- Department of Neurosurgery, Tokyo Metropolitan Tama Medical Center, Fuchu, Tokyo, Japan.
DOI:10.25259/SNI_463_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shotaro Ogawa, Fukutaro Ohgaki, Ryosuke Mizuta, Yasuyuki Furuta, Shigeta Fujitani, So Fujimoto, Takahiro Ota. Two cases of symptomatic common carotid artery occlusion treated by carotid endarterectomy with L-shaped ministernotomy. 03-Jan-2020;11:1
How to cite this URL: Shotaro Ogawa, Fukutaro Ohgaki, Ryosuke Mizuta, Yasuyuki Furuta, Shigeta Fujitani, So Fujimoto, Takahiro Ota. Two cases of symptomatic common carotid artery occlusion treated by carotid endarterectomy with L-shaped ministernotomy. 03-Jan-2020;11:1. Available from: https://surgicalneurologyint.com/surgicalint-articles/9834/
Abstract
Background: Common carotid artery occlusion (CCAO) is rare. Symptomatic lesions are resistant to medical treatment and revascularization are often required, but there is no consensus on the treatment of CCAO. In this paper, two cases of symptomatic CCAO treated by carotid endarterectomy (CEA) with L-shaped ministernotomy, in which the lesions extended to the beginning part of the CCA, are reported.
Case Description: Case 1 involved a 74-year-old man who presented with transient left limb numbness and an abnormal right visual field. Cerebrovascular angiography showed that the right CCA was occluded immediately after its origin and blood was supplied from the posterior circulation. CEA was performed with an L-shaped ministernotomy that allowed exposure of the CCA origin with minimal invasion. There were no complications associated with the sternal incision and he was discharged with a modified Rankin Scale (mRS) score of 0. Case 2 involved a 70-year-old man who presented with left half-blindness. Magnetic resonance imaging showed infarction in the right posterior cerebral artery region and neck echo showed CCA pseudo occlusion just before the carotid bulb. A new infarction in the right middle cerebral artery region developed during hospitalization. CEA with partial sternotomy was performed. The patient was rehabilitated with no deterioration of neurological findings and transferred with an mRS score of 3.
Conclusion: There were no complications resulting from partial sternotomy in the two cases presented. CEA with partial sternotomy could be an effective treatment option for CCAO in which the internal carotid artery is patent and thrombus extends to the proximal CCA.
Keywords: Carotid endarterectomy, Common carotid artery occlusion, Ischemic cerebrovascular disease, Partial sternotomy
BACKGROUND
Common carotid artery occlusion (CCAO) is a relatively rare condition that has been reported to be identified in 1–5% of angiograms for symptomatic cerebrovascular disease.[
Carotid endarterectomy (CEA) with L-shaped ministernotomy was performed in 2 cases of symptomatic CCAO, in which the internal and external carotid artery bifurcations were patent and the lesions extended to the origin of the CCA. We described the actual surgical procedure and its effectiveness along with a literature review.
