- Department of Neurosurgery, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan.
Correspondence Address:
Shanta Thapa, Department of Neurosurgery, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan.
DOI:10.25259/SNI_655_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shanta Thapa, Hitoshi Yamahata, Tomohisa Okada, Masanori Yonenaga, Madan Bajagain, Ryutaro Makino, Ryosuke Hanaya. Spinal intradural solitary fibrous tumor/ hemangiopericytoma with intramedullary invasion mimicking a hemangioblastoma. 30-Sep-2022;13:443
How to cite this URL: Shanta Thapa, Hitoshi Yamahata, Tomohisa Okada, Masanori Yonenaga, Madan Bajagain, Ryutaro Makino, Ryosuke Hanaya. Spinal intradural solitary fibrous tumor/ hemangiopericytoma with intramedullary invasion mimicking a hemangioblastoma. 30-Sep-2022;13:443. Available from: https://surgicalneurologyint.com/surgicalint-articles/11907/
Abstract
Background: Solitary fibrous tumor/hemangiopericytomas (SFT/HPCs) are rare mesenchymal tumors of nonmeningothelial origin that comprises
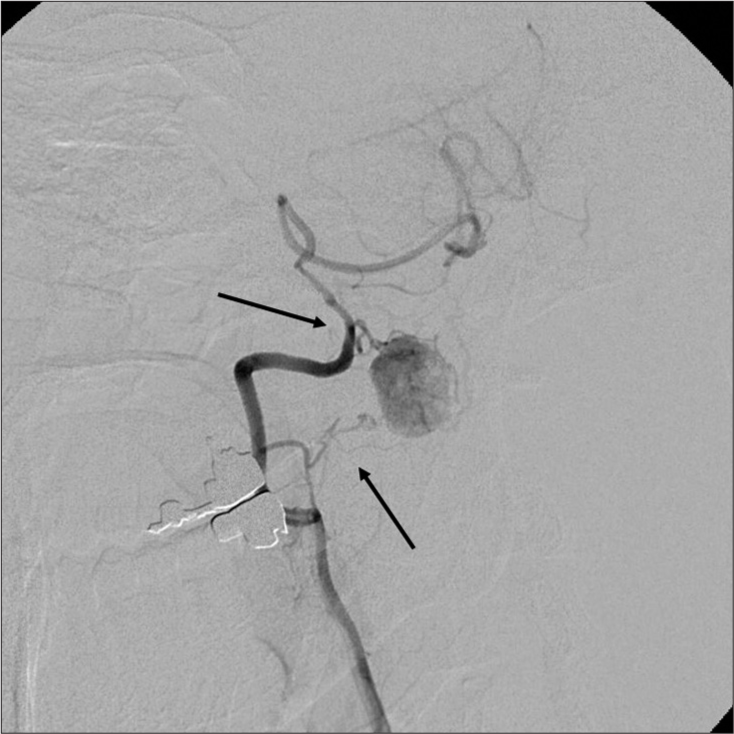
Case Description: A 45-year-old male presented with sleep apnea (apnea-hypopnea index was 17.1 events/hour) and dysesthesias of the right upper and lower extremities. The magnetic resonance demonstrated a heterogeneous intradural extra-axial C1 mass with syringobulbia and syringomyelia. The right vertebral angiography revealed a hypervascular mass (i.e., intense tumor staining). With the preoperative diagnosis of a spinal hemangioblastoma, the patient underwent tumor removal. However, intraoperative findings demonstrated that the ventral component of the tumor was intramedullary without a dural attachment. Further, the histological diagnosis was consistent with SFT/HPC (HPC phenotype). The postoperative course was uneventful, and the patient’s symptoms and the syrinxes spontaneously regressed.
Conclusion: A 45-year-old male presented a rare spinal intradural lesion at C1 appeared to be a spinal hemangioblastoma, but proved to be SFT/HPC (HPC phenotype) with intramedullary invasion.
