- Department of Neuroendovascular Therapy and Neurosurgery, Juntendo University Faculty of Medicine, Bunkyo-ku, Tokyo, Japan.
- Department of Neuroendovascular Therapy, Juntendo University Faculty of Medicine, Bunkyo-ku, Tokyo, Japan.
- Department of Neurosurgery, Juntendo University Faculty of Medicine, Bunkyo-ku, Tokyo, Japan.
Correspondence Address:
Takayuki Kitamura, Department of Neuroendovascular Therapy and Neurosurgery, Juntendo University Faculty of Medicine, 2-1-1 Hongo, Bunkyo-ku, Tokyo 113-8421, Japan.
DOI:10.25259/SNI_1165_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takayuki Kitamura1, Takashi Fujii2, Kenji Yatomi3, Kohsuke Teranishi3, Yumiko Mitome-Mishima3, Hidenori Oishi1. Safety and efficacy of pipeline embolization device treatments for intradural internal carotid artery aneurysms in a single center in a Japanese population. 17-Mar-2023;14:92
How to cite this URL: Takayuki Kitamura1, Takashi Fujii2, Kenji Yatomi3, Kohsuke Teranishi3, Yumiko Mitome-Mishima3, Hidenori Oishi1. Safety and efficacy of pipeline embolization device treatments for intradural internal carotid artery aneurysms in a single center in a Japanese population. 17-Mar-2023;14:92. Available from: https://surgicalneurologyint.com/surgicalint-articles/12195/
Abstract
Background: The pipeline embolization device (PED) is the most common flow diverter device in the world. To date, there have been no reports of treatment outcomes specific to intradural internal carotid artery (ICA) aneurysms. The safety and efficacy of the PED treatments for intradural ICA aneurysms are reported.
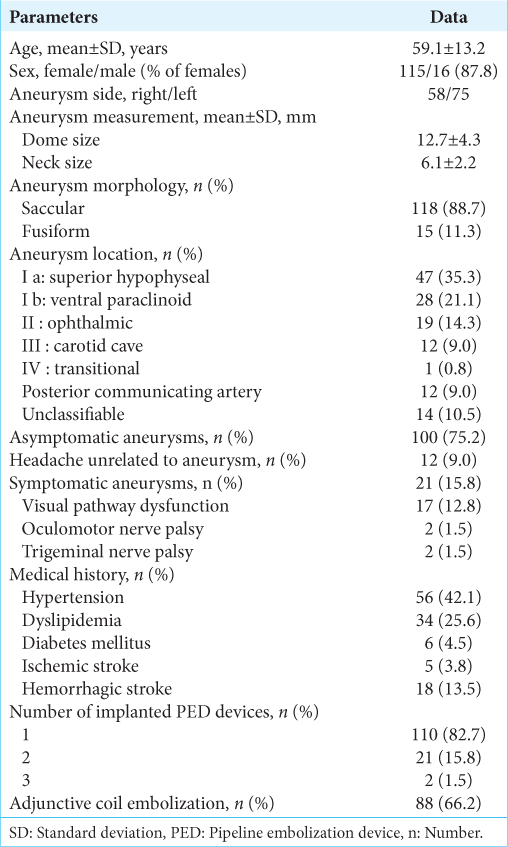
Methods: 131 patients with 133 aneurysms underwent PED treatments for intradural ICA aneurysms. The mean aneurysm dome size and neck length were 12.7 ± 4.3 mm and 6.1 ± 2.2 mm, respectively. We used adjunctive endosaccular coil embolization for 88 aneurysms (66.2%). A total of 113 aneurysms (85%) were angiographically followed up 6 months following the procedure, and 93 aneurysms (69.9%) were followed up for 1 year.
Results: The angiographic outcome at 6 months showed that 94 (83.2%) aneurysms had O’Kelly-Marotta (OKM) grade D, 6 (5.3%) had C, 10 (8.8%) had B, and 3 (2.7%) had A. At 1 year, 82 (88.2%) aneurysms had OKM grade D, 6 (6.5%) had C, 3 (3.2%) had B, and 2 (2.2%) had A. Multivariate analysis showed that aneurysm neck size and adjunctive coiling were statistically significant in aneurysm occlusion status. Major morbidity modified Rankin Scale >2 and mortality rates related to procedures were 3.0% and 0%, respectively. Delayed aneurysm ruptures were not observed.
