- Department of Anesthesiology and Critical Care Ben Arous, University of Tunis El Manar, Tunisia.
- Department of Neurosurgery, Traumatology and Severe Burns Center, Ben Arous, University of Tunis El Manar, Tunisia.
DOI:10.25259/SNI_177_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lotfi Rebai1, Nahed Mahfoudhi1, Nizar Fitouhi1, Mohamed Aziz Daghmouri1, Kamel Bahri2. Intraoperative tranexamic acid use in patients undergoing excision of intracranial meningioma: Randomized, placebo-controlled trial. 14-Jun-2021;12:289
How to cite this URL: Lotfi Rebai1, Nahed Mahfoudhi1, Nizar Fitouhi1, Mohamed Aziz Daghmouri1, Kamel Bahri2. Intraoperative tranexamic acid use in patients undergoing excision of intracranial meningioma: Randomized, placebo-controlled trial. 14-Jun-2021;12:289. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10878
Abstract
Background: Intracranial meningioma resection is associated with substantial intraoperative bleeding. Intraoperative tranexamic acid (TXA) use can reduce bleeding in a variety of surgical procedures. The objective of this study was to evaluate the effects of TXA treatment on blood loss and transfusion requirements in patient undergoing resection of intracranial meningioma.
Methods: We conducted a prospective, randomized double-blind clinical study. The patient scheduled to undergo excision of intracranial meningioma were randomly assigned to receive intraoperatively either intravenous TXA or placebo. Patients in the TXA group received intravenous bolus of 20 mg/kg over 20 min followed by an infusion of 1 mg/kg/h up to surgical wound closure. Efficacy was evaluated based on total blood loss and transfusion requirements. Postoperatively, thrombotic complications, convulsive seizure, and hematoma formation were noted.
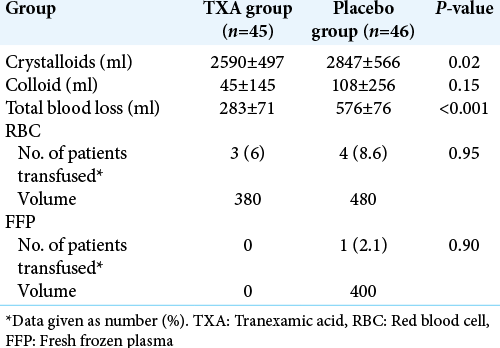
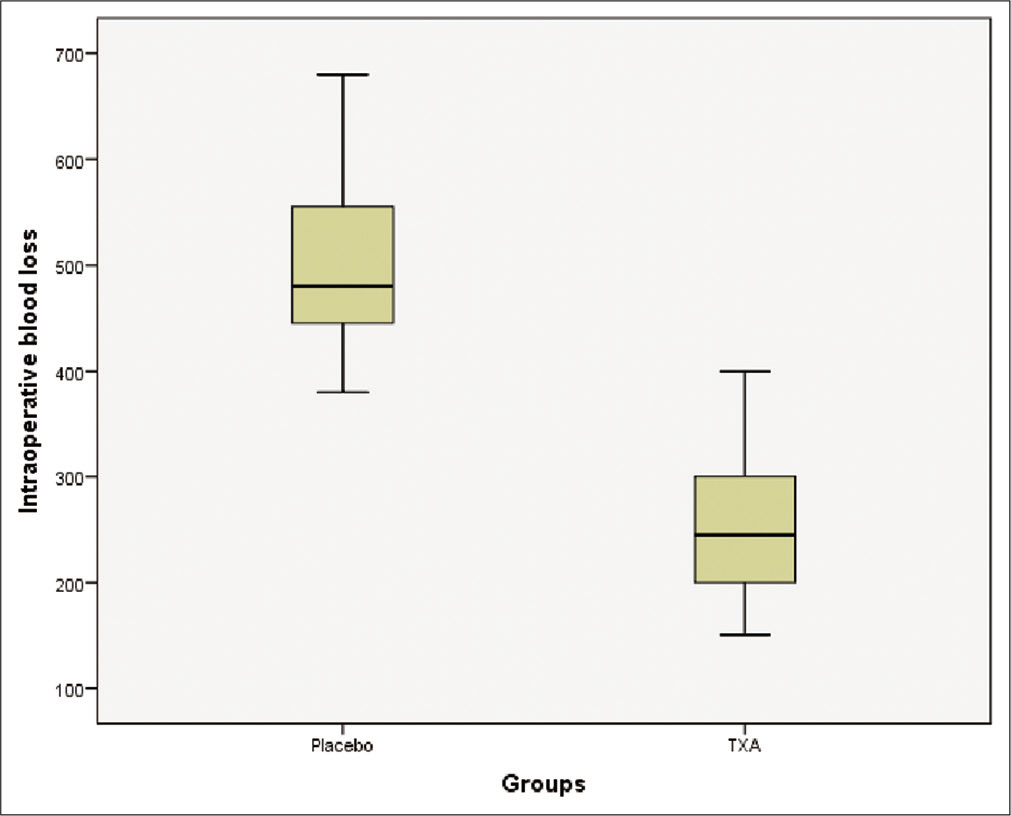
Results: Ninety-one patients were enrolled and randomized: 45 received TXA (TXA group) and 46 received placebo (group placebo). Total blood loss was significantly decreased in TXA group compared to placebo (283 ml vs. 576 ml; P P = 0.95). The incidence of thrombotic complications, convulsive seizure, and hematoma formation was similar in the two groups.
