- Department of Surgery, Division of Neurosurgery, King Saud University, Riyadh, Saudi Arabia.
- Department of Surgery, Imam Abdulrahman Bin Faisal University, Al-Khobar, Eastern Province, Saudi Arabia.
- Department of Pathology, King Saud University, Riyadh, Saudi Arabia.
Correspondence Address:
Sarah A. Basindwah, Department of Surgery, Division of Neurosurgery, King Saud University, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_681_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sarah A. Basindwah1, Basmah S. Alzahrani2, Abdulrazag M. Ajlan1, Hiasham Alkhalidi3. Persistence of communicating hydrocephalus post choroid plexus tumor resection: Case reports and review of literature. 30-Sep-2021;12:483
How to cite this URL: Sarah A. Basindwah1, Basmah S. Alzahrani2, Abdulrazag M. Ajlan1, Hiasham Alkhalidi3. Persistence of communicating hydrocephalus post choroid plexus tumor resection: Case reports and review of literature. 30-Sep-2021;12:483. Available from: https://surgicalneurologyint.com/surgicalint-articles/11145/
Abstract
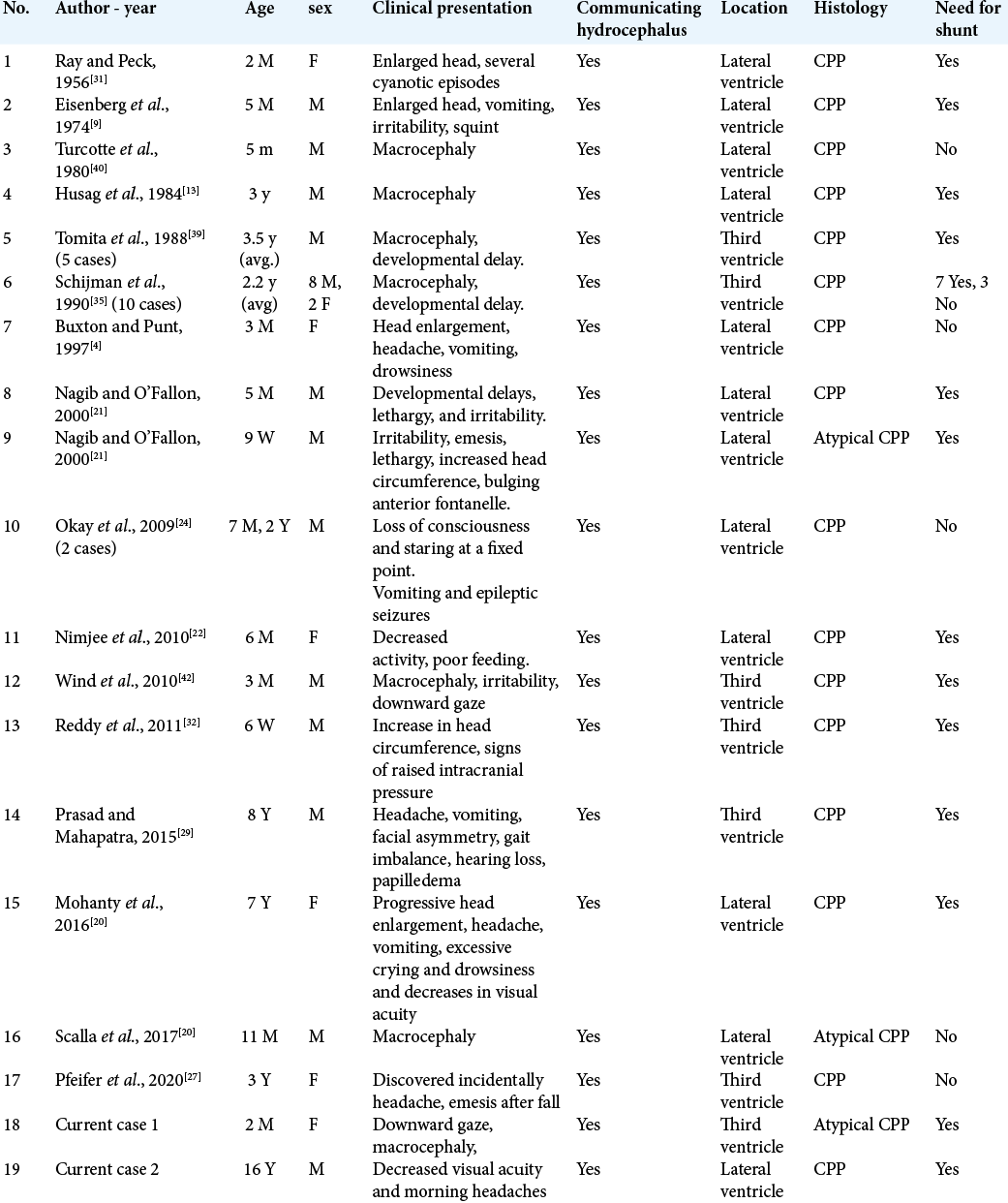
Background: Hydrocephalus is the most common presentation of choroid plexus tumors; it is thought to be caused either by mass effect obstructing the cerebrospinal fluid pathways or secretory properties of the tumor. In these case reports, we present two cases of choroid plexus tumors with persistence of communicating hydrocephalus postoperatively and review similar reports in the literature.
