Screening Thresholds for the Corneal Tomography in Hyperopic Pakistani Population
By Sadia Humayun1, Aisha Fawad2, Mazhar Ishaq1, Quratulain Humayun3, Sabahat Arzoo1, Syed Fawad Mashhadi4Affiliations
doi: 10.29271/jcpsp.2020.09.951ABSTRACT
Objective: To evaluate key corneal tomography parameters for screening mixed astigmatism and hyperopic males and females for refractive surgery and to compare the data to a previously studied myopic group in Pakistani population.
Study Design: Cross-sectional, observational study.
Place and Duration of Study: Armed Forces Institute of Ophthalmology AFIO, National University of Medical Sciences, Rawalpindi, Pakistan, from August 2013 to August 2018.
Methodology: WaveLight Allegro Oculyzer II diagnostic device was used to examine eyes of 106 adult hyperopic patients in order to determine normal values of 20 parameters, which are considered most clinically applicable for refractive surgery screening. Kolmogorov-Smirnov test was used to evaluate normality of data. Results for outliers were displayed as 2.5%, 5%, 95% and 97.5% percentiles.
Results: Two hundred and nine eyes were examined; 110 men and 99 women with overall mean age of 31+11.7 years. Normal mean anterior segment values included: flat simulated keratometry (K1) 42.1±1.84 diopters (D), steep K2 43.8 ± 1.93 D, K maximum 44.4 ± 1.93 D, K mean 42.9 ± 1.75 D, astigmatism -1.3 ± 1.75 D, pachymetry at thinnest point 546.9 ± 33.3 um, front elevation at thinnest point 5.2 ± 3.47 um, and at the back was 14.1 ± 6.60 um, Ambrosio relational thickness maximum 472.0 ± 88.73, progression index (PI) maximum 1.2 ± 0.18, and anterior chamber depth (ACD) 2.7 ± 0.35 mm.
Conclusion: Hyperopic patients had greater front and back elevation and pachymetry but lesser keratometry, anterior chamber depth and chamber volume as compared to myopic patients in Pakistani population. Front and back elevation data in this hyperopic study population was slightly higher than previously published studies.
Key Words: Refractive surgery, Corneal tomography, Screening, Hyperope.
INTRODUCTION
Corneal topography and tomography has revolutionised the diagnosis and management of corneal diseases especially keratoconus.1,2 It is particularly important in screening refractive surgical patients and for evaluating and improving the results of corneal surgical procedures such as laser in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK).3,4
The Pentacam (Oculus Optikgerδte GmbH, Wetzlar, Germany) Scheimpflug imaging device launched in 2004, has become a popular device for calculating a three-dimensional model of the eye from anterior corneal surface to the posterior lens surface in a single scan without contact with the cornea.5,6 Excellent reproducibility and repeatability of this device have been shown in previous studies for the automated measurements of the entire anterior segment structures7,8 It also has a wide range of applications in different procedures like pre- and post-operative evaluation of phakic intra ocular lenses (IOLs), collagen crosslinking, intra-stromal corneal rings, and post-refractive surgery intraocular lens power calculation. Obtaining adequate information and analysing data of anterior segment measurements is essential for the diagnosis and treatment of abnormal ocular conditions. Knowledge of accurate cutoffs and ranges of normal values of anterior segment parameters may prove to be extremely helpful for refractive surgeons. We also know from previous researches that racial and geographical variations might exist.
Currently used pachymetric and elevation normative values in Pentacam Scheimpflug system (Oculus Optikgerate GmbH, Germany) are obtained from varying countries and databases.9 It has been established that myopic normative database for pachymetry, corneal elevation and thickness values were different for Turkey from North and South American population. Similarly, myopic normative values were slightly different in Pakistani population when compared with Turkey, North and South American population.10
Kim et al. found that the hyperopic normal elevation values are different from myopic corneas.11 This leads to the need of finding the normative values for hyperopic corneas, separate from myopic eyes in Pakistani geographic area and location to achieve any clinical significance. United States Food and Drug Administration (FDA) also encourages observation, identification and study of race-specific differences in parameters to increase the effectiveness of medical devices.12 This will help us in better screening of refractive surgery patients and for identification of outliers in Pakistani population.
