- Department of Neurosurgery, Policlinico Riuniti Hospital, Foggia,
- Department of Neurosurgery, Giovanni XXIII Hospital, Bari,
- Department of Neurosurgery, University of Foggia, Foggia, Puglia, Italy.
Correspondence Address:
Francesco Carbone, Department of Neurosurgery, University of Foggia, Foggia, Puglia, Italy.
DOI:10.25259/SNI_1134_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Antonio Colamaria1, Maria Blagia2, Francesco Carbone3, Nicola Pio Fochi3. Blast-related traumatic brain injury: Report of a severe case and review of the literature. 15-Apr-2022;13:151
How to cite this URL: Antonio Colamaria1, Maria Blagia2, Francesco Carbone3, Nicola Pio Fochi3. Blast-related traumatic brain injury: Report of a severe case and review of the literature. 15-Apr-2022;13:151. Available from: https://surgicalneurologyint.com/surgicalint-articles/11532/
Abstract
Background: Traumatic brain injury (TBI) is a well-known brain dysfunction commonly encountered in activities such as military combat or collision sports. The etiopathology can vary depending on the context and bomb explosions are becoming increasingly common in war zones, urban terrorist attacks, and civilian criminal feuds. Blast-related TBI may cause the full severity range of neurotrauma, from a mild concussion to severe, penetrating injury. Recent classifications of the pathophysiological mechanisms comprise five factors that reflect the gravity of the experienced trauma and suggest to the clinician different pathways of injury and consequent pathology caused by the explosion.