Case Description: Case 1: a 2-month-old baby girl presented with bulging fontanelle, sunsetting eyes. Magnetic resonance imaging (MRI) showed large third ventricle mass with communicating hydrocephalus. She underwent complete excision of tumor through transcortical approach with perioperative intraventricular hemorrhage. Hydrocephalus persisted postoperatively and the patient required permanent ventriculoperitoneal (VP) shunt. Case 2: a 16-year-old boy presented decreased visual acuity, papilledema, and morning headaches. MRI showed a tumor in the right ventricle and communicating hydrocephalus. He underwent transparietal resection of the tumor. In both cases, hydrocephalus persisted postoperatively and patients required permanent VP shunt. Review of similar cases showed the majority of cases required permanent shunting.
Conclusion: Choroid plexus tumor patients can present with communicating hydrocephalus that may persist post tumor resection for different etiologies. Careful follow-up to determine the need for cerebrospinal fluid diversion through a permanent VP shunt is important.
Keywords: Choroid plexus papilloma, Communicating hydrocephalus, Cerebrospinal fluid diversion, Pediatric tumors
INTRODUCTION
Choroid plexus papilloma (CPP) is a rare tumor of neuroectodermal origin, accounting for less than 1% of all intracranial neoplasms[
About 70% of CPTs present with hydrocephalus along with symptoms of increased intracranial hypertension, macrocephaly, and developmental delay.[
In this article, we report two cases of third ventricle CPP in an infant and an adolescent with communicating hydrocephalus and review the need for cerebrospinal fluid (CSF) diversion in similar population in literature reports.
CASE DESCRIPTION
Case 1
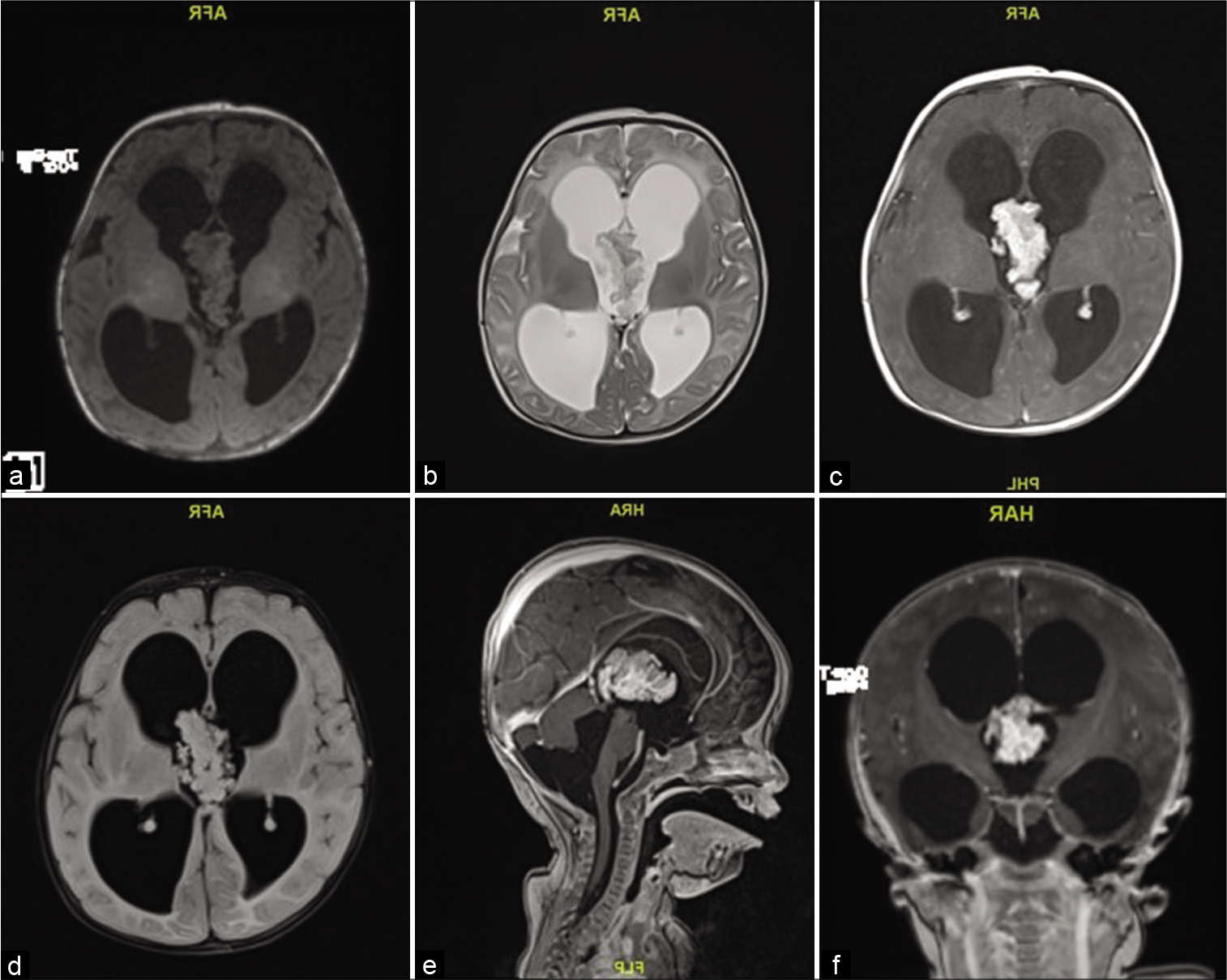
A 2-month-old girl presented with 1 week history of downward gaze, decreased feeding, and irritability. Her head circumference was above the 97th percentile, full fontanelle, and a positive sunset eye sign. Magnetic resonance imaging (MRI) showed a large lobulated mass in the third ventricle, isointense in T2/FLAIR and T1 with avid postcontrast enhancement measuring 38 by 20 by 23 mm with severe communicating hydrocephalus [
An EVD was placed in the ventricular system for draining the excessive CSF, and the tumor was found to be friable, vascular, lobulated mass floating within the CSF of the third ventricle, with its pedicle attached to the roof of the third ventricle. Histopathological examination of the tumor tissue revealed maintained papillary architecture that resembled normal