The objective of this study was to evaluate key corneal tomography parameters for screening mixed astigmatic and hyperopic males and females for refractive surgery and to compare the data to a previously studied myopic group in Pakistani population.
METHODOLOGY
This cross-sectional study was carried out at Armed Forces Institute of Ophthalmology (AFIO), Rawalpindi, which is a tertiary care and major referral centre for refractive surgery patients from all over the country. This study was done from August 2013 to August 2018, after approval from Institutional Review Board and Ethics Committee. Non-probability/convenience sampling was carried out to identify participants from the pool of patients who reported as possible candidates of refractive surgery. One or both the eyes were included in the study after screening the participants for eligibility criteria. Informed consent was taken from all the eligible participants after the nature and consequence of the investigation had been explained to them. At least two weeks prior to corneal imaging, contact lens use was discontinued. The sample size was derived from the formula
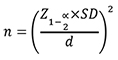
where Z 1-α/2 represents the standard normal coefficient at 95% confidence interval (1.96), SD represents the standard deviation, and d represents the absolute error or precision level (1 µm). Using mean values for each anterior segment parameters that were previously reported in a study.11 A total of 209 eyes of 106 patients between 16 to 58 years of age were recruited in the study.
All participants were examined by two separate observers in a randomised sequence for inclusion or exclusion in this study. The disputed cases were exempted from the study. All participants underwent normal ocular examination, including uncorrected visual acuity (UCVA), corrected distance visual acuity (CDVA), ocular dominance testing, cycloplegic refraction, slit lamp examination, intraocular pressure, fluorescence tear film break-up time (TBUT) and extra-ocular movements. The inclusion criteria were: mixed astigmatism and hyperopic patients with CDVA of 20/40 or better (Spherical equivalent range 0.5 – 8 D). The exclusion criteria were presence of any comorbid medical conditions or ocular pathologies, such as diabetes mellitus, hypertension, glaucoma, previous eye surgery, keratoconus, and family history of keratoconus. Myopia and myopic astigmatism patients were also excluded from the study. To minimise selection bias, the eligible patients were included in the study irrespective of the decision of refractive surgery.
The Allegro Oculyzer II (Wavelight GmbH Erlangen, Germany) diagnostic device was used to examine paired eyes of patients to determine normal values of 20 parameters which are considered most clinically applicable for refractive surgery screening: flat simulated keratometry (K1), steep simulated keratometry (K2), maximum K (Kmax), mean K, astigmatism magnitude, front and back elevation at the thinnest point, front and back elevation at the apex, maximum front and back elevation in the central 4.0 mm, average and maximum progression index13 (PI avg & PI max) which is the measure of the rate of change of corneal thickness, average and maximum Ambrosio Relational thickness14 (ART avg, ART max) (a measure of the thinnest point divided by average/maximum progression index), corneal pachymetry at the apex (Pachy apex), and at thinnest point (Pachy thinnest), difference between apical and thinnest pachymetry measurements, anterior chamber depth (ACD), and chamber volume (CV).
Data was analysed using SPSS version 25.0. Kolmogorov-Smirnov goodness-of-fit test was used to assess the normality of the data. Mean and standard deviations, as well as medians and interquartile range (IQR), were reported for normal and non-normal data, respectively. Frequencies with percentages were used to characterise nominal parameters. Percentiles (2.5, 5.0, 95.0 and 97.5) were computed for each of the parameters to identify outliers.
A sub-group analysis was performed, based on gender, to identify differences in screening thresholds among males and females. Independent sample t-test and Mann-Whitney U-test were used for this purpose.
Furthermore, in order to analyse the differences among the myopic and hyperopic population for the key parameters, Independent sample t-test (student's t-test) was performed for each of the parameters. The level of significance was set at <0.05.
RESULTS
A total of 106 patients (209 eyes) were included in the study. Out of the selected participants, 47.4% were female eyes (n=99) and 52.6% were male eyes (n=110).
