- Department of Neurosurgery, University of Occupational and Environmental Health, Kitakyushu, Fukuoka, Japan.
DOI:10.25259/SNI_843_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroshi Miyachi, Kohei Suzuki, Shohei Nagasaka, Takehiro Kitagawa, Junkoh Yamamoto. Brain ischemia due to direct vascular compression associate with rapid enlargement of unruptured middle cerebral artery aneurysm: A case report. 24-Mar-2021;12:115
How to cite this URL: Hiroshi Miyachi, Kohei Suzuki, Shohei Nagasaka, Takehiro Kitagawa, Junkoh Yamamoto. Brain ischemia due to direct vascular compression associate with rapid enlargement of unruptured middle cerebral artery aneurysm: A case report. 24-Mar-2021;12:115. Available from: https://surgicalneurologyint.com/surgicalint-articles/10664/
Abstract
Background: Acute cerebral infarction is a rare complication resulting from an unruptured cerebral aneurysm (UCA). There is presently no consensus on the optimal strategy for the management of UCAs with cerebral infarctions.
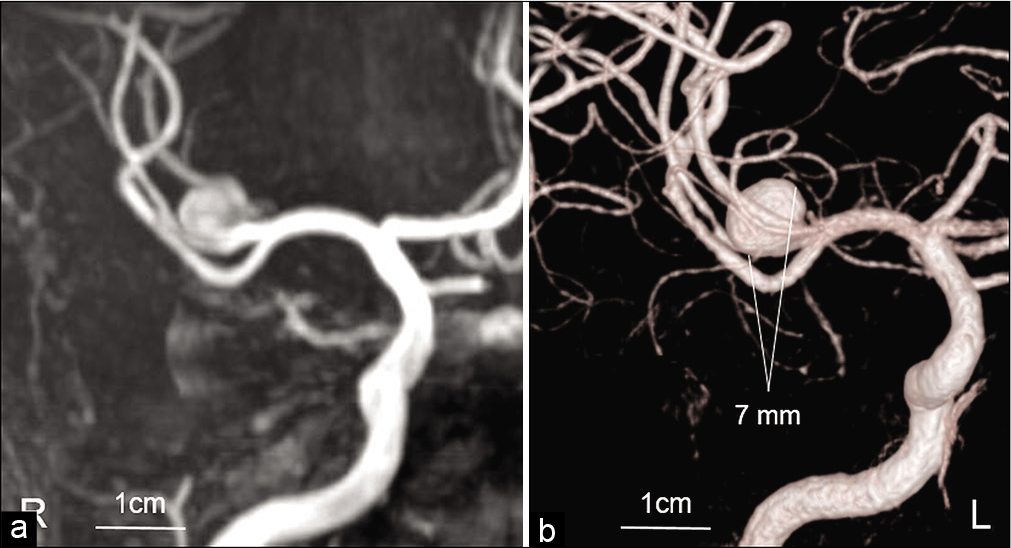
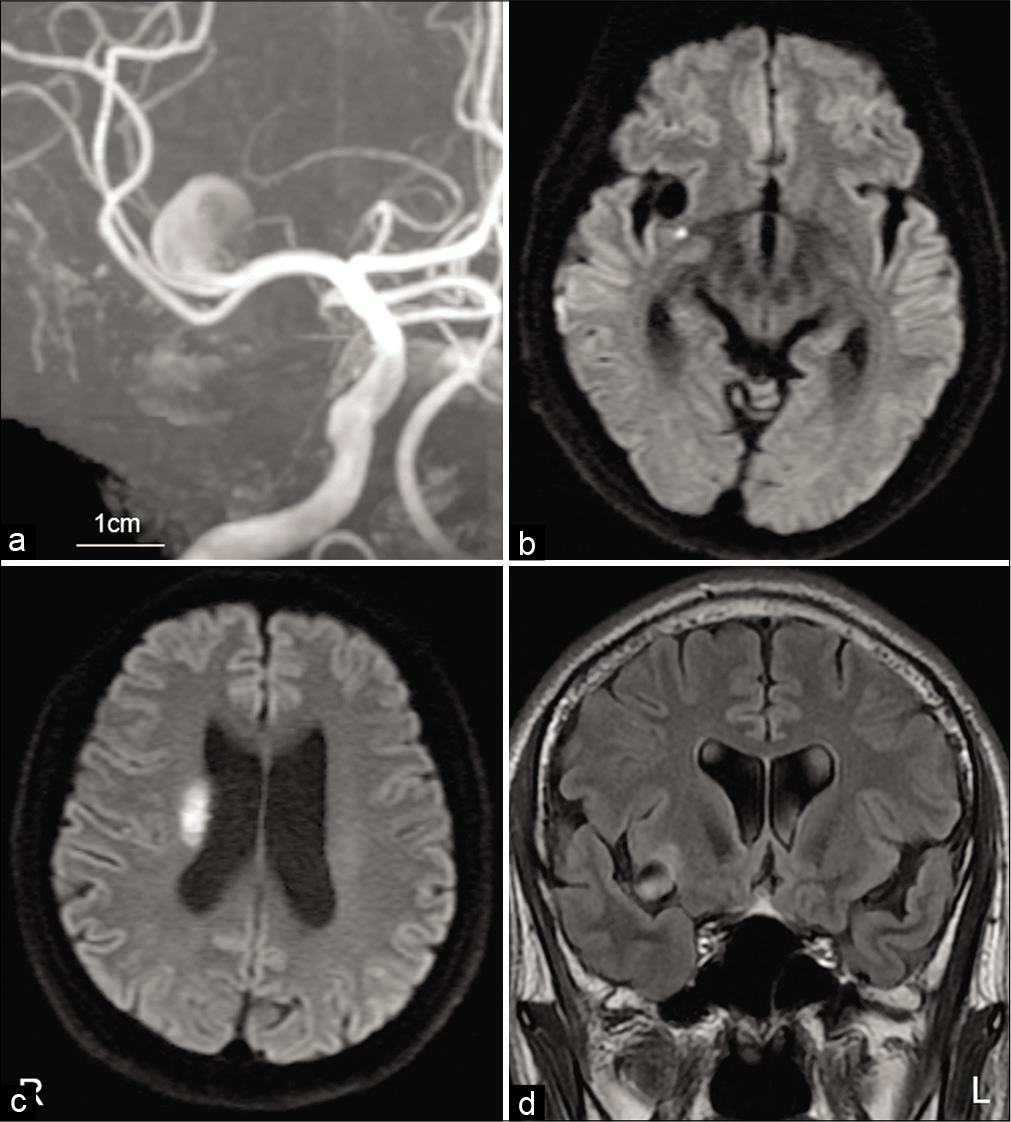
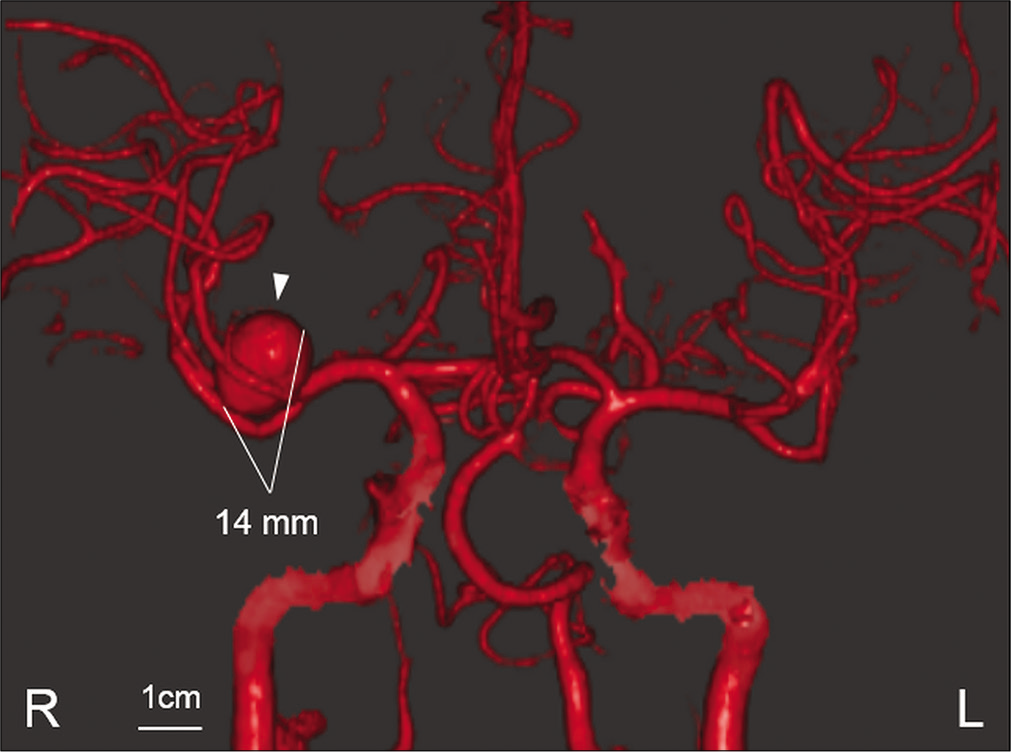
Case Description: A 53-year-old man presented with transient dysarthria and left hemiparesis. Magnetic resonance imaging (MRI) demonstrated the presence of a 7 mm UCA originating from the middle cerebral artery bifurcation, and diffusion-weighted imaging showed no evidence of cerebral infarction. One month