Conclusion: These results reveal that PED treatment of intradural ICA aneurysms is safe and efficacious. The combined use of adjunctive coil embolization not only prevents delayed aneurysm ruptures but also contributes to an increase in the rate of complete occlusion.
Keywords: Adjunctive coil embolization, Complete occlusion, Flow diverter, Intradural aneurysms, Pipeline embolization device
INTRODUCTION
A stent collectively called the flow diverter (FD) has been developed and clinically used as an endovascular treatment device for cerebral aneurysms that are difficult to cure using conventional treatment methods.[
MATERIALS AND METHODS
Case selection
From December 2012 to April 2021, 131 patients with 133 aneurysms underwent PED treatments for intradural ICA aneurysms at our university hospital. A retrospective review of the medical records, outpatient charts, and operative reports was performed. This retrospective study was approved by the Research Ethics Committee, Faculty of Medicine, our University and conducted in accordance with the Declaration of Helsinki (2013).
Antiplatelet therapy
All patients had received dual antiplatelet therapy of 100 mg aspirin and 50–75 mg clopidogrel, per body weight, for at least 10 days before the procedure. Platelet inhibition levels were analyzed on the same day before the procedure or the day preceding the procedure using the VerifyNow P2Y12 assay (Accumetrics, San Diego, CA). The reaction units for aspirin and clopidogrel were targeted at <550 and <230, respectively. If the reaction unit did not reach the target values, the doses of the hyporesponsive antiplatelet drugs were increased up to two fold. Recently, 2.5–3.75 mg prasugrel has been used in patients hyporesponsive to clopidogrel. Postoperative antiplatelet therapy was continued up to 6 months following the procedure with the same dose of clopidogrel and 100 mg of aspirin. Based on angiographic results after 6 months, clopidogrel was gradually reduced, and aspirin alone was used. If complete occlusion is confirmed, aspirin can be tapered off within 1–2 years.
PED
In Japan, clinical trials for the Pipeline (Classic) began in December 2012, and the Pipeline Flex (Flex), with its improved delivery system, were approved by the pharmaceutical affairs bodies (Japanese Ministry of Health, Labour and Welfare) in April 2015. In May 2019, the Pipeline Flex with Shield Technology (Shield) was newly approved. The Flex strand is made of 36 cobalt-chromium alloys and 12 platinum-tungsten alloys, and the Shield is made with this strand metal coated with methacryloyloxyethyl phosphorylcholine (MPC) polymer. The MPC polymer inhibits protein adsorption and cell adhesion and is expected to inhibit platelet adhesion and reduce thrombotic complications as well. Neointima formation occurs earlier with Shield than that with Flex, and that the neointima formation shape is concentric, with no hyperplasia, suggesting uniform intima formation.[
Endovascular procedure
The procedure was performed using the same methods as previously reported; however, after January 2020, the Phenom 27 microcatheter (Medtronic) was used instead of the Marksman microcatheter (Medtronic).[
Radiological follow-up procedure
Catheter angiographies were usually performed postoperatively at 6 months and/or 1 year. The degree of aneurysm occlusion was determined by the O’Kelly-Marotta (OKM) grading scale (A: total filling, B: subtotal filling, C: entry remnant, and D: no filling).[
Statistical analysis
All statistical analyses were performed utilizing EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, v2.13.0). Values are expressed as the mean ± standard deviation and range. The differences between the two groups were analyzed by Fisher’s exact or χ2 tests. We performed multivariate logistic regression analysis for aneurysms with an OKM Grade D and the other variables factors for complete occlusion. Statistical significance was defined as P < 0.05.
