- Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma, United States.
DOI:10.25259/SNI_847_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kylie E. Hagerdon, Lance M. Villeneueve, Christen M. O’Neal, Andrew K. Conner. Resolution of symptoms in idiopathic thalamic pain syndrome after implantation of a cervical and thoracic percutaneous spinal cord stimulator. 10-Feb-2021;12:50
How to cite this URL: Kylie E. Hagerdon, Lance M. Villeneueve, Christen M. O’Neal, Andrew K. Conner. Resolution of symptoms in idiopathic thalamic pain syndrome after implantation of a cervical and thoracic percutaneous spinal cord stimulator. 10-Feb-2021;12:50. Available from: https://surgicalneurologyint.com/surgicalint-articles/10582/
Abstract
Background: Thalamic pain syndrome is classically described as chronic pain after an infarct of the thalamus. It leads to a decrease in the quality of life, especially for patients with inadequate treatment. Supportive imaging, such as a thalamic lesion or infarct, is widely accepted as necessary to diagnose this condition.
Case Description: In this case report, we describe the case of a patient who developed allodynia and hyperesthesia with a hemibody distribution characteristic of thalamic pain syndrome, despite having no clear inciting event or identifiable thalamic lesion. This patient was successfully treated with cervical and thoracic spinal cord stimulation (SCS).
Conclusion: We suggest that this patient may have presented with a non-lesional thalamic pain syndrome, supported by the classic hemibody allodynia and hyperesthesia and the response to SCS. Further, we demonstrate that SCS was an effective method to control this central pain disorder.
Keywords: Non-lesional pain, Spinal cord stimulation, Thalamic pain syndrome
INTRODUCTION
Thalamic pain syndrome, first described by Dejerine and Roussy in 1906, is a distressing and treatment-resistant type of centralized neuropathic pain.[
In addition to the diagnostic challenges, treatment can be problematic as thalamic pain syndrome is commonly refractory to pharmacologic interventions.[
While SCS is an accepted method for treating intractable pain disorders, its clinical utility in treating classic presentations of thalamic pain syndrome is not well understood. In addition, there are few, if any reported cases of non-lesional thalamic pain syndrome without a history of brain injury or ischemia in the current literature. In this case report, we describe a case of non-lesional thalamic pain syndrome with no clear inciting event that was successfully treated with a combination of cervical and thoracic SCS.
CASE PRESENTATION
A 57-year-old right-handed male with a medical history of B12 deficiency, controlled hypertension, and migraine presented to an outside neurologist with an 8-year history of periodic left-sided pain. These attacks had resulted in multiple visits to the emergency room which were unable to determine an etiology. The patient reported periodic soreness and cramping that would begin in his left hand and foot and then radiate to the left shoulder and thigh, respectively. Concurrently, he would have numbness and pain in his left face. While this had started 8 years prior, the symptoms had become less tolerable and had started preventing the patient from carrying out his daily activities. These episodes would occur about once per week and past 3-4 h/episode. During the same time period, the patient complained of bilateral foot numbness that would develop on prolonged standing and also of sexual dysfunction, though neither symptom seemed to be related to the periodic hemianesthesia or paresthesia. The patient’s neurological exam was largely non-focal except for a bilateral Hoffman’s sign.