Case Description: In the present report, the authors describe a case of 26 years old presenting with blast-related severe TBI caused by the detonation of an explosive in an amusement arcade. Surgical decompression to control intracranial pressure and systemic antibiotic treatment to manage and prevent wound infections were the main options available in a civilian hospital.
Conclusion: While numerous studies examined the burden of blast-related brain injuries on service members, few papers have tackled this problem in a civilian setting, where hospitals are not sufficiently equipped, and physicians lack the necessary training. The present case demonstrates the urgent need for evidence-based diagnostic and therapeutic protocols in civilian hospitals that would improve the outcome of such patients.
Keywords: Blast, Civil population, Neurotrauma, Severe traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is characterized by an acute brain injury that occurs as a result of external forces applied to the head. It is estimated that more than 5% of military personnel deployed to Iraq and Afghanistan have suffered from TBI and have exhibited long-term impairment of both cognitive and psychological functions.[
In the present report, a case of 26 years old with blast-related severe TBI presenting with a left frontoparietal fracture and multiple intraparenchymal foreign bodies is described. The management of such cases still poses various challenges, especially in nonmilitary hospitals, where physicians lack sufficient expertise and training.
CASE REPORT
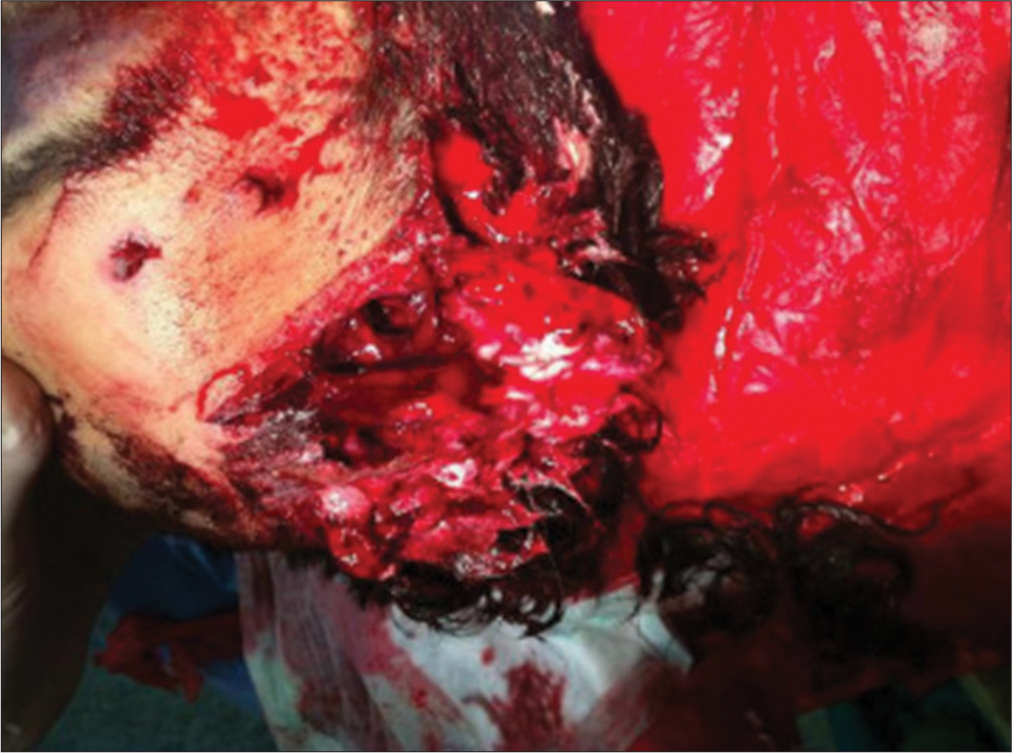
A 26-year-old man was found unconscious in an amusement arcade following the detonation of an unspecified explosive. On his initial assessment, the emergency physician found a Glasgow Coma Scale (GCS) score of 3, a left frontoparietal lacerated and contused wound extending to the frontosphenoidal suture [