later, his transient left hemiparesis recurred, and the patient was admitted to our hospital. Computed tomography angiography showed enlargement of the aneurysm. His left hemiparesis worsened 3 days later. MRI showed cerebral infarction in the area of perforating arteries and further enlargement of the aneurysm with surrounding parenchymal edema. Therefore, the rupture risk was considered to be rarely high and dome clipping was performed immediately. Postoperatively, his neurological status improved without any recurrent brain ischemia.
Conclusion: We report a rare case of a rapidly enlarging aneurysm that presented with cerebral infarction. This is the first report describing aneurysmal sac enlargement that can lead to perforating artery obstruction and brain ischemia. The case illustrates the importance of performing close follow-up examinations to confirm findings that suggest a high rupture risk.
Keywords: Brain ischemia, Direct compression, Rapid enlargement, Suction and decompression technique, Unruptured cerebral aneurysm
INTRODUCTION
Unruptured cerebral aneurysms (UCAs), especially large or giant cerebral aneurysms, are often present with neurological symptoms such as visual disturbances and oculomotor palsy without subarachnoid hemorrhage (SAH).[
These ischemic lesions commonly develop in the distal part of the aneurysm due to thrombotic materials formed within the aneurysmal sac,[
CASE DESCRIPTION
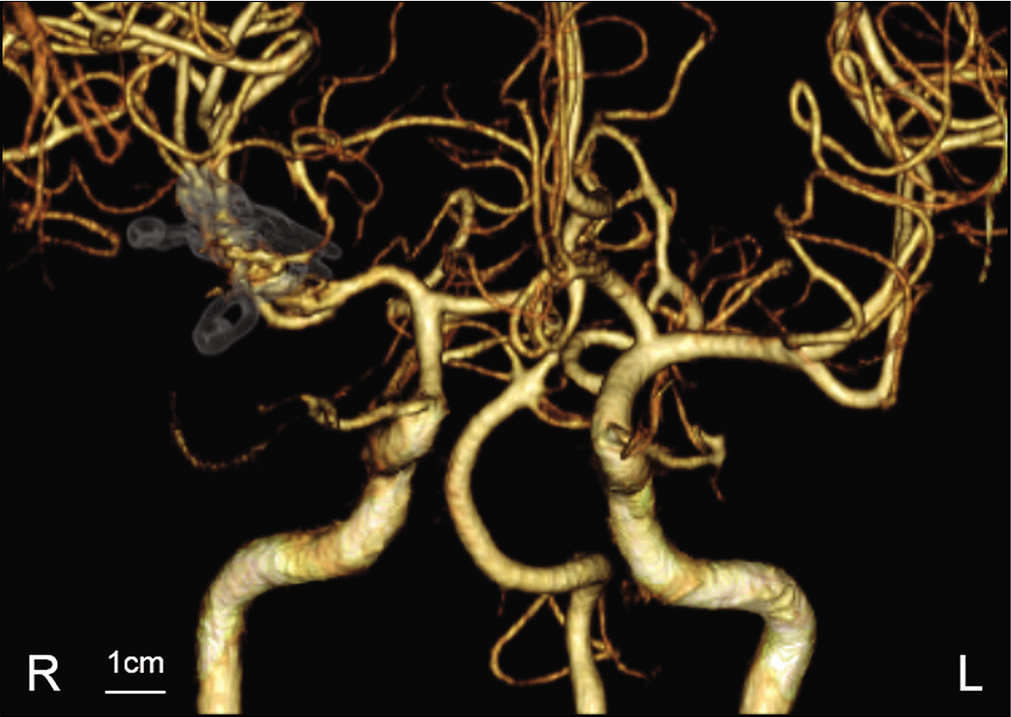
A 53-year-old man with transient dysarthria and left hemiparesis was admitted to our hospital. The patient had recovered from neurological deficits before being transferred to our hospital, and serological examination showed no presence of vascular risk factors. Magnetic resonance imaging (MRI) showed an UCA originating from the MCA bifurcation (7 mm in diameter) without evidence of acute cerebral infarction [
Figure 2:
Magnetic resonance image (MRI) findings immediately after the recurrent transient ischemic attack (TIA). MRA performed immediately after the recurrent TIA detected rapid enlargement of the unruptured cerebral aneurysm (a). MRI showing acute cerebral infarction in the area supplied by a proximal perforating artery (b and c). Fluid-attenuated inversion recovery image showing peripheral edema in insular cortex attached to an enlarged aneurysmal sac (d).
Figure 4:
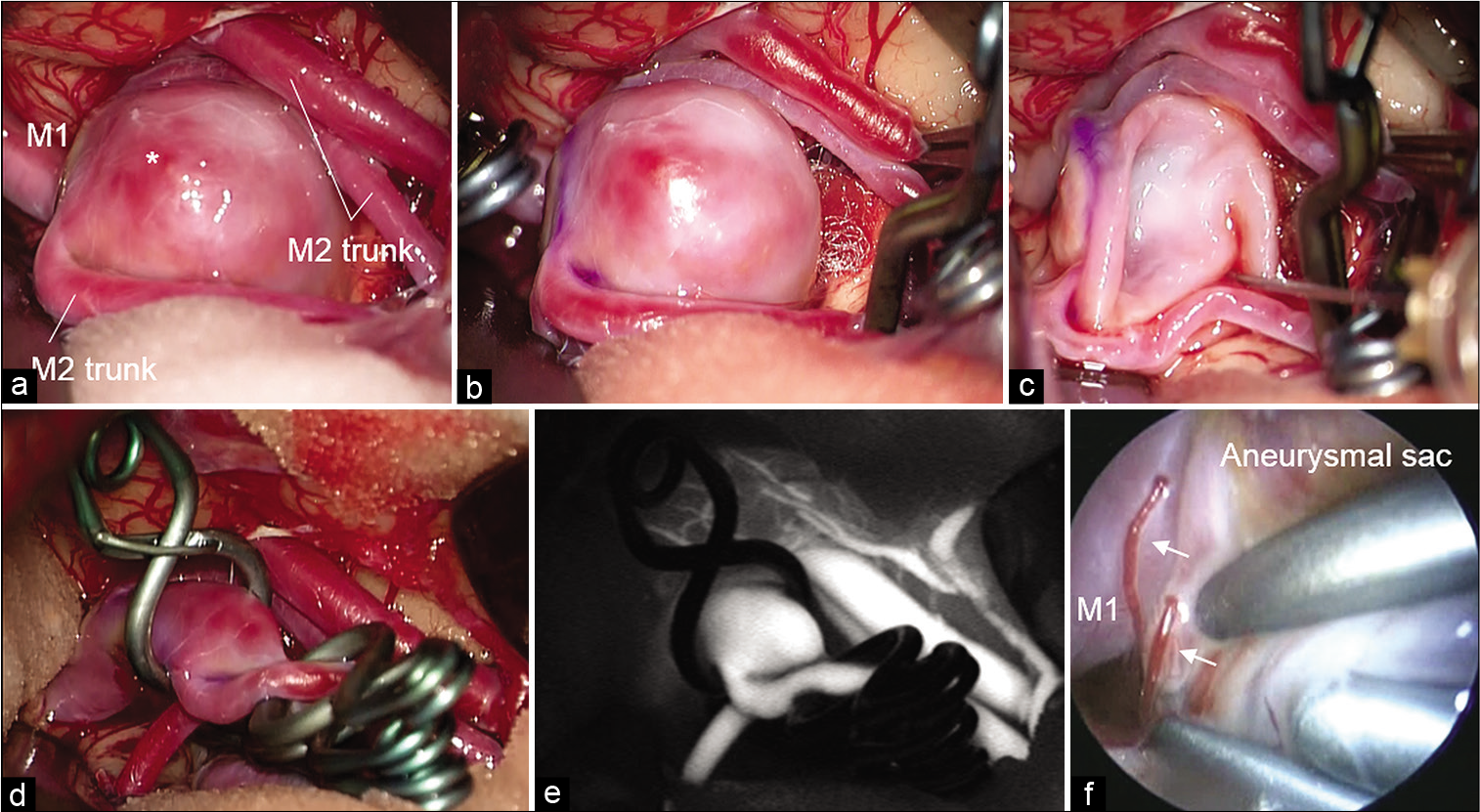
Surgical findings. Aneurysmal sac with bleb (*) and MCA trunks exposed through the pterional approach (a). Temporal clipping of MCA trunks performed, but intimal pressure remained high (b). Aneurysmal sac shrunk using the suction and decompression technique (c). Angioplastic clipping performed to avoid obstruction of the parent artery by multiple clips (d). Indocyanine green imaging (e). Endoscopy showed directly compressed perforating arteries (arrow) with an enlarged aneurysmal sac from behind the shrunk aneurysmal sac (f).
