- Departments of Neurosurgery Service, Hospital de Clínicas de Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazil.
- Departments of Neurosurgery Hospital de Clínicas de Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazil.
- Departments of Neuropathology, Hospital de Clínicas de Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazil.
Correspondence Address:
Rafael Contage Winter
Departments of Neuropathology, Hospital de Clínicas de Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazil.
DOI:10.25259/SNI_528_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rafael Contage Winter1, Apio Claudio Martins Antunes2, Francine Hehn de Oliveira3. The relationship between vascular endothelial growth factor and histological grade in intracranial meningioma. 08-Oct-2020;11:328
How to cite this URL: Rafael Contage Winter1, Apio Claudio Martins Antunes2, Francine Hehn de Oliveira3. The relationship between vascular endothelial growth factor and histological grade in intracranial meningioma. 08-Oct-2020;11:328. Available from: https://surgicalneurologyint.com/surgicalint-articles/10315/
Abstract
Background: Meningioma is the most common benign intracranial neoplasm, accounting for 30% of all primary brain tumors. In 90% of cases, meningiomas are benign. Several aspects of molecular biology, including potential biomarkers, have been studied in attempts to better understand the natural history of meningiomas. Vascular endothelial growth factor (VEGF) is a biomarker responsible for inducing physiological and pathological angiogenesis. VEGF expression has been investigated as a potential predictor of several tumor aspects, including growth rate, recurrence rate, brain tissue invasion, peritumoral edema and surgical prognosis, and also as a marker of histological grade. However, there is no consensus in the literature with respect to the association between this biological factor and meningioma. We digitally analyzed immunohistochemical images using ImageJ software with the aim of correlating VEGF expression with tumor histology.
Methods: Tissue samples from patients presenting with meningioma who had undergone surgical removal between 2007 and 2016 at the Hospital de Clínicas de Porto Alegre (HCPA), in Southern Brazil, were analyzed to identify possible immunohistochemical associations between VEGF and histological grade and subtype.
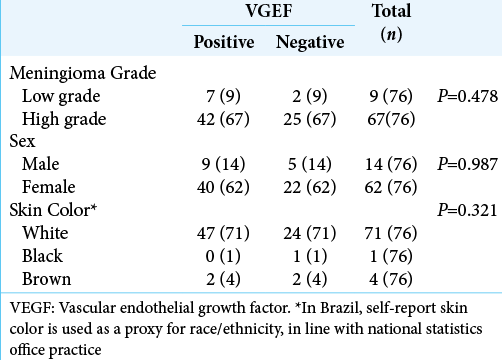
Results: Seventy-six patients were included; 82% were female, mean age was 59.9 years (range: 18–91). No statistically significant associations were found between VEGF expression and histological grade or subtype (P = 0.310).
Conclusion: Our findings suggest that VEGF is frequently present in meningiomas regardless of histological grade and should not be used as a marker of severity or histological grade.
Keywords: Immunohistochemistry, Meningioma, Molecular biology, Vascular endothelial growth factor
INTRODUCTION
In 1922, Harvey Cushing coined the term meningioma to describe neoplasms arising from arachnoidal cells.[
Meningiomas can provoke a diverse set of symptoms, mainly depending on how they affect adjacent neurological structures, either by compression or irritation. Patients who are diagnosed with meningioma do not always need medical treatment. When indicated, the gold standard treatment is surgery, with total resection of the lesion including the involved dura (Simpson Grade I resection).[
Several factors related to the molecular biology of meningiomas have been studied as potential biomarkers for growth rate, likelihood of recurrence, malignant transformation, and invasion of adjacent brain tissue. Despite the considerable number of studies examining these factors, results remain conflicting and inconclusive.[
One such biomarker is vascular endothelial growth factor (VEGF),[
The present study aimed to determine the relationship between VEGF expression and tumor histology determined by digital analysis of immunohistochemical images using ImageJTM software (NIH, USA).