Table I: Normal anterior segment values and their values for outlier.|
Parameters |
Mean (SD) |
Median (IQR) |
Min |
Max |
p-value (K-S test) |
Percentiles |
|||
|
5 |
95 |
2.5 |
97.5 |
||||||
|
K1 (D) |
42.1 (1.84) |
42.1 (2.30) |
37.2 |
48.7 |
0.200 |
38.9 |
45.1 |
38.4 |
45.6 |
|
K2 (D) |
43.6 (1.93) |
43.9 (3.15) |
39.7 |
48.9 |
0.002 |
40.9 |
46.9 |
40.5 |
47.5 |
|
K Mean (D) |
42.9 (1.75) |
43.1 (2.60) |
39.3 |
48.8 |
0.030 |
39.9 |
45.7 |
39.6 |
46.2 |
|
K Max (D) |
44.4 (1.93) |
44.5 (3.10) |
40.0 |
49.2 |
0.052 |
41.2 |
47.4 |
40.8 |
47.9 |
|
Astigmatism (D) |
-1.3 (1.75) |
-1.0 (2.25) |
-5.6 |
5.7 |
<0.001 |
-4.2 |
1.1 |
-4.8 |
2.2 |
|
ACD (mm) |
2.7 (0.35) |
2.7 (0.51) |
1.9 |
3.7 |
0.200 |
2.1 |
3.2 |
2.0 |
3.3 |
|
Front Elev at thinnest point (µm) |
5.2 (3.47) |
5.0(4.0) |
-4.0 |
14.0 |
<0.001 |
0.0 |
11.0 |
-1.0 |
12.0 |
|
Front Elev apex (µm) |
3.8 (2.52) |
4.0 (3.0) |
-2.0 |
13.0 |
<0.001 |
0.0 |
8.0 |
-1.0 |
9.8 |
|
Front Elev max (µm) |
8.4 (4.65) |
7.0 (7.0) |
1.0 |
23.0 |
<0.001 |
3.0 |
17.0 |
2.0 |
19.8 |
|
Back Elev at thinnest point(µm) |
14.1 (6.60) |
14.0 (11.0) |
-2.0 |
31.0 |
<0.001 |
4.0 |
24.0 |
3.0 |
26.8 |
|
Back Elev apex (µm) |
6.7 (5.52) |
6.0 (9.0) |
-4.0 |
23.0 |
<0.001 |
-1.0 |
16.5 |
-2.0 |
19.8 |
|
Back Elev max (µm) |
19.8 (7.37) |
20.0 (12.0) |
4.0 |
36.0 |
0.005 |
8.0 |
33.0 |
6.3 |
34.0 |
|
PI max |
1.2 (0.18) |
1.2 (0.23) |
0.6 |
1.7 |
<0.001 |
0.9 |
1.6 |
0.9 |
1.6 |
|
PI avg |
0.9 (0.14) |
0.9 (0.18) |
0.6 |
1.3 |
0.200 |
0.7 |
1.2 |
0.7 |
1.3 |
|
ART max |
472.0 (88.73) |
471.0 (113) |
291.0 |
891.0 |
0.040 |
332.0 |
620.0 |
319.0 |
661.5 |
|
ART avg |
600.2 (109) |
587.2 (140.9) |
374.2 |
982.7 |
0.004 |
433.3 |
798.7 |
410.4 |
866.6 |
|
Pachy thinnest (µm) |
546.9 (33.33) |
548.0 (45.50) |
467.0 |
628.0 |
0.200 |
488.5 |
601.5 |
480.3 |
609.5 |
|
Pachy apex (µm) |
553.5 (32.67) |
555.0 (47.0) |
473.0 |
632.0 |
0.200 |
500.5 |
607.0 |
483.8 |
616.3 |
|
Difference |
6.6 (3.19) |
6.0 (4.0) |
1.0 |
19.0 |
<0.001 |
3.0 |
12.0 |
2.0 |
15.0 |
|
CV (mm3) |
150.0 (38.16) |
152.0 (66.50) |
73.0 |
236.0 |
0.022 |
89.5 |
204.5 |
83.8 |
226.0 |
|
K1 = flat simulated keratometry, K2 = steep simulated keratometry, K max = maximum keratometry, K mean = mean keratometry, D= Diopters, ACD = anterior chamber depth, Elev = elevation, µm = micrometer, PI = progression index, ART = Ambrosio Relational Thickness, Pachy = pachymetry, max = maximum, avg = average, CV = chamber volume, K-S = Kolmogorov-Smirnov, SD = standard deviation, IQR = interquartile range, Min = minimum. |
|||||||||
Table II: Anterior segment values among hyperopic males and females.