DISCUSSION
Clinical and radiological features of brain ischemia associated with UCA
Brain ischemia associated with UCA is an uncommon complication.[
Mechanism of brain ischemia associated with UCA
The two main mechanisms of brain ischemia associated with UCA have been considered.[
First, distal embolization from intra aneurysmal thrombosis and/or extension of the thrombosis to the parent artery have been considered. Aneurysmal thrombosis is more common in larger UCAs. Factors associated with aneurysmal thrombosis include size and chamber volume to the orifice area, blood stagnation, slow flow, and increased blood flow viscosity. Turbulent flow within the aneurysmal sac leads to endothelial injury. Subsequently, the subendothelial matrix is exposed, which favors platelet deposition and thrombus formation.[
The second considered mechanism are direct compression from the aneurysmal sac to parent artery and these branches, or displacement of the parent artery or adjacent structures. A small number of cases of brain ischemia caused by direct compression of the aneurysmal sac to perforating arteries have also been reported [
Management of brain ischemia associated with UCA
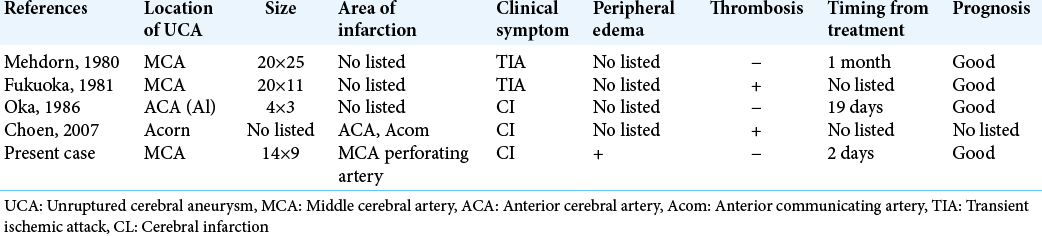
Because of the low frequency of this complication, the management of patients with brain ischemia associated with UCA remains unclear. Based on the previous reports, the risk of ischemic recurrence is low regardless of treatment option, and medical and surgical treatments are not superior to conservative treatment in reducing the risk of ischemic event recurrence.[
Surgical treatment for imminent rupture of UCA presenting with acute brain ischemia
However, to prevent aneurysm rupture, surgical treatment should be considered in the case when imminent rupture is suggested because high frequency of early SAH in patients with UCA after brain ischemia is reported.[
Surgical treatment for large and giant UCA is among the challenging lesions. A previous report described that surgery significantly reduced the mortality (from 31% to 4%) but increased the morbidity (from 8% to 19%) as compared with conservative treatment in patients with giant UCA.[
CONCLUSION
Brain ischemia associated with UCA is a rare complication. Most cases present with distal embolization are associated with aneurysmal thrombosis, whereas rapid enlargement of the UCA can lead to brain ischemia due to direct compression to a nearby parent or perforating artery. Clinicians should be aware of the risk of brain ischemia due to enlargement of the UCA and should consider urgent treatment of the UCA even immediately after brain ischemia. For surgical clipping, several techniques should be considered to ensure safety and success.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Editage (www.editage.com) for English language editing.