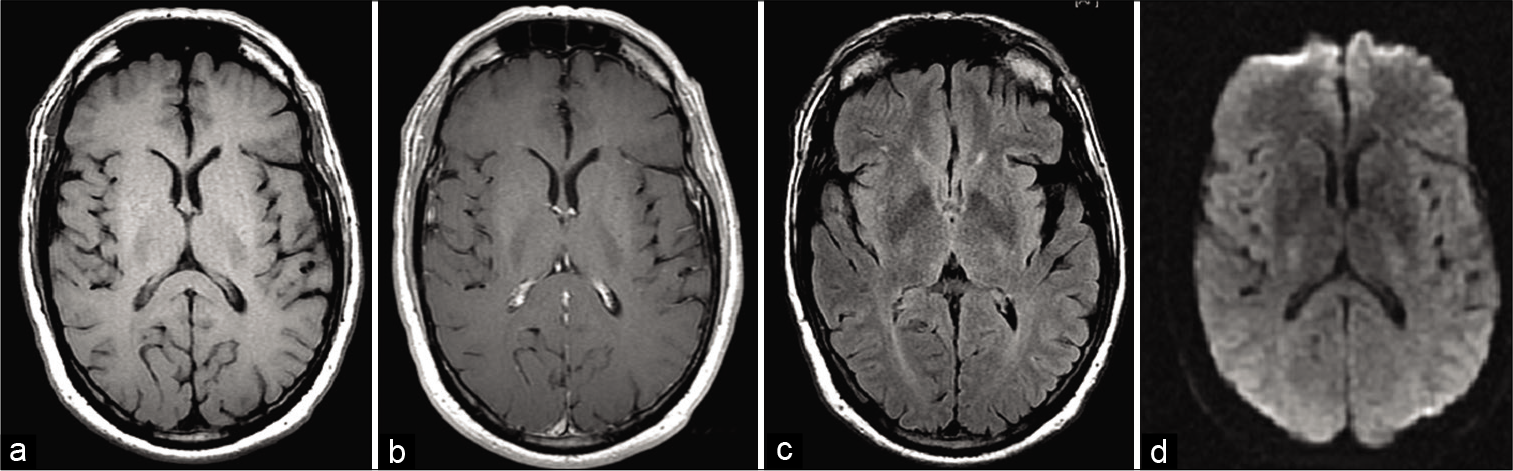
The patient initially had an MRI of the neuroaxis. The MRI brain demonstrated no structural abnormalities, though white matter scattered attenuation consistent with microvascular small vessel disease was noted [
He was referred for consideration of operative intervention, including possible MCS, DBS, or implantation of a spinal cord stimulator. All stimulator options were discussed with the patient, and after much discussion, he elected to undergo a spinal cord stimulator