Conclusion: TXA significantly reduces intraoperative blood loss, but did not significantly reduced transfusion requirements in adults undergoing resection of intracranial meningioma.
Keywords: Blood loss, Blood transfusion, Intracranial meningioma, Intraoperative, Tranexamic acid
INTRODUCTION
Intracranial meningioma accounts for 20–30% of brain tumors.[
Tranexamic acid (TXA) is a synthetic derivative of amino acid lysine that reversibly blocks plasminogen conversion to plasmin and inhibiting fibrin clot dissolution.[
The primary objective of this study was to evaluate the effects of TXA treatment on blood loss and transfusion requirements in patient undergoing resection of intracranial meningioma. The second objective was to evaluate the effects of TXA on perioperative complications and neurological outcome.
METHODS
Study design
This was a single-center, prospective, double-blind, placebo-controlled, randomized study conducted between April 2017 and December 2018 at the Department of Neurosurgery, Traumatology and Severe Burns Center, Tunisia.
Sample size calculation
Sample size calculation was based on our hypothesis that TXA reduces total blood loss compared with placebo. Average blood loss during intracranial meningioma surgery in the previous studies ranged from 340 mL to 1100 mL.[
Study population
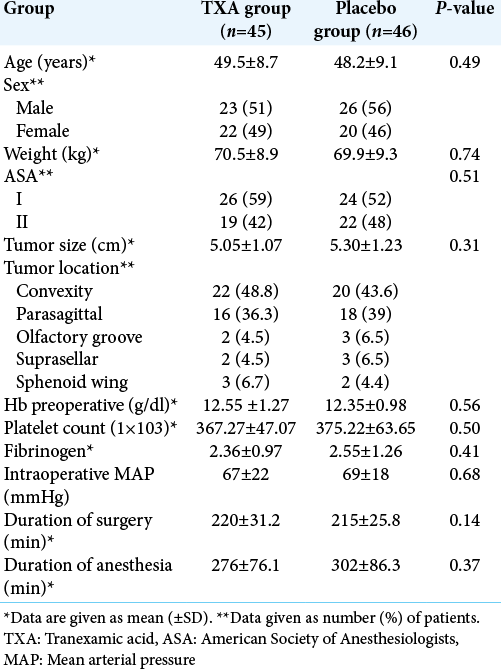
This protocol was approved by the Ethical and Scientific Committee of Traumatology and Severe Burns Center, Tunisia (ESCTSBC/2017/R02). After obtaining written informed consent, 110 consecutive American Society of Anesthesiology Grade I and II patients, in the age group of 18 and 70 years, of either sex undergoing resection of intracranial meningioma were enrolled for the study. The exclusion criteria were as follows: patients who refused to participate in the study, history of allergy to TXA, previous thromboembolic episode, previous seizures, preexisting renal or hepatic disorders, abnormal coagulation parameters (abnormal prothrombin time [PT] or platelet counts), clinical history of bleeding diathesis, current treatment with oral anticoagulant or antiplatelet agents, and pregnant or lactating mothers. In addition, patients planned for preoperative embolization were also not enrolled. All patients were operated by a neurosurgeon with at least 5 years of experience. Demographic data and tumor size were noted. Preoperative laboratory values (baseline value of hemoglobin [Hb], PT, platelets count and fibrinogen) were noted.