Keywords: Hemangioblastoma, Hemangiopericytoma, Intramedullary invasion, Solitary fibrous tumor
INTRODUCTION
Hemangiopericytoma (HPC) and solitary fibrous tumor (SFT) are rare intradural mesenchymal tumors that are difficult to differentiate from meningiomas, schwannomas, ependymomas, astrocytomas, and hemangioblastomas, and constitute < 1% of all central nervous system (CNS) tumors.[
CASE REPORT
Clinical presentation
A 45-year-old male presented with numbness in his right forearm, hand, and leg of 2 months’ duration. He was found to have sleep apnea (i.e., polysomnography [PSG] showed apnea-hypopnea index [AHI] of 17.1 events/ hour [normal range: <5/h]) and a history of hypertension, hypercholesterolemia, asthma, and a lumbar disc herniation. On examination, he had dysesthesias in the right occipital region, the right C5–C7 distributions, the medial right lower leg, and the medial sole of the foot.
MR evaluation
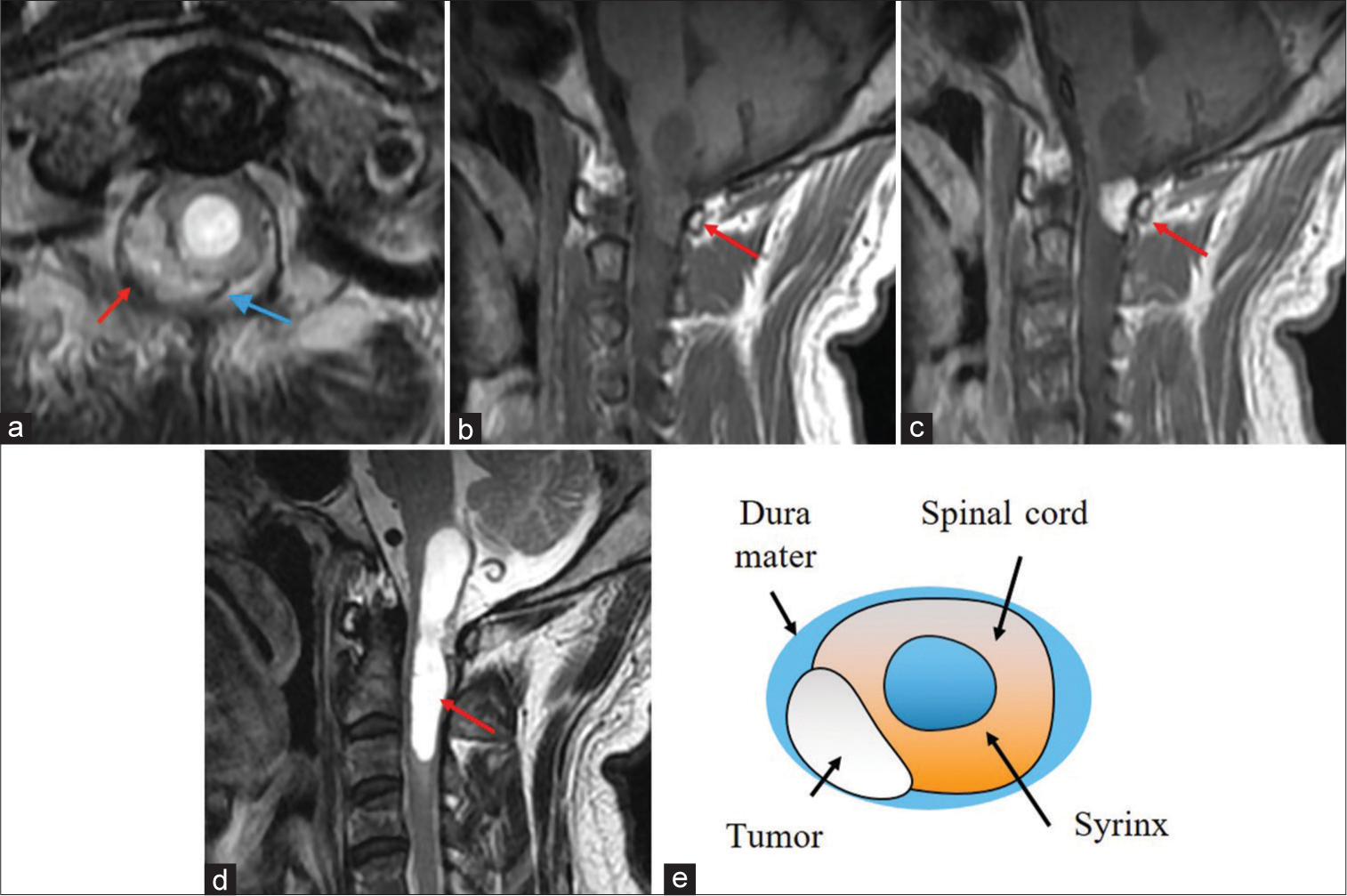
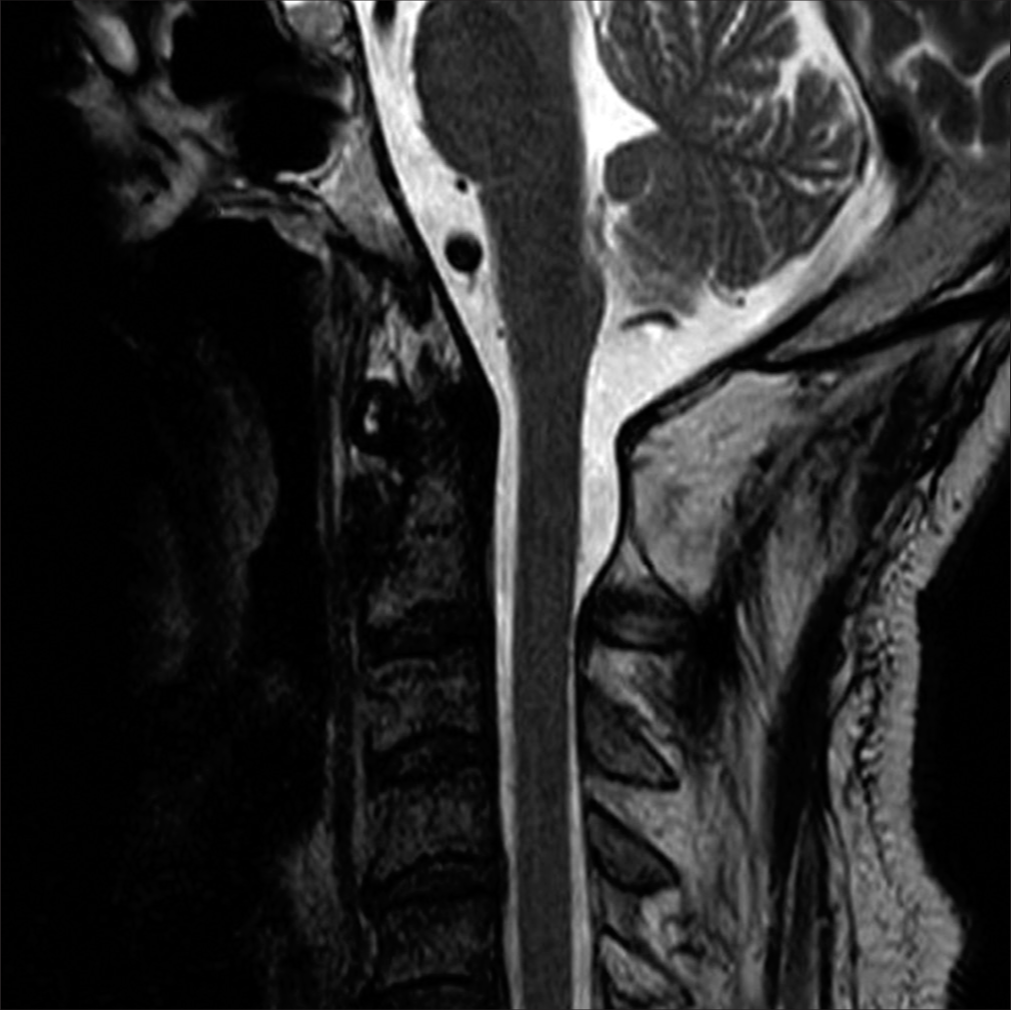
The cervical MR imaging (MRI) revealed a heterogeneously enhancing right-sided dorsal intradural/extramedullary mass with pial/subpial invasion most consistent with a spinal hemangioblastoma at the C1 level. It was isointense on T1 MR and moderately hyperintense on T2 MR studies; flow voids surrounded the tumor [
Figure 1:
T2-weighted axial (a), T1-weighted sagittal (b), and T1-weighted contrast-enhanced sagittal magnetic resonance imaging (c) showing an intradural extramedullary mass at the C1 level (red arrow in (a),(b),(c)). The mass is hyperintense on T2 and isointense on T1 and is heterogeneously enhanced with gadolinium. Flow voids can be seen around the tumor (blue arrow) (a). T2-weighted sagittal MRI reveals a syringobulbia and syringomyelia (red arrow) (d). A scheme demonstrates the anatomical relationship between the tumor and the spinal cord (e). The tumor is an extramedullary mass with pial invasion or a subpial mass with extramedullary extension.