Figure 2:
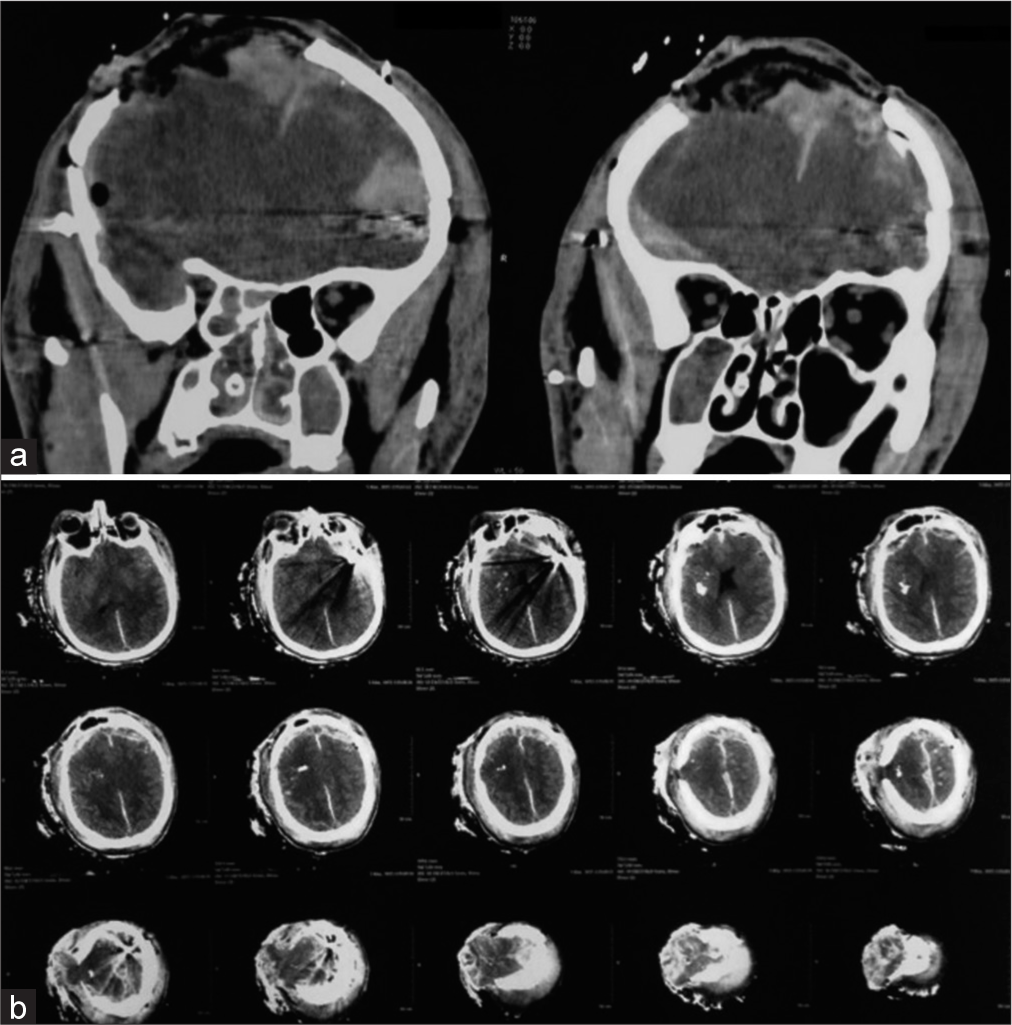
Preoperative CT scans of the head demonstrating (a) a right parietal fracture with underlying frontoparietal pneumocephalus and (b) bilateral multifragmentary burst fractures of the skull accompanied by the presence of intracerebral foreign bodies exhibiting metal density and causing interhemispheric and subarachnoid hemorrhage.
Figure 3:
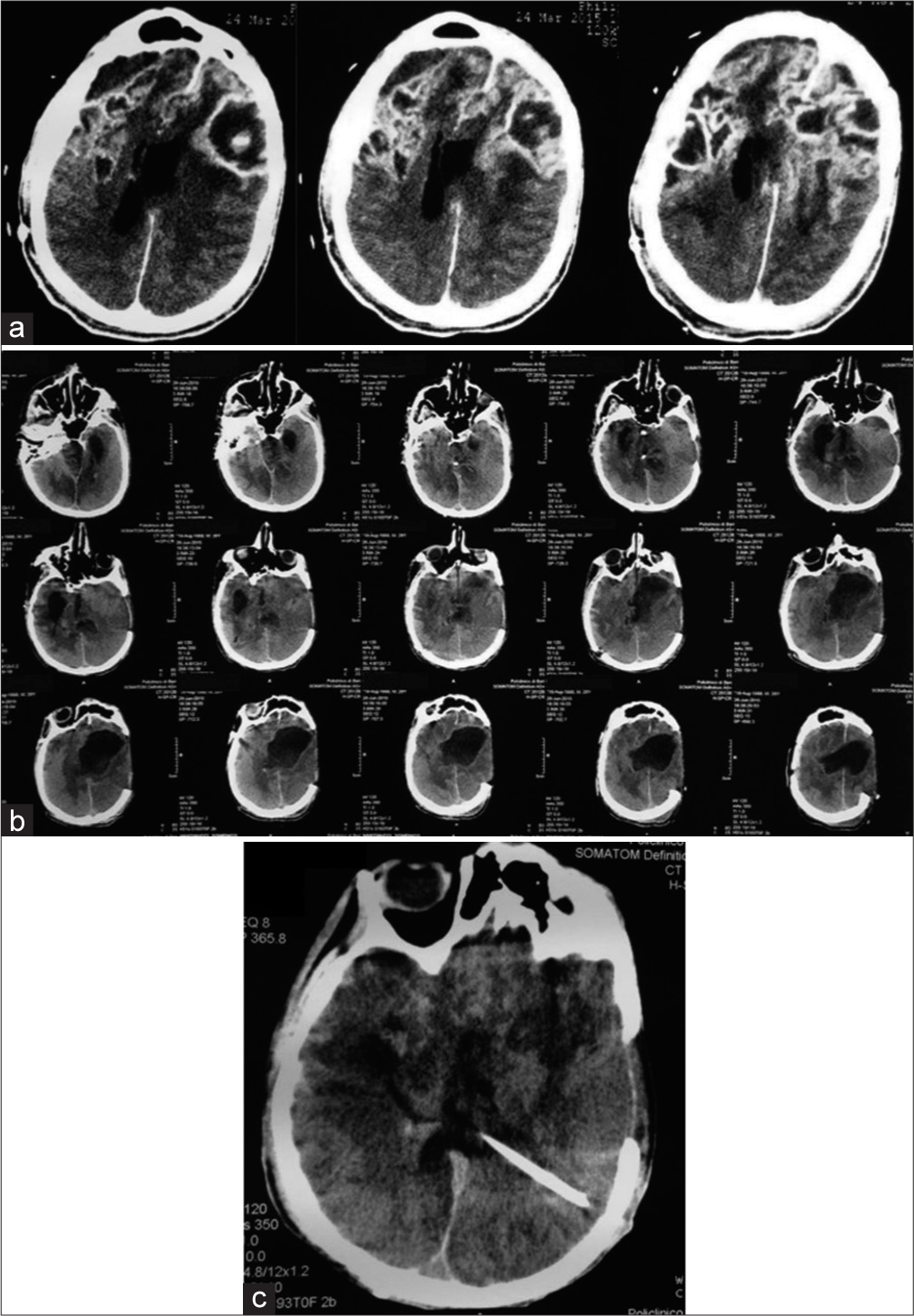
Following the first surgery, the patient developed signs of infection, and a control CT scan of the head showed (a) evidence of abscess formation and wound dehiscence and (b) obstructive hydrocephalus. (c) Subsequent surgical treatment consisted of an enlargement of the first craniotomy and positioning of a ventriculoperitoneal shunt.
DISCUSSION
Definition of a complex entity
TBI is a physical and psychological injury that has been considered a major public health concern for decades, largely associated with motor vehicle crashes, accidental falls, and sports collisions.[
Extent of the burden
In a recent article, Phipps et al.[
Pathophysiology and clinical aspects of blast-related TBI
Recent classifications of blast-related TBI comprise five factors that reflect the gravity of the experienced trauma and suggest to the clinician various mechanisms of injury and consequent pathology caused by the explosion. The majority of deceases are not to be associated with the explosion’s blast wave alone, which most frequently causes ear damage, pulmonary trauma, and concussions. For instance, penetrating injuries are associated with 70% of moderate-to-severe