Randomization and treatment groups
The patients were randomized to receive TXA group or normal saline (placebo group) using a computer-generated random sequence. The choice of TXA dose was based on study by Hooda et al.[
TXA group: Bolus dose of 20 mg/kg (0.5 ml/kg) was administered for 20 min before the surgical incision followed by perfusion of 1 mg/kg/hup to surgical wound closure at completion of surgery.
Placebo group: A saline placebo was administered according to the same TXA group infusion protocol.
Anesthetic management
On admission to the operating room, the patients were placed in the supine position and monitored by cardiac electrical activity, noninvasive blood pressure measurement, and pulse oximetry, with the placement of a peripheral venous catheter at the upper limb. A BIS monitor has been installed if the location of the tumor is not frontal. After verifying the vital constants of the patient, the anesthetic induction was done with remifentanil (0.5 µg/kg over 3 min), propofol (2.5 mg/kg) and cisatracurium (0.15 mg/kg). After intubation, mechanical ventilation was adjusted to have the end-tidal carbon dioxide (EtCO2) between 30 and 35 mmHg. Anesthesia was maintained with remifentanil (0.1–0.4 µg/kg/min), propofol (4 mg/kg/h), and cisatracurium (0.12 mg/kg/h). Intraoperative sedation monitoring is performed by the BIS with intraoperative objectives between 40 and 60. Invasive monitoring of blood pressure was performed by a radial catheter. A subclavian central venous catheter was placed. The temperature was controlled within a range of 36–37°C. Duration of surgery and anesthesia was noted. The record of HR, MAP, SpO2, urine output, and temperature was done every hour.
Measurements of blood loss, fluid therapy, and transfusion strategy
Intraoperatively, the estimation of blood loss is done by the principal investigator and the surgeon without being aware of the treatment given to the patient. Blood loss was determined from the surgical suction bottle (after subtracting the amount of irrigation fluid), soaked sponges, cotton pledges, and the volume of blood in surgical drapes. This measure was done every hour.
Postoperative blood loss corresponded blood volume collected by suction drains at 48 h. Total bleeding was the sum of intra- and postoperative blood loss.
Fluid therapy consisted of administration isotonic crystalloids and colloids guided by monitoring mean arterial pressure and urine output.
Perioperative, transfusion trigger for red blood cells was an Hb concentration <8 g/dl. Fresh frozen plasma was transfused at PT< 60% and platelet concentrate was transfused at platelet count <100,000/mm3.
Postoperative care
All the patients were transferred to the neurosurgical intensive care unit. After monitoring, laboratory investigations were performed (Hb, electrolytes, PT, fibrinogen, and platelet amount) and the decision of extubate or elective ventilation was based on the assessment of the consultant anesthesiologist. Cerebral computed tomography was performed to evaluate tumor resection and hematoma formation. Side effects, such as thrombotic complications or convulsive seizure, were noted during hospitalization and a month later. The Extended Glasgow Outcome Scale (GOSE) was evaluated at the time of discharge of patient from the hospital and was categorized as good recovery (GOSE 7–8), moderate disability (GOSE 5–6), and severe disability (GOSE 1–4). The duration of ICU and hospital stay was recorded.
Primary outcome
The primary outcome was set as the rate of intraoperative hemorrhage. The amount of estimated intraoperative blood loss (in ml), the amount of transfusion (in ml), and postoperative level of hemoglobulin (in g/dl) were recorded.
Statistical analysis
Statistical analysis was performed with SPSS statistical software version 25. The Kolmogorov–Smirnov statistic was used for testing normality for continuous variables. Continuous variables were expressed as mean SD and compared using Student’s t-test or the Mann–Whitney U-test for nonnormal distribution variables. Categorical variables were compared using the Chi-square or Fisher’s test. All statistical analyses were carried out at 5% level of significance and P < 0.05 was considered as statistically significant.
