- Department of Neurosurgery, Division of Pediatric Neurosurgery, Baylor College of Medicine/Texas Children’s Hospital, Houston, Texas,
- Department of Neurosurgery, Division of Pediatric Neurosurgery, Northwestern University Feinberg School of Medicine/Ann and Robert H. Lurie Children’s Hospital, Chicago, IL, USA.
Correspondence Address:
Sandi K. Lam
Department of Neurosurgery, Division of Pediatric Neurosurgery, Northwestern University Feinberg School of Medicine/Ann and Robert H. Lurie Children’s Hospital, Chicago, IL, USA.
DOI:10.25259/SNI_417_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nisha Gadgil, Melissa A. LoPresti, Matthew Muir, Jeffrey M. Treiber, Marc Prablek, Patrick J. Karas, Sandi K. Lam. An update on pediatric surgical epilepsy: Part I. 27-Dec-2019;10:257
How to cite this URL: Nisha Gadgil, Melissa A. LoPresti, Matthew Muir, Jeffrey M. Treiber, Marc Prablek, Patrick J. Karas, Sandi K. Lam. An update on pediatric surgical epilepsy: Part I. 27-Dec-2019;10:257. Available from: https://surgicalneurologyint.com/surgicalint-articles/9821/
Abstract
Epilepsy affects many children worldwide, with drug-resistant epilepsy affecting 20–40% of all children with epilepsy. This carries a significant burden for patients and their families and is strongly correlated with poor cognitive outcomes, depression, anxiety, developmental delay, and impaired activities of daily living. For this reason, we sought to explore the role of pediatric epilepsy surgery and provide an overview of the factors contributing to epilepsy surgery planning and execution. We review the necessary preoperative evaluations, surgical indications, planning considerations, and surgical options to provide a clear pathway in the evaluation and planning of pediatric epilepsy surgery.