Surgery
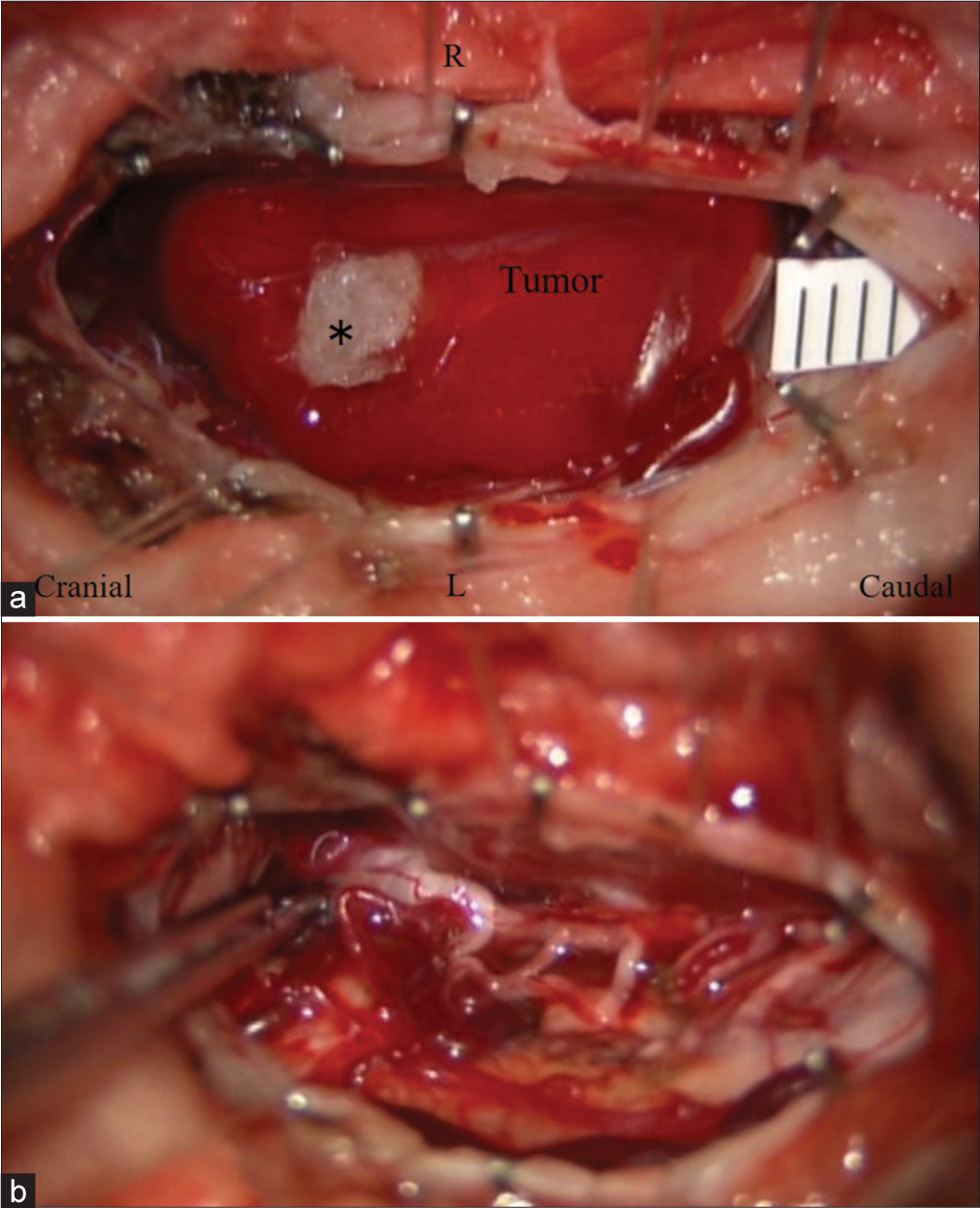
A suboccipital craniotomy, with laminectomy of the atlas, and cephalad laminotomy of C2 (C0-C2) were performed utilizing neurophysiological monitoring. Once the dura was opened, a bright red vascular lesion was identified on the dorsal aspect of the cord. However, the tumor’s ventral component was intramedullary in location. Once the pial feeding vessels were coagulated, the tumor was circumferentially separated and resected en bloc [
Histopathology
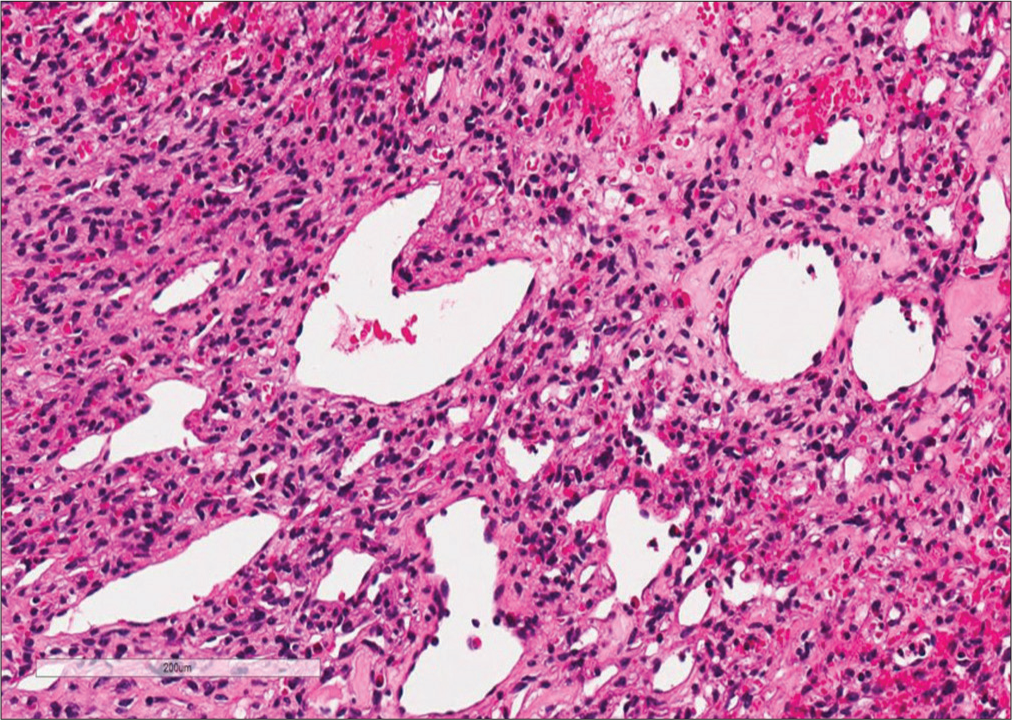
Histopathology confirmed that the tumor was a WHO Grade II SFT/HPC (HPC phenotype). It demonstrated round/ spindle cell proliferation and compact chromatin-stained nuclei that were relatively uniform without mitotic figures (i.e., consistent with a benign