RESULTS
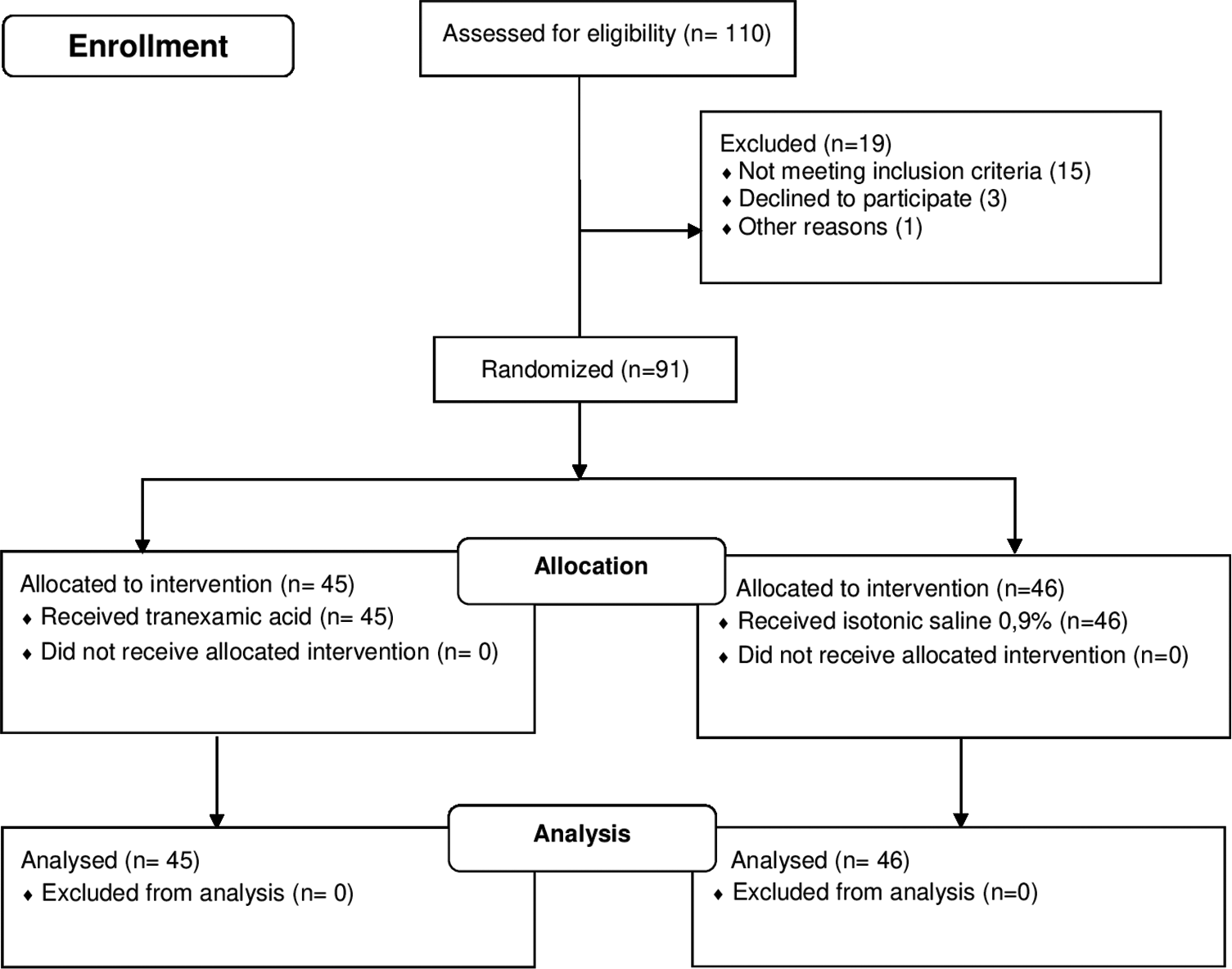
In total, 110 patients were evaluated during the period of study; 91 defined inclusion criteria, were retained in the final sample, and randomized into two groups to receive either TXA or saline (45 in TXA group and 46 in placebo group) [
Demographics
Demographic, anesthesia, and surgery characteristics are summarized in [
Intraoperative blood loss and intravenous fluid management
Intraoperative blood loss, transfusion requirements, and intraoperative fluid management are summarized in [
Postoperative laboratory parameters and complications
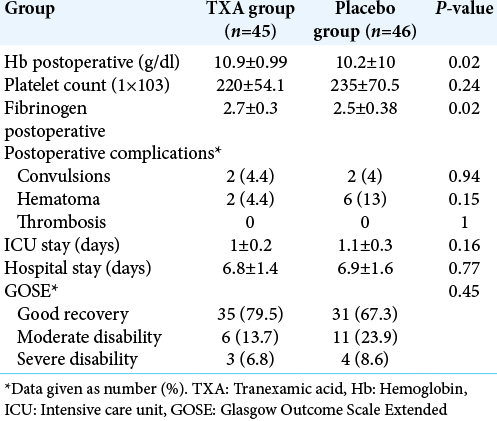
During the postoperative period, Hb count shows statistical difference between the two groups (P = 0.02) but platelet count was comparable between the two groups (P = 0.24). However, the fibrinogen levels were significantly higher in TXA group compared to placebo group (P = 0.02). Incidence of postoperative complications was comparable in the groups. The patient outcome was not affected by intraoperative use of TXA and none of the patient developed side effects. Duration of ICU and hospital stay was also similar in the two groups [
DISCUSSION
In this randomized, double-blind, placebo-controlled study, we demonstrated that the use of TXA resulted in a significant reduction in perioperative bleeding in patient undergoing excision of intracranial meningioma (P < 0.001). There was a mean reduction of blood loss of 293 ml (49%) with the use of TXA as compared to placebo. Nevertheless, we found no difference between the two groups (TXA versus placebo) regarding transfusion requirements (P = 0.95).