CASE DESCRIPTION
Case 1
A 74-year-old man, in 2013, asymptomatic right ICA stenosis was found and followed up, but in June 2017, progressive stenosis, transient left limb numbness, and an abnormal right visual field appeared. He had a previous history of brachytherapy and tumor excision for oral floor cancer in 2011 and 2014. He also had dyslipidemia, hyperuricemia, and smoking history. He was given aspirin 100 mg and bezafibrate 400 mg daily before and after the surgery. Magnetic resonance imaging (MRI) showed an old deep white matter infarct, but no new lesion was noted, and the right ICA-middle cerebral artery (MCA) was poorly visualized on magnetic resonance angiography (MRA). Single-photon emission computed tomography (SPECT) showed no apparent decrease in blood flow. Cerebrovascular angiograms showed that the right CCA was occluded immediately after its origin and blood was supplied from the right vertebral artery to the ICA through the external carotid artery (ECA) [
Figure 1:
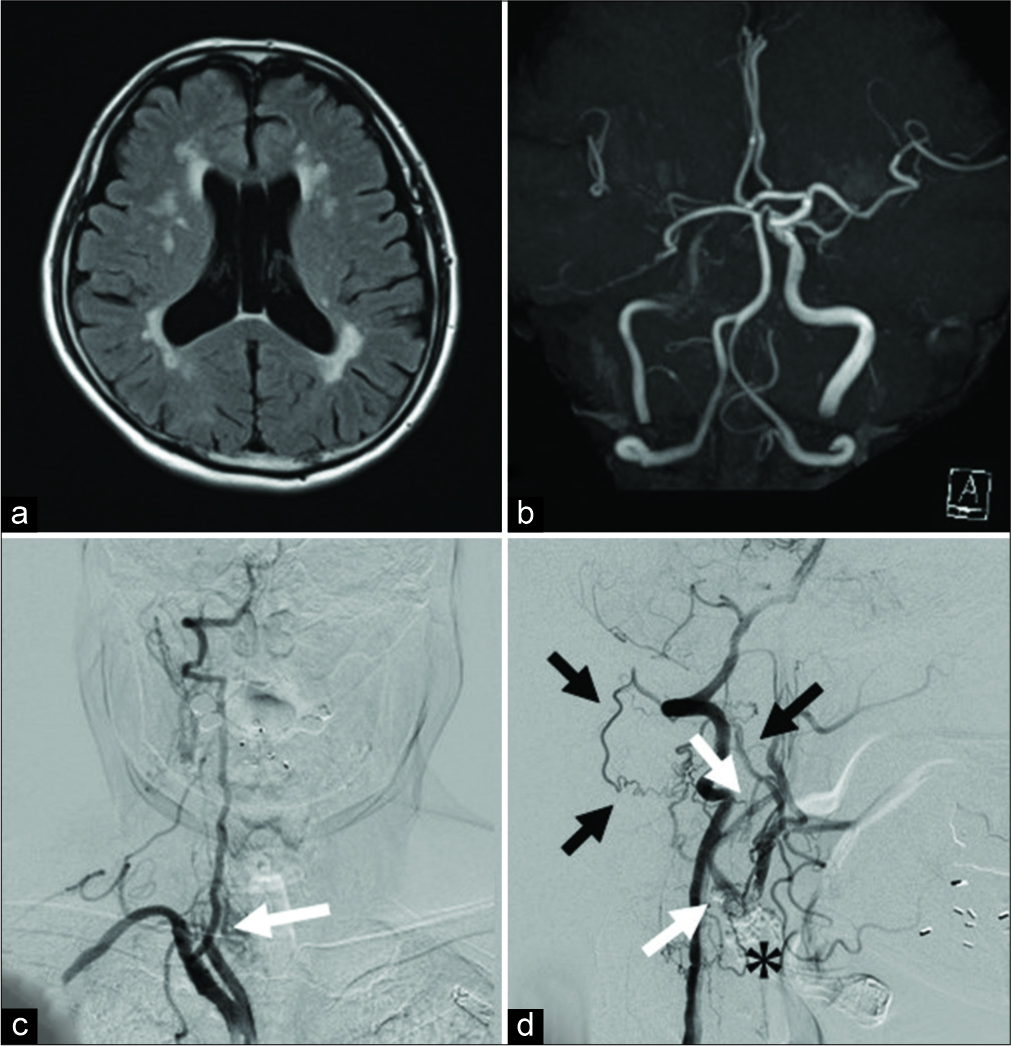
(a) MRI FLAIR image shows old cerebral infarction. (b) MRA shows a decreased blood flow signal in the right ICA and MCA. (c) The right CCA is occluded immediately after the bifurcation at the level of the clavicle. (white arrow). (d) On right vertebral artery angiography, the blood flow is retrograde from the VA segmental artery to the external carotid artery through the occipital artery (black arrow) and antegrade blood flow to the ICA through the carotid blub (*) is observed (white arrow). MRI: Magnetic resonance imaging, FLAIR: Fluid-attenuated inversion recovery, MRA: Magnetic resonance angiography, ICA: Internal carotid artery, MCA: Middle cerebral artery, ECA: External carotid artery, CCA: Common carotid artery, VA: Vertebral artery.