choroid plexus. The papillae were lined by single layer of cuboidal to columnar epithelium, with mild nuclear pleomorphism. Mitosis was easily detectable (more than two mitotic figure per 10 high-power fields). There was no evidence of malignant features or necrosis. The lining epithelial nature was confirmed using Cytokeratin immunohistostaining. Ki-67 proliferative index was increased (up to 8% in scattered foci). The overall findings were in keeping with ACPP (WHO Grade II) [
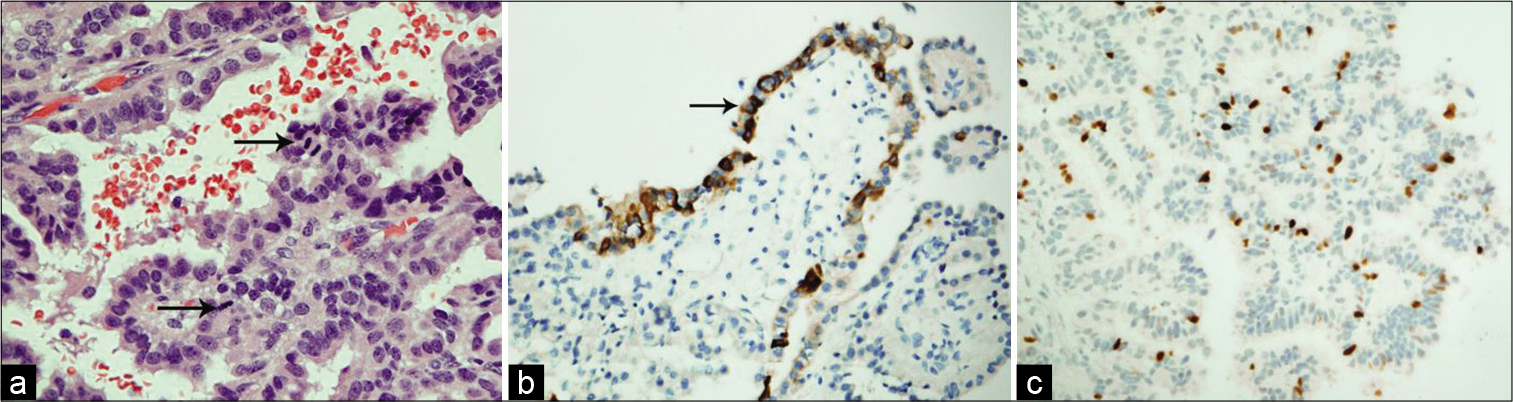
Figure 2:
(a) In this CPP, the cells exhibit mild nuclear pleomorphism and the mitotic figures (arrows) are easily detectable (H and E, ×400). (b) Immunohistochemistry shows reactivity of the epithelial cells to Pan-cytokeratin (PanCK, ×200). (c) Ki-67 proliferative index is estimated up to 8% in some foci (Ki-67, ×200).
The EVD was kept post operatively to try and challenge CSF drainage after recovery and assess the need for a permanent ventriculoperitoneal (VP) shunt.
The patient developed a perioperative and postoperative ventricular hemorrhage, and eventually needed a permanent shunt to divert the excessive CSF and recovered with good function.
Case 2
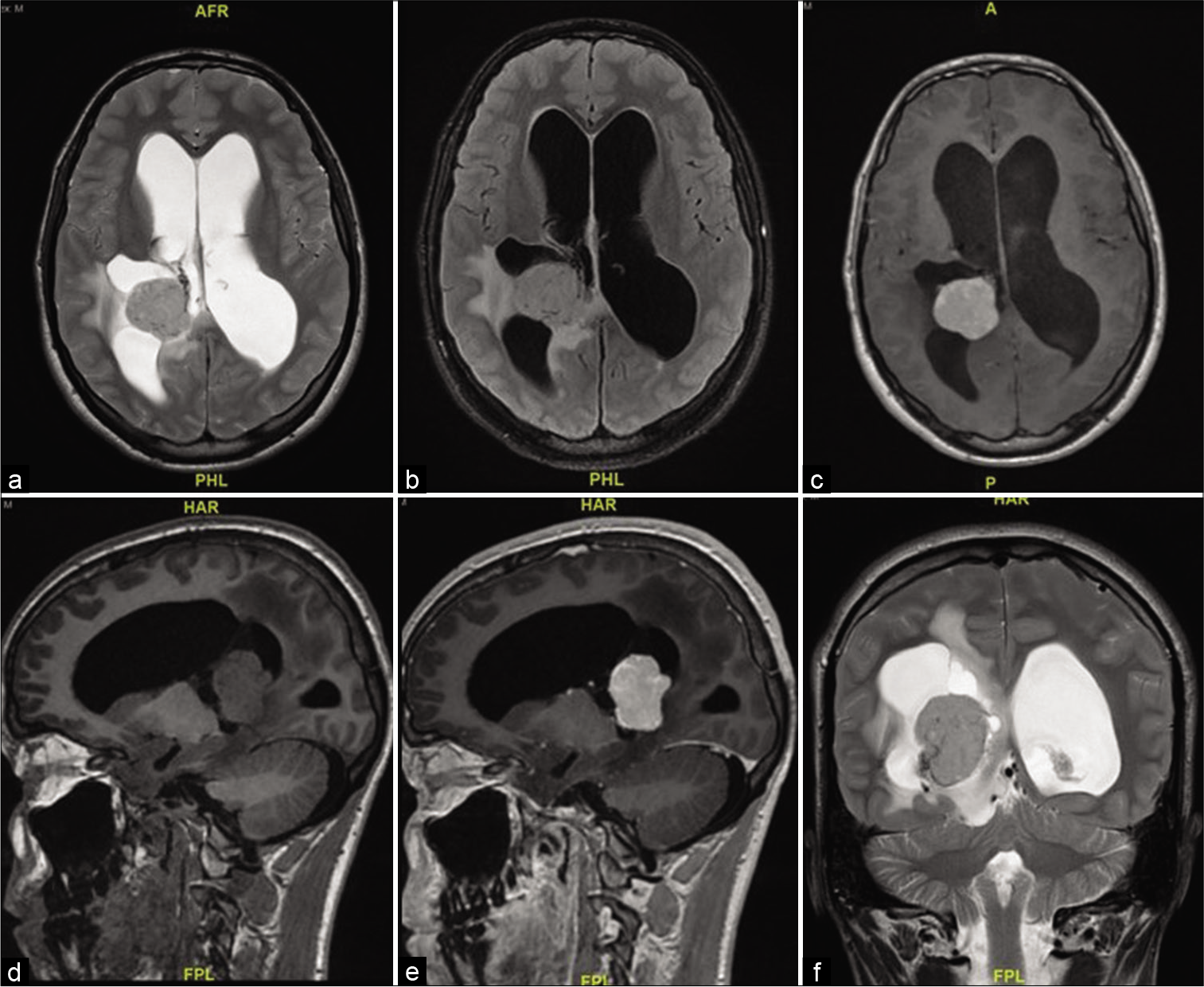