lesion). Immunohistochemistry was positive for CD34 and CD31 and negative for Bcl-2; the Ki-67 index was 2%. In addition, many small and large blood vessels with characteristic Staghorn vasculature were evident throughout [
Postoperative course
Postoperatively, the numbness of the right upper and lower extremities improved. The postoperative PSG showed improvement as his AHI decreased to 0.4 events/h, and his dysphagia disappeared. No further radiation was scheduled due to the complete tumor excision. Eight years later, he has exhibited no tumor recurrence [
DISCUSSION
Case summary
SFT/HPC, nonmeningothelial mesenchymal tumors that are rarely intradural/extramedullary account for <1% of all CNS tumors [
Literature summary
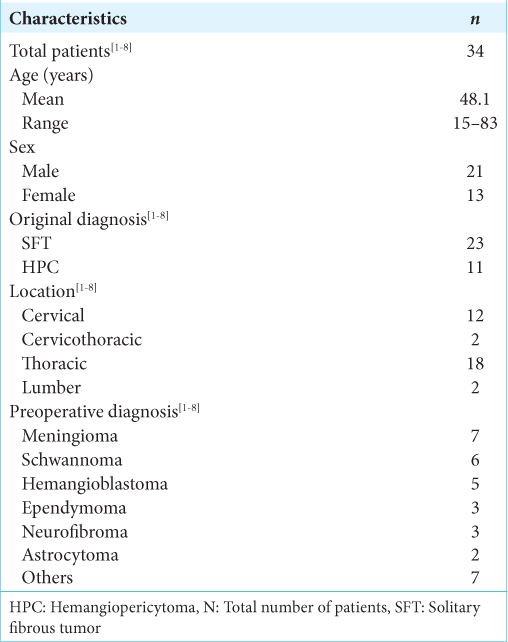
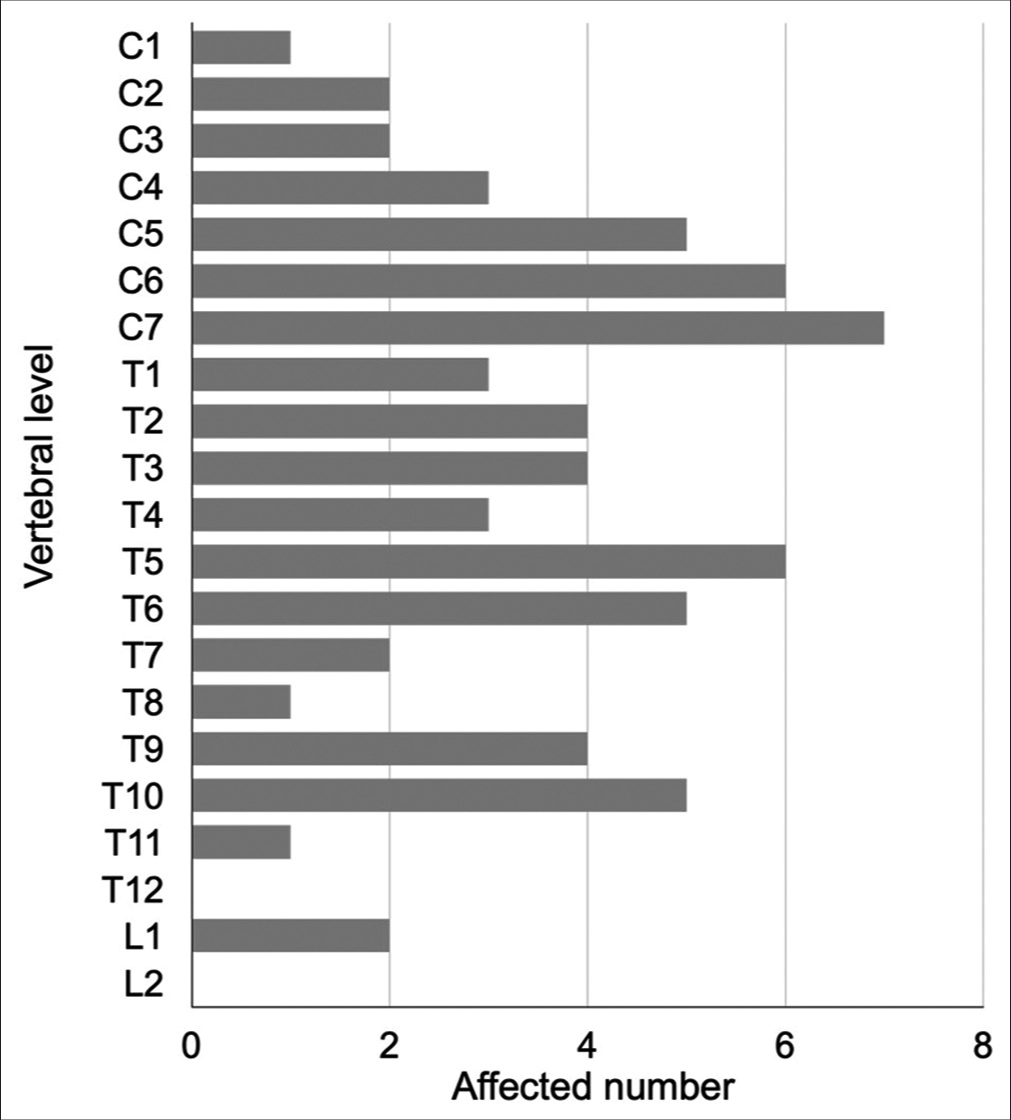