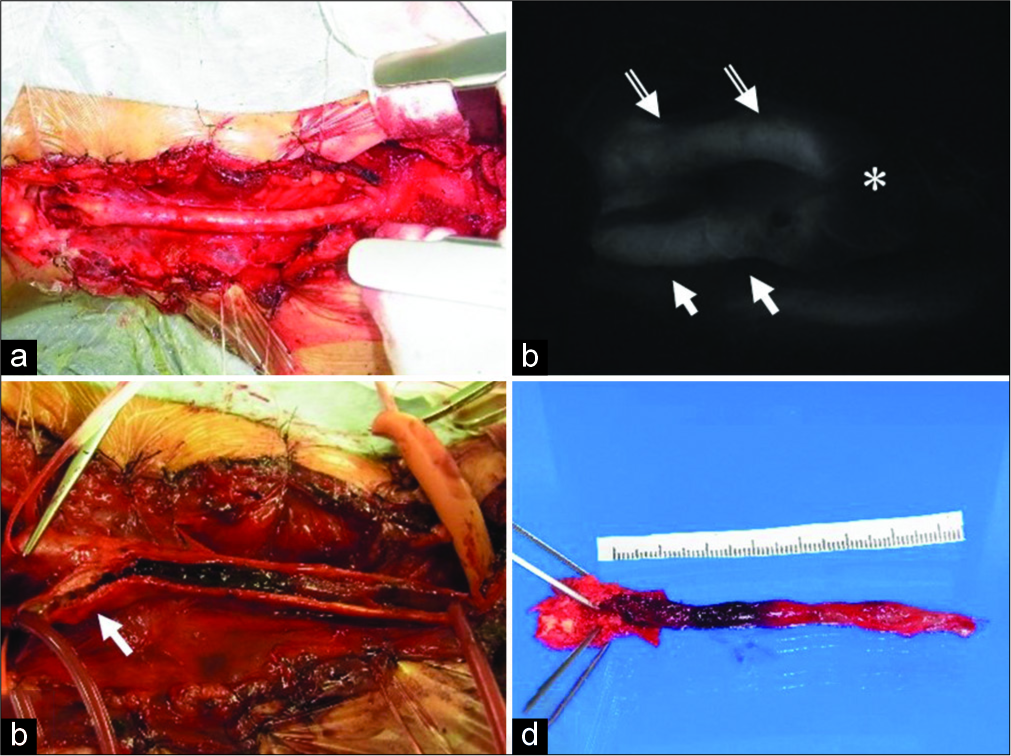
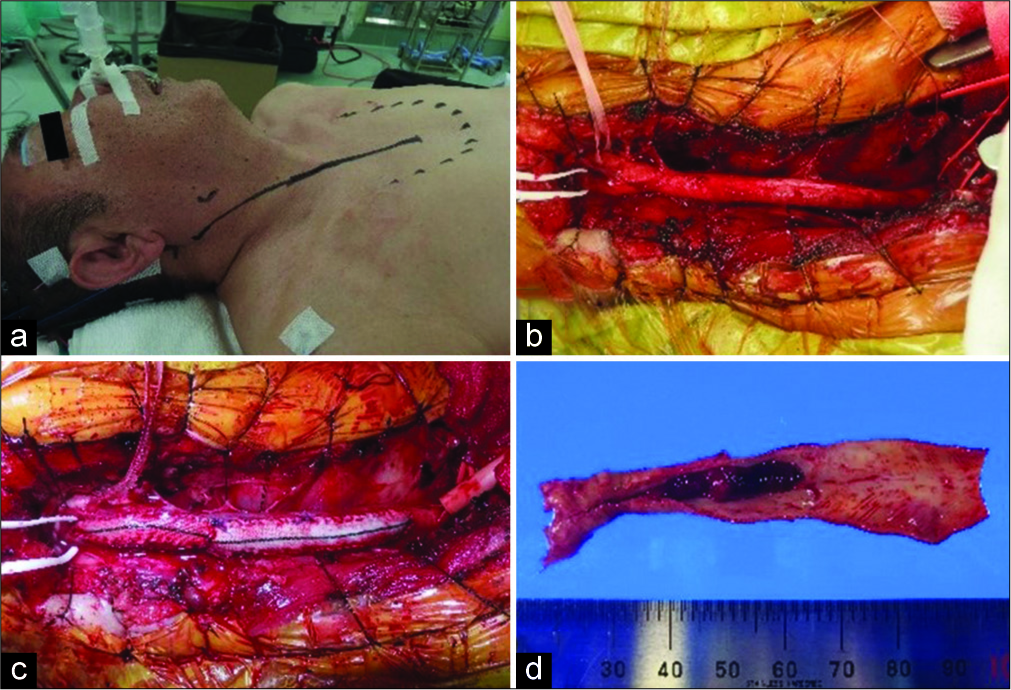
Considering the symptomatic CCA occlusion presenting with transient ischemic attack, CEA was selected as a treatment that could achieve revascularization and plaque removal at the same time. In addition, from the results of cerebral angiography, the occluded site extended to the CCA origin, and the proximal end of the thrombus was secured using a partial sternotomy. Under general anesthesia with transnasal intubation, surgery was started under intraoperative electroencephalogram monitoring. The skin incision along the front edge of the sternocleidomastoid muscle (SCM) was extended to the front chest, and the sternum was cut into an L shape from the median cervical incision to the right second intercostal space, which exposed the CCA origin. The treatment of the precordial region was performed jointly with a cardiovascular surgeon. The proximal end of the plaque was confirmed directly under intraoperative echo and indocyanine green (ICG) video-angiography and blood flow from the ECA to the ICA through the bifurcation was confirmed on ICG video-angiography. The ICA, ECA, and CCA were clamped at sites where no blood clots had occurred, an arteriotomy was placed from the CCA to the ICA, and a shunt tube was inserted. When the lumen was exposed, a long plaque from the beginning of the CCA to the ICA was observed, which was excised in one mass. A hemashield was used to repair the CCA-ICA because primary suture was considered to have a risk of postoperative stenosis. Blood flow was confirmed by Doppler echo and the sternum was fixed with a wire [
Figure 2:
(a) It was possible to expose the brachiocephalic trunk to the CCA bifurcation by cutting the sternum into an L-shaped from the midline of the jugular notch to the right second intercostal space and deploying a thoracotomy device. (b) The distal end of the plaque is confirmed by intraoperative ICG intravenous injection. The blood flow from the ECA (white double arrow) to the ICA (white arrow) through the carotid bulb (*) is confirmed. (c) An arteriotomy from the CCA to the ICA (white arrow) is placed after inserting the shunt tube. When the lumen is observed, a hard plaque near the bifurcation and a long jelly-like thrombus extending to the CCA origin are observed, (d) extracted plaque. Bright plaque with calcification is observed near the carotid bulb and proximal thrombus formation is observed. CCA: Common carotid artery, ICG: Indocyanine green, ECA: External carotid artery, ICA: Internal carotid artery.