A 16-year-old boy presented with 10 days history of decreased visual acuity and morning headaches. He had severe bilateral papilledema Grade IV, and visual acuity of counting fingers from the right eye and 20/400 from the left eye. MRI showed a complex solid cystic lesion in the right ventricle, that is, isointense in T2/FLAIR and T1 and intense contrast enhancement. The mass measured 36 by 29 by 30 mm, with communicating hydrocephalus [
An EVD was inserted to drain excessive CSF out of the ventricles, the tumor was reached through a transparietal approach to the right lateral ventricle. It was found to be firm and vascular. Near-total resection was achieved with a small highly vascular rim left on the temporal horn.
Histopathological assessment of this tumor showed features of a CPP (WHO Grade I). It has the papillary architecture that resembled a normal choroid plexus, with minimal nuclear pleomorphism, very rare mitotic activity, and low Ki-67 proliferative index [
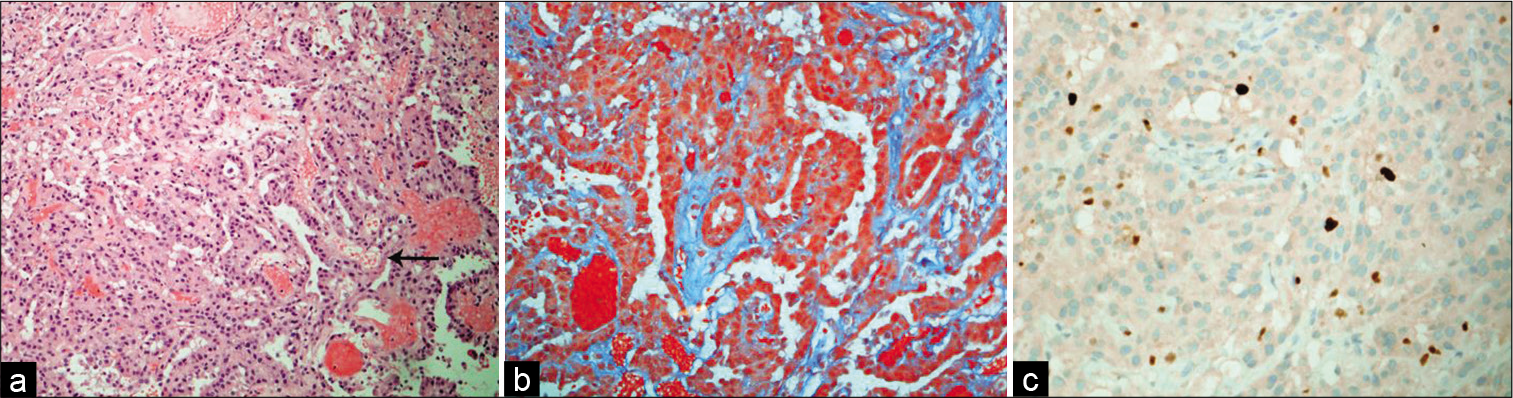
Figure 4:
(a) The tumor exhibits papillary-like architecture that has fibrovascular cores (arrow) and lining by a single layer of cuboid epithelium (H and E, ×100). (b) Trichrome stain shows a collagenous fibrous stroma (green), with overlying epithelial layer (Trichrome, ×200). (c) Ki-67 proliferative index is about 2% (Ki-67, ×200).