blast-induced TBIs and are extensively more invalidating, as a consequence of the wide variety of fragments propelled by the detonation that can lead to fatal outcomes. Further analysis reveals also tertiary mechanisms that comprise bone fractures, traumatic amputations, and collapsing of buildings that result in crushing of the body. Moreover, quaternary injuries may result from burns, asphyxia, and exposure to toxic inhalants usually contained within the explosive.[
Blast-related mTBI is reported to be associated with impairments in sensory and neurocognitive functions, which tend to be most prominent immediately after the injury and usually diminish 3 months after the accident.[
Blast-related severe TBI
As for blast-related severe TBI, this condition is characterized by cerebral edema, intracranial hemorrhage, delayed vasospasm, and pseudoaneurysm formation.[
Clinical management of blast-related severe TBI includes the assessment of the airway, breathing, and circulation and the GCS scores. The patient should undergo a CT scan as soon as possible to evaluate possible brain injuries, such as skull fractures, intracranial hemorrhage, or cerebral edema.
Attention to secondary brain injuries, being SBP < 90 mmHg or SpO2 < 92%, should be paid during prehospital management, as they both appear to increase mortality. Along with these, preintubation in patients with a GCS score <9 is not recommended, as it may result in excessive overventilation, consequent to transient hypoxia.[
Adequate oxygenation in the hospital setting, functional cerebral perfusion, and control of ICP are vital to avoid further damage, given that intracranial hypertension is a common finding in severe TBI.