RESULTS
Clinical characteristics
Radiological outcome
Complications
Major morbidity (modified Rankin Scale, mRS >2) and mortality rates related to these procedures were 3.0% and 0%, respectively. Patients were evaluated by diffusion weighted-imaging (DWI) within 3 days after the procedure. There were 78 aneurysms (58.6%) that showed new high-intensity signals suggestive of microembolic lesions. Symptomatic ischemic complications occurred in 4 patients (3.0%). Two patients suffered a symptomatic stroke immediately following the procedure. DWI on the day of the procedure showed multiple high-intensity signals in the treated area because of distal embolisms and/or parent artery flow insufficiency during the PED deployment. The symptoms of 1 of those patients improved in a short period of time, so the patient was discharged from the hospital. However, the other patient was transferred to another hospital to continue rehabilitation. The other two patients had parent artery occlusions. One had serious symptoms due to sudden PED obstruction the day after the procedure and underwent urgent superficial temporal artery-to-middle cerebral artery (STA-MCA) bypass surgery but was left severely disabled. The other patient had an acute obstruction due to distal parent artery dissection after the PED deployment. That patient also underwent STA-MCA bypass surgery at a later date but was left with serious sequelae. With the exception of these two cases, there were no patients in this case series who had in-stent stenosis of ≥50% or who required retreatment.
Intraparenchymal hemorrhage occurred in one case in which the patient developed a large ipsilateral frontal lobe hematoma on the day following the procedure. The patient had severe hemiplegia and dysphasia but gradually improved and, being able to walk without a cane, was transferred to a rehabilitation hospital. There were no delayed aneurysm ruptures observed in this study.
Although different from the major complications associated with this procedure, a unique complication of delayed hydrocephalus was seen in 2 cases, which has been reported in the literature.[
Cranial nerve dysfunction
Regarding the consequences of cranial nerve dysfunction due to the aneurysm’s mass effect, in 17 aneurysms of visual pathway dysfunction, symptom improvement was obtained in 4 aneurysms (23.5%), no change in 12 (70.6%), and worsening in 1 (5.9%). Of the 2 aneurysms causing oculomotor nerve palsy, 1 case showed improvement and the other case had not yet been evaluated. Of the 2 aneurysms causing trigeminal nerve palsy, 1 case showed no change, and the other case had not yet been evaluated.
DISCUSSION
PED treatment
The PED is the most widely used FD stent in the world, and the first prospective PITA trial was reported in 2011, and many studies have been reported there after. PUFS, IntrePED, and ASPIRe have been reported as major studies of PED treatment, and an analysis of these three studies was reported by Kallmes et al.[
Embolic state after PED implantation
In our study, the analysis was limited to intradural ICA aneurysms, and the complete occlusion rates at 6 months and 1 year were 83.2% and 88.2%, respectively, which are more than equivalent to those reported by Kallmes et al.[
Bender et al. reported that FD treatment with coils accelerates and improves occlusion results without increasing morbidity, with complete occlusion rates as high as 85% at 6 months and 96% at 1 year, respectively.[
The effect of the pipeline shield
In the present study, the Shield was used in 44 aneurysms (33.1%). The SHIELD study reported a high occlusion rate and low rates of neurologic morbidity and mortality.[
Complications after PED implantation
In the present study, major morbidity (mRS >2) and mortality rates related to procedures were 3.0% and 0%, respectively. A previous report analysis of three large PED studies showed major morbidity (5.7%) and mortality (4.0%), a meta-analysis reporting overall FD outcomes showed morbidity (5%) and mortality (4%), and another subsequent report showed ischemic complications (4.1%), hemorrhagic complications (2.9%), neurological sequelae (3.5%), and death (3.4%).[
Change of neurology after PED implantation
It has previously been reported that motor nerve dysfunction tends to improve the neuropathy caused by the mass effect of aneurysms.[
Limitation
The limitation of this study is the single-center retrospective design. This factor must be considered when interpreting the results.