The EVD was kept postoperatively to challenge CSF drainage and assess the need for a permanent shunt, and it was removed 5 days postoperatively.
The patient kept having decreased visual acuity and persistent papilledema 2 weeks postoperatively and a VP shunt was inserted to relieve his persistent high intracranial pressure (ICP) symptoms.
The patient needed a permanent VP shunt and continued to have decreased visual acuity with optic disc gliosis evidence on subsequent images. Five-year follow-up showed no tumor recurrence.
DISCUSSION
Epidemiology
CPPs are benign intraventricular tumors (WHO Grade I) of neuroectodermal origin.[
Hydrocephalus is seen at presentation in >70% of patients, other signs such as increased head circumference, developmental delay, and intracranial hypertension can be commonly seen.[
Clinical presentation
The presentation and clinical course differ between children and adults. CPPs usually present with symptoms headache, diplopia, and ataxia with a more rapid course in adults.[
Massive hydrocephalus causing brain atrophy may be attributed to excessive CSF production of the tumor. Even in cases where those tumors are resected early and a shunt has been placed, hydrocephalus with parenchymal atrophy causing subsequent intellectual disabilities may persist.[
Hydrocephalus causing ventriculomegaly can be surgically adventitious when trying to resect an intraventricular tumor in dilated ventricles. In most cases, hydrocephalus resolves after resection. Shunting is often postponed to postresection to assess the need for a shunt.
Both our patients presented with hydrocephalus and symptoms of high ICP. In an infant, high ICP presents with bulging tense fontanelle and sunsetting eyes. In older children and teenagers, high ICP symptoms are visual changes, headaches, and bilateral papilledema.
In our cases, both patients presented with communicating hydrocephalus that persisted to require a VP shunt after resection.
[
Pathophysiology
Choroid plexus tumors are composed of cuboidal, columnar epithelium resembling that of a normal choroid plexus. The cause of hydrocephalus in CPPs is thought to be either obstructive, communicating, or a combination of both. Obstructive (noncommunicating) hydrocephalus could be caused by the tumor mass obstructing CSF pathways or obstruction of choroid villi due to microhemorrhages from the tumor or chronic arachnoiditis.[
In both of our cases, hydrocephalus persisted even with postoperative gross total resection and both patients required a VP shunt. Intraoperative, intraventricular micro/ macrobleeding may have contributed to occlusion of choroid villi draining CSF.
Pathology
CPP has a nodular, pink cauliflower-like appearance. They can be large enough to fill the whole ventricle. Microscopically, they consist of finger-like papillary projections with a fibrovascular core covered by cuboidal and columnar epithelial cells. ACPP is formed by the same arborizing stems covered by cuboidal or columnar epithelium with higher features of atypia, mitotic figures, and irregular nuclei.[
One variant of CPP is the CPP with villous hypertrophy where there is bilateral ventricular choroid plexus hypertrophy. Patients with this variant may develop shunt resistant hydrocephalus.[
Molecular profiling
To date, there is no evidence of molecular features leading to hydrocephalus formation in CPT patients. There is scant information about the molecular features in CPT’s in the literature. A case study on the molecular changes in CPT’s showed that a germline mutation leading to losses of tumor suppressor genes RB1 and BRCA2 may have led to malignant progression of CPP into atypical CPP.[
Management
Gross total resection is the treatment of choice. As those are highly vascular tumors, blood losses should be compensated early on in surgery, with tumor coagulation and en bloc piecemeal removal.[