trial as it would be the least invasive and allow for evaluation of efficacy before implantation.
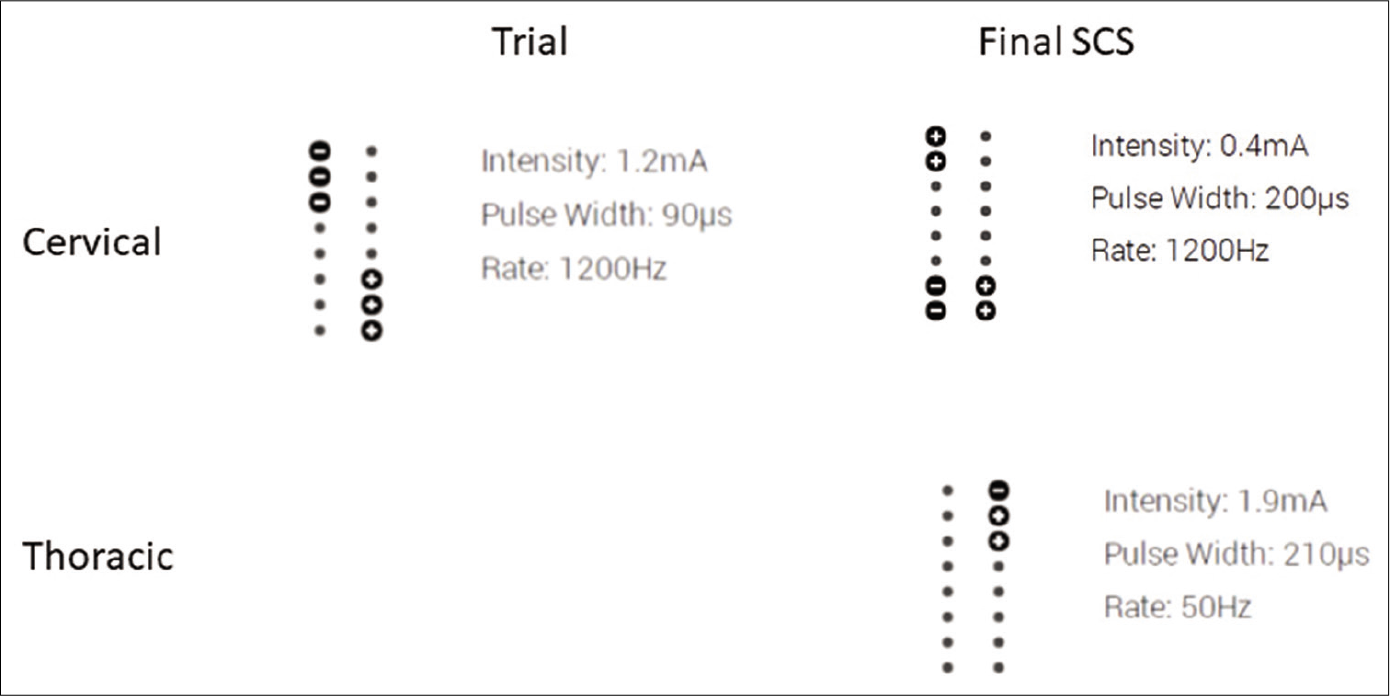
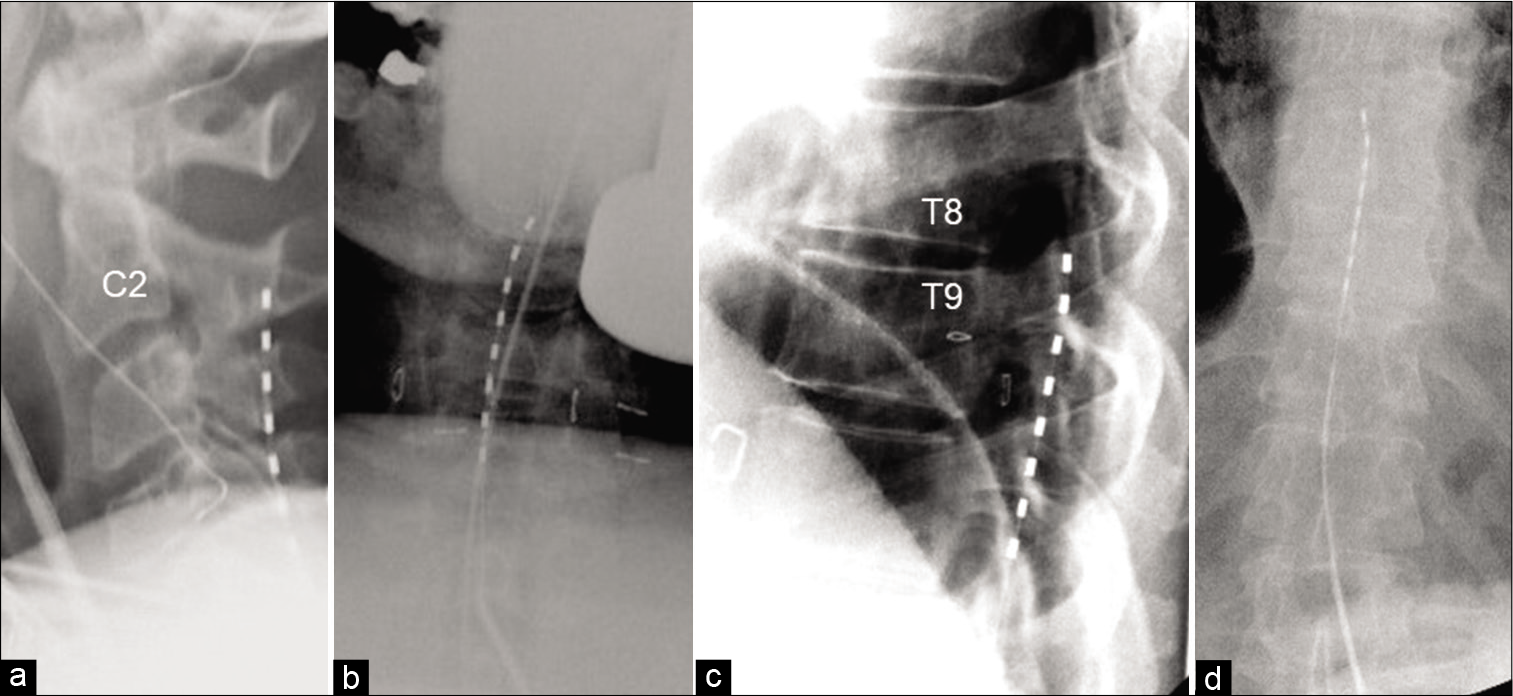
The patient underwent a spinal cord stimulator trial with great results by an independent pain physician after clearing psychiatric screening. The trial stimulator was in the cervical region. The patient reported complete resolution of pain in the left arm, but endorsed continued pain in his left leg. For this reason, the patient