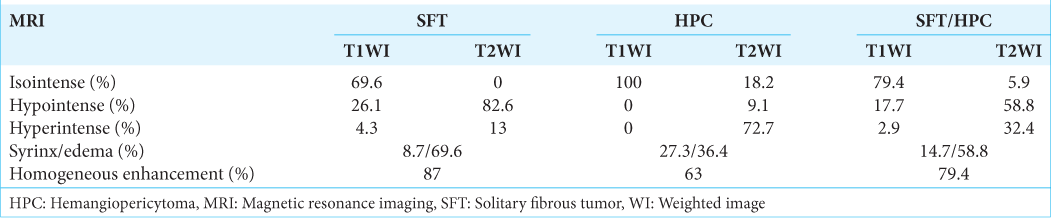
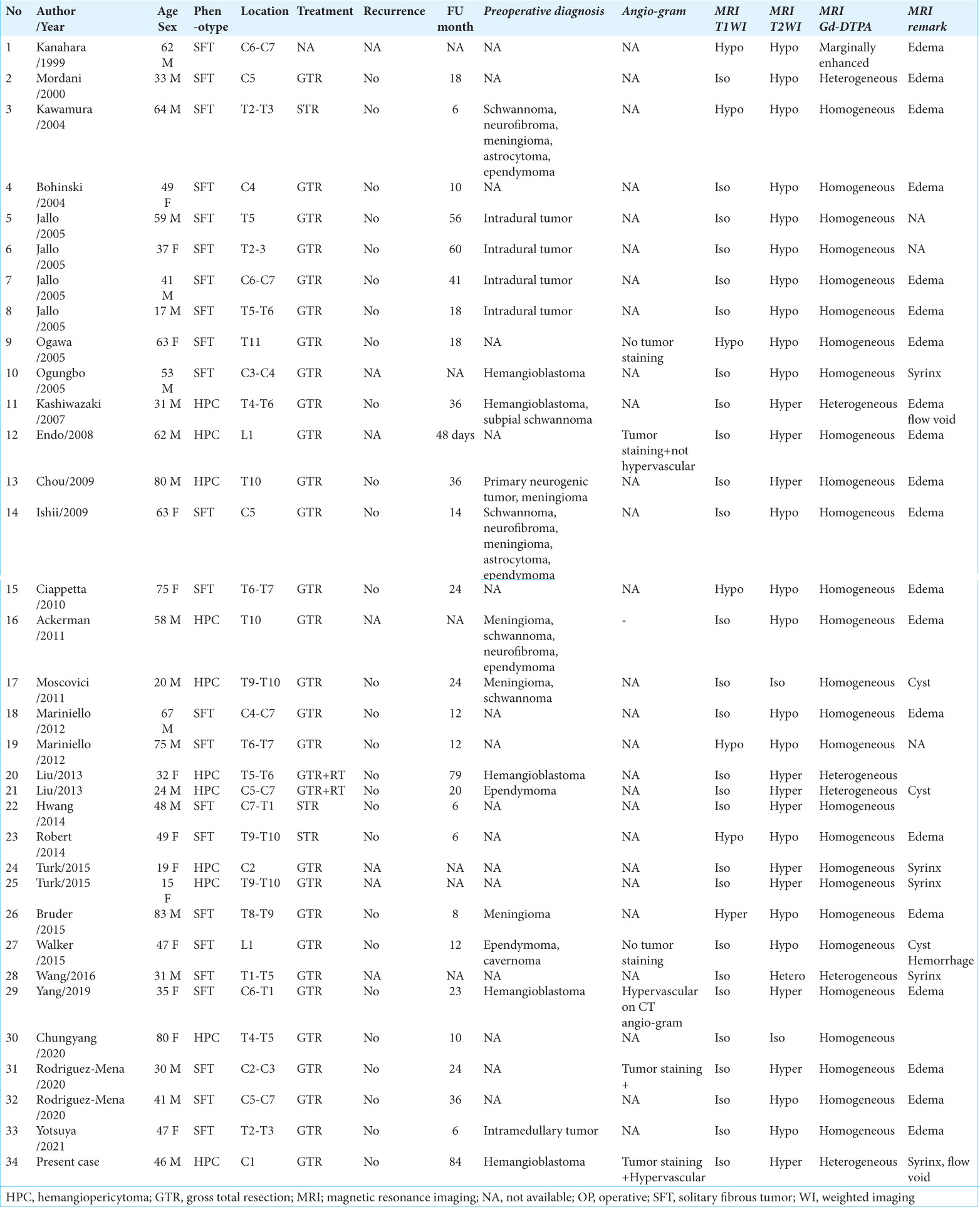
We found 34 cases of SFT/HPC cases (i.e., including this case) in our review of case studies from the literature [
Treatment and long-term prognosis
Following WHO 2016 guidelines, gross total resection (GTR) with radiotherapy is the mainstay for the treatment of the CNS SFT/HPC that typically result in good outcomes [
CONCLUSION
Here, we reported a 45-year-old male with a C1 spinal intradural SFT/HPC (HPC phenotype) with an intramedullary invasion that resulted in MR-documented syringomyelia and syringobulbia. Following GTR, the patient remained asymptomatic 8 years later.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTAL TABLE
References