|
Parameters |
Males Mean±SD / Median (IQR) |
Females Mean±SD / Median (IQR) |
p-value (<0.05) |
|
K1 (D) |
41.9±1.9/41.7 (2.2) |
42.3±1.7/42.4 (2.5) |
0.091t |
|
K2 (D) |
43.6±2.2/43.5 (3.7) |
44.0±1.6/44.5 (2.3) |
0.062ᵁ |
|
K Mean (D) |
42.7±1.9/42.7 (2.7) |
43.1±1.5/43.4 (2.0) |
0.039ᵁ |
|
K max (D) |
44.1±2.1/44.0 (3.5) |
44.6±1.6/45.1 (2.2) |
0.082t |
|
Astigmatism (D) |
-1.3±1.8/-1.1 (2.2) |
-1.2±1.7/-1.0 (2.3) |
0.987ᵁ |
|
ACD (mm) |
2.7±0.4/2.8 (0.6) |
2.6±0.3/2.6 (0.4) |
0.023t |
|
Front Elev at thinnest point (µm) |
5.1±3.6/5.0 (4.0) |
5.3±3.4/5.0 (5.0) |
0.623ᵁ |
|
Front Elev apex (µm) |
3.7±2.3/4.0 (3.0) |
3.9±2.8/4.0 (3.0) |
0.782ᵁ |
|
Front Elev max (µm) |
8.6±5.1/7.0 (7.0) |
8.3±4.1/8.0 (6.0) |
0.948ᵁ |
|
Back Elev at thinnest point (µm) |
12.6±6.8/11.0 (10.0) |
15.8±6.0/17.0 (8.0) |
0.001ᵁ |
|
Back Elev apex (µm) |
5.6±5.1/5.0 (7.0) |
7.8±5.8/7.0 (8.0) |
0.008ᵁ |
|
Back Elev max (µm) |
18.7±7.8/18.0 (13.3) |
21.0±6.6/21.0 (10.0) |
0.024ᵁ |
|
PI max |
1.2±0.2/1.2 (0.2) |
1.2±0.2/1.2 (0.3) |
0.468ᵁ |
|
PI avg |
0.9±0.1/0.9 (0.2) |
0.9±0.1/0.9 (0.19) |
0.200t |
|
ART max |
477.3±96.1/480.3 (116.9) |
466.1±79.8/467.5 (109.0) |
0.579ᵁ |
|
ART avg |
608.9±111.3/602.8 (137.5) |
590.6±106.3/562.9 (137.0) |
0.202ᵁ |
|
Pachy thinnest (µm) |
547.6± 31.7/550.0 (46.3) |
546.3±35.2/546.0 (46.0) |
0.767t |
|
Pachy apex (µm) |
554.1±31.0/556.5 (47.5) |
553.0±34.6/554.0 (46.0) |
0.819t |
|
Difference |
6.4±3.0/6.0 (4.0) |
6.8±3.4/6.0 (5.0) |
0.639ᵁ |
|
CV (mm3) |
157.4±40.4/158.5 (65.8) |
141.8±33.8/138.0 (53.0) |
0.004ᵁ |
|
K1 = flat simulated keratometry, K2 = steep simulated keratometry, K max = maximum keratometry, K mean = mean keratometry, D= Diopters, ACD = anterior chamber depth, Elev = elevation, µm = micrometer, PI = progression index, ART = Ambrosio Relational Thickness, Pachy = pachymetry, max = maximum, avg = average, CV = chamber volume, SD = standard deviation, t = Independent sample t-test, U= Mann-Whitney U-test. |
|||
The mean age of the participants was 31+ 11.7 years with a range of 16-58 years. Mean age for males (27.9 ± 10.19 years) and females (34.4 ± 11.82 years) was found to be statistically different from each other (p <0.001). Majority of the sample, around 60%, was under 33 years of age.