It is known that intraoperative hemorrhage in neurosurgery, specifically during resection of intracranial meningioma, often requires large volume of blood products transfusions. Oka et al.[
To reduce blood loss and transfusion requirements, several techniques have been suggested and investigated but none of these strategies are free of complications.[
In our study, the dose of TXA administered was 20 mg/kg for 20 min before surgical incision followed by perfusion of 1 mg/ kg/h until closure of the skin. This same protocol was used in the study by Hooda et al.[
In our study, postoperatively, the decrease in Hb was greater in the control group but the rest of the biological parameters including platelet count, TP, and fibrinemia, did not show a statistically significant difference between the two groups. These results are consistent with Hooda et al. study.[
The evaluation of the safety of TXA in our study was essential, especially since it was already a relatively high risk surgery for postoperative complications. Convulsions have been described particularly in the use of high-dose TXA in cardiac surgery.[
In our study, no thromboembolic event was noted in all patients, both groups included. This result has been found in similar studies.[
In our series, there was no significant difference between the two groups in the length of stay in intensive care or the total duration of hospitalization. Moreover, as for the risk of occurrence of postoperative hematoma, the number of patients who had this complication was lower in the TXA group (only one patient versus four), but the difference between the two groups was considered statistically no significant (P = 0.6), and this is due to the reduction of intraoperative bleeding and consequently a better local hemostasis. Our results were similar to the studies cited above.[
To the best of our knowledge, our study is the largest clinical trial which evaluating the efficacy of TXA treatment on blood loss and transfusion requirements in patient undergoing resection of intracranial meningioma. Our study has some limitations. The most important is related to the estimation of blood loss by the anesthesiologist; it was a dependent operator parameter and therefore subject to a margin of error. Another important limitation is the dose of TXA which was arbitrary according to the studies conducted in traumatic brain injury.
CONCLUSION
The current study demonstrates that TXA significantly decreases intraoperative and total blood loss, but no significant reduction in blood transfusion in the TXA group compared with placebo, in patients undergoing resection of intracranial meningioma. The rate of thrombotic complications and convulsion in the TXA group was similar to that observed in patients receiving placebo, with a tendency to reduce the incidence of postoperative hematoma.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adler Ma SC, Brindle W, Burton G, Gallacher S, Hong FC, Manelius I. Tranexamic acid is associated with less blood transfusion in off-pump coronary artery bypass graft surgery: A systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2011. 25: 26-35
2. Anker-Møller T, Troldborg A, Sunde N, Hvas AM. Evidence for the use of tranexamic acid in subarachnoid and subdural hemorrhage: A systematic review. Semin Thromb Hemost. 2017. 43: 750-8
3. Chen L, Li D, Lu Y, Hao B, Cao Y. Preoperative embolization versus direct surgery of meningiomas: A meta-analysis. World Neurosurg. 2019. 128: 62-8
4. Shakur H, Roberts I, Bautista R, Caballero J, Coats T. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet. 2010. 376: 23-32
5. Dahmani S, Orliaguet GA, Meyer PG, Blanot S, Renier D, Carli PA. Perioperative blood salvage during surgical correction of craniosynostosis in infants. Br J Anaesth. 2000. 85: 550-5
6. Dai Z, Chu H, Wang S, Liang Y. The effect of tranexamic acid to reduce blood loss and transfusion on off-pump coronary artery bypass surgery: A systematic review and cumulative meta-analysis. J Clin Anesth. 2018. 44: 23-31