References
1. Calviere L, Viguier A, Da Silva NA, Cognard C, Larrue V. Unruptured intracranial aneurysm as a cause of cerebral ischemia. Clin Neurol Neurosurg. 2011. 113: 28-33
2. Cohen JE, Itshayek E, Gomori JM, Grigoriadis S, Raphaeli G, Spektor S. Spontaneous thrombosis of cerebral aneurysms presenting with ischemic stroke. J Neurol Sci. 2007. 254: 95-8
3. Date I. Symptomatic unruptured cerebral aneurysms: Features and surgical outcome. Neurol Med Chir (Tokyo). 2010. 50: 788-99
4. Fukuoka S, Suematsu K, Nakamura J, Matsuzaki T, Satoh S, Hashimoto I. Transient ischemic attacks caused by unruptured intracranial aneurysm. Surg Neurol. 1982. 17: 464-7
5. Guillon B, Daumas-Duport B, Delaroche O, Warin-Fresse K, Sevin M, Herisson F. Cerebral ischemia complicating intracranial aneurysm: A warning sign of imminent rupture?. AJNR Am J Neuroradiol. 2011. 32: 1862-5
6. Inoue T, Shimizu H, Fujimura M, Saito A, Tominaga T. Annual rupture risk of growing unruptured cerebral aneurysms detected by magnetic resonance angiography. J Neurosurg. 2012. 117: 20-5
7. Lonjon M, Pennes F, Sedat J, Bataille B. Epidemiology, genetic, natural history and clinical presentation of giant cerebral aneurysms. Neurochirurgie. 2015. 61: 361-5
8. McLaughlin N, Bojanowski MW. Unruptured cerebral aneurysms presenting with ischemic events. Can J Neurol Sci. 2008. 35: 588-92
9. Mehdorn HM, Chater NL, Townsend JJ, Darroch JD, Perkins RK, Lagger R. Giant aneurysm and cerebral ischemia. Surg Neurol. 1980. 13: 49-57
10. Nakase H, Shin Y, Kanemoto Y, Ohnishi H, Morimoto T, Sakaki T. Long-term outcome of unruptured giant cerebral aneurysms. Neurol Med Chir (Tokyo). 2006. 46: 379-84
11. Oka H, Oki H, Ohbayashi M, Fukami T. A case of unruptured A1 aneurysm causing ischemic compression to the medial proximal striate artery. No Shinkei Geka. 1986. 14: 1613-7
12. Pahl FH, De Oliveira MF, Ferreira NP, De Macedo LL, Brock RS, De Souza VC. Perianeurysmal edema as a predictive sign of aneurysmal rupture. J Neurosurg. 2014. 121: 1112-4
13. Profeta G, De Falco R, Ambrosio G, Profeta L. Endoscope-assisted microneurosurgery for anterior circulation aneurysms using the angle-type rigid endoscope over a 3-year period. Childs Nerv Syst. 2004. 20: 811-5
14. Qureshi AI, Mohammad Y, Yahia AM, Luft AR, Sharma M, Tamargo RJ. Ischemic events associated with unruptured intracranial aneurysms: Multicenter clinical study and review of the literature. Neurosurgery. 2000. 46: 282-9
15. Takeda R, Kurita H. “Mass Reduction” clipping technique for large and complex intracranial middle cerebral artery aneurysm. World Neurosurg. 2019. 125: 150-5
16. Toh K, Suzuki K, Miyaoka R, Kitagawa T, Saito T, Nakano Y. Giant cerebral aneurysm in a patient with cowden syndrome treated with surgical clipping. World Neurosurg. 2019. 126: 336-40