, 41 patients were randomly selected to receive treatment (experimental group, n1 = 42) and the rest did not (control group, n2 = 42).
• Methods: a randomized controlled clinical trial using four peripheral intravenous infusions of 1×106 UC-MSC/kg, at a rate of one every week and follow-up for 12 months.
• Variables: primary, endoscopic CD severity index (CDEIS), CDAI, Harvey-Bradshaw index (HBI), corticosteroid dose, and adverse events. As for secondaries, blood cell analyses of liver and renal function, coagulation, and D-dimer.
• Results:
o On efficacy: significant decrease in CDEIS (p < .01), CDAI (p < .01), HBI (p < .05) and corticosteroids (p < .05) of the UC-MSC group relative to the control group. Concomitant PF improved in six patients in the UC-MSC group and did not improve in seven patients in the control group. No patient achieved complete remission of fistulas.
o On safety: No major adverse events occurred (only 4 patients with fever and 7 with upper respiratory tract reinfection in the experimental group).
• Conclusion: UC-MSC therapy effectively and safely improves the disease status of perianal fistulizing CD receiving a stable dose of steroids, but its long-term effects remain to be investigated.
Study 5 [25]
• Title: A systematic review and meta-analysis of mesenchymal stem cell injections for the treatment of perianal Crohn's disease: progress made and future directions.
• Author and year: Lightner, et al. (2018).
• Objectives: To systematically review the literature to determine the safety and efficacy of MSCs for the treatment of refractory fistulizing perianal CD.
• Sampling: eleven studies met the inclusion criteria and were included in the systematic review. In all cases, randomized controlled clinical trials were analyzed and analyses were based on a statistical comparison of the treated group with the control group.
• Method: a meta-analysis. Sources were consulted in the PubMed, Cochrane Library Central Register of Controlled Trials, and Embase databases. Using 2 independent observers, papers reporting the safety and efficacy of MSCs for the treatment of perianal CD were included.
• Variables: combined remission of fistulous CD (clinical healing of orifices) after administration of MSCs, irrespective of their source of extraction.
• Results:
o On efficacy: MSCs are associated with improved healing between 6 and 24 weeks of treatment (p = 0.04; OR = 3.06) and between 24 and 52 weeks (p = 0.08; OR = 2.37).
o On safety: No relevant increases in mild (p = 0.81; OR = 1.07) or serious (p = 0.04; OR = 0.53) adverse events were detected in subjects treated with MSC.
• Conclusion: Improved efficacy and absence of mild or severe adverse events in MSC-treated subjects compared to control subjects.
Study 6 [26]
• Title: Long-term efficacy and safety of stem cell therapy (C x 601) for complex perianal fistulas in patients with Crohn's disease.
• Author and year: Panés, et al (2018).
• Objectives: To analyze the long-term efficacy and safety of allogeneic expanded adipose tissue-derived stem cells (C x 601) in patients with CD and PF.
• Sampling: 212 patients with refractory and draining complex CD and PF, who did not respond adequately to conventional treatments, from 49 Israeli hospitals and 7 European countries. 107 from the experimental group (n1 = 107) and 105 from the control group (n2 = 105).
• Methods: randomized, controlled, stratified (unmedicated, anti-TNF-α or immunosuppressive patients) and double-blinded (two independent specialists) trial, with the administration of a single local dose of C x 601 allogeneic, expanded adipose-derived MSCs (eASCs) (experimental groups) and a placebo dose (control group). Outcomes were assessed at 6, 12, 18, 24, 36, and 52 weeks after MSC transplantation.
• Variables: As primary variables, combined remission (closure of all external openings > 2 cm) and clinical remission (absence of draining fistulas) were considered one year after starting treatment. As secondary, CDAI, PDAI indices, IBDQ questionnaire scores, and relapses.
• Results:
o On efficacy: at week 24, significant differences in combined remission were found between the two groups (p = .021, 51.5% experimental group vs. 35.6% control group). At week 52, these differences increased further (p = .010, 56.3% vs. 38.6% respectively) and significance was also found in clinical remission (p = .013, 59.2% vs. 41.6%). However, no significance was found for relapse, despite a higher incidence in the experimental group (p = .052, 75% vs. 55.9%), nor between strata (p = .34).
o On safety: safety was maintained at week 52, with no adverse effects in 76.7% of the treated group and 72.5% of the placebo group, of which were severe in 24.3% and 20.6% respectively, the most common being abscesses and PF, followed by proctalgia (8.7% and 8.8%). Tolerability was maintained one year after transplantation and no deaths occurred.