7. Deva AK, Hopper RA, Landecker A, Flores R, Weiner H, McCarthy JG. The use of intraoperative autotransfusion during cranial vault remodeling for craniosynostosis. Plast Reconstr Surg. 2002. 109: 58-63
8. Dewan Y, Komolafe EO, Mejía-Mantilla JH, Perel P, Roberts I, Shakur H. CRASH-3-tranexamic acid for the treatment of significant traumatic brain injury: Study protocol for an international randomized, double-blind, placebo-controlled trial. Trials. 2012. 13: 87
9. Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, El-Dawlatly A. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: A prospective, randomized, double-blind, placebo-controlled study. Spine. 2008. 33: 2577-80
10. Fearon JA, Weinthal J. The use of recombinant erythropoietin in the reduction of blood transfusion rates in craniosynostosis repair in infants and children. Plast Reconstr Surg. 2002. 109: 2190-6
11. Furtmüller R, Schlag MG, Berger M, Hopf R, Huck S, Sieghart W. Tranexamic acid, a widely used antifibrinolytic agent, causes convulsions by a gamma-aminobutyric acid (A) receptor antagonistic effect. J Pharmacol Exp Ther. 2002. 301: 168-73
12. Hooda B, Chouhan RS, Rath GP, Bithal PK, Suri A, Lamsal R. Effect of tranexamic acid on intraoperative blood loss and transfusion requirements in patients undergoing excision of intracranial meningioma. J Clin Neurosci. 2017. 41: 132-8
13. Jennings JD, Solarz MK, Haydel C. Application of tranexamic acid in trauma and orthopedic surgery. Orthop Clin North Am. 2016. 47: 137-43
14. Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ. 2012. 344: e3054
15. Kietpeerakool C, Supoken A, Laopaiboon M, Lumbiganon P. Effectiveness of tranexamic acid in reducing blood loss during cytoreductive surgery for advanced ovarian cancer. Cochrane Database Syst Rev. 2016. 2016: CD011732
16. Li C, Gong Y, Dong L, Xie B, Dai Z. Is prophylactic tranexamic acid administration effective and safe for postpartum hemorrhage prevention? : A systematic review and meta-analysis. Medicine (Baltimore). 2017. 96: e5653
17. MacGillivray RG, Tarabichi SB, Hawari MF, Raoof NT. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: A prospective, randomized double blind study. J Arthroplasty. 2011. 26: 24-8
18. Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010. 110: 350-3
19. Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017. 376: 136-48
20. Nikolaou VS, Masouros P, Floros T, Chronopoulos E, Skertsou M, Babis GC. Single dose of tranexamic acid effectively reduces blood loss and transfusion rates in elderly patients undergoing surgery for hip fracture: A randomized controlled trial. Bone Joint J. 2021. 103-B: 442-8
21. Oka K, Tsuda H, Kamikaseda K, Nakamura R, Fukui M, Nouzuka Y. Meningiomas and hemorrhagic diathesis. J Neurosurg. 1988. 69: 356-60
22. Riffaud L, Mazzon A, Haegelen C, Hamlat A, Morandi X. Surgery for intracranial meningiomas after 80 years. Presse Méd. 2007. 36: 197-202
23. Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L. The CRASH-2 trial: A randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013. 17: 1-79
24. Saraf S, McCarthy BJ, Villano JL. Update on meningiomas. Oncologist. 2011. 16: 1604-13
25. Sun Q, Li J, Chen J, Zheng C, Liu C, Jia Y. Comparison of intravenous, topical or combined routes of tranexamic acid administration in patients undergoing total knee and hip arthroplasty: A meta-analysis of randomised controlled trials. BMJ Open. 2019. 9: e024350
26. Tsuda H, Oka K, Noutsuka Y, Sueishi K. Tissue-type plasminogen activator in patients with intracranial meningiomas. Thromb Haemost. 1988. 60: 508-13
27. Vel R, Udupi B, Prakash MS, Adinarayanan S, Mishra S, Babu L. Effect of low dose tranexamic acid on intra-operative blood loss in neurosurgical patients. Saudi J Anaesth. 2015. 9: 42-8
28. Weng S, Wang W, Wei Q, Lan H, Su J, Xu Y. Effect of tranexamic acid in patients with traumatic brain injury: A systematic review and meta-analysis. World Neurosurg. 2019. 123: 128-35
29. 30. Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P. Tranexamic acid in hip fracture surgery: A randomized controlled trial. Br J Anaesth. 2010. 104: 23-30