MATERIALS AND METHODS
We analyzed tumor tissue samples from patients presenting with meningioma who had undergone surgery between 2007 and 2016 at the Hospital de Clínicas de Porto Alegre (HCPA), a university hospital in southern Brazil. Patients were included if they had not undergone preoperative embolization or been diagnosed with neurofibromatosis. The hospital’s Research Ethics Committee approved this study under registration number 854.176 on October 28, 2014, and also waived the obligation to obtain informed consent from patients.
Hematoxylin and eosin (H and E)-stained slides were examined by a pathologist, and VEGF expression was analyzed immunohistochemically (ab1316, Abcam, USA, mouse monoclonal, clone VG-1, 1:2000 dilution). A BenchMark ULTRA automated platform was used to process samples (Ventana Medical Systems/Roche Diagnostics, USA), and ultraView Universal DAB Detection Kits were used to view reactions (Ventana Medical Systems/Roche Diagnostics, USA, catalogue no. 760-500).
Slides for immunohistochemical analysis were photographed under ×400 with an Olympus QColor5 camera (Olympus Corporation, Japan) coupled to an Olympus BX5 microscope (Olympus Corporation, Japan). The field photographed had been selected in advance by the pathologist. Images were digitally captured with QCapture software, version 2.81.0 (Quantitative Imaging Corporation, USA), using the same parameters for all slides and maintaining the same contrast settings for each sample. These images were digitally analyzed using ImageJ software, as described by Varghese, Bukhari, and Malhorta, based on Ruifrok’s study.[
Statistical analysis
The results of the immunohistochemical analysis of VEGF expression were compared to tumor grade and histological subtypes. The sample size of 76 specimens was calculated considering a difference of 30% VEGF expression in relation to tumor grade, to achieve statistical power of 80% and a 5% significance level. Proportions were compared using Pearson’s Chi-square test or Fisher’s exact test. Results were considered significant if P < 0.05, and SPSS software, version 21.0, was used for the analyses.
RESULTS
The study sample comprised 76 patients, representative of a typical population of patients with meningiomas with respect to age, sex, and histological grade. The mean age of patients was 59.9 (SD = 15.6) years. The youngest patient was 18 years old and the oldest was 91. There were 62 women and 14 men.
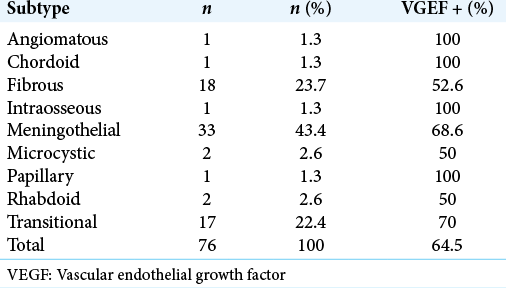
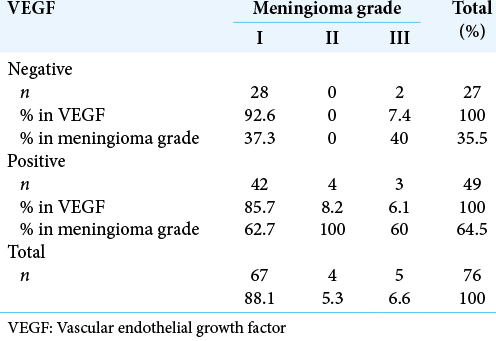
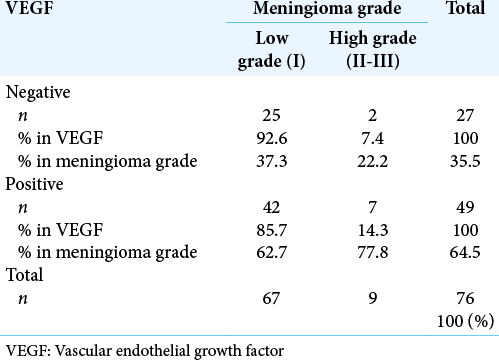
A total of 76 tumors were assessed; 67 were Grade I (88.2%), 4 were Grade II (5.3%), and 5 were Grade III (6.5%) [
DISCUSSION
The natural history of meningioma is still unclear and course is unpredictable. Studies have employed molecular biology to search for factors that could be considered potential biomarkers of accelerated tumor growth rate, aggressive tumor behavior, and tumor grading and could, therefore, be used to guide more effective treatment in early stages and differential management of different tumor types.