The most common complications of surgery include perioperative bleeding, pneumocephalus, subdural fluid collection, or persistence of hydrocephalus.[
Management for ACPP may require postoperative chemotherapy. Long-term survival was seen with surgery and chemotherapy together in ACPP compared to surgery alone in four out of four patients.[
Prognosis
CPPs have a favorable prognosis. Most patients who undergo gross total resection as a primary treatment survive.[
CONCLUSION
CPPs can present with communicating hydrocephalus that may persist post tumor resection. Patients often need CSF diversion through a permanent VP shunt. Although different etiologies were investigated, the cause of persistence of communicating hydrocephalus is not yet known and is still managed by shunt placement.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abdulkader MM, Mansour NH, van Gompel JJ, Bosh GA, Dropcho EJ, Bonnin JM. Disseminated choroid plexus papillomas in adults: A case series and review of the literature. J Clin Neurosci. 2016. 32: 148-54
2. Anselem O, Mezzetta L, Grangé G, Zerah M, Benard C, Marcou V. Fetal tumors of the choroid plexus: Is differential diagnosis between papilloma and carcinoma possible?. Ultrasound Obstet Gynecol. 2011. 38: 229-32
3. Burger PC. Tumors of the central nervous system. AFIP Atlas Tumor Pathol. 2007. 252: 270-3
4. Buxton N, Punt J. Choroid plexus papilloma producing symptoms by secretion of cerebrospinal fluid. Pediatr Neurosurg. 1997. 27: 108-11
5. Dandy WE. Diagnosis, localization and removal of tumors of the third ventricle. Bull Johns Hopkins Hosp. 1922. 33: 188-9
6. di Rocco C, Iannelli A. Poor outcome of bilateral congenital choroid plexus papillomas with extreme hydrocephalus. Eur Neurol. 1997. 37: 33-7
7. Dohrmann GJ, BucY PC. Human choroid plexus: A light and electron microscopic study. J Neurosurg. 1970. 33: 506-16
8. Due-Tønnessen B, Helseth E, Skullerud K, Lundar T. Choroid plexus tumors in children and young adults: Report of 16 consecutive cases. Childs Nerv Syst. 2001. 17: 252-6
9. Eisenberg HM, McComb JG, Lorenzo AV. Cerebrospinal fluid overproduction and hydrocephalus associated with choroid plexus papilloma. J Neurosurg. 1974. 40: 381-5
10. Ellenbogen RG, Winston KR, Kupsky WJ. Tumors of the choroid plexus in children. Neurosurgery. 1989. 25: 327-35
11. Gupta P, Sodhi KS, Mohindra S, Saxena AK, Das A, Khandelwal N. Choroid plexus papilloma of the third ventricle: A rare infantile brain tumor. J Pediatr Neurosci. 2013. 8: 247-9
12. Husag L, Costabile G, Probst C. Persistent hydrocephalus following removal of choroid plexus papilloma of the lateral ventricle. Neurochirurgia. 1984. 27: 82-5
13. Husag L, Wieser H, Probst C. Unilateral hydrocephalus due to membranous occlusions of the foramen of Monro (author’s transl). Acta Neurochir (Wien). 1976. 33: 183-212
14. Johnson DL. Management of choroid plexus tumors in children. Pediatr Neurosurg. 1989. 15: 195-206
15. Lena G, Genitori L, Molina J, Legatte JR, Choux M. Choroid plexus tumours in children. Review of 24 cases. Acta Neurochir (Wien). 1990. 106: 68-72
16. Longatti P, Basaldella L, Orvieto E, Dei Tos A, Martinuzzi A. Aquaporin(s) expression in choroid plexus tumours. Pediatr Neurosurg. 2006. 42: 228-33
17. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
18. McGirr SJ, Ebersold MJ, Scheithauer BW, Quast LM, Shaw EG. Choroid plexus papillomas: Long-term follow-up results in a surgically treated series. J Neurosurg. 1988. 69: 843-9
19. Milhorat TH, Hammock MK, Davis DA, Fenstermacher JD. Choroid plexus papilloma. Pediatr Neurosurg. 1976. 2: 273-89
20. Mohanty S, Rout SS, Sarangi GS, Devi K. Choroid plexus papilloma arising from the temporal horn with a bilateral hypersecretory hydrocephalus: A case report and review of literature. World J Oncol. 2016. 7: 51-6
21. Nagib MG, O’Fallon MT. Lateral ventricle choroid plexus papilloma in childhood: Management and complications. Surg Neurol. 2000. 54: 366-72