Table I shows normal anterior segment values of 20 tomographic parameters chosen. It also shows the results for the parameters chosen to identify outliers which included: 5.0, 95.0, 2.5 and 97.5 percentiles. Statistically significant differences among hyperopic males and females were found for back elevation at thinnest point, back elevation at apex, ACD and CV (Table II). Back elevations were higher in females while ACD and CV were higher in males. No significant difference was observed among the two groups for rest of the parameters.
Table III: Anterior segment values among hyperopic and myopic patients.
|
Parameters |
Mean (SD) |
p-value (<0.05) |
|
|
Hyperopia (n=209) |
Myopia (n=895) |
||
|
K1 (D) |
42.1±1.85 |
42.9±1.44 |
<0.001 |
|
K2 (D) |
43.8±1.94 |
44.0±1.64 |
0.100 |
|
K Mean (D) |
42.9±1.76 |
43.4±2.10 |
<0.001 |
|
K max (D) |
44.4±1.93 |
44.6±1.56 |
0.114 |
|
Astigmatism (D) |
-1.3±1.76 |
-0.96±0.97 |
0.022 |
|
ACD (mm) |
2.7±0.35 |
3.2±0.28 |
<0.001 |
|
Front Elev at thinnest point (µm) |
5.2±3.47 |
4.1±1.97 |
<0.001 |
|
Front Elev apex (µm) |
3.8±2.52 |
3.7±1.80 |
0.718 |
|
Front Elev max (µm) |
8.4±4.65 |
5.5±2.37 |
<0.001 |
|
Back Elev at thinnest point (µm) |
14.1±6.61 |
7.6±4.47 |
<0.001 |
|
Back Elev apex (µm) |
6.7±5.53 |
3.82±4.10 |
<0.001 |
|
Back Elev max (µm) |
19.8±7.38 |
12.1±5.19 |
<0.001 |
|
PI max |
1.2±0.19 |
1.2±0.17 |
0.002 |
|
PI avg |
0.9±0.14 |
1.0±3.01 |
0.339 |
|
ART max |
472.0±88.69 |
484.5±85.44 |
0.065 |
|
ART avg |
600.2±109 |
592.8±96.61 |
0.373 |
|
Pachy thinnest (µm) |
546.9±33.33 |
542.2±30.23 |
0.061 |
|
Pachy apex (µm) |
553.5±32.67 |
545.4±30.08 |
0.001 |
|
Difference |
6.6±3.19 |
3.2±1.82 |
<0.001 |
|
CV (mm3) |
150.0±38.16 |
197.7±31.10 |
<0.001 |
Significant differences among myopic and hyperopic patients were found for K1, K mean, astigmatism, Pachy Apex, front and back elevations at thinnest points, front elevation maximum, back elevation at apex and max, PI max, CV and ACD (Table III). No significant differences were observed among the two groups for rest of the parameters.
DISCUSSION
Refractive surgery is a rapidly evolving field. With each passing day, patient’s expectation and physician’s quest for accuracy and precision are rising. Correct screening of patients prior to refractive surgery is the key to success in this field. Newer and more advanced tomographic evaluation is becoming common practice for preoperative assessment of refractive surgery patients in order to minimise avoidable postsurgical complications. Normative database specific for hyperopic population is now being used as suggested by different studies. We studied normative database in hyperopic Pakistani population to highlight any significant differences from previous studies.