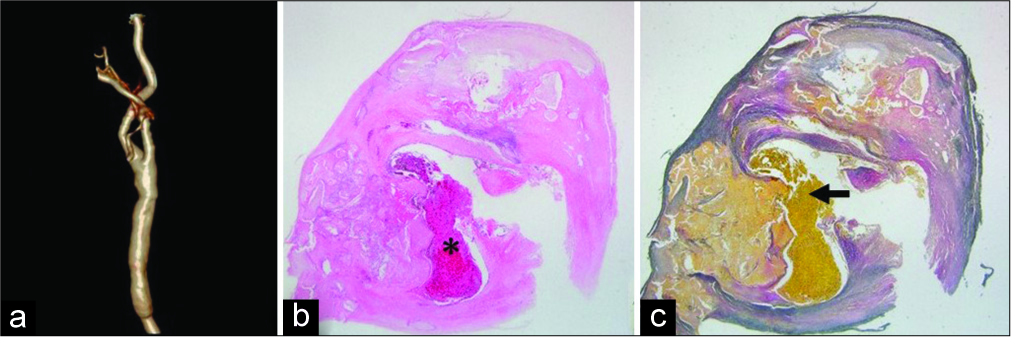
On magnetic resonance diffusion-weighted imaging after surgery, no new infarction appeared, and visualization of the main artery was improved on MRA. Computed tomography angiography confirmed good patency of the CCA. Pathological findings showed fibrous thickening and calcification of the blood vessel wall near the carotid bulb, and the lumen was narrowed. The elastic fibers in the vascular lumen were torn and ulceration was observed. Red thrombus was observed near the bifurcation, but on the central side, white thrombus rich in fiber formation was observed [
Figure 3:
(a) On 3DCTA, good patency of the vessels is confirmed. (b) HE staining. (×12.5) Fibrous thickening and calcification of the blood vessel wall are observed, and the lumen is narrowed (*). (c) EVG staining (×12.5) the elastic fiber in the lumen is torn and ulceration is observed (black arrow). The thrombus in the CCA is a red thrombus near the bifurcation, but a white thrombus rich in fiber formation is seen on the central side. CTA: Computed tomography angiography, HE staining: Hematoxylin eosin staining, EVG staining: Elastica van Gieson staining, CCA: Common carotid artery, 3DCTA: Three-dimensional computed tomography angiography.
There were no complications associated with the sternal incision and the patient was discharged on postoperative day (POD) 11 with a modified Rankin Scale (mRS) score of 0. There was no new cerebral infarction during the 18 months of follow-up after surgery and no CCA restenosis was observed.
Case 2
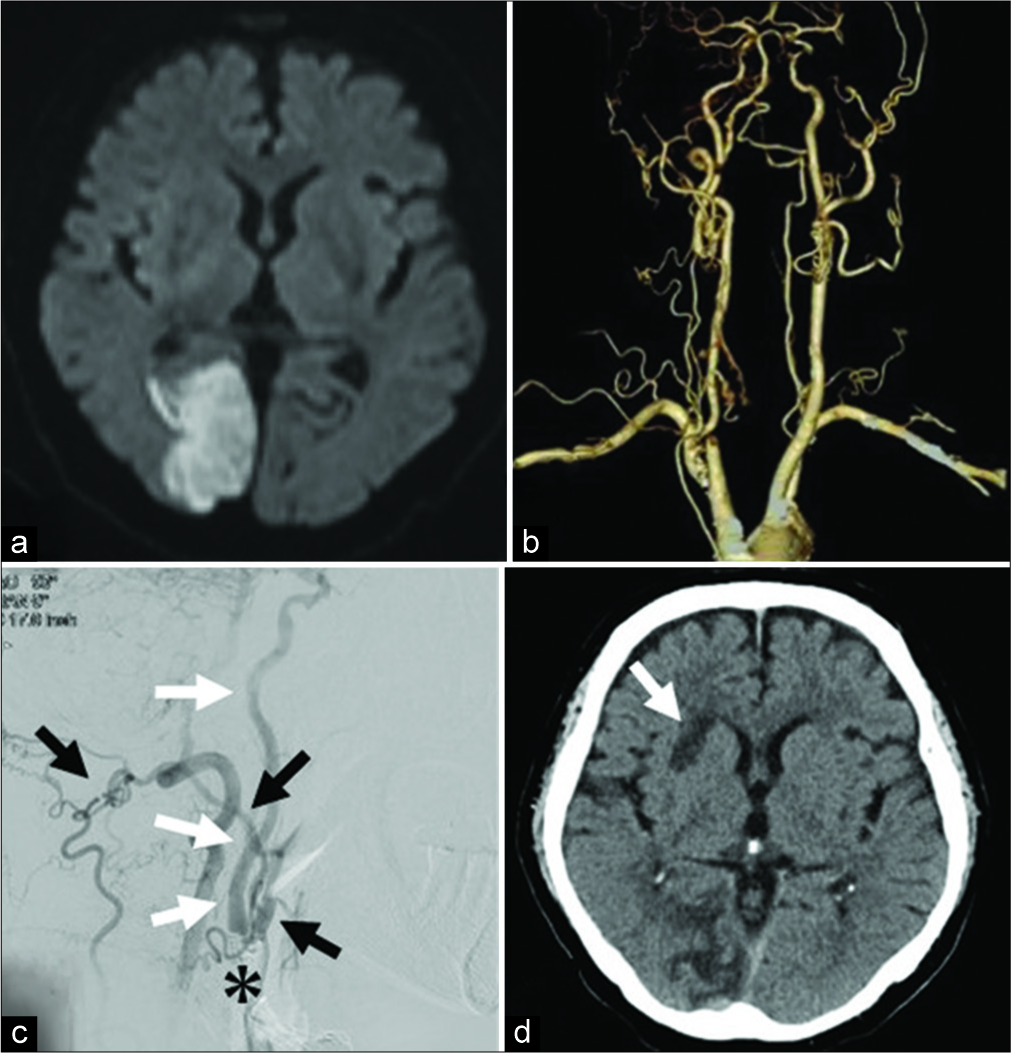