1. Bisceglia M, Galliani C, Giannatempo G, Lauriola W, Bianco M, D’Angelo V. Solitary fibrous tumor of the central nervous system: A 15-year literature survey of 220 cases (August 1996-July 2011). Adv Anat Pathol. 2011. 18: 356-92
2. Bruder M, Tews D, Mittelbronn M, Capper D, Seifert V, Marquardt G. Intramedullary solitary fibrous tumor-a benign form of hemangiopericytoma? Case report and review of the literature. World Neurosurg. 2015. 84: 189.e7-12
3. Giannini C, Rushing E, Hainfellner J, Bouvier C, Figarella-Branger D, von Deimling A, Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.editors. Solitary fibrous tumor/hemangiopericytoma. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC; 2016. p. 249-54
4. Jankovic D, Hanissian A, Rotim K, Splavski B, Arnautovic KI. Novel clinical insights into spinal hemangioblastoma in adults: A systematic review. World Neurosurg. 2021. 158: 1-10
5. Liu HG, Yang AC, Chen N, Yang J, Qiu XG, Zhang JG. Hemangiopericytomas in the spine: Clinical features, classification, treatment, and long-term follow-up in 26 patients. Neurosurgery. 2013. 72: 16-24 discussion 24
6. Türk CÇ, Kara NN, Süren D, Özdöl Ç, Gediz T, Yıldız S. Distinctive characteristic features of intramedullary hemangiopericytomas. Asian Spine J. 2015. 9: 522-8
7. Wang J, Zhao K, Han L, Jiao L, Liu W, Xu Y. Solitary fibrous tumor/hemangiopericytoma of the spinal cord: A retrospective single-center study of 16 cases. World Neurosurg. 2019. 123: e629-38
8. Yi X, Xiao D, He Y, Yin H, Gong G, Long X. Spinal solitary fibrous tumor/hemangiopericytoma: A clinicopathologic and radiologic analysis of eleven cases. World Neurosurg. 2017. 104: 318-29