Kim et al. reported normative data based in their hyperopic American population of 100 eyes.11 The average pachymetry was 545 ± 33.2 µm at the thinnest point and 550 ± 33.0 µm at the apex. Front elevation values at the apex and thinnest points were 0.4 ± 1.9 µm and -0.1 ± 2.2 µm, respectively. Back elevation values at the apex and thinnest points were 5.7 ± 3.6 µm and 10.6± 5.7 µm, respectively. In this study, average pachymetry was 546.9 ± 33.33 µm at the thinnest point and 553.5 ± 32.67 µm at the apex. No significant difference was observed in these parameters. Front elevation values at the apex and thinnest points in this study were 3.8±2.52 µm and 5.2 ± 3.47 µm. These values were significantly higher than values stated by Kim et al. Back elevation values at the apex and thinnest points were 6.7 ± 5.53 µm and 14.1 ± 6.61 µm, respectively. Back elevation at thinnest point was significantly higher than Kim et al. values while no significant difference was found between back elevation at apex11. They also reported no significant difference between hyperopic and myopic pachymetry measurements but front and back elevation at the apex and thinnest point were of greater significance which was comparable to this study except for front elevation at apex which was not statistically significant in our groups.
Murata et al. analysed the anterior segment of 55 refractive surgery candidates using the noncontact, three dimensional analyser Pentacam on Brazilian population.15 The anterior chamber volume 146.6 ± 32.86 mm3, ACD 2.8 ± 0.38 mm and corneal thickness were 550.5±29.49 µm in their study group. In the present study, CV 150.0±38.16 mm3, ACD 2.7 ± 0.35 mm and corneal thickness were 553.5±32.67 µm. However, the difference was not significant when compared with Murata et al. They also observed that patients with hyperopia had greater mean corneal volume and pachymetry and lesser anterior chamber depth and volume when compared with myopic patients, which was comparable to our study.
Hashemi et al. assessed the anterior segment measurements in a sample of 283 Iranian population according to their refractive status, out of which 20 were hyperopic subjects.16 They found that myopic eyes had steeper corneas and significantly higher anterior chamber measurements than hyperopic eyes, which was comparable to this study. The back elevation max and PI Avg in our study were 19.8 ± 7.37 µm and 0.93 ± 0.14, which was significantly higher than the Iranian hyperopic group (15.4 ± 5.4 µm and 0.8 ± 0.1). However, no significant differences were observed when compared minimum corneal thickness, K max, K mean, and ACD between hyperopic patients of both studies.
Statistical analysis showed significant differences between measurements when hyperopic population was compared to myopic population in our study. The difference in K1, K Mean, ACD, Astigmatism, PI max, Pachy apex and CV were significant. Moreover, front and back elevation at thinnest point, Front elevation max, Back elevation at apex and max were highly significant (p<0.001).
In summary, front and back elevation data in this hyperopic study population was slightly higher than previously published studies. Moreover, calculated tomographic values among myopic and hyperopic population were significantly different for few parameters. However, the study is limited due to smaller sample size; and further studies with larger population would help validate the initial results.
CONCLUSION
Hyperopic patients had greater front and back elevation and pachymetry, but lesser keratometry, anterior chamber depth and chamber volume as compared to myopic patients. However, it is recommended that existing screening thresholds for Pentacam should be continued for Pakistani population with special considerations to the differences highlighted in this study. Larger cohort from Pakistan is required before region-specific thresholds may be recommended.
ETHICAL APPROVAL:
Approval from the Hospital Ethical Review Committee of Armed Forces Institute of Ophthalmology was taken prior to the commencement of this research work in accordance with the principles of Declaration of Helsinki.
PATIENTS’ CONSENT:
Written informed consent for collection, analysis and publication of data was taken from every participant of this study.
CONFLICT OF INTEREST:
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION:
SH: Contributed towards conception, design and drafting of the research work.
AF: Revisited the article critically for its important content.
MI: Gave the final approval for the manuscript.
QH, SA, SFM: Contributed towards analysis, acquisition and interpretation of data, respectively.
REFERENCES
- Ambrósio R, Belin MW. Imaging of the cornea: Topography vs tomography. J Refract Surg 2010; 26(11):847-9. doi: 10.3928/1081597X-20101006-01.
- Imbornoni LM, McGhee CNJ, Belin MW. Evolution of keratoconus: From diagnosis to therapeutics. Klin Monbl Augenheilkd 2018; 235(6):680-8. doi: 10.1055/s-0044- 100617.