requested placement of an additional lead in the thoracic spine. The patient’s trial spinal cord stimulator settings are included in [
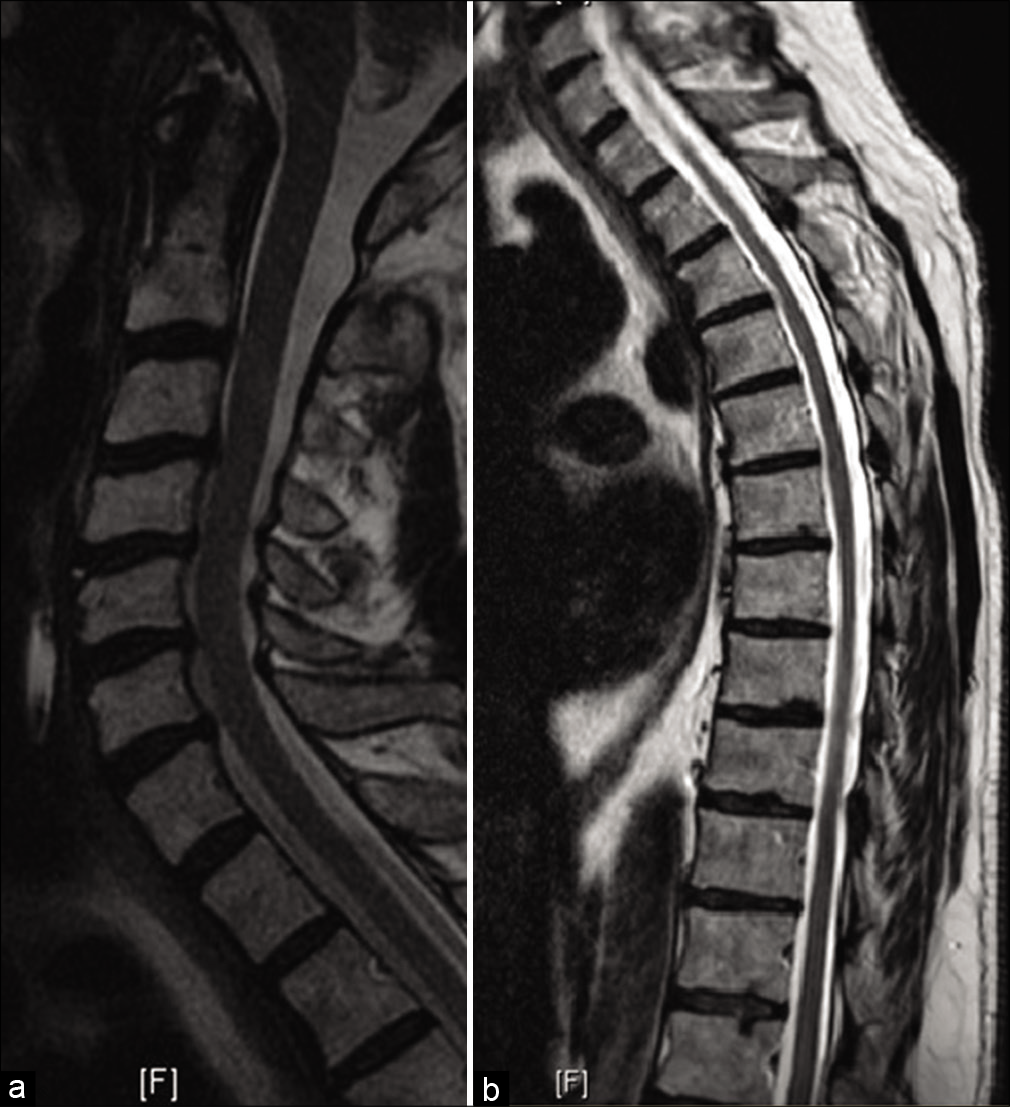
The preoperative cervical and thoracic images are included in [
During his 1-week postoperative appointment, he reported significant pain reduction and good coverage in his left-hemibody including his leg. At 6-months follow-up, he reported continued pain relief with approximately 95% reduction in his pain following permanent SCS implantation.