A 70-year-old man, the patient had a history of angina pectoris, glaucoma (blind right eye), hypertension, dyslipidemia, and smoking 20 cigarettes/day for 30 years. He was given aspirin 81 mg, atorvastatin 10 mg, and ethyl icosapentate 1800 mg daily perioperatively. A new infarction in the right posterior cerebral artery region was observed on MRI, which had resulted in left half-blindness. Although the main artery was preserved on MRA, the neck echo showed CCA pseudo-occlusion just before the carotid bulb and low-echo plaque in the lumen. SPECT showed no apparent decrease in blood flow. Cerebrovascular angiography, as in Case 1, showed that the CCA was occluded immediately after branching from the brachiocephalic trunk, and blood was supplied from the posterior circulation to the ICA via the ECA. A new infarction in the right MCA region developed during hospitalization and was considered symptomatic CCA occlusion [
Figure 4:
(a) MRI at the first visit shows a new infarction in the right PCA region. (b) On CTA, the right CCA is occluded immediately after branching from the brachiocephalic trunk and the appearance beyond the carotid bulb is maintained. (c) On angiography, the carotid bulb (*) is patent as in Case 1 and the ICA (white arrow) is visualized from the VA through the ECA (black arrow). (d) A new infarction in the right basal ganglia (white arrow) has developed during the examination, which was considered to be artery to artery embolization from the CCA occlusion site. MRI: Magnetic resonance imaging, PCA: Posterior cerebral artery, CTA: Computed tomography angiography, CCA: Common carotid artery, ICA: Internal carotid artery, VA: Vertebral artery, ECA: External carotid artery.