22. Nimjee SM, Powers CJ, McLendon RE, Grant GA, Fuchs HE. Single-stage bilateral choroid plexectomy for choroid plexus papilloma in a patient presenting with high cerebrospinal fluid output: Case report. J Neurosurg Pediatr. 2010. 5: 342-5
23. Ogiwara H, Dipatri AJ, Alden TD, Bowman RM, Tomita T. Choroid plexus tumors in pediatric patients. Br J Neurosurg. 2012. 26: 32-7
24. Okay O, Dağlıoğlu E, Yakıcıer C, Üren D, Dalgıç A, Ergüngör F. Choroid plexus papillomas in two siblings: Case report. Turk Neurosurg. 2009. 19: 281-4
25. Passariello A, Tufano M, Spennato P, Quaglietta L, Verrico A, Migliorati R. The role of chemotherapy and surgical removal in the treatment of Choroid Plexus carcinomas and atypical papillomas. Childs Nerv Syst. 2015. 31: 1079-88
26. Pencalet P, Sainte-Rose C, Lellouch-Tubiana A, Kalifa C, Brunelle F, Sgouros S. Papillomas and carcinomas of the choroid plexus in children. J Neurosurg. 1998. 88: 521-8
27. Pfeifer CM, Wong K, Malireddy R, van Tassel D, Veltkamp DL, Cornejo P. Third ventricle choroid plexus papilloma: 2 cases. Radiol Case Rep. 2020. 15: 454-6
28. Pollock I, Schor N, Martinez A, Towbin R. Bobble-head doll syndrome and drop attacks in a child with a cystic choroid plexus papilloma of the third ventricle. J Neurosurg. 1995. 83: 729-32
29. Prasad GL, Mahapatra AK. Case series of choroid plexus papilloma in children at uncommon locations and review of the literature. Surg Neurol Int. 2015. 6: 151
30. Raimondi AJ.editors. Theoretical principles; art of surgical techniques. Pediatric Neurosurgery. New York: Springer-Verlag; 1987. p. 73-109
31. Ray BS, Peck FC. Papilloma of the choroid plexus of the lateral ventricles causing hydrocephalus in an infant. J Neurosurg. 1956. 13: 317-22
32. Reddy D, Gunnarsson T, Scheinemann K, Provias J, Singh S. Combined staged endoscopic and microsurgical approach of a third ventricular choroid plexus papilloma in an infant. Minim Invasive Neurosurg. 2011. 54: 264-7
33. Rekate H, Erwood S, Brodkey J, Chizeck H, Spear T, Ko W. Etiology of ventriculomegaly in choroid plexus papilloma. Pediatr Neurosurg. 1985. 12: 196-201
34. Safaee M, Oh MC, Bloch O, Sun MZ, Kaur G, Auguste KI. Choroid plexus papillomas: Advances in molecular biology and understanding of tumorigenesis. Neuro Oncol. 2013. 15: 255-67
35. Schijman E, Monges J, Raimondi A, Tomita T. Choroid plexus papillomas of the III ventricle in childhood. Childs Nerv Syst. 1990. 6: 331-4
36. Souweidane MM, Johnson JH, Lis E. Volumetric reduction of a choroid plexus carcinoma using preoperative chemotherapy. J Neurooncol. 1999. 43: 167-71
37. Tabori U, Shlien A, Baskin B, Levitt S, Ray P, Alon N. TP53 alterations determine clinical subgroups and survival of patients with choroid plexus tumors. J Clin Oncol. 2010. 28: 1995-2001
38. Thomas C, Ruland V, Kordes U, Hartung S, Capper D, Pietsch T. Pediatric atypical choroid plexus papilloma reconsidered: Increased mitotic activity is prognostic only in older children. Acta Neuropathol. 2015. 129: 925-7
39. Tomita T, McLone DG, Flannery AM. Choroid plexus papillomas of neonates, infants and children. Pediatr Neurosci. 1988. 14: 23-30
40. Turcotte J, Copty M, Bedard F, Michaud J, Verret S. Lateral ventricle plexus papilloma and communicating hydrocephalus. Surg Neurol. 1980. 13: 143-6
41. Welch K, Strand R, Bresnan M, Cavazzuti V. Congenital hydrocephalus due to villous hypertrophy of the telencephalic choroid plexuses: Case report. J Neurosurg. 1983. 59: 172-5
42. Wind JJ, Bell RS, Bank WO, Myseros JS. Treatment of third ventricular choroid plexus papilloma in an infant with embolization alone: Case report. J Neurosurg Pediatr. 2010. 6: 579-82
43. Wolff J, Sajedi M, Brant R, Coppes M, Egeler R. Choroid plexus tumours. Br J Cancer. 2002. 87: 1086-91
44. Yankelevich M, Finlay JL, Gorsi H, Kupsky W, Boue DR, Koschmann CJ. Molecular insights into malignant progression of atypical choroid plexus papilloma. Cold Spring Harb Mol Case Stud. 2021. 7: a005272