• Conclusion: C x 601 treatment is safe and effective in closing external openings one year after trans-plantation, although it is still not free of adverse events.
Study 7 [27]
• Title: Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for perianal fistulas in Crohn's disease.
• Author and year: Barnhoorn et al. (2020).
• Objectives: To provide a clinical evaluation of 4 years of allogeneic BM-MSC treatment of perianal CD fistulas.
• Sampling: 21 patients from Leiden University Medical Center with refractory perianal fistulizing CD.
• Methods: randomised double-blind study in a 5:2 fashion in three groups: received locally and BM-MSC, 0.9% NaCl solution, 5% human albumin with 1 × 10 7 [cohort 1, n = 5], 3×107 [cohort 2, n = 5], or 9×107 [cohort 3, n = 5] or cell-free solution [placebo group, n = 6].
• Variables: As primary variables, fistula closure and anti-human leukocyte antigen [HLA] antibodies by pelvic MRI and rectoscopy. As secondary, PDAI and CDAI index, adapted and short-form Vaizey fecal incontinence score [SF]-36, and IBDQ Questionnaire.
• Results:
o On efficacy: in cohort 2 all fistulae closed 4 years after BM-MSC therapy. In cohort 1 they closed in 63% of cases, in cohort 3 43%, and the placebo group 0% (no fistula closure). In none of the patients anti-HLA antibodies could be detected 24 weeks and 4 years later. Pelvic MRI showed significantly shorter fistula tracts in the long term.
o On safety: no serious therapy-related adverse events were found, although 4 subjects developed perianal abscesses, 3 showed CD activity in the last 4 years, 5 were treated for infections and 1 showed B-cell lymphoproliferative disease in the rectum.
• Conclusion: Treatment with MSC (allogeneic BM-MSC) used to cure CD-associated PF is also, in the long term, effective and safe therapy for a majority of patients.
Study 8 [28]
• Title: Efficacy of freshly harvested autologous adipose tissue injection in perianal fistulas in patients with Crohn's disease.
• Author and year: Dige, et al. (2019).
• Aims: To investigate the effects of injecting freshly harvested autologous adipose tissue MSCs into the PFs of patients with CD.
• Sampling: 21 patients with fistulizing CD.
• Method: prospective interventional clinical trial with a single experimental group.
• Variables: complete closure of PFs6 months after the last injection (no symptoms of discharge, no external opening visible in the perineum, and no internal opening by rectal examination).
• Results:
o On efficacy: 57% showed complete healing of PFs, 14% ceased fistular discharge and only 5% reported small discharge. In 10 patients with trans-sphincteric or inter-sphincteric fistulae, complete fistula healing occurred in 9 patients and only one gracile fistula in the remaining patient. Of the 12 patients with complete fistula healing, 43% required 1 injection, 10% 2 injections, and the rest (5%) 3 injections.
o On safety: the most frequent adverse effects were proctalgia after treatment for a few days, small abscesses, and minor bleeding during liposuction
• Conclusion: injection of freshly harvested autologous adipose tissue is safe and completely cures PF in 57% of patients.
Study 9 [29]
• Title: Allogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn's disease.
• Author and year: Molendijk, et al. (2015).
• Aims: To evaluate the effects of local administration of bone marrow-derived MSCs (BM-MSCs) from healthy donors in patients with fistulizing CD.
• Sampling: 21 patients with refractory perianal fistulizing CD treated at Leiden University Medical Centre.
• Method: a double-blind, placebo-controlled study was randomly assigned to 3 experimental groups treated with BM-MSC injections of 1 × 107 (n = 5, group 1), 3×107 (n = 5, group 2), and 9×107 (n = 5, group 3) or placebo (saline, n = 6).
• Variables: as primary, fistula healing at 6, 12, and 24 weeks after injection, in the absence of discharge and < 2 cm of fluid accumulated at week 12.
• Results:o On efficacy: healing at week 6 of 60.0% in group 1, 80.0% in group 2, and 20.0% in group 3, versus 16.7% in the placebo group, although differences were not statistically significant between-group 2 and control (p = .08). At week 12, the cure was observed in 40.0% of group 1, 80.0% of group 2, and 20.0% of group 3 versus 33.3% of the placebo group. These effects remained until week 24 and increased to 80.0% in group 1. At week 6, 44.4% of group 1 showed healing of the PF, 85.7% of group 2, 28.6% of group 3, versus 22.2% in the placebo group, with significant differences between the placebo group and group 2 (p = .04). At week 12, 33.3% of group 1 healed PF, 85.7% of group 2, and 28.6% of group 3, compared to 33.3%, with effects maintained until week 24, and even increased in 66.7% of group 1, with significant differences between group 2 and placebo at weeks 12 and 24 (p = .06).
o On Safety: No notable adverse effects were found.