New blood vessels develop to supply tumors through neoangiogenesis, which is a crucial process for tumor cell growth. Since VEGF is a major inducer of angiogenesis, VEGF expression is believed to be essential for tumor development.[
Yamasaki et al. correlated VEGF expression with recurrence of Grade I meningioma after macroscopic total resection and found that high levels of VEGF expression were significantly associated with tumor recurrence, suggesting that this factor is one of the main predictors of recurrence.[
In contrast, and consistent with our findings, Baxter et al. prospectively assessed 175 patients and found no relationship between VEGF expression and histological meningioma grade.[
VEGF plays an important role in the neoangiogenesis of tumor cells, as demonstrated by several studies relating it to microvessel density in meningioma.[
We did not detect associations between VEGF expression and meningioma grade in our samples. Although VEGF is present in meningioma and is significantly involved in vascular development and central nervous system maintenance and repair, the effect most probably depends on other angiogenic factors.
CONCLUSION
VEGF and other factors of molecular biology have become essential to understanding the natural history of brain tumors, helping in the choice of appropriate treatment and follow-up decisions for patients presenting with meningioma. Our study suggests that there is no association between VEGF expression and meningioma grade. The limited sample size may be a contributing factor to the negative association and a very large sample size may be needed to detect associations. In view of the conflicting results reported in the literature, future studies with larger samples should focus on investigating the possible role of VEGF in tumor behavior.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Algire G.editors. The Biology of Melanomas. New York: Academy of Sciences Press; 1947. p.
2. Barresi V. Angiogenesis in meningiomas. Brain Tumor Pathol. 2011. 28: 99-106
3. Baxter DS, Orrego A, Rosenfeld JV, Mathiesen T. An audit of immunohistochemical marker patterns in meningioma. J Clin Neurosci. 2014. 21: 421-6
4. Cushing H, Eisennhardt L.editors. Meningiomas: Their Classification, Regional Behaviour, Life History, and Surgical End Results. Springfield, Illinois: Charles C. Thomas; 1938. p.
5. Denizot Y, de Armas R, Caire F, Moreau JJ, Pommepuy I, Truffinet V. The quantitative analysis of bFGF and VEGF by ELISA in human meningiomas. Mediators Inflamm. 2006. 2006: 36376
6. Dharmalingam P, Kumar VR, Verma SK. Vascular endothelial growth factor expression and angiogenesis in various grades and subtypes of meningioma. Indian J Pathol Microbiol. 2013. 56: 349-54
7. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997. 18: 4-25
8. Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989. 339: 58-61
9. Folkman J. Tumour angiogenesis: Therapeutic implications. N Engl J Med. 1971. 285: 1182-6
10. Goldman CK, Bharara S, Palmer CA, Vitek J, Tsai JC, Weiss HL. Brain edema in meningiomas is associated with increased vascular endothelial growth factor expression. Neurosurgery. 1997. 40: 1269-77
11. Hai J, Li ST, Lin Q, Pan QG, Gao F, Ding MX. Vascular endothelial growth factor expression and angiogenesis induced by chronic cerebral hypoperfusion in rat brain. Neurosurgery. 2003. 53: 963-72
12. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996. 86: 353-64
13. Hou J, Kshettry VR, Selman WR, Bambakidis NC. Peritumoral brain edema in intracranial meningiomas: The emergence of vascular endothelial growth factor-directed therapy. Neurosurg Focus. 2013. 35: E2
14. Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007. 8: 610-22