Keywords: Drug-resistant epilepsy, Epilepsy surgery, Pediatric
INTRODUCTION
Epilepsy is estimated to affect 10.5 million children worldwide.[
Surgical treatment of DRE has been shown to be safer and more efficacious compared to medical management.[
With advances in technology, there are now multiple indications for the different types of surgery to address pediatric epilepsy. We describe, in Part I, practices and advances in diagnostic workup and surgical strategies.
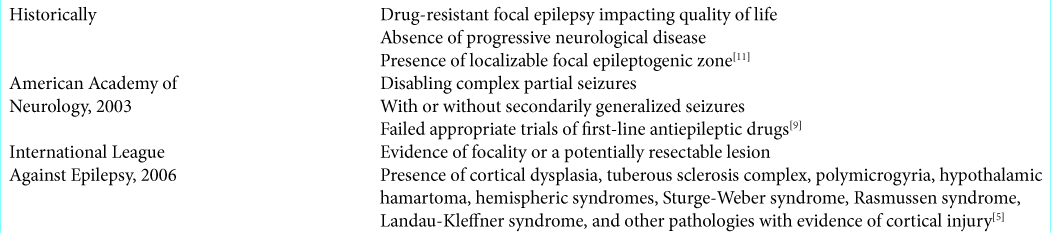
SURGICAL INDICATIONS
Surgical indications have evolved overtime to encompass a wider variety of epilepsy types, applying epilepsy surgery to more patients.
SURGICAL PLANNING
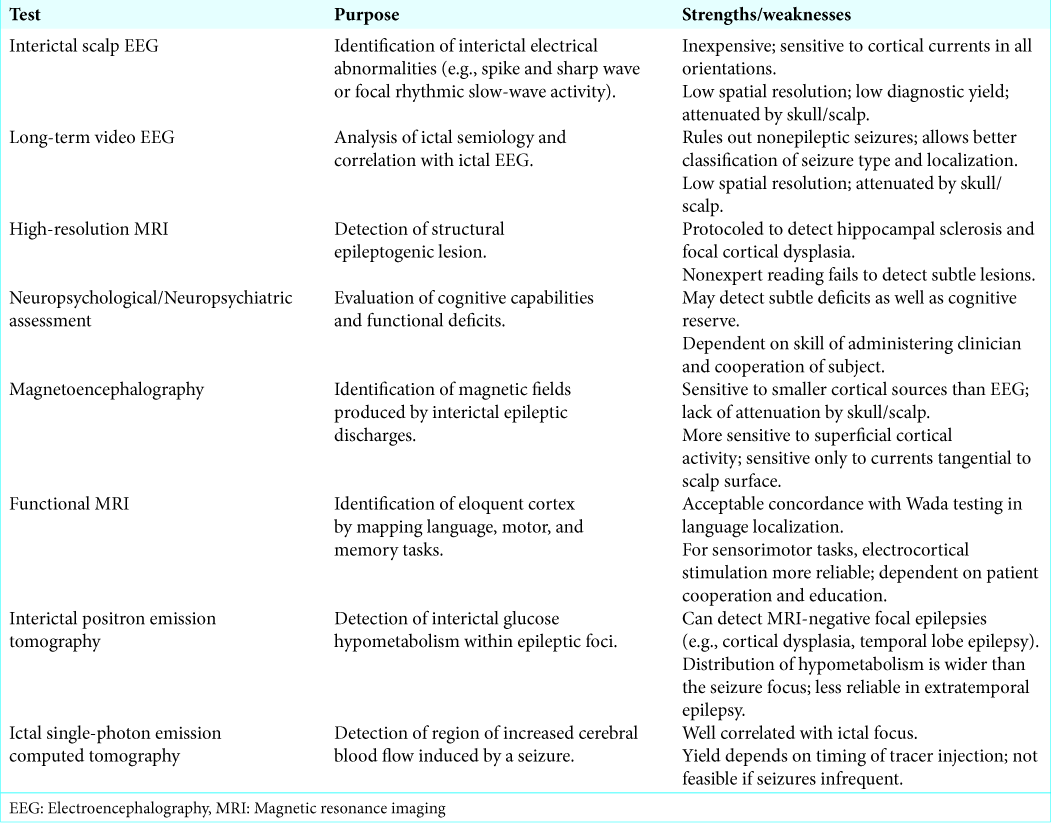
Presurgical evaluation identifies the EZ, correlating it with function. Stepwise evaluation should include a detailed clinical history, interictal scalp electroencephalography (EEG), long-term video EEG, high-resolution structural magnetic resonance imaging (MRI), and neuropsychological/ neuropsychiatric assessment[
Where the EZ cannot be characterized with noninvasive testing, or noninvasive testing yields contradictory results, Phase 2 assessment utilizing intracranial EEG monitoring may be pursued. Implantation of subdural grids and depth electrodes allows more accurate localization of the EZ than scalp EEG. Functional zones may be identified through cortical stimulation mapping. However, invasive electrocorticography may carry a complication rate of up to 20% (e.g., intracranial hematoma).[
For those who are not candidates or have failed surgery, vagus nerve stimulation may palliatively reduce seizures by 50–75%.[