• Conclusion: Injection of 3 × 10 (7) MSCs appeared to promote healing of perianal fistulas in the absence of serious adverse effects.
Study 10 [30]
• Title: Efficacy and safety of mesenchymal/stromal stem cell therapy for inflammatory bowel diseases: an updated systematic review.
• Author and year: Ko, Johnson, and Dave (2021).
• Objectives: to update a previously published systematic review by Dave et al. regarding the efficacy and safety of treatments.
• Sampling: 32 peer-reviewed articles, letters, or abstracts that met the inclusion/exclusion criteria (treatment of patients with inflammatory bowel disease involving MSCs or MSC-containing tissue products and reporting efficacy and safety).
• Method: A systematic review of impact clinical trials.
• Variables: Short- and long-term outcomes on PF healing (closure and absence of drainage).
• Results:
o On efficacy: 3 sources reported short-term effects without blinding or placebo group, using allogeneic adipose MSCs (Allo-ASCs) and autologous adipose MSCs (Auto-ASCs). 13 sources reported long-term effects using Alo-ASC, Allo-BM-MSC or Auto-ASC. The rest reported effects on luminal CD.
o On safety: No serious adverse effects were described, the most frequent being the occurrence of fistulas, anal abscesses, and/or proctalgia.
• Conclusion: There is strong evidence for the efficacy and safety of local MSC injections in patients with fistulising CD. Despite the heterogeneity of the results, partly due to the different methodologies employed, MSC therapy is a promising alternative to the failure of conventional treatments.
Summary of study results
Of the sample of selected articles reporting on the efficacy and safety of the use of MSCs for the treatment of PF in CD, 80% were clinical trials, 10% were meta-analyses and 10% were systematic reviews.
Of the 80% of clinical studies, 5 articles (62.5%) conducted randomized controlled trials with one or more experimental groups (treated with MWC) and a control group (placebo administration), while 3 articles (37.5%) and 1 review article (10% of the total) conducted trials with a single experimental group where the evolution of the disease was analyzed over several measurement points.
Also, 6 articles (60%) used in vitro cultures of expanded allogeneic transplanted MSCs, 2 articles (20%) used auto-genic or autologous MSCs and one article (10%) reported the use of both types of transplantation.
In 60% of the studies, MSCs from adipose tissue were used, in 20% from bone marrow, in 10% from the umbilical cord and in the rest (10%) the results from these three sources are reported.
In almost all articles, combined remission (closure or cure) of PFs was used as a primary variable and clinical remission and response, the occurrence of relapses, various activity indices (CDAI, PDAI, Vaizey, etc.), and assessment of patients' quality of life (IBDQ) were used as secondary variables.
The efficacy of the therapies was strongly certified in 5 articles (50%), partially certified (some of the secondary variables did not show significant improvements) in 4 articles (40%), and unclear in only one article (10%). Presence of anal stenosis, no improvement in clinical response, presence of relapses, or scarcity of short-term effects is reported.
Finally, safety was established in 7 articles (70%), i.e. no significant adverse effects were found, and in the rest (30%) it was not clear, with reports of proctalgia, abscesses, secretions, reinfections, fever, or protumorigenic effects of varying severity, depending on the origin of the MSCs, the methodology used and the specialization of the experimenters in the application of the treatments, among others.
MSC therapies for treating PF due to complex refractory fistulizing CD are still in the experimental phase but are proving to be a promising alternative to conventional treatment failures, as they are effective in a high percentage of patients treated, as well as safe since most the adverse effects found are null or mild. The variability between the results found may be due, in part, to the different methodologies used in the treatments, the origin of the sources of MSC extraction, and the degree of specialization of the professionals involved in these therapies.
This study, among others, opens up a wide field of research with different uses for mesenchymal stem cell treatment, such as the use of mesenchymal stem cells in intestinal anastomosis to prevent leakage. This offers hope for both patients and healthcare professionals who face complex patient challenges daily.
This study has some limitations, such as the selection criteria. We have not included articles to which we did not have access for different reasons. In addition, there is heterogeneity between the different studies. They are investigations carried out in different contexts, with patients with characteristics that are not necessarily similar or even with different results, which could affect the outcome of the review.
- Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn's disease. Dis Mon. 2018 Feb;64(2):20-57. doi: 10.1016/j.disamonth.2017.07.001. Epub 2017 Aug 18. PMID: 28826742.
- Figueroa C. Epidemiología de la enfermedad inflamatoria intestinal. Rev Med Clin Las Condes. 2019;30(4):257-261. DOI: 10.1016/j.rmclc.2019.06.003.
- Bastida G, Garrido A, Valero E, del Pozo P. Enfermedad de Crohn. Medicine 2020;13(11):603-612.
- Veauthier B, Hornecker JR. Crohn's Disease: Diagnosis and Management. Am Fam Physician. 2018;98(11):661-669. PMID: 30485038.
- Ballester MP, Boscá-Watts MM, Mínguez M. Enfermedad de Crohn. Med Clin. 2018;151(1):26-33. Disponible en: https://www.sciencedirect.com/science/article/pii/S0025775317308448
- Lopez N, Ramamoorthy S, Sandborn WJ. Recent advances in the management of perianal fistulizing Crohn's disease: lessons for the clinic. Expert Rev Gastroenterol Hepatol 2019;13(6):563-577. DOI: 10.1080/17474124.2019.1608818.
- Grimstad T, Carlsen A, Karlsen LN. Medical management of fistulising Crohn's disease. Tidsskr Nor Laegeforen. 2019 Jan 9;139(1). English, Norwegian. doi: 10.4045/tidsskr.17.1055. PMID: 30644688.
- Vetter M, Neurath MF. Treatment Perspectives in Crohn's Disease. Digestion. 2018;98(3):135-142. doi: 10.1159/000488449. Epub 2018 Jun 5. PMID: 29870990.
- Xu M, Yang B, Chen H, Gu Y, Li Y. Analysis of Risk Factors for Anorectal Stenosis of Perianal Fistulizing Crohn's Disease. Med Sci Monit. 2020 Feb 28;26:e920243. doi: 10.12659/MSM.920243. PMID: 32109226; PMCID: PMC7063846.
- González N. Situación Actual en el Tratamiento de la Enfermedad de Crohn Perianal. Archivos de coloproctología 2019;2(2):4-33. DOI: https://doi.org/10.26754/ojs_arcol/arch_colo.201923794.
- Mao F, Tu Q, Wang L, Chu F, Li X, Li HS, Xu W. Mesenchymal stem cells and their therapeutic applications in inflammatory bowel disease. Oncotarget. 2017 Jun 6;8(23):38008-38021. doi: 10.18632/oncotarget.16682. PMID: 28402942; PMCID: PMC5514968.
- Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016 Aug 31;7(1):125. doi: 10.1186/s13287-016-0363-7. PMID: 27581859; PMCID: PMC5007684.
- Assis-Ribas T, Forni MF, Winnischofer SMB, Sogayar MC, Trombetta-Lima M. Extracellular matrix dynamics during mesenchymal stem cells differentiation. Dev Biol. 2018 May 15;437(2):63-74. doi: 10.1016/j.ydbio.2018.03.002. Epub 2018 Mar 12. PMID: 29544769.
- Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017 Aug 25;18(9):1852. doi: 10.3390/ijms18091852. PMID: 28841158; PMCID: PMC5618501.
- Andrzejewska A, Lukomska B, Janowski M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells. 2019 Jul;37(7):855-864. doi: 10.1002/stem.3016. Epub 2019 Apr 30. PMID: 30977255; PMCID: PMC6658105.
- Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019 Sep;76(17):3323-3348. doi: 10.1007/s00018-019-03125-1. Epub 2019 May 4. PMID: 31055643.
- Zuo W, Xie B, Li C, Yan Y, Zhang Y, Liu W, Huang J, Chen D. The Clinical Applications of Endometrial Mesenchymal Stem Cells. Biopreserv Biobank. 2018 Apr;16(2):158-164. doi: 10.1089/bio.2017.0057. Epub 2017 Dec 21. PMID: 29265881; PMCID: PMC5906727.
- Ryu JS, Jeong EJ, Kim JY, Park SJ, Ju WS, Kim CH, Kim JS, Choo YK. Application of Mesenchymal Stem Cells in Inflammatory and Fibrotic Diseases. Int J Mol Sci. 2020 Nov 7;21(21):8366. doi: 10.3390/ijms21218366. PMID: 33171878; PMCID: PMC7664655.
- Markov A, Thangavelu L, Aravindhan S, Zekiy AO, Jarahian M, Chartrand MS, Pathak Y, Marofi F, Shamlou S, Hassanzadeh A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther. 2021 Mar 18;12(1):192. doi: 10.1186/s13287-021-02265-1. PMID: 33736695; PMCID: PMC7971361.