CONCLUSION
Our results show that PED treatment of intradural ICA aneurysms is safe and effective. In addition, the combined use of adjunctive coil embolization not only prevents delayed aneurysm rupture but also contributes to an increase in the high rate of complete aneurysmal occlusions.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
Hidenori Oishi receives a donation in the form of a research fund to the endowed chair of his departments and about 1 million yen yearly from Medtronic Co., Ltd.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adeeb N, Moore JM, Wirtz M, Griessenauer CJ, Foreman PM, Shallwani H. Predictors of incomplete occlusion following pipeline embolization of intracranial aneurysms: Is it less effective in older patients?. AJNR Am J Neuroradiol. 2017. 38: 2295-300
2. Al-Rodhan NR, Piepgras DG, Sundt TM. Transitional cavernous aneurysms of the internal carotid artery. Neurosurgery. 1993. 33: 993-6 discussion 997-8
3. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G. Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology. 2013. 267: 858-68
4. Bender MT, Jiang B, Campos JK, Lin LM, Beaty N, Vo CD. Single-stage flow diversion with adjunctive coiling for cerebral aneurysm: Outcomes and technical considerations in 72 cases. J Neurointerv Surg. 2018. 10: 843-50
5. Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A. Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review. Neuroradiol J. 2015. 28: 365-75
6. Brinjikji W, Kallmes DF, Cloft HJ, Lanzino G. Age-related outcomes following intracranial aneurysm treatment with the pipeline embolization device: A subgroup analysis of the IntrePED registry. J Neurosurg. 2016. 124: 1726-30
7. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke. 2013. 44: 442-7
8. Caroff J, Tamura T, King RM, Lylyk PN, Langan ET, Brooks OW. Phosphorylcholine surface modified flow diverter associated with reduced intimal hyperplasia. J Neurointerv Surg. 2018. 10: 1097-101
9. Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S. The warfarin-aspirin symptomatic intracranial disease study. Neurology. 1995. 45: 1488-93
10. Fischer S, Vajda Z, Perez MA, Schmid E, Hopf N, Bazner H. Pipeline embolization device (PED) for neurovascular reconstruction: Initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology. 2012. 54: 369-82
11. Gentric JC, Darsaut TE, Makoyeva A, Salazkin I, Raymond J. The success of flow diversion in large and giant sidewall aneurysms may depend on the size of the defect in the parent artery. AJNR Am J Neuroradiol. 2014. 35: 2119-24
12. Guedon A, Thepenier C, Shotar E, Gabrieli J, Mathon B, Premat K. Predictive score for complete occlusion of intracranial aneurysms treated by flow-diverter stents using machine learning. J Neurointerv Surg. 2021. 13: 341-6
13. Hanel RA, Kallmes DF, Lopes DK, Nelson PK, Siddiqui A, Jabbour P. Prospective study on embolization of intracranial aneurysms with the pipeline device: The PREMIER study 1 Year results. J Neurointerv Surg. 2020. 12: 62-6
14. Kallmes DF, Brinjikji W, Boccardi E, Ciceri E, Diaz O, Tawk R. Aneurysm study of pipeline in an observational registry (ASPIRe). Interv Neurol. 2016. 5: 89-99
15. Kallmes DF, Brinjikji W, Cekirge S, Fiorella D, Hanel RA, Jabbour P. Safety and efficacy of the Pipeline embolization device for treatment of intracranial aneurysms: A pooled analysis of 3 large studies. J Neurosurg. 2017. 127: 775-80
16. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007. 38: 2346-52
17. Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S. International retrospective study of the pipeline embolization device: A multicenter aneurysm treatment study. AJNR Am J Neuroradiol. 2015. 36: 108-15
18. Kan P, Siddiqui AH, Veznedaroglu E, Liebman KM, Binning MJ, Dumont TM. Early postmarket results after treatment of intracranial aneurysms with the pipeline embolization device: A U.S. multicenter experience. Neurosurgery. 2012. 71: 1080-7 discussion 1087-8
19. Kulcsar Z, Houdart E, Bonafe A, Parker G, Millar J, Goddard AJ. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol. 2011. 32: 20-5