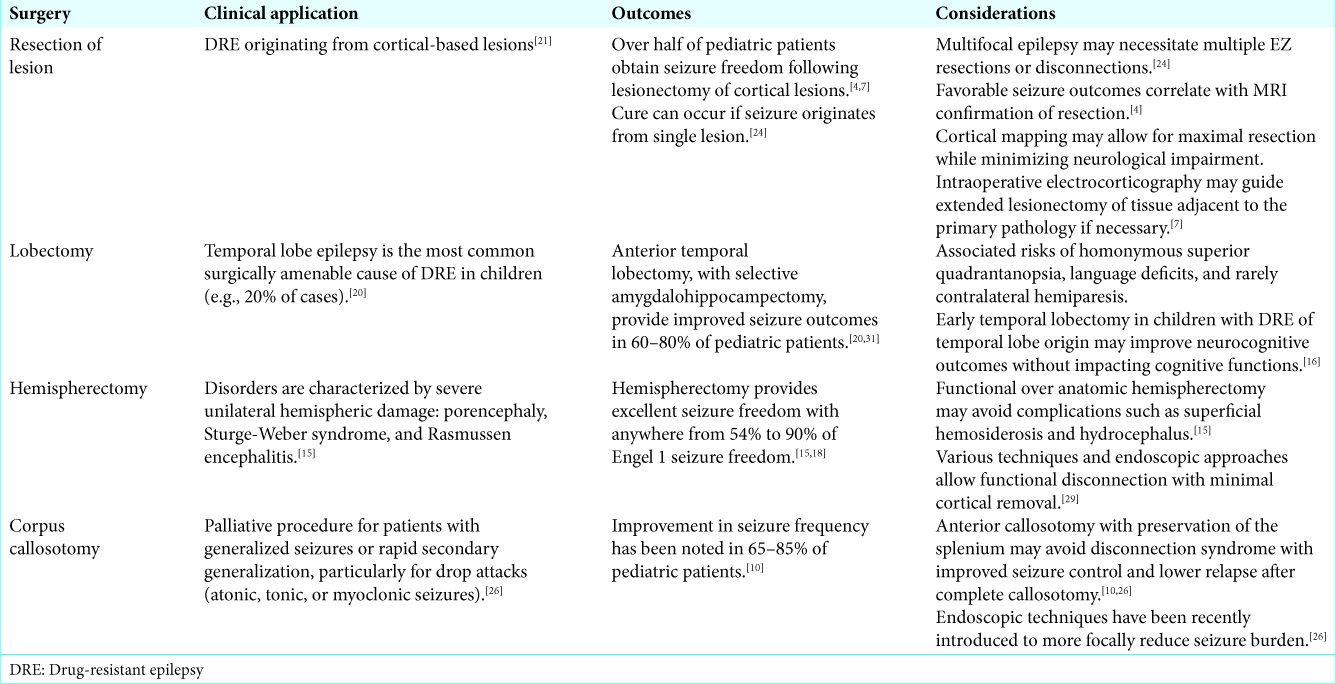
SURGICAL OPTIONS
Several surgical options exist based on the seizure type, lesion type, size and location, and EZ characteristics. Lesionectomy is favored for singular cortical-based lesions and can be curative. Lobectomy is used for more focal lesions and proven superior in cases of temporal lobe epilepsy over medical management (Class I evidence).[
SURGICAL CONSIDERATIONS IN PEDIATRICS
Pediatric epilepsy is more diverse in etiology and semiology with migrational disorders, congenital epileptic syndromes, and extratemporal epileptogenic foci more common in children. Therefore, cortical excisions and hemispherectomies are perhaps more common than temporal lobectomies in the pediatric population versus adults. In addition, DRE impacts neurodevelopment in children. Early surgical intervention limits the time on intolerable medications, minimizes cognitive delays and learning disabilities, and improves psychomotor development.[
IDENTIFICATION OF CANDIDATES FOR EPILEPSY SURGERY
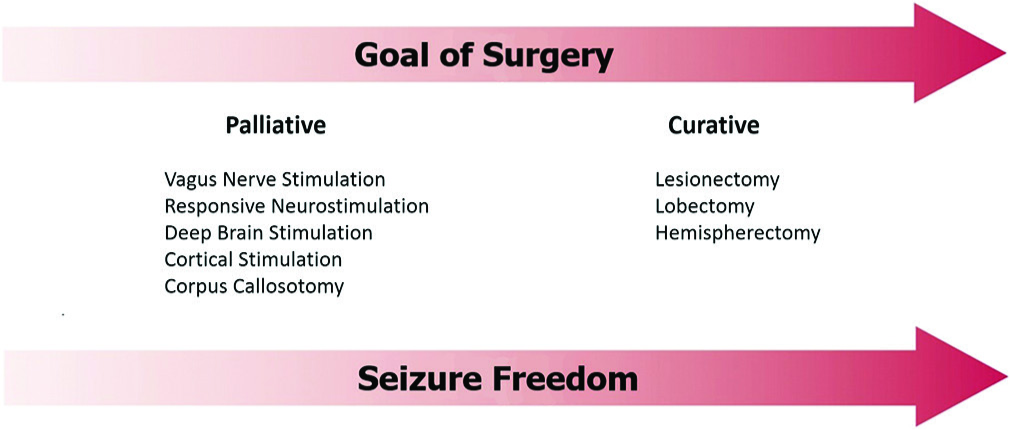
It is critical to identify the best candidates for epilepsy surgery. The goals include cure or palliation and may warrant a variety of open versus stereotactic techniques [
Despite the growing appreciation for the deleterious developmental and psychosocial effects of pediatric DRE, there are too few surgical referrals,[
CONCLUSION
Here, we reviewed, summarized, and synthesized important practices and advances in diagnostic workup and surgical strategies of epilepsy surgery. Future increased awareness of the role of epilepsy surgery in children with DRE is critical to increase the breadth of impact.