That was classified as large vessel atherothrombotic stroke in TOAST classification because the patient was not at risk for cardiogenic infarction and had no lab abnormality strongly suggestive of other etiologies such as hypercoagulable state.[
CEA with an L-shaped ministernotomy was performed. As in Case 1, the plaque was removed as a lump by exposing the CCA origin to the ICA. Postoperative MRI showed no new infarction and no complications resulting from partial sternotomy were observed [
Figure 5:
(a) Intraoperative electroencephalogram monitoring is set up under general anesthesia with nasal intubation. A skin incision of about 20 cm extends from the front edge of the SCM to the front chest. (b) As in Case 1, the right CCA origin is exposed by cutting the sternum partially and deploying a thoracotomy device. The CCA, ICA, and ECA are each secured with vascular tape. (c) A vessel wall is reconstructed using two hemashield patches so that the vessel does not become narrowed by suturing, (d) extracted plaque. Long-distance plaque formation is observed. It is also accompanied by luminal thrombus formation. SCM: Sternocleidomastoid muscle, CCA: Common carotid artery, ICA: Internal carotid artery, ECA: External carotid artery.
After initial rehabilitation, the patient was transferred on POD 22 with no deterioration of neurological findings (mRS score 3) for further rehabilitation. Two months after the operation, the patient was discharged home, and at 3-month follow-up, there was no recurrence of cerebral infarction or CCA restenosis, and his mRS score was 1.
DISCUSSION
We reported a novel surgical procedure, the modified CEA with an L-shaped ministernotomy for symptomatic CCAO in two cases. CCAO is a relatively rare condition,[
For surgical treatment, it is necessary to determine the reconstruction method based on the presence or absence of involvement of the ICA and ECA. Riles et al. classified the CCAO into four types: [
The advantage of CEA for CCAO is that plaque can be removed and revascularization achieved at the same time. If artery to artery embolization is suspected, as in this case, there is a possibility of recurrence of cerebral infarction with only bypass surgery, and CEA seems to be particularly appropriate. In bypass surgery, anastomoses from the subclavian artery or axillary artery to the ICA or CCA through venous grafts are often performed, but the procedure is complicated, and there are reports of restenosis of the anastomosis portion.[
There are several points of concern for performing CEA. First, the cervical ICA must be patent and is limited to Type 1A or Type 1C in the Riles classification. The ICA patency rate in CCAO varies in reports (11–73%)[
Second, the proximal end of the plaque can be secured in the operative field using ICG video-angiography during surgery, and it was possible to confirm the position of the proximal and distal ends of the plaque in the present cases. Previous reports of CEA for CCAO were often limited to those where the thrombus did not reach the proximal portion of the CCA. Although there are reports of retrograde CEA using a ring stripper that does not require confirmation of the proximal end, there are many problems, such as perforation and dissection of the brachiocephalic trunk and aorta, and early restenosis,[
In the present cases, partial sternotomy was performed to allow exposure of the CCA bifurcation from the brachiocephalic trunk with minimal invasion. This technique has been widely accepted as a partial upper hemisternotomy or L-shaped ministernotomy in the field of thoracic surgery,[
In the present two cases, there were no complications due to partial sternotomy, but this technique requires a long skin incision from the neck to the front chest, and there is a risk of bleeding and postoperative pain associated with the sternal incision. The invasiveness of this approach is clearly greater than that of normal CEA. When it is assumed that the normal CEA cannot secure the proximal end of the thrombus in the CCA, CEA combined with partial sternotomy is effective, and appropriate case selection by sufficient imaging evaluation before surgery is important.