- Ambrósio R, Valbon BF, Faria-Correia F, Ramos I, Luz A. Scheimpflug imaging for laser refractive surgery. Curr Opin Ophthalmol 2013; 24(4):310-20. doi: 10.1097/ICU.0b013 e3283622a94.
- Motlagh MN, Moshirfar M, Murri MS, Skanchy DF, Momeni-Moghaddam H, Ronquillo YC, et al. Pentacam® corneal tomography for screening of refractive surgery candidates: A review of the literature, part I. Med Hypothesis Discov Innov Ophthalmol 2019 Fall; 8(3):177-203.
- Meyer JJ, Gokul A, Vellara HR, Prime Z, McGhee CN. Repeatability and agreement of orbscan II, pentacam hr, and Galilei tomography systems in corneas with keratoconus. Am J Ophthalmol 2017; 175:122-8. doi: 10.1016/j.ajo.2016.12.003.
- Hamer CA, Buckhurst H, Purslow C, Shum GL, Habib NE, Buckhurst PJ. Comparison of reliability and repeatability of corneal curvature assessment with six keratometers. Clin Exp Optom 2016; 99(6):583-9. doi: 10.1111/cxo.12329.
- Sadigh AL, Aali TA, Sadeghi A. Outcome of intrastromal corneal ring segment relative to depth of insertion evaluated with scheimpflug image. J Curr Ophthalmol 2015; 27(1-2):25-31. doi: 10.1016/j.joco.2015.09.009.
- Xu K, Hao Y, Qi H. Intraocular lens power calculations using a Scheimpflug camera to measure corneal power. Biotech Histochem 2014; 89(5):348-54. doi: 10.3109/10520295. 2013.867532.
- Feng MT, Kim JT, Ambrósio R, Belin MW, Grewal SPS, Yan W, et al. International values of central pachymetry in normal subjects by rotating scheimpflug camera. Asia Pac J Ophthalmol (Phila) 2012; 1(1):13-8. doi: 10.1097/APO. 0b013 e31823e58da.
- Humayun S, Fawad A, Humayun Q, Arzoo S, Ishaq M. Screening thresholds for the corneal tomography in a myopic pakistani population. J Coll Physicians Surg Pak 2019; 29(2):128-132. doi: 10.29271/jcpsp.2019.02.128.
- Kim JT, Cortese M, Belin MW, Ambrosio R Jr, Khachikian SS. Tomographic normal values for corneal elevation and pachymetry in a hyperopic population. J Clin Exp Ophthalmol 2011; 2:130.
- Guidance for industry. Collection of Race and Ethnicity Data in Clinical Trials. U.S. Department of Health and Human Services, Food and Drug Administration, 2005. Available at: http://www.fda.gov/downloads/Regulatory information/Guidances/ucm 126396.pdf. Document issued on October 26, 2016
- Ambrósio R, Faria-Correia F, Ramos I, Valbon BF, Lopes B, Jardim D, et al. Enhanced screening for ectasia susceptibility among refractive candidates: The role of corneal tomography and biomechanics. Curr Ophthalmol Rep 2013; 1:28-38. DOI 10.1007/s40135-012-0003-z
- Ambrosio R Jr, Caiado AL, Guerra FP, Louzada R, Sinha RA, Luz A, et al. Novel pachymetric parameters based on corneal tomography for diagnosing keratoconus. J Refract Surg 2011; 27(10):753-8. doi: 10.3928/1081597X- 20110721-01.
- Murata C, Mallmann F, Yamazaki E, Campos M. Anterior ocular segment study with the Scheimpflug rotational camera in refractive surgery candidates. Arq Bras Oftalmol 2007; 70(4):619-24. doi: 10.1590/s0004-274920 07000400012.
- Hashemi M, Falavarjani KG, Aghai GH, Aghdam KA, Gordiz A. Anterior segment study with the pentacam scheimpflug camera in refractive surgery candidates. Middle East Afr J Ophthalmol 2013; 20(3):212-6. doi: 10.4103/0974- 9233.114793.