References
1. Asarnow RF, LoPresti C, Guthrie D, Elliott T, Cynn V, Shields WD. Developmental outcomes in children receiving resection surgery for medically intractable infantile spasms. Dev Med Child Neurol. 1997. 39: 430-40
2. Benbadis SR, Geller E, Ryvlin P, Schachter S, Wheless J, Doyle W. Putting it all together: Options for intractable epilepsy: An updated algorithm on the use of epilepsy surgery and neurostimulation. Epilepsy Behav. 2018. 88S: 33-8
3. Berg AT. Identification of pharmacoresistant epilepsy. Neurol Clin. 2009. 27: 1003-13
4. Choi SA, Kim SY, Kim H, Kim WJ, Kim H, Hwang H. Surgical outcome and predictive factors of epilepsy surgery in pediatric isolated focal cortical dysplasia. Epilepsy Res. 2018. 139: 54-9
5. Cross JH, Jayakar P, Nordli D, Delalande O, Duchowny M, Wieser HG. Proposed criteria for referral and evaluation of children for epilepsy surgery: Recommendations of the subcommission for pediatric epilepsy surgery. Epilepsia. 2006. 47: 952-9
6. Desai VR, Vedantam A, Lam SK, Mirea L, Foldes ST, Curry DJ. Language lateralization with resting-state and task-based functional MRI in pediatric epilepsy. J Neurosurg Pediatr. 2018. 23: 171-7
7. Dhiman V, Rao S, Sinha S, Arimappamagan A, Mahadevan A, Bharath RD. Outcome of lesionectomy in medically refractory epilepsy due to non-mesial temporal sclerosis (non-MTS) lesions. Clin Neurol Neurosurg. 2013. 115: 2445-53
8. Engel J Jr. The current place of epilepsy surgery. Curr Opin Neurol. 2018. 31: 192-7
9. Engel J, Wiebe S, French J, Sperling M, Williamson P, Spencer D. Practice parameter: Temporal lobe and localized neocortical resections for epilepsy: Report of the quality standards subcommittee of the American academy of neurology, in association with the American epilepsy society and the American. Epilepsia. 2003. 44: 741-51
10. Graham D, Tisdall MM, Gill D. Corpus callosotomy outcomes in pediatric patients: A systematic review. Epilepsia. 2016. 57: 1053-68
11. Green RC, Adler JR, Erba G. Review article: Epilepsy surgery in children. J Child Neurol. 1988. 3: 155-66
12. Guerrini R. Epilepsy in children. Lancet. 2006. 367: 499-524
13. Jonas R, Nguyen S, Hu B, Asarnow RF, LoPresti C, Curtiss S. Cerebral hemispherectomy: Hospital course, seizure, developmental, language, and motor outcomes. Neurology. 2004. 62: 1712-21
14. Karsy M, Guan J, Ducis K, Bollo RJ. Emerging surgical therapies in the treatment of pediatric epilepsy. Transl Pediatr. 2016. 5: 67-78
15. Kim JS, Park EK, Shim KW, Kim DS. Hemispherotomy and functional hemispherectomy: Indications and outcomes. J Epilepsy Res. 2018. 8: 1-5
16. Lee YJ, Kang HC, Kim HD, Kim DS, Shim KW, Eom S. Neurocognitive function in children after anterior temporal lobectomy with amygdalohippocampectomy. Pediatr Neurol. 2015. 52: 88-93
17. Lüders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W. The epileptogenic zone: General principles. Epileptic Disord. 2006. 8: S1-9
18. Otsuki T, Yoshimoto T. Surgical treatment of intractable epilepsy in children: Indication for resective surgery. Epilepsia. 2000. 41: 26-7
19. Papanicolaou AC, Rezaie R, Narayana S, Choudhri AF, Babajani-Feremi A, Boop FA. On the relative merits of invasive and non-invasive pre-surgical brain mapping: New tools in ablative epilepsy surgery. Epilepsy Res. 2018. 142: 153-5
20. Radhakrishnan A, Menon R, Abraham M, Vilanilam G, Sharma S, Thomas B. Predictors of outcome after surgery in 134 children with drug-resistant TLE. Epilepsy Res. 2018. 139: 150-6
21. Ramírez-Molina JL, Di Giacomo R, Mariani V, Deleo F, Cardinale F, Uscátegui-Daccarett AM. Surgical outcomes in two different age groups with Focal Cortical Dysplasia Type II: Any real difference?. Epilepsy Behav. 2017. 70: 45-9
22. Rathore C, Radhakrishnan K. Concept of epilepsy surgery and presurgical evaluation. Epileptic Disord. 2015. 17: 19-31
23. Ravindra VM, Sweney MT, Bollo RJ. Recent developments in the surgical management of paediatric epilepsy. Arch Dis Child. 2017. 102: 760-6
24. Roth J, Carlson C, Devinsky O, Harter DH, Macallister WS, Weiner HL. Safety of staged epilepsy surgery in children. Neurosurgery. 2014. 74: 154-62
25. Shen A, Quaid KT, Porter BE. Delay in pediatric epilepsy surgery: A caregiver’s perspective. Epilepsy Behav. 2018. 78: 175-8
26. Smyth MD, Vellimana AK, Asano E, Sood S. Corpus callosotomy-Open and endoscopic surgical techniques. Epilepsia. 2017. 58: 73-9
27. Sugano H, Arai H. Epilepsy surgery for pediatric epilepsy: Optimal timing of surgical intervention. Neurol Med Chir (Tokyo). 2015. 55: 399-406
28. Tanganelli P, Ferrero S, Colotto P, Regesta G. Vagus nerve stimulation for treatment of medically intractable seizures. Evaluation of long-term outcome. Clin Neurol Neurosurg. 2002. 105: 9-13
29. Taugher B, Richards M. Functional hemispherectomy. Axone. 1992. 14: 29-32
30. Vale FL, Pollock G, Dionisio J, Benbadis SR, Tatum WO. Outcome and complications of chronically implanted subdural electrodes for the treatment of medically resistant epilepsy. Clin Neurol Neurosurg. 2013. 115: 985-90
31. Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001. 345: 311-8