Blood in the basilar cisterns might suggest a higher severity of the lesion, whereas a subarachnoid hemorrhage, revealed with the CT scan, usually leads to hyperemia and severe edema in the acute period.[
Vascular damage, unexplained variations of the neurological status, or sudden alteration observed by ICU monitoring imply the need for angiography or transcranial Doppler, due to the high risk of pseudoaneurysms formation and cerebral vasospasm.[
Early decompressive craniotomy has been a major factor in the improvement of survival and outcomes for patients with these severe injuries. Extensive unilateral or bilateral decompressive craniotomies are often needed to treat gross brain swelling and increased ICP. Cranioplasty could represent a viable option after the craniotomy; however, a latency of 3 months is recommended following evidence of infection. Ventriculostomy or lumbar drain can be performed if there is evidence of concomitant CSF leaks. Besides surgical treatment, hypertonic saline solutions and mild hypothermia (34–36°C) seem to have a beneficial effect on delayed intracranial hypertension.[
Summary of the present case
In the present report, a case of a 26-year-old male with blast-related severe TBI that occurred in a civilian setting is described. On his initial assessment, the patient presented in a comatose state with a left frontoparietal lacerated and contused wound, hypotension, and tachycardia. A head CT scan revealed multifragmentary burst fractures of the skull bilaterally accompanied by the presence of intracerebral foreign bodies, indicating that both primary and secondary explosion-related mechanisms had concurred in the damage. Emergency surgical treatment consisted of a left frontotemporal decompressive craniotomy with ligation and transection of the anterior third of the superior sagittal sinus and microscopic resection of intraparenchymal foreign bodies. Subsequent reoperation was needed as the patient soon exhibited signs of infection and obstructive hydrocephalus, and intravenous and intrathecal antibiotic treatment was initiated. Unfortunately, the patient showed progressive worsening of the neurological condition and died 1 month after the accident.
CONCLUSION
Blast-related TBI has come to represent a serious concern in both the military and the civilian settings. The effects of a bomb blast on the human brain depend on many factors, such as blast energy, distance from the blast, and body position at the moment of explosion, therefore, resulting in damage of variable gravity. Management of this kind of trauma may be challenging, especially for severe TBI occurring in the civilian setting. In the present report, the authors describe the case of a 26-year-old patient presenting in a comatose state with a left frontoparietal lacerated and contused wound, hypotension, and tachycardia. Notwithstanding surgical and antibiotic emergency treatment, the patient died 1 month after the accident. Therefore, it appears clear that early neurosurgical and pharmacological treatment is crucial to favor the outcome of these patients, although evidence-based treatment algorithms are still needed.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Armonda RA, Bell RS, Vo AH, Ling G, DeGraba TJ, Crandall B. Wartime traumatic cerebral vasospasm: Recent review of combat casualties. Neurosurgery. 2006. 59: 1215-25
2. Bauer D, Tung ML, Tsao JW. Mechanisms of traumatic brain injury. Semin Neurol. 2015. 35: e14-22
3. Chandra N, Sundaramurthy A, Kobeissy FH.editors. Acute pathophysiology of blast injury from biomechanics to experiments and computations: Implications on head and polytrauma. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Ch. 18. Boca Raton, FL: CRC Press/ Taylor and Francis; 2015. p.