DISCUSSION
In this report, we demonstrate a unique case of a patient who developed hemibody allodynia and hyperesthesia comparable to thalamic pain syndrome, despite having no clear precipitating event or identifiable thalamic lesion. Using SCS, we provided significant and sustained pain relief for this patient, allowing him to return to his normal daily activities. We suggest the patient in this case presented with a nonlesional thalamic pain syndrome, evidenced by the classic hemibody allodynia and hyperesthesia and the response to SCS.
The ambiguity of the presentation and overlap with other pain disorders led to an 8-year delay in diagnosis and effective treatment of thalamic pain syndrome in this patient. Early identification of atypical pain could have prevented a delay in treatment, thereby increasing quality of life. For patients identified as having atypical pain, early referral to a neurologist specializing in pain syndromes is necessary to optimize patient care.
The differential diagnoses for this patient included thalamic pain syndrome, chronic pain syndrome, complex regional pain syndrome, idiopathic peripheral neuropathy, lateral medullary infarction, multiple sclerosis, a brain mass, and syringomyelia. Since no identifiable brain or spinal cord abnormalities were present on imaging, structural causes such as multiple sclerosis, brain mass, and syringomyelia were ruled out. The distribution of this patient’s pain affected solely the left-hemibody, thereby ruling out idiopathic peripheral neuropathy and lateral medullary syndrome. Although this patient had allodynia and hyperesthesia, the lack of an identifiable trigger largely negated a diagnosis of chronic pain syndrome and complex regional pain syndrome. In addition, this patient had no autonomic or vasomotor symptoms, further suggesting a diagnosis other than complex regional pain syndrome. Therefore, thalamic pain syndrome was favored as the classification of the patient’s syndrome because he had long-term allodynia and hyperesthesia in a distribution characteristic of thalamic pain syndrome, despite the lack of an identifiable lesion.[
Treatment for this unique condition also presented unique considerations. Recent works have demonstrated the effectiveness of SCS for treating central pain syndrome.[
However, the patient in this report experienced complete left-hemibody pain of the face, arm, and leg, with the most disabling pain localized to his arm and leg. After attempting medical management without successful pain reduction, the patient decided to undergo operative intervention. Cortical stimulation, DBS, and SCS could all address different aspects of the syndrome; however, cortical and DBS provided some major concerns for this patient given the invasive nature. The successful trial of a spinal cord stimulator offered a minimally invasive option, which added reassurance of a permanent spinal cord stimulator benefiting this patient. [
CONCLUSION
In this report, we demonstrate the unique case of a patient developing allodynia and hyperesthesia with a hemibody distribution characteristic of thalamic pain syndrome, despite having no clear inciting event. Due to the classic hemibody distribution of allodynia and hyperesthesia, we suggest this patient may have non-lesional thalamic pain syndrome. Further, we demonstrate that SCS was an effective method to control this central pain disorder with characteristic features thalamic pain syndrome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aly MM, Saitoh Y, Hosomi K, Oshino S, Kishima H, Yoshimine T. Spinal cord stimulation for central poststroke pain. Neurosurgery. 2010. 67: ons206-12