20. Lin N, Brouillard AM, Krishna C, Mokin M, Natarajan SK, Sonig A. Use of coils in conjunction with the pipeline embolization device for treatment of intracranial aneurysms. Neurosurgery. 2015. 76: 142-9
21. Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: The Buenos Aires experience. Neurosurgery. 2009. 64: 632-42 discussion 642-33; quiz N636
22. Maragkos GA, Ascanio LC, Salem MM, Gopakumar S, Gomez-Paz S, Enriquez-Marulanda A. Predictive factors of incomplete aneurysm occlusion after endovascular treatment with the Pipeline embolization device. J Neurosurg. 2019. 132: 1598-605
23. Matsuda Y, Chung J, Lopes DK. Analysis of neointima development in flow diverters using optical coherence tomography imaging. J Neurointerv Surg. 2018. 10: 162-7
24. Murthy SB, Shah S, Rao CP, Bershad EM, Suarez JI. Treatment of unruptured intracranial aneurysms with the pipeline embolization device. J Clin Neurosci. 2014. 21: 6-11
25. Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011. 32: 34-40
26. Nossek E, Chalif DJ, Chakraborty S, Lombardo K, Black KS, Setton A. concurrent use of the pipeline embolization device and coils for intracranial aneurysms: technique, safety, and efficacy. J Neurosurg. 2015. 122: 904-11
27. O’Kelly CJ, Krings T, Fiorella D, Marotta TR. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. 2010. 16: 133-7
28. Oishi H, Fujii T, Suzuki M, Takano N, Teranishi K, Yatomi K. Usefulness of silent MR angiography for intracranial aneurysms treated with a flow-diverter device. AJNR Am J Neuroradiol. 2019. 40: 808-14
29. Oishi H, Mishima Y, Yatomi K, Teranishi K, Suzuki K, Fujii T. Defective endothelialization of pipeline embolization device after flow diverter therapy: An autopsy case report. NMC Case Rep J. 2021. 8: 33-7
30. Oishi H, Teranishi K, Yatomi K, Fujii T, Yamamoto M, Arai H. Flow diverter therapy using a pipeline embolization device for 100 unruptured large and giant internal carotid artery aneurysms in a single center in a Japanese population. Neurol Med Chir (Tokyo). 2018. 58: 461-7
31. Peschillo S, Caporlingua A, Resta MC, Peluso JP, Burdi N, Sourour N. Endovascular treatment of large and giant carotid aneurysms with flow-diverter stents alone or in combination with coils: A multicenter experience and long-term follow-up. Oper Neurosurg (Hagerstown). 2017. 13: 492-502
32. Ravindran K, Salem MM, Alturki AY, Thomas AJ, Ogilvy CS, Moore JM. Endothelialization following flow diversion for intracranial aneurysms: A systematic review. AJNR Am J Neuroradiol. 2019. 40: 295-301
33. Rice H, Galdamez MM, Holtmannspotter M, Spelle L, Lagios K, Ruggiero M. Periprocedural to 1-year safety and efficacy outcomes with the pipeline embolization device with Shield technology for intracranial aneurysms: A prospective, post-market, multi-center study. J Neurointerv Surg. 2020. 12: 1107-12
34. Rouchaud A, Brinjikji W, Lanzino G, Cloft HJ, Kadirvel R, Kallmes DF. Delayed hemorrhagic complications after flow diversion for intracranial aneurysms: A literature overview. Neuroradiology. 2016. 58: 171-7
35. Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: A single-center experience with long-term follow-up results. AJNR Am J Neuroradiol. 2012. 33: 1436-46
36. Siddiqui AH, Kan P, Abla AA, Hopkins LN, Levy EI. Complications after treatment with pipeline embolization for giant distal intracranial aneurysms with or without coil embolization. Neurosurgery. 2012. 71: E509-513 discussion E513
37. Szikora I, Berentei Z, Kulcsar Z, Marosfoi M, Vajda ZS, Lee W. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: The budapest experience with the pipeline embolization device. AJNR Am J Neuroradiol. 2010. 31: 1139-47
38. Teranishi K, Yatomi K, Mitome-Mishima Y, Sugiyama N, Yamamoto M, Oishi H. Delayed hydrocephalus after combined treatment with pipeline embolization device and platinum coil for large unruptured intracranial aneurysm: A report of two cases. J Neuroendovasc Ther. 2018. 12: 148-52
39. Velioglu M, Kizilkilic O, Selcuk H, Kocak B, Tureci E, Islak C. Early and midterm results of complex cerebral aneurysms treated with Silk stent. Neuroradiology. 2012. 54: 1355-65