- Mishra VK, Shih HH, Parveen F, Lenzen D, Ito E, Chan TF, Ke LY. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells. 2020 May 6;9(5):1145. doi: 10.3390/cells9051145. PMID: 32384763; PMCID: PMC7291143.
- Agencia Española de Medicamentos y productos Sanitarios. Informe de Posicionamiento Terapéutico de Darvadstrocel (Alofisel®) en el tratamiento de fístulas perianales complejas en enfermedad de Crohn. Ministerio de Sanidad, Consumo y Bienestar Social. 2019;50/2019 V1.
- Laureti S, Gionchetti P, Cappelli A, Vittori L, Contedini F, Rizzello F, Golfieri R, Campieri M, Poggioli G. Refractory Complex Crohn's Perianal Fistulas: A Role for Autologous Microfragmented Adipose Tissue Injection. Inflamm Bowel Dis. 2020 Jan 6;26(2):321-330. doi: 10.1093/ibd/izz051. PMID: 31220252; PMCID: PMC6943693.
- Carvello M, Lightner A, Yamamoto T, Kotze PG, Spinelli A. Mesenchymal Stem Cells for Perianal Crohn's Disease. Cells. 2019 Jul 23;8(7):764. doi: 10.3390/cells8070764. PMID: 31340546; PMCID: PMC6679174.
- Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical Cord Mesenchymal Stem Cell Treatment for Crohn's Disease: A Randomized Controlled Clinical Trial. Gut Liver. 2018 Jan 15;12(1):73-78. doi: 10.5009/gnl17035. PMID: 28873511; PMCID: PMC5753687.
- Lightner AL, Wang Z, Zubair AC, Dozois EJ. A Systematic Review and Meta-analysis of Mesenchymal Stem Cell Injections for the Treatment of Perianal Crohn's Disease: Progress Made and Future Directions. Dis Colon Rectum. 2018 May;61(5):629-640. doi: 10.1097/DCR.0000000000001093. PMID: 29578916.
- Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Diez MC, Tagarro I, Leselbaum A, Danese S; ADMIRE CD Study Group Collaborators. Long-term Efficacy and Safety of Stem Cell Therapy (C x 601) for Complex Perianal Fistulas in Patients with Crohn's Disease. Gastroenterology 2018 citado 10 abr 2021];154(5):1334-1342. DOI: 10.1053/j.gastro.2017.12.020.
- Barnhoorn MC, Wasser MNJM, Roelofs H, Maljaars PWJ, Molendijk I, Bonsing BA, Oosten LEM, Dijkstra G, van der Woude CJ, Roelen DL, Zwaginga JJ, Verspaget HW, Fibbe WE, Hommes DW, Peeters KCMJ, van der Meulen-de Jong AE. Long-term Evaluation of Allogeneic Bone Marrow-derived Mesenchymal Stromal Cell Therapy for Crohn's Disease Perianal Fistulas. J Crohns Colitis. 2020 Jan 1;14(1):64-70. doi: 10.1093/ecco-jcc/jjz116. PMID: 31197361; PMCID: PMC6930001.
- Dige A, Hougaard HT, Agnholt J, Pedersen BG, Tencerova M, Kassem M, Krogh K, Lundby L. Efficacy of Injection of Freshly Collected Autologous Adipose Tissue Into Perianal Fistulas in Patients With Crohn's Disease. Gastroenterology. 2019 Jun;156(8):2208-2216.e1. doi: 10.1053/j.gastro.2019.02.005. Epub 2019 Feb 14. PMID: 30772343.
- Molendijk I, Bonsing BA, Roelofs H, Peeters KC, Wasser MN, Dijkstra G, van der Woude CJ, Duijvestein M, Veenendaal RA, Zwaginga JJ, Verspaget HW, Fibbe WE, van der Meulen-de Jong AE, Hommes DW. Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn's Disease. Gastroenterology. 2015 Oct;149(4):918-27.e6. doi: 10.1053/j.gastro.2015.06.014. Epub 2015 Jun 25. PMID: 26116801.
- Ko JZ, Johnson S, Dave M. Efficacy and Safety of Mesenchymal Stem/Stromal Cell Therapy for Inflammatory Bowel Diseases: An Up-to-Date Systematic Review. Biomolecules. 2021 Jan 11;11(1):82. doi: 10.3390/biom11010082. PMID: 33440772; PMCID: PMC7827559.