15. Jensen R, Lee J. Predicting outcomes of patients with intracranial meningiomas using molecular markers of hypoxia, vascularity, and proliferation. Neurosurgery. 2012. 71: 146-56
16. Jensen RL. Brain tumor hypoxia: Tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neurooncol. 2009. 92: 317-35
17. Kaley T, Barani I, Chamberlain M, McDermott M, Panageas K, Raizer J. Historical benchmarks for medical therapy trials in surgery-and radiation-refractory meningioma: A RANO review. Neuro Oncol. 2014. 16: 829-40
18. Korkolopoulou P, Patsouris E, Konstantinidou AE, Pavlopoulos PM, Kavantzas N, Boviatsis E. Hypoxiainducible factor 1alpha/vascular endothelial growth factor axis in astrocytomas. Associations with microvessel morphometry, proliferation and prognosis. Neuropathol Appl Neurobiol. 2004. 30: 267-78
19. Lamszus K, Lengler U, Schmidt NO, Stavrou D, Ergün S, Westphal M. Vascular endothelial growth factor, hepatocyte growth factor/scatter factor, basic fibroblast growth factor, and placenta growth factor in human meningiomas and their relation to angiogenesis and malignancy. Neurosurgery. 2000. 46: 938-48
20. Lee SH, Lee YS, Hong YG, Kang CS. Significance of COX-2 and VEGF expression in histopathologic grading and invasiveness of meningiomas. APMIS. 2014. 122: 16-24
21. Machein MR, Plate KH. VEGF in brain tumors. J Neurooncol. 2000. 50: 109-20
22. Maiuri F, de Caro MB, Esposito F, Cappabianca P, Strazzullo V, Pettinato G. Recurrences of meningiomas: Predictive value of pathological features and hormonal and growth factors. J Neurooncol. 2007. 82: 63-8
23. McDermott M, Meningiomas WC, Youmans J.editorsNeurological Surgery. Philadelphia, PA: WB Saunders; 1996. p. 2782-825
24. Moazzam AA, Wagle N, Zada G. Recent developments in chemotherapy for meningiomas: A review. Neurosurg Focus. 2013. 35: E18
25. Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neurooncol. 2000. 50: 1-15
26. Pistolesi S, Boldrini L, Gisfredi S, de Ieso K, Camacci T, Caniglia M. Angiogenesis in intracranial meningiomas: Immunohistochemical and molecular study. Neuropathol Appl Neurobiol. 2004. 30: 118-25
27. Pistolesi S, Fontanini G, Camacci T, de Ieso K, Boldrini L, Lupi G. Meningioma-associated brain oedema: The role of angiogenic factors and pial blood supply. J Neurooncol. 2002. 60: 159-64
28. Platt SR. Angiogenesis and cerebral neoplasia. Vet Comp Oncol. 2005. 3: 123-38
29. Reszec J, Hermanowicz A, Rutkowski R, Turek G, Mariak Z, Chyczewski L. Expression of MMP-9 and VEGF in meningiomas and their correlation with peritumoral brain edema. Biomed Res Int. 2015. 2015: 646853
30. Risau W. Mechanisms of angiogenesis. Nature. 1997. 386: 671-4
31. Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001. 23: 291-9
32. Sakuma T, Nakagawa T, Ido K, Takeuchi H, Sato K, Kubota T. Expression of vascular endothelial growth factor-A and mRNA stability factor HuR in human meningiomas. J Neurooncol. 2008. 88: 143-55
33. Salokorpi N, Yrjänä S, Tuominen H, Karttunen A, Heljasvaara R, Pihlajaniemi T. Expression of VEGF and collagen XVIII in meningiomas: Correlations with histopathological and MRI characteristics. Acta Neurochir (Wien). 2013. 155: 989-96
34. Schmid S, Aboul-Enein F, Pfisterer W, Birkner T, Stadek C, Knosp E. Vascular endothelial growth factor: The major factor for tumor neovascularization and edema formation in meningioma patients. Neurosurgery. 2010. 67: 1703-8
35. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957. 20: 22-39
36. Varghese F, Bukhari AB, Malhotra R, Abhijit D. IHC profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014. 9: e96801
37. Vranic A, Popovic M, Cör A, Prestor B, Pizem J. Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: A study of 86 patients. Neurosurgery. 2010. 67: 1124-32
38. Yamasaki F, Yoshioka H, Hama S, Sugiyama K, Arita K, Kurisu K. Recurrence of meningiomas. Cancer. 2000. 89: 1102-10