2. Boccard SG, Pereira EA, Moir L, van Hartevelt TJ, Kringelbach ML, FitzGerald JJ. Deep brain stimulation of the anterior cingulate cortex: Targeting the affective component of chronic pain. Neuroreport. 2014. 25: 83-8
3. Cesaro P, Mann MW, Moretti JL, Defer G, Roualdes B, Nguyen JP. Central pain and thalamic hyperactivity: A single photon emission computerized tomographic study. Pain. 1991. 47: 329-36
4. Guedon A, Thiebaut JB, Benichi S, Mikol J, Moxham B, Plaisant O. Dejerine-roussy syndrome: Historical cases. Neurology. 2019. 93: 624-9
5. Jang SH, Lee J, Yeo SS. Central post-stroke pain due to injury of the spinothalamic tract in patients with cerebral infarction: A diffusion tensor tractography imaging study. Neural Regen Res. 2017. 12: 2021-4
6. Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: Clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009. 8: 857-68
7. Lempka SF, Malone DA, Hu B, Baker KB, Wyant A, Ozinga JG. Randomized clinical trial of deep brain stimulation for poststroke pain. Ann Neurol. 2017. 81: 653-63
8. Levi V, Cordella R, D’Ammando A, Tringali G, Dones I, Messina G. Dorsal anterior cingulate cortex (ACC) deep brain stimulation (DBS): A promising surgical option for the treatment of refractory thalamic pain syndrome (TPS). Acta Neurochir (Wien). 2019. 161: 1579-88
9. Lopez JA, Torres LM, Gala F, Iglesias I. Spinal cord stimulation and thalamic pain: Long-term results of eight cases. Neuromodulation. 2009. 12: 240-3
10. Parmar VK, Gee L, Smith H, Pilitsis JG. Supraspinal stimulation for treatment of refractory pain. Clin Neurol Neurosurg. 2014. 123: 155-63
11. Smits H, van Kleef M, Holsheimer J, Joosten EA. Experimental spinal cord stimulation and neuropathic pain: Mechanism of action, technical aspects, and effectiveness. Pain Pract. 2013. 13: 154-68
12. Sokal P, Harat M, Malukiewicz A, Kiec M, Switonska M, Jablonska R. Effectiveness of tonic and burst motor cortex stimulation in chronic neuropathic pain. J Pain Res. 2019. 12: 1863-9
13. Sokal P, Harat M, Zielinski P, Furtak J, Paczkowski D, Rusinek M. Motor cortex stimulation in patients with chronic central pain. Adv Clin Exp Med. 2015. 24: 289-96
14. Son BC, Kim DR, Kim HS, Lee SW. Simultaneous trial of deep brain and motor cortex stimulation in chronic intractable neuropathic pain. Stereotact Funct Neurosurg. 2014. 92: 218-26
15. Tanei T, Kajita Y, Noda H, Takebayashi S, Nakatsubo D, Maesawa S. Efficacy of motor cortex stimulation for intractable central neuropathic pain: Comparison of stimulation parameters between post-stroke pain and other central pain. Neurol Med Chir (Tokyo). 2011. 51: 8-14
16. Treister AK, Hatch MN, Cramer SC, Chang EY. Demystifying poststroke pain: From etiology to treatment. PM R. 2017. 9: 63-75
17. Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993. 78: 393-401
18. Urits I, Gress K, Charipova K, Orhurhu V, Freeman JA, Kaye RJ. Diagnosis, treatment, and management of dejerine-roussy syndrome: A comprehensive review. Curr Pain Headache Rep. 2020. 24: 48
19. Ward M, Mammis A. Deep brain stimulation for the treatment of dejerine-roussy syndrome. Stereotact Funct Neurosurg. 2017. 95: 298-306
20. Willoch F, Schindler F, Wester HJ, Empl M, Straube A, Schwaiger M. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: A [11C] diprenorphine PET study. Pain. 2004. 108: 213-20