CONCLUSION
We performed CEA with an L-shaped ministernotomy for symptomatic CCAO. The two reported cases had a good postoperative course and there were no complications resulting from partial sternotomy. CEA combined with partial sternotomy could be an effective treatment option for CCAO, in which the thrombus extends to the proximal CCA.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993. 24: 35-41
2. Aguiar ET, Lederman A, Matsunaga P. Ring-stripping retrograde common carotid endarterectomy: Case report. Sao Paulo Med J. 2002. 120: 154-7
3. Belczak S, Mulatti GC, Abrão SR, da Silva ES, Aun R, Puech-Leão P. Common carotid artery occlusion: A single-center experience in 40 cases. Int J Angiol. 2016. 25: 39-43
4. Belkin M, Mackey WC, Pessin MS, Caplan LR, O’Donnell TF. Common carotid artery occlusion with patent internal and external carotid arteries: Diagnosis and surgical management. J Vasc Surg. 1993. 17: 1019-27
5. Collice M, D’Angelo V, Areno O. Surgical treatment of common carotid occlusion. Neurosurgery. 1983. 12: 515-24
6. Fry WR, Martin JD, Clagett GP, Fry WJ. Extrathoracic carotid reconstruction: The subclavian-carotid artery bypass. J Vasc Surg. 1992. 15: 83-8
7. Fukuda H, Yamana N, Suzuki T, Andou M, Fukumitsu R, Iwasaki K. Low flow bypass using radial artery interposition graft for symptomatic chronic common carotid artery occlusion. Surg Cereb Stroke (Jpn). 2014. 42: 427-31
8. Gilmanov D, Bevilacqua S, Murzi M, Cerillo AG, Gasbarri T, Kallushi E. Minimally invasive and conventional aortic valve replacement: A propensity score analysis. Ann Thorac Surg. 2013. 96: 837-43
9. Hashimoto Y, Kin S, Haraguchi K, Niwa J. Two symptomatic cases of common carotid artery occlusion. Jpn J Neurosurg. 2005. 14: 347-51
10. Hass WK, Fields WS, North RR, Kircheff II, Chase NE, Bauer RB. Joint study of extracranial arterial occlusion. II. Arteriography, techniques, sites, and complications. JAMA. 1968. 203: 961-8
11. Hasumi T, Hoshi F, Kawamura M, Saito Y. L-shaped mini-sternotomy combined with a supraclavicular approach for resection of a cervico-mediastinal tumor: Three case reports. Jpn J Chest Surg. 2018. 25: 589-94
12. Inoue T, Tsutsumi K, Adachi S, Tanaka S, Kunii N, Indo M. Direct and primary carotid endarterectomy for common carotid artery occlusion. Report of 2 cases. Surg Neurol. 2008. 69: 620-6
13. Kader I, Jones SM, Harrison C, Miteff F, Kumar S. Common carotid artery occlusion presenting with recurrent syncopal episodes. Neurol Int. 2016. 8: 6822-
14. Klonaris C, Kouvelos GN, Kafeza M, Koutsoumpelis A, Katsargyris A, Tsigris C. Common carotid artery occlusion treatment: Revealing a gap in the current guidelines. Eur J Vasc Endovasc Surg. 2013. 46: 291-8
15. Linni K, Aspalter M, Ugurluoglu A, Hölzenbein T. Proximal common carotid artery lesions: Endovascular and open repair. Eur J Vasc Endovasc Surg. 2011. 41: 728-34
16. Nagasawa S, Tanaka H, Kawanishi M, Ohta T. Contralateral external carotid-to-external carotid artery (half-collar) saphenous vein graft for common carotid artery occlusion. Surg Neurol. 1996. 45: 138-41
17. Pintér L, Cagiannos C, Bakoyiannis CN, Kolvenbach R. Hybrid treatment of common carotid artery occlusion with ring-stripper endarterectomy plus stenting. J Vasc Surg. 2007. 46: 135-9
18. Riles TS, Imparato AM, Posner MP, Eikelboom BC. Common carotid occlusion. Assessment of the distal vessels. Ann Surg. 1984. 199: 363-6
19. Semsroth S, Matteucci Gothe R, Raith YR, de Brabandere K, Hanspeter E, Kilo J. Comparison of Two Minimally Invasive Techniques and Median Sternotomy in Aortic Valve Replacement. Ann Thorac Surg. 2017. 104: 877-83