4. de la Plata CD, Hart T, Hammond FM, Frol AB, Hudak A, Harper CR. Impact of age on long-term recovery from traumatic brain injury. Arch Phys Med Rehabil. 2008. 89: 896-903
5. DuBose JJ, Barmparas G, Inaba K, Stein DM, Scalea T, Cancio LC. Isolated severe traumatic brain injuries sustained during combat operations: Demographics, mortality outcomes, and lessons to be learned from contrasts to civilian counterparts. J Trauma. 2011. 70: 11-6
6. Goodrich GL, Flyg HM, Kirby JE, Chang CY, Martinsen GL. Mechanisms of TBI and visual consequences in military and veteran populations. Optom Vis Sci. 2013. 90: 105-12
7. Heinzelmann M, Reddy SY, French LM, Wang D, Lee H, Barr T. Military personnel with chronic symptoms following blast traumatic brain injury have differential expression of neuronal recovery and epidermal growth factor receptor genes. Front Neurol. 2014. 5: 198
8. Kontos AP, Kotwal RS, Elbin RJ, Lutz RH, Forsten RD, Benson PJ. Residual effects of combat-related mild traumatic brain injury. J Neurotrauma. 2013. 30: 680-6
9. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: A brief overview. J Head Trauma Rehabil. 2006. 21: 375-8
10. Lew HL, Pogoda TK, Baker E, Stolzmann KL, Meterko M, Cifu DX. Prevalence of dual sensory impairment and its association with traumatic brain injury and blast exposure in OEF/OIF veterans. J Head Trauma Rehabil. 2011. 26: 489-96
11. Ling G, Bandak F, Armonda R, Grant G, Ecklund J. Explosive blast neurotrauma. J Neurotrauma. 2009. 26: 815-25
12. Magone MT, Kwon E, Shin SY. Chronic visual dysfunction after blast-induced mild traumatic brain injury. J Rehabil Res Dev. 2014. 51: 71-80
13. Mu W, Catenaccio E, Lipton ML. Neuroimaging in blast-related mild traumatic brain injury. J Head Trauma Rehabil. 2017. 32: 55-69
14. Neipert L, Pastorek NJ, Troyanskaya M, Scheibel RS, Petersen NJ, Levin HS. Effect of clinical characteristics on cognitive performance in service members and veterans with histories of blast-related mild traumatic brain injury. Brain Inj. 2014. 28: 1667-74
15. Phipps H, Mondello S, Wilson A, Dittmer T, Rohde NN, Schroeder PJ. Characteristics and impact of US military blast-related mild traumatic brain injury: A systematic review. Front Neurol. 2020. 11: 559318
16. Pogoda TK, Hendricks AM, Iverson KM, Stolzmann KL, Krengel MH, Baker E. Multisensory impairment reported by veterans with and without mild traumatic brain injury history. J Rehabil Res Dev. 2012. 49: 971-84
17. Rohling ML, Binder LM, Demakis GJ, Larrabee GJ, Ploetz DM, Langhinrichsen-Rohling J. A meta-analysis of neuropsychological outcome after mild traumatic brain injury: Re-analyses and reconsiderations of Binder et al. (1997), Frencham et al. 2005 and Pertab et al. (2009). Clin Neuropsychol. 2011. 25: 608-23
18. Rosenfeld JV, McFarlane AC, Bragge P, Armonda RA, Grimes JB, Ling GS. Blast-related traumatic brain injury. Lancet Neurol. 2013. 12: 882-93
19. Silverberg ND, Iverson GL. Expert panel survey to update the American congress of rehabilitation medicine definition of mild traumatic brain injury. Arch Phys Med Rehabil. 2021. 102: 76-86
20. Smits M, Hunink MG, van Rijssel DA, Dekker HM, Vos PE, Kool DR. Outcome after complicated minor head injury. AJNR Am J Neuroradiol. 2008. 29: 506-13
21. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974. 2: 81-4
22. Vakhtin AA, Calhoun VD, Jung RE, Prestopnik JL, Taylor PA, Ford CC. Changes in intrinsic functional brain networks following blast-induced mild traumatic brain injury. Brain Inj. 2013. 27: 1304-10
23. Vella MA, Crandall ML, Patel MB. Acute management of traumatic brain injury. Surg Clin North Am. 2017. 97: 1015-30
24. Walsh DV, Capó-Aponte JE, Jorgensen-Wagers K, Temme LA, Goodrich G, Sosa J. Visual field dysfunctions in warfighters during different stages following blast and nonblast mTBI. Mil Med. 2015. 180: 178-85